Chapter 112 Corpus Callosotomy
Indications and Techniques
In 1940, William P. van Wagenen and R. Yorke Herren described a small series of patients with various disruptive lesions of the corpus callosum.1 Their report highlighted the phenomenologic distinction between generalized convulsive seizures in individuals with tumors near the intact callosum and only partial seizures following insidious invasion of the corpus callosum months to years later. The hypothesis that interruption of the corpus callosum could alter the bihemispheric spread of seizure activity in epileptic individuals led to the simple yet elegant conclusion that surgical division of the corpus callosum might confine epileptogenic foci to a single hemisphere and thus ameliorate secondary seizure generalization. At the time of their writing, the practice of excising regions of brain tissue to eliminate seizures had been firmly established, although with varying rates of success.2 Nevertheless, the historical significance of van Wagenen and Herren’s careful observations cannot be overstated. They set the stage for the first corpus callosotomy lesions performed with the intent of stopping seizure propagation to the contralateral hemisphere in individuals with intractable epilepsy, marking a critical advancement in the field of modern epilepsy surgery.
In the 10 cases of corpus callosotomy that constituted van Wagenen and Herren’s landmark report, there was significant reduction in seizure frequency and with few permanent adverse effects.3 Curiously, however, the practice of corpus callosotomy for treatment of epilepsy was rarely used until over 20 years later, when the next case reports of corpus callosotomy in humans appeared and the procedure began to gain acceptance within the neurosurgical community.4–6 The procedure itself has undergone various technical modifications, including total versus partial callosotomy, anterior versus posterior approaches, single versus multistaged operations and even radiosurgical callosotomy.7,8 Furthermore, 21st-century technology for less invasive neurosurgical approaches, such as vagal nerve stimulation,9–11 and the advent of novel antiepileptics have led to reconsideration of the role of surgical corpus callosotomy for patients having intractable seizures without a focally resectable lesion. However, the safety and efficacy of this procedure have been substantiated in over 60 years of empiric and retrospective studies in humans and experimental studies in animals.1,4,6,12–14 Van Wagenen and Herren’s seminal work directly contributed to the first theoretical interpretation of “split-brain” syndromes by Andrew J. Akelaitis,15–17 and eventually culminated in the awarding of a Nobel Prize to the neuropsychologist Roger Sperry for his innovative tachistoscopic experiments on disconnected patients.18 Sperry’s insights subsequently led to more detailed anatomical and physiological studies of callosal function, which have helped shape our contemporary understanding of the functional lateralization of the cerebral hemispheres.
Modern Indications
Corpus callosotomy was developed specifically for treatment of generalized seizures, which can include numerous abnormal movement phases (tonic, clonic, and atonic). Of these variations, drop attacks (both tonic and atonic) remain the primary indication for corpus callosotomy, as repeated studies have shown superior efficacy for this seizure type.7,19–21 Nevertheless, all forms of generalized seizures, including tonic, clonic, tonic-clonic, myoclonic and absence seizures, have been reported to respond favorably to corpus callosotomy.22 In some studies, corpus callosotomy has also been reported to reduce partial seizures as well, although at significantly lower rates. 23 Additionally, callosotomy has been reported to reduce seizures in several childhood epilepsy disorders including Rasmussen’s encephalitis24,25 and Lennox-Gastaut syndrome,26,27 as well as infantile hemiplegia and frontal lobe epilepsy.19
In most modern epilepsy surgery centers, corpus callosotomy is not considered a primary surgical treatment in patients who might benefit from focal resection of epileptogenic regions.28 Controversy remains regarding whether forms of mental retardation should preclude patients from callosotomy,7,12 although intelligence quotient has not been found to significantly predict outcome following callosotomy.29 Patients with crossed dominance have been reported to have less favorable outcomes, presumably because these patients require cortical interconnections for routine function to a more significant extent than those without crossed dominance.19
Preoperative Evaluation
