Coronary artery stents
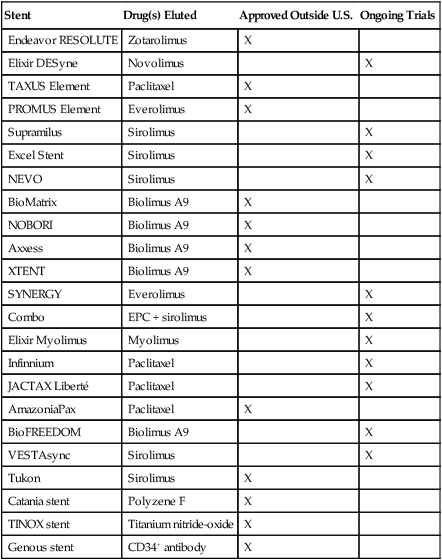
Coronary artery stents were first developed in the 1980s and are now placed in most percutaneous coronary interventions (PCIs). Interventional cardiologists have a wide choice of stents for implantation. The choices range from bare-metal stents (BMSs) and drug-eluting stents (DESs) (Table 144-1) that are widely used in contemporary practice to new stents, such as DESs with novel coatings, biodegradable stents, DESs with biodegradable polymers, DESs that are polymer free, dedicated bifurcation stents, and self-expanding stents. A number of DESs are currently undergoing study or are available outside the United States (Table 144-2).
Table 144-1
U.S. Food and Drug Administration–Approved Drug-Eluting Stents
| Stent | Manufacturer | Drug Eluted |
| Cypher | J&J Cordis | Sirolimus |
| TAXUS Express and Liberté | Boston Scientific | Paclitaxel |
| Endeavor | Medtronic | Zotarolimus |
| Xience V | Guidant, Abbott | Everolimus |
Table 144-2
Metallic Stents Available Outside the United States or Undergoing Clinical Evaluation
| Stent | Drug(s) Eluted | Approved Outside U.S. | Ongoing Trials |
| Endeavor RESOLUTE | Zotarolimus | X | |
| Elixir DESyne | Novolimus | X | |
| TAXUS Element | Paclitaxel | X | |
| PROMUS Element | Everolimus | X | |
| Supramilus | Sirolimus | X | |
| Excel Stent | Sirolimus | X | |
| NEVO | Sirolimus | X | |
| BioMatrix | Biolimus A9 | X | |
| NOBORI | Biolimus A9 | X | |
| Axxess | Biolimus A9 | X | |
| XTENT | Biolimus A9 | X | |
| SYNERGY | Everolimus | X | |
| Combo | EPC + sirolimus | X | |
| Elixir Myolimus | Myolimus | X | |
| Infinnium | Paclitaxel | X | |
| JACTAX Liberté | Paclitaxel | X | |
| AmazoniaPax | Paclitaxel | X | |
| BioFREEDOM | Biolimus A9 | X | |
| VESTAsync | Sirolimus | X | |
| Tukon | Sirolimus | X | |
| Catania stent | Polyzene F | X | |
| TINOX stent | Titanium nitride-oxide | X | |
| Genous stent | CD34+ antibody | X |
Ulrich Sigwart placed the first stent in 1986. This BMS proved to be effective as a rescue device for patients who were in imminent danger of vessel closure and, thus, reduced the number of patients undergoing emergency coronary artery bypass grafting. However, the risk of subacute thrombotic coronary occlusion hindered the further development of these stents. Coronary artery stenting finally became widely accepted in 1994 after evidence showed that stenting was safe with the use of dual antiplatelet therapy (typically, aspirin and a platelet P2Y12 receptor antagonist). By 1999, the placement of coronary artery stents made up more than 80% of PCIs. The risk of subacute thrombosis remained, and a new iatrogenic problem developed of in-stent neointimal hyperplasia, which resulted in 20% to 30% restenosis rates, stimulating the development of the DES. The risk of stent thrombosis after the placement of either a BMS or DES can be reduced by implementation of platelet P2Y12 receptor antagonist therapy in combination with aspirin therapy. Box 144-1 lists definitions of stents, complications with their use, and related types of therapy.
Complications
Thrombosis, hemorrhage, myocardial infarction, stroke, and contrast nephropathy
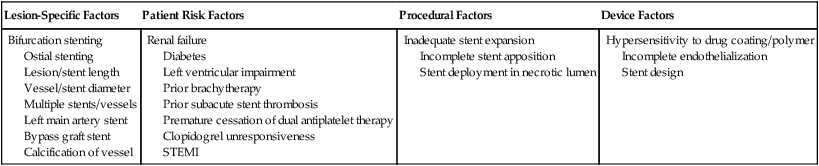
Stent thrombosis has surfaced as the major safety concern following the placement of coronary artery stents in practice today. A review of published data indicates no difference between DES and BMS in terms of risk of early or late stent thrombosis (0.1% and 0.9%, respectively). However, the risk for very late stent thrombosis with a DES is much higher than with a BMS (0.6-0.7% vs. 0.0-0.2%, respectively). The data also indicate that the risk of stent thrombosis is higher in patients treated with a DES for off-label use compared with patients treated for on-label use. The exact mechanism remains unclear, but several factors have been implicated. Some risk factors associated with early or late stent thrombosis include early cessation of dual antiplatelet therapy, clopidogrel unresponsiveness, complexity of lesions, multistent implantation, small lesion diameter, and lesions longer than 28 to 30 mm. Risk factors associated with very late stent thrombosis include factors such as renal failure and prior brachytherapy (Table 144-3). Other complications of PCI include hemorrhage, MI, stroke, and contrast-induced nephropathy.
Table 144-3
Risk Factors for Stent Thrombosis
| Lesion-Specific Factors | Patient Risk Factors | Procedural Factors | Device Factors |
| Bifurcation stenting Ostial stenting Lesion/stent length Vessel/stent diameter Multiple stents/vessels Left main artery stent Bypass graft stent Calcification of vessel |
Renal failure Diabetes Left ventricular impairment Prior brachytherapy Prior subacute stent thrombosis Premature cessation of dual antiplatelet therapy Clopidogrel unresponsiveness STEMI |
Inadequate stent expansion Incomplete stent apposition Stent deployment in necrotic lumen |
Hypersensitivity to drug coating/polymer Incomplete endothelialization Stent design |