Chapter 5 Evaluating Cosmeceutical Efficiency
INTRODUCTION
Box 5.1 provides an alphabetical listing of the instruments that have been used over the years to noninvasively measure cosmeceutical effects on human skin. This is a very active area of research and there are a number of methods currently being developed.
Box 5.1 Instruments that have been used to noninvasively evaluate the skin of human volunteers
Rather than discuss the various instruments in alphabetical order as they are presented in Box 5.1, a better approach is to start with those methods that measure aspects of the skin that are directly related to how the dermatologist and/or patients evaluate skin condition. That is, primarily to look with their eyes and feel with their fingers. Other instrumental techniques measure properties that cannot readily be appreciated by either visual or tactile means. These include assessments based on physiologic processes such as blood flow or transepidermal water loss rates.
INSTRUMENTAL METHODS THAT ARE RELATED TO VISUAL ASSESSMENTS
• Image analysis
One of the more popular claims currently being made for most cosmeceuticals is that they are ‘antiaging and help restore a more youthful skin’, or words to that effect. One highly desirable outcome of such a treatment would be to reduce the appearance of facial wrinkles, such as those in the crow’s feet region. Although such changes can be documented by standardized clinical photographs, it is more desirable to cast a replica of the skin surface by using a silicone rubber impression material, such as Silflo. Figure 5.1 shows representative specimens obtained from individuals with varying degrees of photodamage; the differences in wrinkle depth are readily appreciated. However, by using optical profilometry one can objectively measure changes in skin surface topography due to effective cosmeceutical treatments. This technique involves computer imaging techniques in which a digitized image is taken of the replica that is illuminated from a fixed low angle. This causes various surface features to be highlighted or shadowed in such a way that a graphic representation of the surface topography can be generated and subsequently analyzed for wrinkling, roughness, and other textural features (Fig. 5.2).
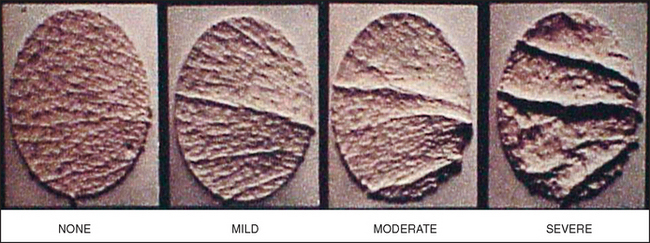
Fig. 5.1 Representative skin specimens obtained from individuals with varying degrees of photodamage
This is but one example of how computerized image analysis can be used to objectively extract quantitative information from images. Box 5.2 provides a listing of some of the more common applications of image analysis that have been used to study skin structure and function. The basic rule seems to be that anything that can be seen by the unaided eye can easily be measured. Moreover, by using specialized lighting techniques such as Wood’s lamp illumination, things that cannot be directly visualized can be detected and measured in the specially created images.
• Skin coloration
The devices that are currently being employed in experimental dermatology, skin pharmacology, toxicology, and cosmetic science to measure skin color changes fall into two distinct types of instrument as shown in Box 5.3. In one category we have the tristimulus colorimeters, which are based on the three-dimensional L*a*b* color space (CIELAB). L*a*b* allows any color to be mathematically described by its hue (position on the color wheel), value (lightness), and chroma (saturation). These would include the Minolta ChromaMeter and the MicroColor of Dr. Bruno Lange GmbH which have seen widespread use for the quantification of erythema in the study of irritant dermatitis due to exposure to detergents, topical corticoid activity in the vasoconstriction test, and for measuring the percutaneous penetration of vasodilators such as nicotinic acid.
Other types of instrument are the DermaSpectrophotometer (Cortex Technology) and the Erythema Meter (Dia-Stron Ltd) which are based on the two-wavelength method of Diffey et al. These instruments emit green and red light and measure the reflected light from the skin surface. Because changes in skin redness will greatly affect the absorption of green light but will have very little effect on that of red light, an erythema index can be calculated. Since increased melanin pigmentation will lead to an increased absorption of both red and green light, a melanin index can be computed in a similar fashion.
INSTRUMENTAL METHODS RELATED TO TACTILE ASSESSMENTS
• Dermal firmness/elasticity
Another characteristic change which is well documented in photoaged skin is the loss of firmness and elasticity due to structural changes in collagen and elastin. Over the years, a wide variety of instruments have been developed to objectively measure the biomechanical properties of the skin. Box 5.4 lists some of the more popular devices. Although there are fundamental differences in how they interact with the skin, the basic approach is the same for all of them, i.e. load the skin in a standard fashion and measure the subsequent deformation. These changes in deformation represent a change in skin elasticity and firmness, which can be altered by cosmeceuticals.
