57 Congestive Heart Failure
• Acute decompensated heart failure can be manifested as volume overload, acute diastolic dysfunction, and low cardiac output.
• Identifying and addressing the precipitants of the decompensation are as important as treating the decompensation itself.
• Biomarkers such as B-type natriuretic peptide and the inactive N-terminal fragment of B-type natriuretic peptide can assist in making the diagnosis, but it is important to understand the limitations of these tests.
• In patients with volume overload, diuretics remain the cornerstone of therapy.
• Not all patients with acute decompensated heart failure are significantly volume-overloaded; overaggressive diuresis risks hypotension and worsening renal function.
• Nitrates are first-line therapy for patients in whom an acute reduction in cardiac preload and afterload is desired.
• Inotropic therapy should not be routinely instituted unless the patient is in cardiogenic shock.
• For patients in respiratory distress, noninvasive support (continuous positive airway pressure or bilevel positive airway pressure) may reduce the need for endotracheal intubation.
Epidemiology
With the aging of the U.S. population and improved survival after myocardial infarction, the prevalence of heart failure is on the rise.1 At the same time, advances in medical therapy are allowing patients with heart failure to live longer. In 2008, 5.7 million Americans were estimated to have heart failure, with approximately 670,000 new cases diagnosed that year. Heart failure contributes to nearly 300,000 deaths per year, and costs associated with the treatment of heart failure exceed $30 billion annually. Heart failure accounts for nearly 1 million inpatient admissions per year and represents the primary reason for hospitalization in the growing elderly population. Approximately four of every five patients hospitalized for heart failure initially come to the emergency department (ED) for treatment.
Evidence-based literature for ED management of heart failure lags behind that of other emergency conditions, such as acute coronary syndrome and stroke. The number of large, randomized controlled clinical trials is small, and most practice guidelines, such as those from the Heart Failure Society of America and the European Society of Cardiology, rely heavily on consensus statements.2,3 A recent American Heart Association scientific statement highlighted the significant gaps in knowledge and the lack of evidence-based approaches to the management of heart failure in the ED.4 In contrast, data from the Acute Decompensated Heart Failure National Registry (ADHERE) and the Acute Heart Failure Global Survey of Standard Treatment (ALARM-HF) registry have provided important insight into the clinical characteristics and actual patterns of care of these patients.5,6
In addition to the paucity of controlled clinical trial data, there remains confusion about terminology. Heart failure refers to the clinical syndrome that can result from any structural or functional cardiac disorder that impairs the ability of the ventricle to fill with or eject blood. Causes of chronic heart failure are numerous and diverse (Box 57.1), but in the United States the majority of cases arise as a consequence of coronary artery disease and long-standing hypertension. The term acute heart failure is reserved for the presence of acute signs and symptoms of heart failure in an individual without previously known structural or functional cardiac disease. Examples of acute heart failure are massive ST-segment elevation myocardial infarction, acute papillary muscle rupture, and fulminant myocarditis. Much more commonly, a patient comes to the ED with worsening symptoms of known chronic heart failure, in common parlance a “heart failure exacerbation.” The term acute decompensated heart failure (ADHF) has been adopted to describe this phenomenon, whereby a patient with an established diagnosis of heart failure experiences increasing signs and symptoms of the disease after a period of relative stability.
Pathophysiology
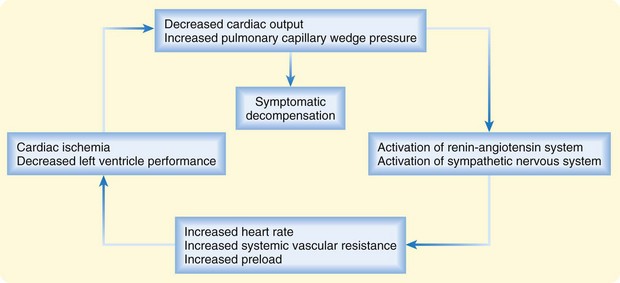
In patients with chronic heart failure, inadequacy of cardiac function sets in motion a common set of compensatory mechanisms based on the Frank-Starling relationship and characterized by elevated sympathetic tone, fluid and salt retention, and ventricular remodeling. These adaptations can allow heart failure to remain stable (or “compensated”) for a time but also provide the final common pathway for decompensation—a downward spiral that can accelerate in response to a particular precipitant or stress (Fig. 57.1). High circulating levels of aldosterone, vasopressin, epinephrine, and norepinephrine can become maladaptive when tachycardia and vasoconstriction compromise intrinsic left ventricular (LV) performance and worsen myocardial oxygen balance. Deteriorating LV function can result in further neurohormonal activation and self-perpetuation of this adverse cycle. Acute decompensation can develop over a period of minutes, hours, or days and can range in severity from mild symptoms of volume overload or decreased cardiac output to frank pulmonary edema or cardiogenic shock.
