30 Congestive Heart Failure in the Fetus
I. INTRODUCTION
1. Fetal echocardiography can now diagnose many forms of congenital heart disease and assess the prognosis of cardiac lesions based on their anatomy and presentation in utero.
2. However, the presence of signs of fetal heart failure, such as hydrops or valvar regurgitation, makes the assessment of prognosis more difficult.
3. A tool for this assessment is the cardiovascular profile score, which combines ultrasonic markers of fetal cardiovascular unwellness based on univariate parameters that have been correlated with perinatal mortality.
4. This profile could then become the heart failure score and could potentially be used in much the same way as, and in combination with, the biophysical profile score.
5. This chapter presents a straightforward method for rapid evaluation of the fetus with possible fetal congestive heart failure (CHF).
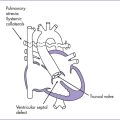
II. FETAL CIRCULATION AND HEART FAILURE
1. Fetal ventricles pump blood in parallel rather than in series.
2. Three shunts (ductus venosus, foramen ovale, and ductus arteriosus) allow the fetal heart to work with two parallel rather than one series circulation.
3. The left ventricle (LV) pumps to the aorta and upper body, and the right ventricle (RV) pumps to the ductus arteriosus and the lower body and placenta.
4. The lungs have a high resistance in utero, and the placenta fulfills the role of oxygenating the blood and ridding the body of wastes.
5. Highly oxygenated blood from the placenta passes to the ductus venosus, where a portion bypasses the liver and passes predominantly to the left atrium (LA).
6. Deoxygenated blood from the upper body passes to the tricuspid valve and then to the ductus arteriosus and lungs.
7. Deoxygenated blood from the inferior vena cava (VC) and the right hepatic veins is directed to the right atrium (RA) and predominantly to the tricuspid valve.
8. The IVC connects directly to the foramen ovale and the superior portion of the atrial septum, the crista dividens, which overlies the IVC, effectively dividing it into two streams.
9. Right and left atrial pressures are almost equal because of the presence of the foramen ovale, and right and left ventricular pressures are equal due to the ductus arteriosus.
III. FACTORS AFFECTING PERINATAL CARDIAC OUTPUT
1. Fetal myocardium develops less active tension than the adult’s at similar muscle lengths. Structural differences, such as less T-tubular system and fewer organized myofibrils, are observed, but there are also differences in calcium uptake into the sarcoplasmic reticulum.
2. Decreased sympathetic innervation in the immature myocardium could influence the stress response of the myocardium.
3. Fetal myocytes are smaller, have fewer mitochondria, smaller sarcoplasmic reticulum, fewer myofilaments, fewer α- and β-adrenoceptors, fewer T-tubules, and higher concentrations of DNA, reflecting a larger number of nuclei.
4. Growth or increased work load in the fetus results in hyperplasia of the myocardium, with an increased number of cells. In contrast, after birth, the myocardium increases only by increased cell size or hypertrophy (increased protein content of each cell).
5. In the very immature heart, myofilaments are arranged in a more chaotic way, but they become better organized as gestation advances.
6. The metabolic source of energy for the fetal myocardium is glucose almost exclusively. In adults, fatty acids are the major source of energy for the myocardium.
7. The fetus has a range of heart rates between 50 and 200, at which the stroke volume of the ventricular chambers can adapt to maintain adequate combined ventricular output (CVO) and tissue perfusion. Outside of this range, heart failure will often result.
8. In summary, the major determinant of cardiac output is the afterload of the fetal ventricle. Any influence that raises the impedance to ejection will inversely lower the ventricular stroke volume by the effect on both the systolic and diastolic function of the heart.
V. ETIOLOGY
A. Hydrops fetalis
1. All of these factors contribute to decreased cardiac reserve in response to stress and to a higher susceptibility of the fetus to cardiac failure:
a. The reduced ability of the fetal heart to contract and to generate force.
b. The lower myocardial compliance and the diminished Starling mechanism.
c. The higher dependence of cardiac output on heart rate.
2. All of these factors favor fluid movement out of the capillary into tissue, causing fluid accumulation in fetal tissue:
a. The younger the fetus, the higher its extracellular water content and the lower its tissue pressure.
b. Fluid movement between intravascular and extravascular space is dependent on intra- and extravascular hydrostatic and oncotic pressure and the fluid filtration coefficient.
