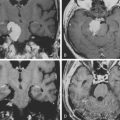
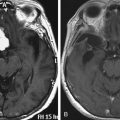
Chapter 6 Cortical and Subcortical Brain Mapping
The first goal of brain surgery, especially in neuro-oncology, is to optimize the extent of resection (EOR) of the lesion. Indeed, maximal resection of glioma, when possible, is currently the first treatment to consider, both in low-grade gliomas (LGGs)1 and in high-grade gliomas.2 In the recent series measuring objectively the EOR on repeated postoperative MRI, all of them supported EOR as a statistically significant predictor of overall survival. In WHO grade II gliomas, when no signal abnormality was visible on control MRI, especially on FLAIR-weighted imaging (i.e., the so-called “complete resection”), patients had a significantly longer overall survival compared with patients having any residual abnormality. Interestingly, even in cases of incomplete tumor removal, patients with a greater percentage of resection had a significantly longer overall survival. In addition to the percentage of resection, the postoperative tumor volume is also a predictor of survival, with a significantly longer overall survival when the residue is less than 10 ml (“subtotal resection”) compared with more than 10 ml (“partial resection”).3 In glioblastomas, it was also shown that the complete removal of the enhanced part of the tumor controlled on postsurgical MRI increased the median survival around 17 months, instead of only 12 months if a residual enhancement was left.2
Consequently, to optimize the benefit-to-risk ratio of surgery, an increasing number of authors used functional mapping methods over the last decade. Indeed, considerable interindividual anatomofunctional variability was demonstrated in healthy volunteers.5 Furthermore, this variability is increased in cases of gliomas, due to cerebral plasticity, explaining why many patients have no or only a mild deficit before surgery, especially in slow-growing tumor such as LGG.6 It is thus mandatory, for every patient, to study the cortical functional organization, effective connectivity, and brain plastic potential, in order to tailor the resection according not only to oncologic but also to cortico–subcortical functional boundaries.
Presurgical Functional Brain Mapping: Advances and Pitfalls
Preoperative Neurocognitive Assessment
Gliomas, especially LGG, are usually revealed by inaugural seizures in young patients who have had a normal life, with no or only a mild neurologic deficit. However, recent extensive neuropsychological examinations have demonstrated that most of patients had cognitive disturbances, especially concerning working memory and executive functions.7 This is the reason why a systematic preoperative neurocognitive assessment is now recommended to search the possible neuropsychological deficit not identified by a standard neurologic examination, to adapt the surgical methodology (e.g., functional mapping under local anesthesia) to the results of this assessment, to benefit from a presurgical baseline allowing a comparison with the postsurgical evaluation, and to plan specific functional rehabilitation.
It is nonetheless puzzling to note that these deficits are not more pronounced, despite the frequent location of LGG in the so-called “eloquent areas.” This can be explained by mechanisms of brain reshaping allowing functional compensation in cases of slow-growing lesions. Indeed, it was shown that cerebral remapping was possible, with a recruitment of perilesional or remote areas within the ipsilesional hemisphere and/or recruitment of contra-hemispheric homologous areas. The recent integration of these concepts into the therapeutic strategy has resulted in dramatic changes in the surgical management of LGG patients, with an increase of surgical indications in eloquent regions classically considered “inoperable”.6
Preoperative Functional Neuroimaging: A Necessary Baseline
However, it is worth noting that FNI methods are not yet reliable enough at the individual scale, despite constant improvement efforts, mainly because the results depend on biomathematical models used for reconstruction. Regarding fMRI, correlations with intraoperative electrophysiology demonstrated that the sensitivity of fMRI was currently only around 71% for movement, and from 59% to 100% for language (specificity from 0% to 97%).8 Such discrepancies can be explained by a neurovascular decoupling in cases of glioma (blood-oxygen–level dependence response in the vicinity of gliomas does not reflect the neuronal signal as accurately as it does in healthy tissue), by inadequate tasks (not adapted to the location of the glioma and/or to the neurologic status of the patient), or to methodological problems (e.g., selection of the threshold). As a consequence, there is a risk of false negative and then to operate a patient without intraoperative mapping, although the glioma is actually located in crucial areas for the function, but not detected by preoperative FNI. Moreover, an erroneous interpretation of brain reshaping (“pseudoreorganization”) can be made. Finally, these methods are not able to differentiate the structures essential for the function, which should be surgically preserved, from those which can be functionally compensated and so potentially resected without permanent deficit. Thus, there is a double risk: first, failure to select a patient for surgery while the tumor was operable, and second, to stop the resection prematurely with a lower impact on the natural history of the glioma (or both).
The recent development of the diffusion tensor imaging (DTI) has also allowed the identification of the main bundles and their location in relation to the tumor. However, this new method needs to be validated before it can be used routinely for surgical planning, especially due to the fact that results of DTI, as FNI, strongly depend on the biomathematical models used for the fiber tracking. Indeed, comparison of distinct fiber-tracking software tools found different results, showing that neurosurgeons have to be cautious about applying tractography results intraoperatively, especially when dealing with an abnormal or distorted fiber tract anatomy. Furthermore, correlations between DTI and intrasurgical subcortical stimulation demonstrated that, despite good correspondence, DTI is not yet optimal for mapping language tracts in patients. Negative tractography does not rule out persistence of a fiber tract, especially when invaded by a glioma.9 Moreover, DTI enables study of the sole anatomy of the subcortical pathways, but not their function.
Intrasurgical Functional Brain Mapping: Toward a Hodotopic View of Brain Processing
Intraoperatively, the integration of multimodal imaging into frameless stereotactic surgery was extensively used in the past decade and referred to as “functional neuronavigation.” However, a randomized trial failed to demonstrate significant impact of navigation on postoperative results.10 It can be explained by the limitations of the presurgical neuroimaging detailed above, as well as to the high risk of intraoperative brain shift, due to surgical retraction, mass effect, gravity, extent of resection (especially for voluminous tumors), and cerebrospinal fluid leakage. Several technical improvements have been proposed to reduce the effects of this shift, but their reliability has still to be optimized: combination with intraoperative ultrasound, producing real-time imaging; use of mathematical models based on data from ultrasonography or digital images that track cortical displacement; and intraoperative MRI. Nevertheless, their actual value on the improvement of EOR and preservation of quality of life remains to demonstrate. As a consequence, invasive electrophysiologic investigations currently remain the “gold standard” when operating in eloquent brain structures.
First, the technique of somatosensory- and motor-evoked potentials was extensively used in the past decades for intraoperative identification of the central region. However, its reliability regarding the localization of the rolandic sulcus is not optimal, with accurate localization of the central sulcus reported only 91% to 94%. Estimation of the overall sensitivity and negative predictive value of this method is around 79% and 96%, respectively. Moreover, phase reversal recording identifies only the central sulcus itself, but offers no direct information on the particular distribution of motor function on the adjacent exposed cerebral structures. Also, whereas the method of motor evoked potentials was improved, when recording compound muscle action potentials, only the monitored muscles can be controlled, that is, there is an inability to detect and possibly avoid motor deficits in nonmonitored muscles. Next, monitoring of muscle action potentials does not mean monitoring of complex movements and action adapted to the environment, which is nonetheless the ultimate goal for the patient. Above all, intraoperative-evoked potentials cannot currently be used to map language, memory or other higher functions crucial for patients’ quality of life (for a review, see ref.11).