Although ADHF represents a final common pathway, it is generally triggered by one or more specific precipitants (Box 57.2). Noncompliance with medications or dietary restrictions and myocardial ischemia are believed to be the most common causes of clinical cardiac decompensation. Other cardiovascular precipitants are arrhythmia (atrial fibrillation in particular), acute valvular dysfunction, and hypertensive crisis, but ADHF can also arise as a consequence of noncardiac conditions such as infections, anemia, alcohol withdrawal, uncontrolled diabetes, and thyroid disease.
Presenting Signs and Symptoms
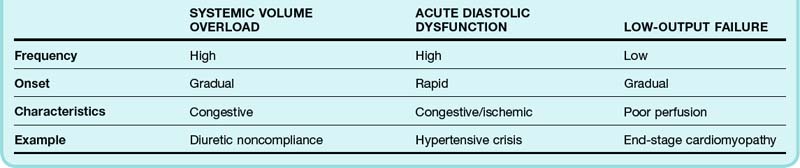
The heterogeneity of the signs and symptoms in patients with ADHF reflects, to some extent, the relative contributions of volume overload, acute diastolic dysfunction, and low cardiac output (Table 57.1). Volume overload, which usually occurs in the setting of medication noncompliance or dietary indiscretion (or both), is classically associated with gradually worsening congestive symptoms. Acute diastolic dysfunction can occur in the setting of myocardial ischemia, tachyarrhythmia, or uncontrolled hypertension and is typically manifested as flash pulmonary edema. Nearly half of all patients admitted to the hospital for ADHF have mild or no impairment in systolic function.5 Overt manifestations of low cardiac output (i.e., hypoperfusion) are not generally seen except in patients with advanced LV dysfunction.
Most patients with ADHF have some degree of dyspnea. However, ADHF can closely mimic many other cardiac, respiratory, and systemic diseases. Historical features such as a history of orthopnea, paroxysmal nocturnal dyspnea, or peripheral edema make the diagnosis of ADHF more likely. The most valuable single piece of historical information to elicit from patients is a previous history of heart failure, myocardial infarction, or coronary artery disease. For example, patients evaluated in the ED because of acute dyspnea are approximately six times more likely to have ADHF if they have previously experienced heart failure (Table 57.2).7
Table 57.2 Sensitivity, Specificity, and Positive Likelihood Ratio of Selected Clinical Findings Associated with Acute Decompensated Heart Failure
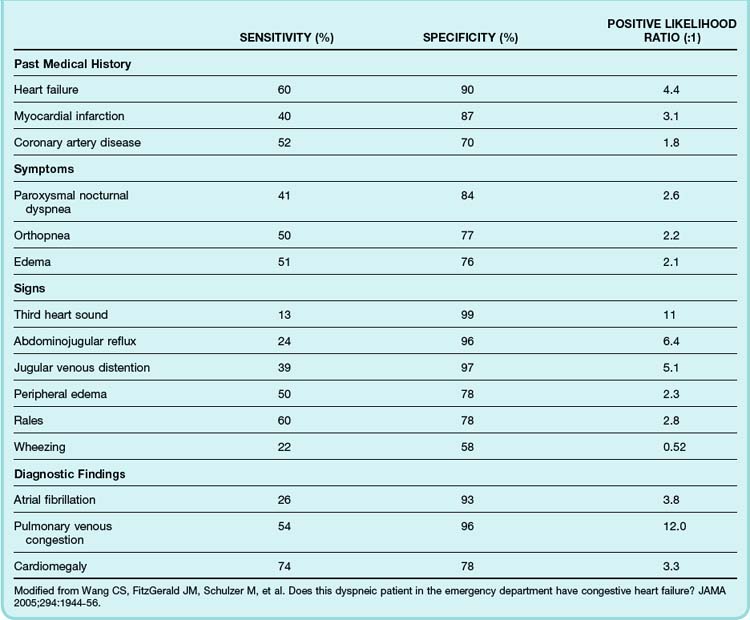
The diagnostic utility of the physical examination has been well studied in the setting of chronic heart failure but less so for ADHF. It should be recognized that in ADHF, physical findings may be misleading because of the rapidly evolving clinical situation. Generally speaking, jugular venous distention, abdominojugular reflux, pedal edema, and an audible third heart sound are specific but insensitive indicators of heart failure, whereas the presence of pulmonary rales has only moderate specificity for heart failure (see Table 57.2).7
Differential Diagnosis and Medical Decision Making