The preoperative assessment of patients considered for corpus callosotomy is comprehensive and should adhere to three general principles. First, seizure intractability30 with multiple anticonvulsants used in combination should be established. Second, a resectable epileptogenic focus should be excluded, since alternative procedures such as lesionectomy or lobectomy have higher seizure control rates. Third, it should be communicated to patients that callosotomy is considered a palliative procedure, and patient expectations and goals for the surgery should be carefully explored. Other specific components of the workup should include a complete physical examination, battery of neuropsychological testing, magnetic resonance imaging of the brain to evaluate structural lesions and corpus callosal anatomy, and EEG with video monitoring of ictal and interictal states.7,19
Surgical Approaches
Rationale
The topographic organization of the corpus callosum follows a bilaterally connected arrangement in the anterior-posterior plane. The anterior portion of the corpus callosum carries fibers connecting frontal cortical regions, including anterior cingulate, premotor, motor and anterior insular areas.10 Secondary generalization is therefore dependent on anterior motor callosal fibers during tonic-clonic and atonic seizures. Posterior fibers transmit somatosensory and visual information from the parietal and occipital lobes as well as information from association cortices.31 Hence, severing these posterior connections can produce a perceptual disconnection syndrome, which is the rationale for preserving the splenium.7 Nevertheless, posterior callosotomy may still be reserved for seizures refractory to anterior section alone.32 Specific patients, such as those with severe, bilateral seizures, may even benefit from initial complete callosotomy, although this has not been proven.7 It should be pointed out that the corpus callosum is one of several midline commissures. Others include the anterior commissure, the dorsal hippocampal commissure, and the massa intermedia of the thalamus. The callosum is the primary pathway through which seizures become secondarily generalized. However, seizure propagation may also be transmitted through these alternative pathways. Presumably, in such cases, these alternate pathways may be the basis for residual seizure activity following callosotomy.
Techniques
Historically, in addition to sectioning the corpus callosum, section of other major commissures have been attempted, including fornicotomy, anterior commissurotomy, and division of the massa intermedia of the thalamus.1,19 Presently, an anterior two thirds callosotomy is the most commonly performed initial procedure.
Patients preparing for surgical callosotomy should be maintained on antiepileptic therapy at preoperative doses. Corticosteroids and appropriate prophylactic antibiotics are given at the beginning of the case.19
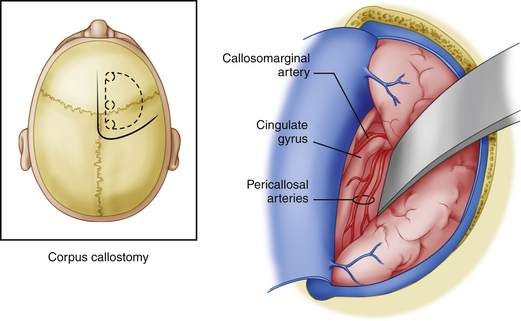
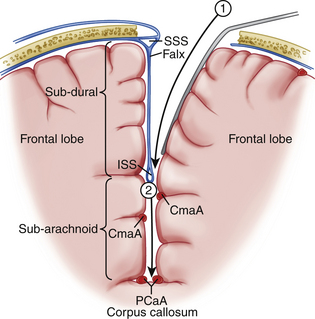
Modern corpus callosotomy techniques are aided by the use of a frameless stereotactic guidance system. This technology assists in precisely mapping the margins of the corpus callosum, especially the rostral-caudal extent. The patient is positioned in the supine position and draped for an incision which will ideally allow for visualization of both genu and splenium without significant brain retraction.7 Various incision shapes have been used, including an S-shape, bicoronal and midline straight incision with a curve toward the side of the craniotomy (preferred by these authors) (Figure 112-1). Lateral decubitus positioning has also been described with gravity assisting in retraction of the dominant hemisphere.19 Most reports use a right-sided approach presuming that this is the language non-dominant hemisphere. Others, including these authors, have used pre-operative Wada tests to determine which hemisphere is non-dominant. These patients have been shown to have a higher than normal incidence of right-sided language function, thereby making a routine right-sided approach somewhat more at risk for postoperative language dysfunction. Up to four burr holes are created, with two parasagittal holes 1 cm from midline, separated by 12 to 15 cm in the anteroposterior plane, and two lateral holes approximately 10 cm from midline.7 This creates a bone flap that gives a direct approach to the interhemispheric fissure with minimal risk of entry into the superior sagittal sinus. The dimensions of the bone flap can be modified with regard to subsequent planned posterior procedures, and can be directed by the frameless stereotactic navigation images. Dural incision is made in relation to the arrangement of draining bridging veins, which should be preserved to reduce the possibility of venous infarction. An acceptable exposure should maintain a 1.5- to 2-cm window between bridging veins.7 The superior sagittal sinus should serve as the dural hinge. Brain relaxation can be achieved using standard techniques such as positioning, CSF drainage during arachnoid dissection, and ventilatory control.19