INSTRUMENTAL METHODS BASED ON PHYSIOLOGIC PROCESSES
• Transepidermal water loss
Another physiologic process that has been extensively measured with instrumentation, which has no visual or tactile counterparts, is transepidermal water loss (TEWL). Measurement of TEWL rates through human skin can be used to noninvasively monitor changes in stratum corneum barrier function (Fig. 5.3). In normal healthy skin, the barrier is quite effective and water loss rates are typically very low. If the barrier is compromised due to pathologic processes or damaged by physical or chemical agents, there will be a corresponding increase in water loss rate that directly relates to the degree of impairment. Conversely, there will be a corresponding decrease in TEWL as the barrier is restored. This means that monitoring changes in TEWL over time not only allows one to evaluate the therapeutic response to different treatments, but also to determine the effectiveness of various prophylactic strategies that might prevent or lessen the injury. Thus, it is not surprising that there is considerable literature dealing with TEWL measurements. Indeed, TEWL measurements were the very first to be reviewed by the Standardization Group of the European Society of Contact Dermatitis.
INSTRUMENTAL MEASUREMENTS BASED ON PHYSICAL PROPERTIES
• Skin hydration
There are many biophysical methods available for measuring the relative hydration state of the stratum corneum. Most, such as those shown in Box 5.5, are based on the electrical properties of the skin surface. It has been shown most notably by Obata and Tagami that the ability of an alternating current to flow through the stratum corneum is an indirect measure of its water content. Higher water content translates into increased electrical conduction.
Barardesca E, Elsner P, Wilhelm K-P, et al, editors. Bioengineering of the skin: methods and instrumentation. Boca Raton: CRC Press, 1995.
Diffey BL, Oliver RJ, Farr PM. A portable instrument for quantifying erythema induced by ultraviolet radiation. British Journal of Dermatology. 1984;111:663–672.
Elsner P, Barardesca E, Maibach H, editors. Bioengineering of the skin: water and the stratum corneum. Boca Raton: CRC Press, 1994.
Elsner P, Barardesca E, Wilhelm K-P, et al, editors. Bioengineering of the skin: skin biomechanics. Boca Raton: CRC Press, 2002.
Fluhr J, Elsner P, Barardesca E, Maibach HI, editors. Bioengineering of the skin: water and the stratum corneum, 2nd edn. Boca Raton: CRC Press. 2005:275–285.
Grove GL. Dermatological applications of the Magiscan image analyzing computer. In: Marks R, Payne PA, editors. Bioengineering and the skin. Lancaster: MTP Press; 1981:173–181.
Grove GL. Techniques for substantiating skin care product claims. In: Kligman AM, Leyden JJ, editors. Safety and efficacy of topically applied drugs and cosmetics. New York: Grune & Stratton; 1982:157–176.
Grove GL. Design of studies to measure skin care product performance. Bioengineering and the Skin. 1987;3:359–373.
Grove GL, Grove MJ. Objective methods for assessing skin surface topography noninvasively. In: Leveque JL, editor. Cutaneous investigation in health and disease. New York: Marcel Dekker; 1989:1–31.
Grove GL, Zerweek C. Hardware and measuring principles: the computerized DermaLab® transepidermal water loss probe. In: Fluhr J, Elsner P, Barardesca E, Maibach HI, editors. Bioengineering of the skin, water and the stratum corneum. Florida: CRC Press; 2005:275–285.
Grove GL, Grove MJ, Leyden JJ. Optical profilometry: an objective method for quantification of facial wrinkles. Journal of the American Academy of Dermatology. 1989;21:631–637.
Grove GL, Grove MJ, Leyden JJ, et al. Skin replica analysis of photodamaged skin after therapy with tretinoin emollient cream. Journal of the American Academy of Dermatology. 1991;25:231–237.
Grove G, Zerweck C, Pierce E. Noninvasive instrumental methods for assessing moisturizers. In: Leyden JJ, Rawlings AV, editors. Skin moisturization. New York: Marcel Dekker; 2002:499–528.
Grove GL, Damia J, Grove MJ, Zerweck C. Suction chamber method for measurement of skin mechanics: the DermaLab. In: Serup J, Jemec JBE, Grove GL, editors. Handbook of non-invasive methods and the skin. 2nd edn. Boca Raton: CRC Press; 2006:593-599. Taylor & Francis Group, London
Kollias N, Stamatas N. Optical noninvasive approaches to diagnosis of skin diseases. Journal of Investigative Dermatology Symposium Proceedings. 2002;7:64–75.
Obata M, Tagami H. A rapid in vitro test to assess skin moisturizers. Journal of the Society of Cosmetic Chemists. 1990;41:235-241.
Pinnagoda J, Tupker RA, Agner T, et al. Guidelines for transepidermal water loss (TEWL) measurement. Contact Dermatitis. 1990;22:164–178.
Serup J, Jemec GBE, editors. Handbook of noninvasive methods and the skin. Boca Raton: CRC Press, 1995.
Shriver MD, Parra EJ. Comparison of narrow-band reflectance spectroscopy and tristimulus colorimetry for measurements of skin and hair color in persons of different biological ancestry. American Journal of Physical Anthropology. 2000;112:17–27.