Even before patients reach the hospital, ADHF is associated with significant morbidity and mortality, including malignant arrhythmias and prehospital cardiac arrest. With few exceptions, the safety and efficacy of prehospital interventions have been poorly studied. Prehospital therapy for decompensated heart failure should be undertaken with caution in light of the relatively high number of inaccurate diagnoses made in the field. In as many as 50% of patients with assumed heart-associated respiratory distress, a different condition is diagnosed once they arrive at the hospital. Despite these concerns, evidence suggests that prehospital therapy for presumed heart failure can prevent serious complications and improve survival, particularly for critically ill patients. For example, prehospital use of continuous positive airway pressure (CPAP) in patients with acute pulmonary edema may avert the need for endotracheal intubation.8
Nitroglycerin appears to be the safest and most effective of the prehospital medications used for presumed pulmonary edema.9 The role of other medications for heart failure in the prehospital setting is less clear. Early administration of furosemide appears to have very little benefit and may result in short-term complications. Prehospital use of morphine sulfate for presumed pulmonary edema has been associated with a higher rate of endotracheal intubation, particularly in patients whose condition turns out to have been misdiagnosed in the field.
Diagnostic Studies
B-Type Natriuretic Peptide and NT-Probnp
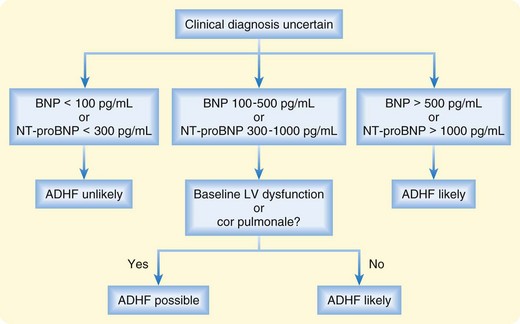
Plasma levels of BNP and NT-proBNP have been shown to be useful in distinguishing between cardiac and noncardiac causes of dyspnea.10,11 Acutely dyspneic patients with plasma BNP levels lower than 100 pg/mL or NT-proBNP levels lower than 300 pg/mL are very unlikely to have ADHF (90% to 99% sensitivity), whereas those with BNP levels higher than 500 pg/mL or NT-proBNP levels higher than 1000 pg/mL are very likely to have ADHF (87% to 95% specificity). Intermediate levels must be interpreted in the clinical context (Fig. 57.2).
In general, emergency physicians (EPs) are about 80% accurate in distinguishing between cardiac and noncardiac causes of dyspnea on clinical grounds. Supplementing clinical acumen with routine BNP or NT-proBNP measurement does increase diagnostic accuracy overall, but as demonstrated in the Breathing Not Properly Multinational Study, the improvement is rather marginal.12 For example, in clear-cut cases, very high or very low values are unlikely to have an effect on diagnosis, whereas in less clear-cut cases, intermediate results are more likely.
BNP and NT-proBNP levels also carry modest prognostic information.13 Although levels at admission correlate only modestly with short-term outcomes, discharge levels are strong independent predictors of death or readmission.