For a partial anterior callosotomy, dissection should begin at the posterior border of the planned resection and proceed anteriorly. The posterior border of resection typically lies within 5 cm from the genu. Some authors also utilize electrical stimulation to map the interhemispheric lower limb motor cortex in estimating the posterior edge of resection, although use of frameless stereotactic guidance makes this less necessary and allows the anesthesiologist to maintain paralysis throughout the microsurgical portion of the procedure.7 In complete callosotomy, visualization of arachnoid covering the vein of Galen and internal cerebral veins establishes the posterior edge of resection. Interhemispheric dissection can be accomplished with self-retaining retractors (Figs. 112-1 and 112-2). When an incomplete falx is encountered, distinct pia and arachnoid layers may not be discernible. In such cases, it is necessary to develop a plane between the cingulate gyri. Complete preservation of one cingulate gyrus with mild retraction damage to the other is preferable to bilateral damage with resultant neurologic deficits. Care should be taken to complete the dissection below the cingulate, as one can mistake a fused cingulum for the callosum.7 This can be avoided with staged use of the frameless stereotactic navigation system which should be very accurate with respect to depth of the callosum even when there is mild sagging and retraction of the superficial brain structures. In addition, experienced neurosurgeons can identify the glistening, white, and relatively avascular corpus callosum. While the dissection is typically accomplished between the paired pericallosal arteries (Fig. 112-3), anatomic variations such as fused or densely adherent arteries can make dissection lateral to both arteries the preferred approach. However, one must be aware of delicate perforators extending from the cingulate gyrus.7 The callosal transection is typically carried out under the microscope using a combination of microaspiration and bipolar electrocautery.19 The inferior edge of dissection should ideally extend to the ependyma but remain extraependymal as to avoid entering the ventricular system. However, complete preservation of the ependymal layer is rarely possible. Complete transection of all commissural fibers should take precedence over preservation of the ependymal layer. The anterior cerebral arteries and anterior commissure typically define the anterior and inferior borders of resection, respectively. Here one encounters anterior cerebral artery perforators, which should be preserved to prevent septal infarcts.7
Surgical Morbidity
Postoperative Complications
Overall, the morbidity of callosotomy is low and is similar to other surgical epilepsy procedures.7,19 Reports of surgical complications, including epidural hematoma, infection, and hydrocephalus are as high as 25% in some series; however, these rarely produce permanent effects.9,10,33 Even more debilitating sequelae such as impaired memory,20 onset of new seizure types,34,35 and neurologic deficits9 are reported as temporary by most authors, usually lasting only a few weeks. High rates of satisfaction as rated by families—83% in one series—are reported after callosotomy.20,36 Deaths are extremely rare occurrences and are usually remote from surgery.7,9,19,22,32,36
Disconnection Syndrome
A definable split-brain or disconnection syndrome associated with callosotomy, especially complete, has been described by authors since the mid-20th century when the procedure was first developed.1,15–17 The syndrome is so named because of observed episodes in which movement and/or perception of stimuli appears to be under the control of one hemisphere without apparent awareness within the opposite hemisphere. For example, in alien hand syndrome (AHS), the nondominant hand may reach for objects or manipulate items without conscious control.37,38 An interesting constellation of disconnection syndromes exist, including SMA syndrome, AHS, dichotic listening suppression, tactile dysnomia, hemispatial neglect, nondominant hand agraphia, alexia without agraphia, and tachistoscopic visual suppression.39 Even in chronic disconnection syndromes, with the exception of AHS, motor or perceptual deficits are not clinically apparent and manifest only with detailed laboratory testing. Nevertheless, disconnection syndromes are reportedly less common following anterior than posterior callosotomy.38,40 Moreover, acute disconnection of the posterior callosum can result in a more permanent syndrome of interhemispheric sensory dissociation.19,32 Some authors have found that staging of complete callosotomy to allow for improvement in postoperative neurologic deficits can reduce long-lasting disconnection responses.23
Language Impairment