Intrasurgical Cortical and Subcortical Electrostimulation Mapping Methods
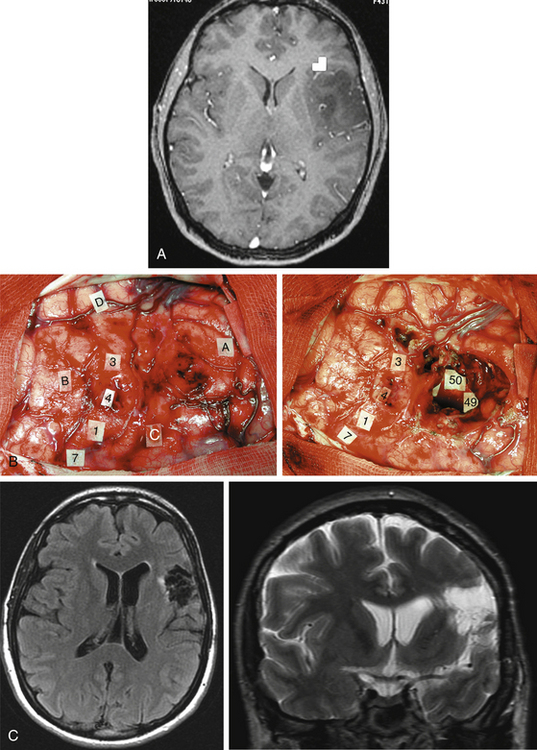
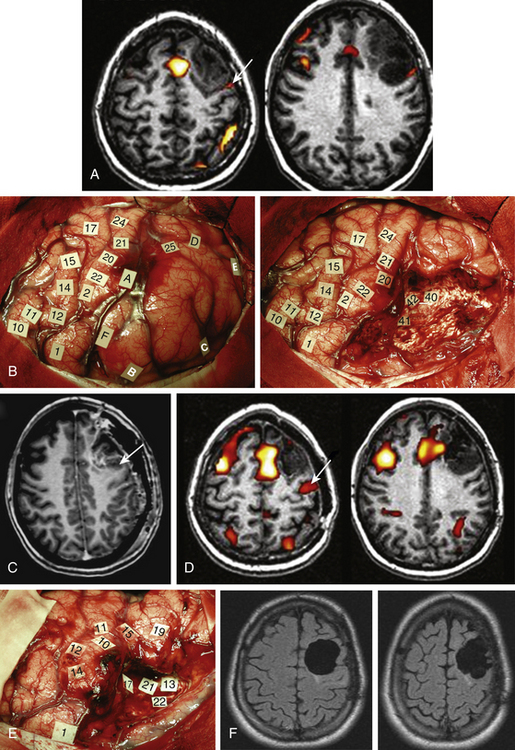
Taking into account the advantages and the limits of these different mapping techniques, more and more neurosurgeons advocate the additional use of intrasurgical electrostimulation mapping (IESM), under general or local anesthesia during surgery in eloquent areas.12,13 Indeed, except for tumors located within the motor structures, the mapping is performed in awake patients. However, as previously mentioned, since movements and action are more complex than single muscle contractions, it is also currently proposed to map the motor function under local anesthesia with active participation of the patient.14 The principle is to use IESM as a focal and transitory virtual lesion to obtain an individual functional map both at cortical and subcortical levels, and to test if a structure involved by a lesion is still crucial for the function—which is, for instance, observed in 15% to 20% of LGG cases. Stimulation of an essential area generates a transient disruption of the task performed by the patient, and this area should be preserved. An individual cortical mapping is thus obtained before the resection, which can be tailored according to functional boundaries (Fig. 6-1). Practically, a bipolar electrode tip spaced 5 mm apart and delivering a biphasic current (pulse frequency 60 Hz, single-pulse phase duration 1 millisecond) is applied to the brain. The current intensity adapted to each patient is determined by progressively increasing the amplitude in 1-mA increments from a baseline of 2 mA until a functional response is elicited, with 6 mA as the upper limit under local anesthesia, and with 16 mA as the upper limit under general anesthesia—with the goal of avoiding the generation of seizures. The patient is never informed when the brain is stimulated. At least one picture presentation without stimulation must separate each stimulation, and no site is stimulated twice in succession to avoid seizures. Each cortical site (size 5 × 5 mm, due to the spatial resolution of the probe) of the entire cortex exposed by the bone flap is tested three times. Indeed, it is admitted nowadays that three trials are sufficient to ensure whether an area is crucial for language, by generating disturbances during its three stimulations, and with normalization of the function as soon as the stimulation is stopped. This limitation of trials and tasks is required by the timing of the surgical procedure, because the patient is awake and can be tired at the end of the resection.
Interestingly, recent series show that the surgical procedure can be simplified by avoiding the use of intraoperative electrocorticography despite an equivalent reliability of electrical mapping and without increasing the rate of seizures.12 However, in cases of stimulation-induced seizures, the use of cold Ringer’s lactate is recommended to abrogate the seizure activity. In addition, some authors emphasized the value of “negative mapping” (no identification of eloquent sites) in the setting of a tailored cortical exposure.13 Although such recommendation is acceptable for high-grade gliomas, since the surgical goal is mainly to remove the enhanced part of the tumor, a negative mapping can be dangerous in surgery of diffuse LGG, especially in nonexpert hands. Indeed, due to the fact that LGG is poorly delineated, the limit of the resection will be essentially guided according to functional criteria. Because negative mapping can be due to false negative for methodologic reasons, it does not guarantee the absence of eloquent sites. In the experience reported by Sanai et al., all four patients with permanent postoperative deficits had no positive sites detected prior to their resections.12 Therefore, other authors continue to promote a wider bone flap, in order to obtain a systematic positive mapping before performing the resection.11,12 Moreover, a positive mapping might also allow an optimization of the EOR, since the resection can be pursued until eloquent areas are encountered, that is, with no margin around the functional structures. A recent study demonstrated that in a consecutive and homogeneous series of 115 LGG in the left dominant hemisphere, the rate of permanent deficit remained lower than 2% despite the absence of margin around the language sites.12
IESM allows the mapping of motor function (possibly under general anesthesia, by inducing involuntary motor response, but also in awake patient by eliciting a disturbance of the movement), somatosensory function (by eliciting dysesthesias described by the patient himself intraoperatively), visual function (by eliciting phosphenes and/or visual field deficit described by the patient), auditivo-vestibular function (by inducing vertigo), language (spontaneous speech, counting, object naming, comprehension, writing, reading, bilingualism, switching from one language to another), and also the mapping of higher-order functions such as calculation, memory, spatial cognition, cross-modal judgment or even emotional processing, by generating transient disturbances if the electrical stimulation is applied at the level of a functional “epicenter.”14 It is crucial that a speech therapist/neuropsychologist/neurologist be present in the operative room, in order to interpret accurately the kind of disorders induced by IESM, for instance speech arrest, anarthria, speech apraxia, phonological disturbances, semantic paraphasia, perseveration, anomia, dysculia, and so on. Thus, IESM is able to identify in real-time the cortical sites essential for the function before the beginning of the resection, in order to both select the best surgical approach and to define the cortical limits of the lesion removal.