3. Faced with a fetus with hydrops fetalis, one must first determine if the hydrops is cardiac, inflammatory, or metabolic. There are several possibilities for the cause of heart failure in the fetus after ruling out fetal infection.
VI. MARKERS OF FETAL MORTALITY
The fetal echocardiographic approach to defining the prognosis of fetal CHF.
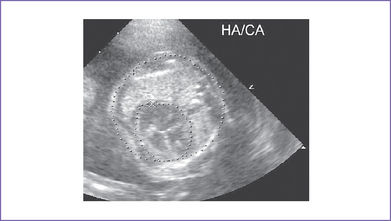
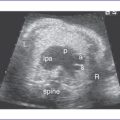
A. Cardiothoracic ratio
VII. VENTRICULAR FUNCTION IN THE FETUS
A. Diagnosis
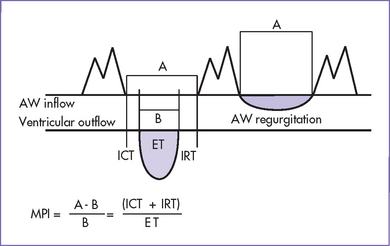
a. Cardiac function can be estimated using the Tei index (myocardial performance index).
b. This is calculated using the filling time of the AV valve and the ejection time of the ventricle.
c. The index is calculated as shown in Figure 30-2.
2. The diagnosis of fetal CHF must be addressed in a clinical fashion similar to that after birth.
3. The classic postnatal clinical tetrad of cardiomegaly, tachycardia, tachypnea, and hepatomegaly has been used in neonates and children. This clinical state in the fetus can be characterized by findings in at least five categories, which are obtained during the ultrasonographic examination.
4. Within specific disease entities, more emphasis is placed on certain areas by the attending physician to predict the prognosis. As always, this information can only account for a portion of the total picture and must be integrated by the attending physician into the diagnostic and treatment plan for the patient.
B. Cardiovascular profile score
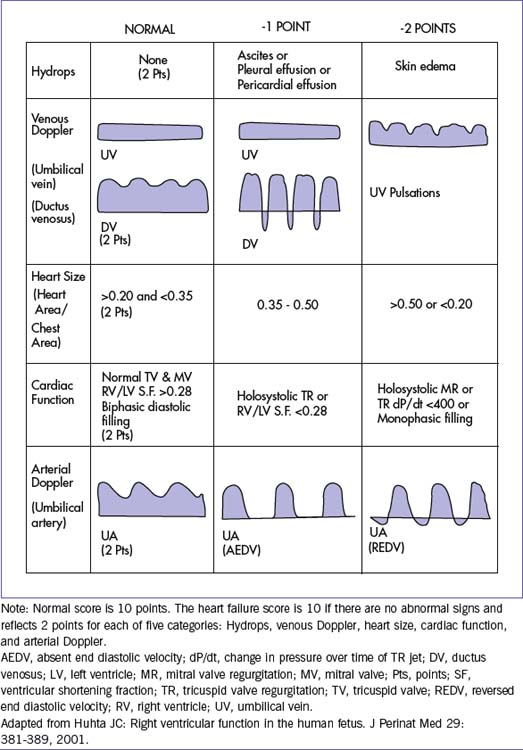
1. Five categories are each worth 2 points in a 10-point scoring system to assess the cardiovascular system. Abnormalities in the cardiovascular profile score can occur before the clinical state of hydrops fetalis. The five categories are:
d. Abnormal myocardial function.
e. Arterial Doppler gives a semiquantitative score of the fetal cardiac well-being and uses known markers by ultrasound that have been correlated with poor fetal outcome (Table 30-1).
2. This profile is normal if the score is 10. Signs of cardiac abnormalities result in a decrease of the score from normal. For example, if there is hydrops with ascites and no other abnormalities, 1 point is deducted for hydrops (ascites but no skin edema), and no points are deducted for the other categories, for a score of 9/10.