Various aphasic states have been observed following callosotomy. Sass studied language impairment in patients undergoing callosotomy and noted two distinct forms. In three right-handed, right hemisphere–dominant patients, agrammatism and dysgraphia were observed following callosotomy. In a fourth patient who was left-handed and left hemisphere–dominant, postoperative dysgraphia with relative sparing of speech was observed.41 Sussman also described a distinct case of buccofacial apraxia causing transient mutism in a patient following complete callosotomy and hippocampal commissurotomy.42 Some reports of postoperative language impairment, including mutism, are difficult to interpret because there is a lack of preoperative language lateralization (Wada tests not performed). Thus, impairments consistent with supplementary motor syndrome with hemiparesis and mutism may result if the right hemisphere is retracted in a right hemisphere–dominant patient. This speaks to the importance of preoperative language lateralization in these patients.
Outcomes
Retrospective analyses have consistently documented efficacy of callosotomy in reducing generalized seizures, especially drop attacks, since the advent of the procedure. Wilson and colleagues were one of the first groups to link akinetic seizure suppression with corpus callosotomy in the early 1980s.12 Oguni and colleagues also noted improvement in 30 out of 43 patients with drop attacks after anterior callosotomy.29 Nine out of 19 patients were completely cured of drop attacks in Rossi’s study.40 Maehara and colleagues showed a cure rate of drop attacks in 42% of pediatric patients and 25% of adult patients.43 In a subsequent study, they found that 85% of patients with drop attacks in their population achieved seizure cessation or a greater than 90% reduction.20 Twelve of 21 patients experienced cure of drop attacks in Kim’s case series,23 while 1 in 16 patients with drop attacks was cured in Turanli’s report.14 Rates of favorable outcomes following callosotomy for drop attacks were 66% and 92% in Rathore’s and Cukiert’s reports, respectively.21,35 Significant seizure reduction was achieved for generalized tonic-clonic (GTC), absence and myoclonic seizures in all of the abovementioned studies as well. In particular, Jenssen and colleagues found reduction of GTC seizure frequency from 6.3 to 1.1 per month in a cohort of nine patients. Also, two out of five patients with absence seizures were cured and one out of three patients with myoclonic epilepsy experienced greater than 90% reduction in seizure frequency.22 Although rates of no improvement or worsened seizures after surgery were very low in these studies, the mean follow-up of patients was limited to a few years in most cases. In a recent meta-analysis of long-term outcomes, Tellez-Zenteno and colleagues found that only 35% of patients in three studies with over 20 patients each and a mean follow-up greater than 5 years were seizure free, signifying that more work in reconciling short- and long-term outcomes following callosotomy is needed.
Several authors have drawn conclusions regarding prognostic factors of outcome following callosotomy. In two studies, preoperative intelligence quotient (IQ) was not found to be a significant predictor of seizure reduction after surgery,13,29 suggesting that cognitive function is relatively preserved after callosotomy. This, however, remains a matter of controversy, as other authors have found that mental retardation (i.e., low preoperative IQ) portends a poor prognosis following the procedure.12 While complete callosotomy carries a higher risk of disconnection syndrome, Fuiks and colleagues found that 5 of 10 patients with a previous, unsuccessful partial callosotomy achieved subsequent reduction in seizure frequency.32 Additionally, Maehara and colleagues demonstrated that complete callosotomy is an independent predictor of satisfaction with callosotomy for drop attacks despite additional side effects. Young age was also shown to be predictive of improvement in overall daily function in that same study.20
Conclusions
Corpus callosotomy is a surgical procedure devised over 70 years ago to alleviate debilitating seizures in individuals with intractable epilepsy. Although the procedure has undergone modifications since its inception, the fundamental concept is to divide the callosum in order to inhibit the electrical spread of epileptiform activity from one hemisphere to the other. As an invasive procedure, corpus callosotomy has demonstrated efficacy in reducing generalized seizures of all types, especially drop attacks, which is the main indication for the procedure today. Nevertheless, it remains a palliative procedure,44 and preoperative evaluation should focus on definitively excluding a resectable focus amenable to curative therapies. Current approaches emphasize partial rather than total callosotomy due to long-standing disconnection syndromes that may emerge. Overall, corpus callosotomy results in a high rate of patient satisfaction precisely because it is extremely effective in moderating the most devastating seizure conditions. It should therefore remain a viable treatment option for patients with proven intractable epileptic conditions with no focally resectable or lateralizable lesion.