Another major issue is the use of subcortical mapping throughout the resection, in addition to the cortical mapping before the lesion removal.11,12 Brain lesion studies have taught that damage of the white matter pathways generated more severe deficit than cortical injury. Therefore, the subcortical tracts subserving motor, somatosensory, visual, auditivovestibular, language, and cognitive functions must be detected during the lesion removal, in order to preserve anatomofunctional connectivity while optimizing the EOR, that is, to pursue the resection until eloquent pathways are detected. Interestingly, according to the same principle as that described at the cortical level, IESM can also identify eloquent subcortical structures. It allows the study of anatomofunctional connectivity by directly and regularly stimulating the white matter tracts and deep gray nuclei throughout the resection, and by eliciting functional response when in contact with deep crucial areas (Fig. 6-1). Furthermore, IESM enables a better understanding of the brain connectivity, showing that dynamic cerebral processing is underlain by parallel distributed and interactive networks, the so-called “hodology.”15 This connectionist view also opens the door to the concept of cerebral plasticity, crucial in LGG surgery.
One of the major advantages of IESM for brain mapping in adult patients is that it intrinsically does not cause any false negatives—if the methodology is rigorously applied as detailed previously. Indeed, IESM is highly sensitive for detecting the cortical and axonal eloquent structures, and it also provides a unique opportunity to study brain connectivity, since each area responsive to stimulation is in fact an input gate into a large-scale network, rather than an isolated discrete functional site. IESM, however, also has a limitation, as its specificity is suboptimal. Indeed, IESM may lead to interpretation that a structure is crucial, due to the induction of a transient functional response when stimulated, whereas this effect is caused by backward spreading of the electrostimulation along the network to an essential area, and/or the stimulated region can be functionally compensated thanks to long-term brain plasticity mechanisms. In brief, although IESM is still the gold standard for brain mapping, due to the risk of “false positives,” its combination with new methods such as perioperative FNI and biomathematical modeling is now mandatory, to clearly differentiate those networks that are actually indispensable to function from those that can be compensated.16
IESM: New Insights into Dynamic Functional Organization of the Brain
Anatomofunctional Organization of Supplementary Motor Area
The supplementary motor area (SMA), namely the frontomesial area located in front of the primary motor area of the inferior limb, is involved in the planning of the movement. Its resection induces the classical “SMA syndrome.” This syndrome is characterized by a complete akinesia and even mutism in cases of lesions of the left dominant SMA, which occurs approximately 30 min following the end of the resection, as observed in awake patients. Then, this syndrome suddenly and spontaneously resolves around the 10th day following surgery, even if some rehabilitation is often needed during 1 to 3 months in order to allow a truly complete functional recovery. Using preoperative fMRI, it has been shown that the occurrence of this syndrome was not related to the volume of the frontal resection, but directly to the removal of a specific structure called the “SMA-proper,” detectable on the preoperative FNI. Thus, on the basis of the presurgical fMRI, it is now possible to predict, before surgery, if an SMA syndrome will occur or not postoperatively, and to inform the patient and his family.17 Moreover, by coupling preoperative fMRI, the pattern of clinical deficit after surgery, and the extent of resection on the postoperative MRI, the existence of a somatotopy within the SMA-proper has been demonstrated—namely, from anterior to posterior: the representation of language (at least in the dominant hemisphere), of the face, then the superior limb, and then the inferior limb (immediately in front of the paracentral lobule). As a consequence, it is also possible to predict before SMA resection the severity and the pattern of the postoperative transient deficit (e.g., only mutism, or mutism and akinesia of the superior limb, or akinesia of the entire hemibody). This has an important impact in planning rehabilitation.
Role of Insula in Language and Swallowing
Although the insular lobe is also frequently involved in tumors, particularly LGGs, this structure was poorly studied over a long period of time for technical reasons. In fact, the insula is an anatomical, cytoarchitectonic, and functional interface between the allocortex and neocortex. Recent FNI studies have enhanced understanding of this multimodal lobe in many functions, in particular in language. Indeed, preoperative fMRI has regularly showed an activation of the anterior insular cortex in the dominant hemisphere during language tasks, as reported in healthy volunteers. Moreover, these results were confirmed by IESM, which induced language disorders, and more specifically articulatory disturbances when applied on the insular cortex, supporting the role of this structure in the complex planning of speech, as previously suggested in stroke studies.18 These data have important implications for the neurosurgeon, since in left dominant (frontotemporo) insular lesions, resection carries a high risk of being incomplete. Moreover, following resection of LGG involving the right nondominant insulo-opercular structures, the induction of a transient Foix-Chavany-Marie syndrome can be observed, that is, a bilateral facio-linguo-pharyngo-laryngal palsy, with a reversible inability for the patient to speak and swallow.
Functional Organization of Broca’s Area
Using IESM, it was shown that the classical “Broca’s area” was not basically involved in speech production, but was in fact implied in several language processings: its posterior part (pars opercularis) is more involved in phonological processing, its superior part (pars triangularis) is implied in syntactic processing, and its anterior part (pars orbitaris) is more involved in a large semantic network that overlays the inferior fronto-occipital fasciculus.19 Interestingly, these results provided by IESM are in accordance with those obtained using fMRI, as shown in a recent meta-analysis of the literature.5
Role of Premotor Cortex in Language
Although many studies have allowed a better clarification of the implication of this structure in motor function, its participation in language remains poorly understood. Interestingly, it has demonstrated using IESM that stimulation of the dominant dorsal premotor area (namely the structure lateral to the SMA, in front of the primary motor area of the hand), induced anomia when stimulated. On the other hand, stimulation of the dominant ventral premotor cortex regularly elicited anarthria.12 These results give strong arguments in favor of the involvement of the dorsal premotor cortex in the naming network, in accordance with fMRI studies which have suggested that this region could participate to lexical retrieval and that its engagement might be related to conceptual category; and the involvement of the ventral premotor cortex in the planning of articulation, explaining why lesion studies have reported that damage of the “lower motor cortex” induced speech apraxia (i.e., aphemia).
Functional Organization of Wernicke’s Area
Concerning lesions located within dominant temporal posterior areas, tasks adapted to test comprehension during IESM have been developed. For instance, a triad of pictures is shown, and the patient is asked to pair them by naming two pictures with conceptual links, such as a pyramid and a palm tree. Interestingly, several sites within the posterior part of the superior temporal gyrus specifically elicited an anomia without comprehension disorders when stimulated, although other sites within the same gyrus elicited only comprehension disorders with preservation of the ability to name, and other areas generated only phonological disturbances. These results give some arguments in favor of the complexity of the functional organization of Wernicke’s area (in accordance with fMRI results) with its participation not only in comprehension, but also in naming phonological processing.5
Role of the Right Supramarginal Gyrus and Posterior Temporal Areas in Spatial Awareness
The use of a line bisection task during awake surgery in patients with a lesion involving the right parietotemporal junction enables the mapping the areas involved in the spatial awareness. A significant rightward deviation is usually observed during the stimulation of the anteroinferior part of the supramarginal gyrus and the caudal part of the superior temporal gyrus. In other words, a transient and reproducible left neglect is induced by electrical inactivation of cortical sites essential for the visuospatial integration, at the level of the right parietotemporal junction. If these eloquent areas are preserved, the patients show no signs of neglect when examined a few days after surgery. These findings demonstrate that the supramarginal gyrus and the caudal part of the superior temporal gyrus, at least in the right hemisphere, are critical to the symmetrical processing of the visual scene in humans.20
Role of the Left Dorsolateral Cortex in Judgment
For lesions located within the left dominant prefrontal cortex, tasks of cross-modal (visual-verbal) congruent and incongruent judgment have been performed in awake patients. Visual and auditory stimuli were presently simultaneously, both referring to the same item (congruence condition), or not (semantic or phonemic incongruent condition). It was shown that brain areas not involved in naming processing elicited a reproducible deficit of incongruent judgment when stimulated, especially at the level of the left dorsolateral prefrontal region, even if an interindividual variability was observed, as for other functions.21 Preservation of such executive functions is essential for the daily life, in particular regarding decision making and planning of complex strategy.