3. The cardiovascular profile score (see Table 30-1) is composed of 2 points in each of the five categories used in serial studies to provide a method of uniform physiologic assessment.
a. By taking a multivariate approach, this type of multifactorial score can combine assessment of direct and indirect markers of cardiovascular function.
b. The initial validation of the cardiovascular profile score in hydrops was shown by Falkensammer and colleagues (2001).
c. Hofstaetter and colleagues (2006) measured the cardiovascular profile score in 59 hydropic fetuses.
d. Makikallio and colleagues (2008) studied 75 growth-restricted fetuses.
VIII. MEDICAL TREATMENT OF FETAL HEART FAILURE
A. Diagnosis
1. Echocardiography has long depended on the shortening of the ventricles to assess the systolic function. However, it is known that the shortening is inversely proportional to the afterload of the heart, and intense vasoconstriction and redistribution of flow are the rule in fetal CHF.
2. Better methods for assessing cardiac work, including pressure and flow data, are needed.
a. Fetal dP/dt (change in pressure per change in time) measurement is feasible when valvar regurgitation is present.
b. Doppler assessment of diastolic function may be useful in detecting compromise of the myocardial function.
3. The diagnosis of fetal anemia can be made using the middle cerebral artery peak velocity. With anemia, the cardiac output is increased, with a reduced oxygen-carrying capacity.
B. Intervention
1. Treatment of fetal cardiovascular problems can be classified into five of the most common subgroups based on the etiology of CHF.
a. Abnormal peripheral impedances causing redistribution of flow and growth failure.
b. High output due to anemia, arteriovenous fistula, agenesis of the ductus venosus with connection of the umbilical vein to a systemic vein other than in the liver, or acardiac twin pregnancy.
c. Primary or secondary valvar regurgitation.
d. Heart failure due to myocardial dysfunction.
e. Tachycardia or bradycardia.
2. Interventions aimed at improving the effective cardiac output are also aimed at prolonging the pregnancy and preventing prematurity and prenatal asphyxia.
3. The rapidity with which a disease progresses, determines the urgency with which treatment should occur. This is because the myocardial response to increased wall stress will be either adequate or inadequate depending on the severity and timing and duration of the insult, the coronary perfusion, the nutritional state of the fetus, and the other problems in the pregnancy.
C. Digoxin
1. Treatment with digoxin for evidence of decreased ventricular shortening is controversial.
a. Digoxin is known to decrease the catecholamine response to CHF, and if there is diastolic dysfunction in the fetus, digoxin can improve filling and can lower filling pressures.
b. If the afterload is high, then an increase in oxygen consumption could result from increased inotropy without improved myocardial perfusion.
2. Terbutaline appears to have promise as an inotropic and chronotropic agent, but studies of the possible negative effects on the fetal myocardium are needed. At present, we use digoxin for fetal cardiac failure caused by arrhythmias and high output states such as fistula and anemia when the CVP score is 7 or less.
3. In a recent case of acardiac twinning where the normal fetus was supporting two circulations, digoxin appeared to improve cardiac function and result in a prolonged and successful gestation for the normal twin.
4. Digoxin has been used in such circumstances due to its antiadrenergic benefits and the significant experience that has been gained about its safety in pregnancy.
5. We use lanoxin (Lanoxicaps) 0.2 mg orally two to four times per day based on maternal serum levels. We use a trough level of 1.0-2.0 to avoid any maternal side effects. In fetuses with arteriovenous fistula and heart failure, we also use digoxin to support the heart.
6. When myocardial dysfunction is seen without an obvious cause and fetal infection has been excluded, we consider that an inherited form of cardiomyopathy of either the left or right ventricle can present in utero. We use digoxin for these patients if there is no sign of ventricular ectopy or tachycardia.
D. Dexamethasone
1. In pregnancies where the mother has significant levels of anti-Rho and anti-La antibodies, we recommend dexamethasone 4 mg daily if there are signs of valvar regurgitation, heart block, valvulitis, myocardial dysfunction, myocardial echogenicity, or effusion.
2. Early use of this medication can prevent progression of heart block and myocardial injury.
E. Other therapy
1. The usual treatment of placental dysfunction is designed to improve the vascular impedance of the placenta and to increase the flow of oxygenated blood to the fetus.