Akelaitis A. A study of gnosis, praxis, and language following section of the corpus callosum and anterior commissure. J Neurosurg. 1944;1:94-102.
Akelaitis A. Studies on the corpus callosum. II. The higher visual functions in each homonymous field following complete section of the corpus callosum. Arch Neurol Psych. 1941;45:788-796.
Akelaitis A. Studies on the corpus callosum. VII. Study of language functions (tactile and visual lexia and graphia) unilaterally following section of the corpus callosum. J Neuropathol Exp Neurol. 1943;2:226-262.
Andersen B., et al. Corpus callosotomy: seizure and psychosocial outcome. A 39-month follow-up of 20 patients. Epilepsy Res. 1996;23(1):77-85.
Asadi-Pooya A.A., et al. Corpus callosotomy. Epilepsy Behav. 2008;13(2):271-278.
Assal F., Schwartz S., Vuilleumier P. Moving with or without will: functional neural correlates of alien hand syndrome. Ann Neurol. 2007;62(3):301-306.
Berg A.T., Kelly M.M. Defining intractability: comparisons among published definitions. Epilepsia. 2006;47(2):431-436.
Bogen J.E., Fisher E.D., Vogel P.J. Cerebral commissurotomy. A second case report. Jama. 1965;194(12):1328-1329.
Cukiert A., et al. Extended, one-stage callosal section for treatment of refractory secondarily generalized epilepsy in patients with Lennox-Gastaut and Lennox-like syndromes. Epilepsia. 2006;47(2):371-374.
Enam S. History of epilepsy surgery. Pakistan J Neurol Sci. 2002;2(2):129.
Gates J. Corpus callosotomy: its place in modern surgical decision making. In: Miller J.W., editor. Epilepsy Surgery: Principles and Controversies. New York: Informa Healthcare; 2005:552-555.
Jea A., et al. Corpus callosotomy in children and the disconnection syndromes: a review. Childs Nerv Syst. 2008;24:685-692.
Kim D.S., et al. The surgical effect of callosotomy in the treatment of intractable seizure. Yonsei Med J. 2004;45(2):233-240.
Lucas T.II, West G. Corpus callosotomy: indications, surgical procedures, and outcomes. In: Miller J.W., editor. Epilepsy Surgery: Principles and Controversies. New York: Informa Healthcare; 2005:541-551.
Luessenhop A.J., Dela Cruz T.C., Fenichel G.M. Surgical disconnection of the cerebral hemispheres for intractable seizures. Results in infancy and childhood. JAMA. 1970;213(10):1630-1636.
Maehara T., et al. Surgical treatment of children with medically intractable epilepsy—outcome of various surgical procedures. Neurol Med Chir (Tokyo). 1996;36(5):305-309.
Mathews M.S., Linskey M.E., Binder D.K., William P. van Wagenen and the first corpus callosotomies for epilepsy. J Neurosurg. 2008;108(3):608-613.
Nei M., et al. Refractory generalized seizures: response to corpus callosotomy and vagal nerve stimulation. Epilepsia. 2006;47(1):115-122.
Oguni H., et al. Anterior callosotomy in the treatment of medically intractable epilepsies: a study of 43 patients with a mean follow-up of 39 months. Ann Neurol. 1991;30(3):357-364.