IESM: Study of the Subcortical Connectivity
Subcortical Motor Pathways
In precentral lesion, after detection and preservation of the primary motor cortical areas using IESM, it is also important to detect the corresponding descending motor pathways using subcortical stimulation, and their somatotopy, that is, the different fibers of the corona radiata, with the pyramidal bundles of the lower limb medially, of the upper limb and of the face more laterally. As at the cortical level, these subcortical motor fibers constitute the posterior and deep functional limits of the resection, until the opening of the ventricle. The pyramidal pathways may also be identified at the level of the posterior limb of the internal capsule, in particular in cases of (fronto-temporo-)insular lesion, in which the deep boundaries of the resection are given when subcortical stimulation induce motor responses in the inferior part of the corona radiata up to the superior part of the mesencephalic peduncles.11
Subcortical Somatosensory Pathways
In the same way, the thalamo-cortical somatosensory pathways and their somatotopy can be identified by IESM, which induces dysesthesias in awake patients in cases of retrocentral tumors.11
Subcortical Language Pathways
In left dominant precentral lesion, after identification of the motor and language cortical sites within the pre-rolandic and inferior frontal gyri (Broca’s area), IESM also enables detection of different language pathways.12 Medially, IESM can identify the fasciculus subcallosal medialis (running from the SMA and cingulate gyrus to the head of the caudate nucleus), which elicits transient transcortical motor aphasia during its stimulation; this tract is involved in the initiation of language. Posteriorly, the fibers coming from the premotor ventral cortex must be detected, inducing anarthria when stimulated: this pathway is crucial for speech production. More laterally, the operculo-insular connections should also be detected, by generating a complete speech arrest during stimulation; these connections are particularly involved in speech planning.
In addition to these locoregional language pathways, IESM also allows the detection of long-distance–association pathways, with first of all, the deep part of the superior longitudinal fasciculus, namely the arcuate fasciculus (AF). Indeed, in patients with a lesion involving the left insula and/or left inferior frontal gyrus, it is possible to identify the anterior part of AF, located within the anterior floor of the external capsule (under the superior part of the insula) and also under the posterior part of the so-called Broca’s area (namely the pars opercularis and pars triangularis of the inferior frontal gyrus). Stimulation induces transient symptoms classically observed in conduction aphasia, associating phonemic paraphasia and repetition disorders. In the same way, AF must also be detected at the level of its posterosuperior loop, located under the supramarginal gyrus, in patients operated on for a left posterior parietal lesion. The same symptoms associating phonemic paraphasias and repetition disorders are induced during stimulation. Again, AF is detected in posterior temporal lesions, the posterior part of its posterior funiculus corresponding to the anterior functional limit of the resection. Finally, the anterior part of the anterior funiculus of AF must also have been used as the posterior functional boundary of left dominant anterior and mid-temporal lobectomy.12 Interestingly, AF seems also to subserve a wide network involved in language switching (from a native language to another language or vice versa): IESM can disrupt such function, crucial to detection and preservation in bilingual patients.
In addition to the AF, there is a lateral part of the supero-longitudinal fasciculus. In particular, in patients harboring a left retrocentral supra-sylvian lesion, IESM detects not only the language cortical sites at the level both of the ventral premotor cortex in front of the tumor and of the supramarginal gyrus and/or angular gyrus behind it, but also a fronto-parietal subcortical network inducing speech apraxia when stimulated. This operculo-opercular loop might underlie the anatomofunctional connectivity of the working memory circuit. Indeed, as recently demonstrated by DTI, this loop corresponds to the anterior segment of an indirect pathway of the classical superior longitudinal fasciculus, which runs parallel and lateral to the AF, by connecting Broca’s territory with the inferior parietal lobe. It seems that this tract might be involved in the vocalization of semantic content. Therefore, this example illustrates well that IESM and DTI can be combined in order to better understand the anatomofunctional connectivity of the brain.15
In addition to this “dorsal phonological root,” IESM has provided arguments supporting the likely role of the inferior fronto-occipital fasciculus (IFOF) in the semantic system, that is, the “ventral semantic root.” Indeed, in patients with a lesion involving the frontal structures immediately in front and above the Broca’s area—namely, the pars orbitaris of the left inferior frontal gyrus and the dorsolateral prefrontal area—the anterior part of the IFOF has been identified under these regions, by eliciting semantic paraphasias during subcortical stimulation. In the same way, IFOF has also been detected, by inducing the same symptoms (i.e., semantic paraphasias) when stimulated, in its intermediate part located within the anterior floor of the internal capsule (in front and inferiorly to the AF, and behind and superiorly to the uncinate fasciculus), in surgery for left insular lesion. Again, IFOF has been detected in left temporal LGG, by eliciting semantic disorders when stimulated, and it constituted the deep limit of the resection (above the roof of the temporal horn of the ventricle).19
Interestingly, it was demonstrated that stimulation of the anterior part of the inferior longitudinal fasciculus, in front of the visual word form area (i.e., the basal part of the temporo-occipital junction, involved in high-level visual processing such as reading), as well as stimulation of the uncinate fasciculus, never generated transient language disturbances, and thus could be removed with no aphasia. It seems that this indirect pathway from the temporo-occipital areas to the prefrontal region, with a relay at the level of the temporal pole (temporo-occipital area, inferior longitudinal fasciculus, temporal pole, uncinate fasciculus, orbito-frontal and prefrontal areas) can be compensated by the direct pathways constituted by the IFOF.12
In addition, IESM also allows the mapping of the deep gray nuclei, sometimes invaded by tumors such as LGG. Indeed, stimulation of the head of the dominant caudate in patients harboring a frontomesial LGG coming in contact of the striatum in the depth, generally generates perseverations namely the repetition of the previous item while the next item is presented to the patient. These results give further arguments in favor of an inhibitor role of the caudate in the control of cognition. Equally, it is important to map the lateral part of the dominant lentiform nucleus, at the end of the resection of insular tumors. Lentiform stimulation induces anarthria, supporting the likely role of this structure in the planning of articulation, in association with the insula and ventral premotor cortex.12
Finally, it is also important to use IESM for language, both at cortical and subcortical levels, for lesion involving the right hemisphere in right-handed or even ambidextrous patients, due to the bilateral distribution of language.
Subcortical Pathway Involved in Spatial Awareness
Using a task of line bisection during awake surgery in patients harboring a lesion within the right parieto-temporal junction (as previously described at the cortical level), IESM must also detect the white matter tracts implied in spatial processing, in order to avoid postoperative left neglect. During the stimulation of part II of the superior longitudinal fasciculus, a significant rightward deviation is regularly observed.20 As a consequence, it seems that this parieto-frontal pathway subserves spatial awareness, and that a lesion at its level may generate a permanent left neglect. Stimulation of the right superior longitudinal facsiculus may also induce vertigo by disrupting a large network between the parieto-insular vestibular cortex and the visual and the sensory-motor areas.