2. With bed rest, improved nutrition or maternal oxygen, placental function can improve.
3. Tocolytic medications might relax the placenta and improve its function.
4. Myocardial support for advanced growth restriction has not been proposed, partly because the validation of diagnostic methods is lacking.
a. Studies of ventricular ejection force in growth restriction have shown that both ventricles have decreased ejection force.
b. Advanced heart failure in this setting, with severely decreased arterial PaO2 and poor nutrition, are manifested by nonspecific signs of increased RV and RA size, atrial reversal in the venous Doppler pattern, and altered forward flow velocities.
5. Laser treatment of the twin-to-twin communications or cord ligation with acardiac twins can be applied to improve cardiac failure.
6. With anemia, it is possible to transfuse the fetus via the umbilical vein.
7. When there is cardiomegaly (see earlier for criteria), it is rational to use transplacental treatment of the fetus to support the myocardium if the pregnancy will be continuing long enough to get medication to therapeutic levels.
8. When fetal valvar regurgitation is present on a congenital basis, it could be useful to decrease the afterload of the fetal ventricles as is done in infants with a similar problem. However, medications that reduce the afterload, such as angiotensin-converting enzyme (ACE) inhibitors, are known to be dangerous to the fetus in pregnancy. Reduction of catecholamine levels could have a similar effect, and digoxin could be useful in this situation.
IX. TAKE-HOME MESSAGE
1. The challenge in diagnosing fetal heart failure can be summarized as the difficulty in knowing how well the fetal myocardium is performing under changing loading conditions.
2. By combining information from the obstetrics and cardiology evaluations, the perinatal cardiologist can assess the likelihood that the function abnormality is transient or permanent.
3. The etiology cannot always be known, but the differential between infectious, inherited, congenital, or toxic causes can be tested.
4. The cardiovascular profile score can be used to identify a cardiac cause of hydrops and to grade the severity of fetal heart failure.
5. Serial studies using the cardiovascular profile score are necessary to derive value from this test.
Falkensammer CB, Paul J, Huhta JC. Fetal congestive heart failure: Correlation of Tei-Index and cardiovascular-score. J Perinat Med. 2001;29:390-398.
Gudmundsson S, Huhta JC, Wood DC, et al. Venous Doppler ultrasonography in the fetus with non-immune hydrops. Am J Obstet Gynecol. 1991;164:33-37.
Hofstaetter C, Hansmann M, Eik-Nes SH, et al. J Matern Fetal Neonatal Med. 2006;19(7):407-413.
Huhta JC. Right ventricular function in the human fetus. J Perinat Med. 2001;29:381-389.
Johnson P, Sharland G, Allan LD, et al. Umbilical venous pressure in nonimmune hydrops fetalis: Correlation with cardiac size. Am J Obstet Gynecol. 1992;167:1309-1313.
Makikallio K, Rasanen J, Makikallio T, et al. Human fetal cardiovascular profile score and neonatal outcome in intrauterine growth restriction. Ultrasound Obstet Gynecol. 2008;31(1):48-54.
Makikallio K, Vuolteenaho O, Jouppila P, Rasanen J. Association of severe placental insufficiency and systemic venous pressure rise in the fetus with increased neonatal cardiac troponin T levels. Am J Obstet Gynecol. 2000;183:726-731.
Respondek M, Kammermeier M, Ludomirsky A, et al. The prevalence and clinical significance of fetal tricuspid valve regurgitation with normal heart anatomy. Am J Obstet Gynecol. 1994;171:1265-1270.
Respondek M, Respondek A, Huhta JC, Wilczynski J. 2D echocardiographic assessment of the fetal heart size in the 2nd and 3rd trimester of uncomplicated pregnancy. European J Ob-Gyn Repro Bio. 1992;44:185-188.
Sharif DS, Huhta JC, Moise KJ, et al. Changes in fetal hemodynamics with terbutaline treatment and premature labor. J Clin Ultrasound. 1990;18:85-89.
Tulzer G, Gudmundsson S, Rotondo KM, et al. Doppler in the evaluation and prognosis of fetuses with tricuspid regurgitation. J Matern Fetal Invest. 1991;1:15-18.
Tulzer G, Gudmundsson S, Wood DC, et al. Doppler in non-immune hydrops fetalis. Ultrasound Obstet Gynecol. 1994;4:279-283.