Sass K.J., et al. Postcallosotomy language impairments in patients with crossed cerebral dominance. J Neurosurg. 1990;72(1):85-90.
Sussman N.M., et al. Mutism as a consequence of callosotomy. J Neurosurg. 1983;59(3):514-519.
Tomasch J. Size, distribution, and number of fibres in the human corpus callosum. Anat Rec. 1954;119(1):119-135.
Turanli G., et al. Outcome and long term follow-up after corpus callosotomy in childhood onset intractable epilepsy. Childs Nerv Syst. 2006;22(10):1322-1327.
Van Wagenen W., Herren R. Surgical division of commissural pathways in the corpus callosum: relation to spread of an epileptic attack. Arch Neurol Psychiatry. 1940;44:740-759.
Wong T.T., et al. Corpus callosotomy in children. Childs Nerv Syst. 2006;22(8):999-1011.
1. Van Wagenen W., Herren R. Surgical division of commissural pathways in the corpus callosum: relation to spread of an epileptic attack. Arch Neurol Psychiatry. 1940;44:740-759.
2. Enam S. History of epilepsy surgery. Pakistan J Neurol Sci. 2002;2(2):129.
3. Mathews M.S., Linskey M.E., Binder D.K., William P. van Wagenen and the first corpus callosotomies for epilepsy. J Neurosurg. 2008;108(3):608-613.
4. Bogen J.E., Fisher E.D., Vogel P.J. Cerebral commissurotomy. A second case report. JAMA. 1965;194(12):1328-1329.
5. Luessenhop A.J. Interhemispheric commissurotomy: (the split brain operation) as an alternate to hemispherectomy for control of intractable seizures. Am Surg. 1970;36(5):265-268.
6. Luessenhop A.J., Dela Cruz T.C., Fenichel G.M. Surgical disconnection of the cerebral hemispheres for intractable seizures. Results in infancy and childhood. JAMA. 1970;213(10):1630-1636.
7. Asadi-Pooya A.A., et al. Corpus callosotomy. Epilepsy Behav. 2008;13(2):271-278.
8. Eder H.G., et al. Gamma knife radiosurgery for callosotomy in children with drug-resistant epilepsy. Childs Nerv Syst. 2006;22(8):1012-1017.
9. Nei M., et al. Refractory generalized seizures: response to corpus callosotomy and vagal nerve stimulation. Epilepsia. 2006;47(1):115-122.
10. Wong T.T., et al. Corpus callosotomy in children. Childs Nerv Syst. 2006;22(8):999-1011.
11. Rosenfeld W.E., Roberts D.W. Tonic and atonic seizures: what’s next—VNS or callosotomy? Epilepsia. 2009;50(Suppl 8):25-30.
12. Wilson D.H., Reeves A.G., Gazzaniga M.S. “Central” commissurotomy for intractable generalized epilepsy: series two. Neurology. 1982;32(7):687-697.
13. Mamelak A.N., et al. Corpus callosotomy: a quantitative study of the extent of resection, seizure control, and neuropsychological outcome. J Neurosurg. 1993;79(5):688-695.
14. Turanli G., et al. Outcome and long term follow-up after corpus callosotomy in childhood onset intractable epilepsy. Childs Nerv Syst. 2006;22(10):1322-1327.
15. Akelaitis A. Studies on the corpus callosum. II. The higher visual functions in each homonymous field following complete section of the corpus callosum. Arch Neurol Psych. 1941;45:788-796.
16. Akelaitis A. Studies on the corpus callosum. VII. Study of language functions (tactile and visual lexia and graphia) unilaterally following section of the corpus callosum. J Neuropathol Exp Neurol. 1943;2:226-262.
17. Akelaitis A. A study of gnosis, praxis, and language following section of the corpus callosum and anterior commissure. J Neurosurg. 1944;1:94-102.
18. Gazzaniga M.S., Sperry R.W. Language after section of the cerebral commissures. Brain. 1967;90(1):131-148.
19. Lucas T.II, West G. Corpus callosotomy: indications, surgical procedures, and outcomes. In: Miller J.W., editor. Epilepsy Surgery: Principles and Controversies. New York: Informa Healthcare; 2005:541-551.