In summary, the vision of the neural basis of cognition begins to shift from a localizationist and then an associationist view toward a “hodologic” organization (i.e., dynamic parallel large-scale networks able to compensate themselves). Indeed, from Lichtheim to Geschwind, cognitive functions such as language were conceived in associationist terms of centers and pathways, the general assumption being that visual and auditory linguistic information were processed in localized cortical regions with the serial passage of information between regions through white matter tracts. Currently, an alternative hodologic account is proposed, in which language is conceived as resulting from parallel distributed processing performed by distributed groups of connected neurons rather that individual centers15 In contrast to the serial model of language in which one processing must be finished before the information enters another level of processing, these new models of “independent networks” state that different processing can be performed simultaneously with interactive feedback. Interestingly, the recent methodologic advances in DTI as well as intraoperative cortico-subcortical electrical mapping have enabled direct observation in humans of the anatomofunctional connectivity that underlies linguistic functions, supporting and completing Mesulam’s large-scale neural network model of language. In particular, it seems that there are at least two parallel pathways, namely the dorsal phonologic stream and the ventral semantic stream, which converge to a common final tract allowing speech production. Furthermore, this entire network is modulated by cortico-striato-pallido-thalamo-cortical loops. Of course, it is worth noting that the goal of this new concept is not to substitute the “cortical centers” (topology) with “subcortical pathways” (hodology), but rather to envision the common interactive processing of both gray and white matters (“hodotopy”).15 The next step to progress in the understanding of brain connectivity might be a more accurate analysis of the interactions between the language circuit and networks underlying other cognitive functions, in particular the visuospatial component in which the role of the superior longitudinal fasciculus has been emphasized, as well as emotional and behavioral aspects. Such a multimodality approach seems to represent a unique opportunity to move toward an integrative model of the various functions. In this way, recent advances in biomathematical modeling of the electrophysiologic and hemodynamic signals, which allow a reliable study of the activity time course within the neuronal networks via the analysis of the synchrony (the so-called “chrono-architecture”), may open a new door to the effective connectivity, that is, the influence that one neural system exerts over another.
Consequently, it is crucial for neurosurgeons to improve their knowledge of anatomofunctional connectivity, and thus to integrate more easily and more systematically the concept of subcortical mapping in surgical strategy. This is desirable because the gliomas, in essence, involve both cortical and subcortical structures, and thus they may alter connectivity. Next, lesions of the white matter may elicit more severe permanent deficits than cortical damage. In addition, such a hodologic view may explain why some epicenters considered essential for language in a localizationist model—for instance, Broca’s area—can be in certain conditions involved by a tumor (or even surgically removed) with no aphasia due to a functional compensation within a large distributed network (i.e., so-called brain plasticity).22
IESM: New Insights into Brain Plasticity
As early as the beginning of the 19th century, two opposing perspectives on central nervous system function were suggested. First, the theory of equipotentiality hypothesized that the entire brain, or at least one complete hemisphere, was implied in the practice of a functional task. Conversely, the theory of “localizationism,” in which each part of the brain was supposed to correspond to a specific function, was built following the seminal description of “phrenology.” Progressively, frequent reports of lesional studies led into an intermediate view, namely a brain organized in highly specialized functional areas, called “eloquent” regions (such as the central, Broca’s, and Wernicke’s areas), for which any lesion gives rise to major irrevocable neurologic deficits, and in “nonfunctional” structures, with no clinical consequences when injured. Based on these first anatomofunctional correlations, and despite descriptions by certain pioneers of postlesional recovery, the dogma of a static functional organization of the brain was dominant for a long time, that is, inability to compensate any injury involving the so-called eloquent areas. However, through regular reports of improvement in functional status following damage to cortical and/or subcortical structures considered as “critical,” this view of a “fixed” central nervous system was called into question. Consequently, many investigations were performed, initially in vitro and in animals, and then more recently in humans since the development of FNI, in order to study the mechanisms underlying these compensatory phenomena, known as cerebral plasticity.
Cerebral plasticity can be defined as the continuous processings that allow short-, middle-, and long-term remodeling of neuronosynaptic organization, in order to optimize the functioning of the networks of the brain during phylogenesis, ontogeny, physiological learning and following lesions involving the peripheral as well as the central nervous system. Several hypotheses about the pathophysiologic mechanisms underlying plasticity have been considered. At a microscopic scale, these mechanisms seem to be essentially represented by synaptic efficacy modulations, unmasking of latent connections, phenotypic modifications, synchrony changes, and neurogenesis. At a macroscopic scale, diaschisis, functional redundancies, crossmodal plasticity with sensory substitution and morphologic changes may be involved. Moreover, the behavioral consequences of such cerebral phenomena have been analyzed in humans in the last decade, both in physiology (ontogeny and learning) and in pathology. In particular, the ability to recover after a lesion of the nervous system, and the patterns of functional reorganization within an eloquent area and/or within distributed networks, allowing such compensation have been extensively studied.23
Interestingly, the field of slow-growing cerebral tumors such as LGGs, has demonstrated that large amounts of cerebral tissue could be removed, inside or outside the so-called eloquent areas, with impressive recovery, with no permanent detectable functional consequences.6,22 Such knowledge combined to the use of IESM allows better study of the dynamic reorganization of the eloquent maps induced by LGGs at the individual scale, and thus opens new surgical indications in classically “inoperable” areas.
Preoperative Plasticity
As already mentioned, it could seem surprising that numerous patients harboring a brain tumor, especially an LGG, typically have only mild functional deficits, in spite of the frequent invasion of eloquent structures. This means that these slow-growing lesions have likely induced progressive functional brain reshaping, as suggested by preoperative FNI. Interestingly, reorganization patterns may differ among patients, a very important concept for the neurosurgeon with the goal to optimize both indications of surgery and surgical planning.6,23 Indeed, despite the limitations of the preoperative FNI previously detailed, these methods have shown that three kinds of preoperative functional redistribution are possible in patients without any deficit. In the first, due to the infiltrative feature of gliomas, function still persists within the tumor, thus with a very limited chance to perform a fair resection without inducing postoperative sequelae. In the second, eloquent areas are redistributed around the tumor, thus with a reasonable chance to perform at least a near-total resection despite a likely immediate transient deficit, but with secondary recovery within a few weeks to months. In the third, a preoperative compensation by remote areas already exists within the lesional hemisphere and/or by the contralateral homologous; consequently, the chance of performing a real total resection of this kind of glioma is very high, with only a slight and very transient deficit. Therefore, in cases of brain lesions involving eloquent areas, plasticity mechanisms seem to be based on an hierarchically organized model: first, with intrinsic reorganization within injured areas (indice of favorable outcome); and second, when this reshaping is not sufficient, other regions implicated in the functional network are recruited, in the ipsilateral hemisphere (close and even remote to the damaged area) and then in the contralateral hemisphere if necessary.23
Intraoperative Plasticity
Intraoperative stimulation before any resection has allowed the confirmation of the existence of a functional reshaping induced by brain lesions, notably with a possible remapping of the motor homunculus and also a reorganization of the language sites. Moreover, acute reorganization of functional maps was equally observed during the resection, likely due to the surgical act itself which can generate a locoregional hyperexcitability, as has been demonstrated in head injury.6 Indeed, in several patients harboring a frontal lesion, although stimulation of the precentral gyrus induced motor responses only at the level of a limited number of cortical sites before resection, an acute unmasking of redundant motor sites located within the same precentral gyrus and eliciting the same movements than the previous adjacent sites when stimulated, was observed immediately following lesion removal. Acute unmasking of redundant somatosensory sites was also regularly observed within the retrocentral gyrus in patients operated on for a parietal glioma. Furthermore, it was equally possible to detect a redistribution within a more larger network involving the whole rolandic region, that is, with unmasking of a functional homologous area located in the precentral gyrus for the first cortical representation and in the retrocentral gyrus for its redundancy (or vice versa). Finally, intraoperative mapping also has a prognostic value concerning the postoperative recovery: a positive response means that the patient will recover.
Postoperative Plasticity
The mechanisms of such a plasticity induced by surgical resection within eloquent areas were also studied by performing postoperative FNI once patients had recovered preoperative functional status. In particular, several patients were examined following resection of gliomas involving the SMA, which elicited a transient postsurgical SMA syndrome (see above). Functional MRI showed, in comparison to the preoperative FNI, the occurrence of activations of the SMA and premotor cortex contralateral to the lesion: the contrahemispheric homologous area thus participated to the postsurgical functional compensation.17
IESM: Therapeutic Implications in Oncologic Neurosurgery
Incorporating individual plastic potential in surgical strategy for gliomas, especially LGGs, has the following goals: extend the indications of resection in eloquent structures previously considered inoperable; maximize the extent of glioma removal, by performing the resection according to (dynamic) functional boundaries, and minimizing the risk of postoperative permanent neurologic deficits or even improving quality of life.23
Consequently, several surgical series showed that it was possible to remove LGGs invading the following eloquent brain structures (Fig. 6-2):
• SMA resection: As previously mentioned, all patients recover, and postoperative fMRI has supported functional compensation by the contralateral SMA and premotor cortex as well as by the ipsilesional primary motor cortex.17
• Insular resection: Despite hemiparesis after right insula removal, likely because this region is a nonprimary motor area, and transient speech disturbances following left dominant insula resection, all patients recover—except in rare cases of deep stroke.18 Moreover, it was possible in right nondominant fronto-temporo-insular LGGs involving the deep gray nuclei, to remove the clautrum without any cognitive disorders (despite its suggested role in consciousness), and also to remove the invaded striatum without inducing motor deficit or movement disorders. This compensation can be explained by a recruitment of parallel subcortical circuits such as pallido-luyso-pallidal, strio-nigro-striate, cortico-strio-nigro-thalamo-cortical, and cortico-luysal networks.
• S1 resection: The first results using pre- and postoperative FNI have suggested the possible recruitment of “redundant” eloquent sites around the cavity within the postcentral gyrus. In accordance with IESM data, results show unmasking of redundant somatosensory sites during resection, likely explained by the decrease of the cortico-cortical inhibition. The recruitment of the second somatosensory area or posterior parietal cortex, primary motor area (M1) (due to strong anatomofunctional connections between the pre- and retro-central gyri), and contralateral primary somatosensory area are also possible factors in recovery.
• Resection of the (dominant) parietal posterior lobe can be performed without inducing any sequelae, and even with a possible improvement in comparison to preoperative status, especially using the pointing task.22
• Resection of nondominant M1 of the face: Recovery of the usual transient central facial palsy, with a potential Foix-Chavany-Marie syndrome when the insula is also involved, is likely explained by the disinhibition of the contralateral homologous sites, via the transcallosal pathways.6
• Resection of M1 of the upper limb: On the basis of the existence of multiple cortical motor representations in humans using fMRI and IESM, the compensation of the motor function could be explained by the recruitment of parallel networks within M1, allowing the superior limb area removal, eventually using two consecutive surgeries in order to induce durable remapping following the first one.
• Broca’s area resection: Language compensation may be rooted in the recruitment of adjacent regions, and in particular the pars orbitaris of the inferior frontal gyrus, the dorsolateral prefrontal cortex, and the insula.23
• Temporal language area resection: Language compensation following left dominant temporal resection could be explained by the fact that this function seems to be organized with multiple parallel networks.5 Consequently, beyond the recruitment of areas adjacent to the surgical cavity, the long term reshaping could be related to progressive involvement of (1) remote regions within the left dominant hemisphere, such as the posterior part of the superior temporal gyrus, the pars triangularis of inferior frontal gyrus, or other left frontolateral regions; and (2) the contralateral right nondominant hemisphere due to a transcallosal disinhibition phenomenon.2
Postoperative Functional Mapping: Toward Multiple-Stages Surgical Approach
Postoperative Functional and Oncologic Results
A dramatic improvement of the surgical results was provided by advances in IESM. First, it has been demonstrated that the use of IESM has allowed to significantly increase the surgical indications in eloquent areas which were classically considered as “inoperable.”4
In addition, despite a frequent transitory neurologic worsening in the immediate postoperative period (due to the attempt to perform a maximal tumor removal according to cortico-subcortical functional limits using IESM), leading to a specific functional rehabilitation, more than 98% of patients recovered the same status than before surgery after glioma resection within eloquent brain areas guided by functional mapping, and returned to a normal social and professional life12,13 Even more, at least in LGG, 15% to 20% of patients can improve in comparison to their preoperative neurologic and neuropsychological assessment, and 80% of patients with presurgical intractable epilepsy can benefit from relief of seizures.18 In other words, LGG surgery is currently not only able to preserve brain functions but may also improve patients’ quality of life as demonstrated by extensive neurocognitive assessment performed after the surgical resection. These data support the existence of additional brain plasticity mechanisms occurring after the operation, likely facilitated by a systematic and adapted rehabilitation.23 Interestingly, this rate of less than 2% of sequelae is very reproducible among the teams using IEMS worldwide. In comparison, in series that did not use IEMS, the rate of sequelae ranged from 13% to 27.5%, with a mean around 19% (for a review, see ref. 4).
Finally, a comparative of LGG resection performed without or with IESM showed that the EOR was significantly increased thanks to IESM, along with better functional results following resection within eloquent areas.3,4 Indeed, since IESM allows identification of the cortical and subcortical eloquent structures individually, it seems logical to perform a resection according to functional boundaries. The resection is continued until the functional structures are detected by IESM, and not before, in order to optimize the EOR without increasing the risk of permanent deficit.
Moreover, while extensive resection is still controversial in neuro-oncology, especially concerning LGG, current surgical results support the positive impact of such a “maximal” treatment strategy, with a benefit in the natural history of the tumors that seems to be directly related to the EOR.1–4
Multiple-Stages Surgical Approach and the Role of Serial Mapping
However, the price to pay to obtain such favorable functional results is sometimes to perform incomplete resection of the glioma when the tumor has invaded areas still crucial for the function. A new concept recently proposed is to use postoperative FNI—since it can be easily repeated due to its noninvasive feature—when the patient has totally recovered, in order to compare the new maps to those obtained before surgery. Indeed, even if this method has the limitations detailed previously, subtraction between a pre- and post-operative acquisition may nonetheless show a possible additional functional reshaping due to the (1) resection itself, (2) rehabilitation, and (3) regrowth of the residual tumor (as before surgery).