20. Maehara T., Shimizu H. Surgical outcome of corpus callosotomy in patients with drop attacks. Epilepsia. 2001;42(1):67-71.
21. Rathore C., et al. Outcome after corpus callosotomy in children with injurious drop attacks and severe mental retardation. Brain Dev. 2007;29(9):577-585.
22. Jenssen S., et al. Corpus callosotomy in refractory idiopathic generalized epilepsy. Seizure. 2006;15(8):621-629.
23. Kim D.S., et al. The surgical effect of callosotomy in the treatment of intractable seizure. Yonsei Med J. 2004;45(2):233-240.
24. Rasmussen T., Olszewski J., Lloydsmith D. Focal seizures due to chronic localized encephalitis. Neurology. 1958;8(6):435-445.
25. Rasmussen T. Further observations on the syndrome of chronic encephalitis and epilepsy. Appl Neurophysiol. 1978;41(1-4):1-12.
26. Lennox W. The petit mal epilepsies. JAMA. 1945;129:1069-1074.
27. Gastaut H., et al. Childhood epileptic encephalopathy with diffuse slow spike-wave (otherwise known as “petit mal variant”) or Lennox syndrome. Epilepsia. 1966;7:139-179.
28. Benifla M., et al. Multiple subpial transections in pediatric epilepsy: indications and outcomes. Childs Nerv Syst. 2006;22(8):992-998.
29. Oguni H., et al. Anterior callosotomy in the treatment of medically intractable epilepsies: a study of 43 patients with a mean follow-up of 39 months. Ann Neurol. 1991;30(3):357-364.
30. Berg A.T., Kelly M.M. Defining intractability: comparisons among published definitions. Epilepsia. 2006;47(2):431-436.
31. Tomasch J. Size, distribution, and number of fibres in the human corpus callosum. Anat Rec. 1954;119(1):119-135.
32. Fuiks K.S., et al. Seizure outcome from anterior and complete corpus callosotomy. J Neurosurg. 1991;74(4):573-578.
33. Shimizu H. Our experience with pediatric epilepsy surgery focusing on corpus callosotomy and hemispherotomy. Epilepsia. 2005;46(Suppl 1):30-31.
34. Sorenson J.M., et al. Corpus callosotomy for medically intractable seizures. Pediatr Neurosurg. 1997;27(5):260-267.
35. Cukiert A., et al. Extended, one-stage callosal section for treatment of refractory secondarily generalized epilepsy in patients with Lennox-Gastaut and Lennox-like syndromes. Epilepsia. 2006;47(2):371-374.
36. Andersen B., et al. Corpus callosotomy: seizure and psychosocial outcome. A 39-month follow-up of 20 patients. Epilepsy Res. 1996;23(1):77-85.
37. Assal F., Schwartz S., Vuilleumier P. Moving with or without will: functional neural correlates of alien hand syndrome. Ann Neurol. 2007;62(3):301-306.
38. Geschwind D.H., et al. Alien hand syndrome: interhemispheric motor disconnection due to a lesion in the midbody of the corpus callosum. Neurology. 1995;45(4):802-808.
39. Jea A., et al. Corpus callosotomy in children and the disconnection syndromes: a review. Childs Nerv Syst. 2008;24:685-692.
40. Rossi G.F., et al. Callosotomy for severe epilepsies with generalized seizures: outcome and prognostic factors. Acta Neurochir (Wien). 1996;138(2):221-227.
41. Sass K.J., et al. Postcallosotomy language impairments in patients with crossed cerebral dominance. J Neurosurg. 1990;72(1):85-90.
42. Sussman N.M., et al. Mutism as a consequence of callosotomy. J Neurosurg. 1983;59(3):514-519.
43. Maehara T., et al. Surgical treatment of children with medically intractable epilepsy—outcome of various surgical procedures. Neurol Med Chir (Tokyo). 1996;36(5):305-309.
44. Gates J. Corpus callosotomy: its place in modern surgical decision making. In: Miller J.W., editor. Epilepsy Surgery: Principles and Controversies. New York: Informa Healthcare; 2005:552-555.