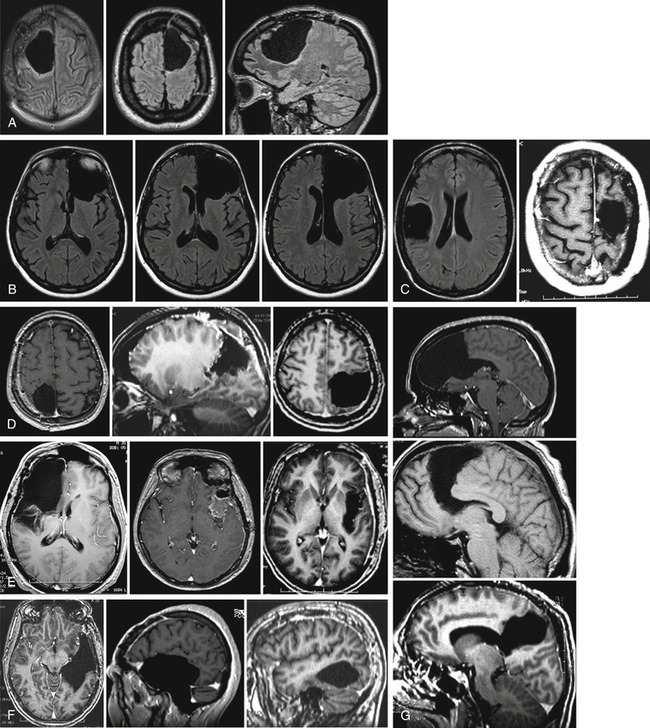
Interestingly, recent series have demonstrated that such remapping was not a theoretical concept, but a concrete reality. Postoperative FNI performed some months or years following the surgery clearly showed a new recruitment of perilesional areas and/or remote regions within the ipsilesional hemisphere and/or a recruitment of contralateral structures.17 On the basis of these data, a second surgery was proposed in patients who continued to lead a normal life, before the occurrence of new symptoms (except possible seizures), only because of an increase in glioma size. The second surgery was also conducted using intraoperative cortical and subcortical mapping, in order to validate the mechanisms of brain reshaping hypothesized but not proven by preoperative FNI, before performing the additional resection24 (Fig. 6-3). Preliminary results supported the efficacy and the safety of such reoperation for LGGs not totally removed during a first surgery, due to their location within eloquent areas. Indeed, in this recent experience, 74% of resections were complete or subtotal (less than 10 ml of residue) following the second operation, despite no additional serious neurologic deficit; on the contrary, there was improvement of neurologic status in 16% of cases. Again, the seizures were reduced or disappeared in 82% of patients with epilepsy before the second operation. The median time between the two operations was 4.1 years, and all patients were still alive with a median follow-up of 6.6 years despite an initial incomplete resection. Therefore, these original data demonstrated that thanks to mechanisms of cerebral plasticity, it is possible to reoperate patients with an LGG involving eloquent areas with minimal morbidity and increased EOR. However, 58% of tumors had already progressed to high-grade glioma at the time of the second surgery, raising the problem of the timing of reoperation. It was thus suggested to “over-indicate” an early reintervention in order to anticipate the second surgery before anaplastic transformation.25
Interestingly, one can currently consider to perform postoperative FNI after rehabilitation and recovery following a second surgery in order to open the door to a possible third or even fourth resection several years after the previous operations. The goal is both to allow the patient to enjoy a normal life as well as to increase overall survival. It is also possible to integrate surgeries within a dynamic therapeutic strategy, including chemotherapy and radiotherapy, especially when a wide removal is not possible for functional reasons.3 To this end, neoadjuvant chemotherapy was recently advocated for LGGs, with the goal of inducing tumor shrinkage before an operation or a reoperation.3
Conclusions and Perspectives
Brain surgery may now benefit from important technical developments in the field of functional mapping, using complementary noninvasive methods of FNI and invasive IESM. Such recent advances have enabled better understanding of the eloquent brain organization for each patient, in order to integrate the concept of inter-individual anatomofunctional variability in surgical strategy. Furthermore, intraoperative real-time subcortical stimulation, in association with cortical mapping, gives a unique opportunity to study the so-called “effective connectivity,” since it allows on-line correlations between discrete and transient “virtual” lesions, which can be performed at each place of a distributed network (each cortical and subcortical sites being perfectly identified anatomically using 3D MRI) and their functional consequences (accurately analyzed by a speech therapist along the surgical procedure). Combination of these intraoperative anatomofunctional data with those provided by DTI (subcortical anatomic data), magnetoencephalography (temporal data), and fMRI (perioperative functional data) could enable one to elaborate individual and predictive models of the functioning of neurono-synaptic circuits, that is, to open a new door to hodotopy.15 Such correlations with IESM, which remains the gold standard regarding functional brain mapping, can also enable to validate the noninvasive method of neuroimaging, especially the new technique of DTI.9
Moreover, such connectionist models may lead to a better knowledge of the dynamic potential of spatiotemporal reorganization of the parallel and interactive networks, namely the mechanisms of brain plasticity, thought to play a major role of functional compensation in slow-growing tumors and in their surgical resection. In this way, individual plastic potential could be better understood using repeated intraoperative mappings combined to postsurgical NFI, and then possibly guiding specific postoperative rehabilitation program in order to optimize the quality of functional recovery.6,22–24
In practice, in order to evolve toward a multistage surgical approach (i.e., second or third surgery more extensive than the first one in cases of initial incomplete resection within crucial areas), a dynamic strategy has to be envisaged for functional neuroimaging.24,25 The goal is to switch from a “static” use of a unique preoperative FNI assessment (limited technique with lack of reliability), to longitudinal studies based on repetition of the FNI before and after surgical resection(s), with the goal to analyze a possible brain reshaping at the individual scale, and to select the candidates to reoperation(s). The next step is now to use biomathematical models able to examine brain functional interaction through effective connectivity, in order to attempt to predict before surgery the patterns of postsurgical remapping at the individual scale on the basis of the data provided by the preoperative FNI.
Desmurget M., Bonnetblanc F., Duffau H. Contrasting acute and slow growing lesions: a new door to brain plasticity. Brain. 2007;130:898-914.
Duffau H. Lessons from brain mapping in surgery for low-grade glioma: insights into associations between tumour and brain plasticity. Lancet Neurol. 2005;4:476-486.
Duffau H. Brain plasticity and tumors. Adv Tech Stand Neurosurg. 2008;33:3-33.
Duffau H. The anatomo-functional connectivity of language revisited: new insights provided by electrostimulation and tractography. Neuropsychologia. 2008;4:927-934.
Duffau H. A personal consecutive series of surgically treated 51 cases of insular WHO Grade II glioma: advances and limitations. J Neurosurg. 2009;110:696-708.
Duffau H. Surgery of low-grade gliomas: towards a “functional neurooncology.”. Curr Opin Oncol. 2009;21:543-549.
Duffau H. Awake surgery for nonlanguage mapping. Neurosurgery. 2010;66:523-528.
Duffau H., Capelle L., Denvil D., et al. Usefulness of intraoperative electrical subcortical mapping during surgery for low-grade gliomas located within eloquent brain regions: functional results in a consecutive series of 103 patients. J Neurosurg. 2003;98:764-778.
Duffau H., Gatignol P., Mandonnet E., et al. New insights into the anatomo-functional connectivity of the semantic system: a study using cortico-subcortical electrostimulations. Brain. 2005;128:797-810.
Duffau H., Gatignol P., Mandonnet E., et al. Contribution of intraoperative subcortical stimulation mapping of language pathways: a consecutive series of 115 patients operated on for a WHO grade II glioma in the left dominant hemisphere. J Neurosurg. 2008;109:461-471.
Duffau H., Lopes M., Arthuis F., et al. Contribution of intraoperative electrical stimulations in surgery of low grade gliomas: a comparative study between two series without 1985-96 and with 1996-2003 functional mapping in the same institution. J Neurol Neurosurg Psychiatry. 2005;76:845-851.
Gil Robles S., Gatignol P., Lehéricy S., et al. Long-term brain plasticity allowing multiple-stages surgical approach for WHO grade II gliomas in eloquent areas: a combined study using longitudinal functional MRI and intraoperative electrical stimulation. J Neurosurg. 2008;109:615-624.
Giussani C., Roux F.E., Ojemman J., et al. Is preoperative functional magnetic resonance imaging reliable for language areas mapping in brain tumor surgery? Review of language functional magnetic resonance imaging and direct cortical stimulation correlation studies. Neurosurgery. 2010;66:113-120.
Krainik A., Duffau H., Capelle L., et al. Role of the healthy hemisphere in recovery after resection of the supplementary motor area. Neurology. 2004;62(8):1323-1332.
Leclercq D., Duffau H., Delmaire C., et al. Comparison of diffusion tensor imaging tractography of language tracts and intraoperative subcortical stimulations. J Neurosurg. 2010;112:503-511.
Mandonnet E., Winkler P.A., Duffau H. Direct electrical stimulation as an input gate into brain functional networks: principles, advantages and limitations. Acta Neurochir (Wien). 2010;152:185-193.
Martino J., Taillandier L., Moritz-Gasser S., et al. Re-operation is a safe and effective therapeutic strategy in recurrent WHO grade II gliomas within eloquent areas. Acta Neurochir (Wien). 2009;151:427-436.
Plaza M., Gatignol P., Cohen H., et al. A discrete area within the left dorsolateral prefrontal cortex involved in visual-verbal incongruence judgment. Cereb Cortex. 2008;18:1253-1259.
Sanai N., Mirzadeh Z., Berger M.S. Functional outcome after language mapping for glioma resection. N Engl J Med. 2008;358:18-27.
Smith J.S., Chang E.F., Lambom K.R., et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol. 2008;26:1338-1345.
Stummer W., Pichlmeier U., Meinel T., et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7:392-401.
Teixidor P., Gatignol P., Leroy M., et al. Assessment of verbal working memory before and after surgery for low-grade glioma. J Neurooncol. 2007;81:305-313.
Thiebaut de Schotten M., Urbanski M., Duffau H., et al. Direct evidence for a parietal-frontal pathway subserving spatial awareness in humans. Science. 2005;309:2226-2228.
Vigneau M., Beaucousin V., Herve P.Y., et al. Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. Neuroimage. 2006;30:1414-1432.
Willems P.W., Taphoorn M.J., Burger H., et al. Effectiveness of neuronavigation in resecting solitary intracerebral contrast-enhancing tumors: a randomized controlled trial. J Neurosurg. 2006;104:360-368.
1. Smith J.S., Chang E.F., Lambom K.R., et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol. 2008;26:1338-1345.
2. Stummer W., Pichlmeier U., Meinel T., et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7:392-401.
3. Duffau H. Surgery of low-grade gliomas: towards a “functional neurooncology.”. Curr Opin Oncol. 2009;21:543-549.
4. Duffau H., Lopes M., Arthuis F., et al. Contribution of intraoperative electrical stimulations in surgery of low grade gliomas: a comparative study between two series without 1985-96 and with 1996-2003 functional mapping in the same institution. J Neurol Neurosurg Psychiatry. 2005;76:845-851.
5. Vigneau M., Beaucousin V., Herve P.Y., et al. Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. Neuroimage. 2006;30:1414-1432.
6. Duffau H. Lessons from brain mapping in surgery for low-grade glioma: insights into associations between tumour and brain plasticity. Lancet Neurol. 2005;4:476-486.
7. Teixidor P., Gatignol P., Leroy M., et al. Assessment of verbal working memory before and after surgery for low-grade glioma. J Neurooncol. 2007;81:305-313.
8. Giussani C., Roux F.E., Ojemman J., et al. Is preoperative functional magnetic resonance imaging reliable for language areas mapping in brain tumor surgery? Review of language functional magnetic resonance imaging and direct cortical stimulation correlation studies. Neurosurgery. 2010;66:113-120.
9. Leclercq D., Duffau H., Delmaire C., et al. Comparison of diffusion tensor imaging tractography of language tracts and intraoperative subcortical stimulations. J Neurosurg. 2010;112:503-511.
10. Willems P.W., Taphoorn M.J., Burger H., et al. Effectiveness of neuronavigation in resecting solitary intracerebral contrast-enhancing tumors: a randomized controlled trial. J Neurosurg. 2006;104:360-368.
11. Duffau H., Capelle L., Denvil D., et al. Usefulness of intraoperative electrical subcortical mapping during surgery for low-grade gliomas located within eloquent brain regions: functional results in a consecutive series of 103 patients. J Neurosurg. 2003;98:764-778.
12. Duffau H., Gatignol P., Mandonnet E., et al. Contribution of intraoperative subcortical stimulation mapping of language pathways: a consecutive series of 115 patients operated on for a WHO grade II glioma in the left dominant hemisphere. J Neurosurg. 2008;109:461-471.
13. Sanai N., Mirzadeh Z., Berger M.S. Functional outcome after language mapping for glioma resection. N Engl J Med. 2008;358:18-27.
14. Duffau H. Awake surgery for nonlanguage mapping. Neurosurgery. 2010;66:523-528.
15. Duffau H. The anatomo-functional connectivity of language revisited: new insights provided by electrostimulation and tractography. Neuropsychologia. 2008;4:927-934.
16. Mandonnet E., Winkler P.A., Duffau H. Direct electrical stimulation as an input gate into brain functional networks: principles, advantages and limitations. Acta Neurochir (Wien). 2010;1152:185-193.
17. Krainik A., Duffau H., Capelle L., et al. Role of the healthy hemisphere in recovery after resection of the supplementary motor area. Neurology. 2004;62(8):1323-1332.
18. Duffau H. A personal consecutive series of surgically treated 51 cases of insular WHO Grade II glioma: advances and limitations. J Neurosurg. 2009;110:696-708.
19. Duffau H., Gatignol P., Mandonnet E., et al. New insights into the anatomo-functional connectivity of the semantic system: a study using cortico-subcortical electrostimulations. Brain. 2005;128:797-810.
20. Thiebaut de Schotten M., Urbanski M., Duffau H., et al. Direct evidence for a parietal-frontal pathway subserving spatial awareness in humans. Science. 2005;309:2226-2228.
21. Plaza M., Gatignol P., Cohen H., et al. A discrete area within the left dorsolateral prefrontal cortex involved in visual-verbal incongruence judgment. Cereb Cortex. 2008;18:1253-1259.
22. Desmurget M., Bonnetblanc F., Duffau H. Contrasting acute and slow growing lesions: a new door to brain plasticity. Brain. 2007;130:898-914.
23. Duffau H. Brain plasticity and tumors. Adv Tech Stand Neurosurg. 2008;33:3-33.
24. Gil Robles S., Gatignol P., Lehéricy S., et al. Long-term brain plasticity allowing multiple-stages surgical approach for WHO grade II gliomas in eloquent areas: a combined study using longitudinal functional MRI and intraoperative electrical stimulation. J Neurosurg. 2008;109:615-624.
25. Martino J., Taillandier L., Moritz-Gasser S., et al. Re-operation is a safe and effective therapeutic strategy in recurrent WHO grade II gliomas within eloquent areas. Acta Neurochir (Wien). 2009;151:427-436.