Treatment
Priorities of Treatment
Noninvasive Respiratory Support
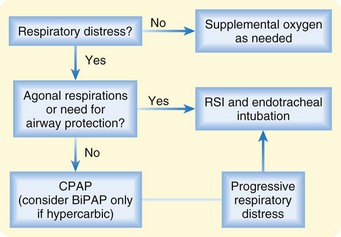
For patients with respiratory distress in whom intubation is not immediately required, noninvasive respiratory support via CPAP or bilevel positive airway pressure (BiPAP) should be instituted (Fig. 57.3). Although the decision to initiate noninvasive respiratory support may depend on a variety of factors, the presumption is that the earlier therapy is instituted, the greater the likelihood of averting intubation. Success also depends on appropriate patient selection. Patients with unstable cardiac rhythms or cardiogenic shock are generally believed to not be candidates for a noninvasive approach. Likewise, in the setting of severe myocardial ischemia or infarction, full ventilatory support may be preferable to decrease myocardial oxygen demand.
CPAP improves lung mechanics by recruiting atelectatic alveoli, improving pulmonary compliance, and reducing the work of breathing. At the same time, particularly in patients with heart failure, CPAP improves hemodynamics by reducing preload and afterload, thereby enhancing LV performance. Pooled data from several randomized, controlled clinical trials suggest that the use of CPAP (at 5 to 10 mm Hg) in patients with respiratory distress caused by ADHF reduces the frequency of endotracheal intubation and may be associated with lower mortality.14
Pharmacologic Therapy
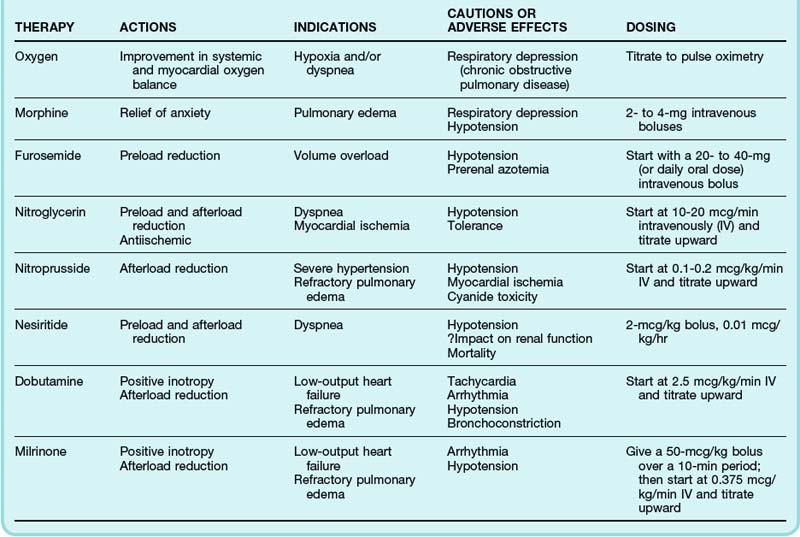
The twin objectives of pharmacologic therapy for ADHF are relief of pulmonary congestion and improvement in systemic tissue perfusion. Strategies to achieve these goals involve reducing preload and enhancing LV function while aiming to maintain or even improve myocardial oxygen balance (Table 57.3).
Diuretics
Depending on a patient’s clinical condition and previous use of diuretics, an initial intravenous (IV) dose of furosemide, 20 to 200 mg, is typically administered. For patients already receiving diuretic therapy, a common strategy is to begin with the usual daily dose given as an IV bolus. A recently published multicenter, prospective, double-blind, randomized trial comparing a strategy of low-dose furosemide (equivalent to the patient’s previous oral dose) versus high-dose furosemide (2.5 times the previous oral dose) in 308 patients with ADHF found no significant differences in patients’ global assessment of symptoms or in the change in renal function over the first 72 hours.15 A high-dose strategy was associated with greater diuresis, but median length of hospital stay was not significantly different. In the same trial, patients randomized to bolus IV therapy (every 12 hours) or the same dose of furosemide delivered via continuous IV infusion had no significant difference in outcome.
Nitrates
Nitrates have been shown to be both safe and effective for the treatment of ADHF, particularly in the context of acute pulmonary edema.16 When compared with placebo therapy in the Vasodilation in the Management of Acute Congestive Heart Failure (VMAC) trial, IV nitroglycerin resulted in better dyspnea scores, but the study was not powered to demonstrate differences in morbidity or mortality.17
Nesiritide
A number of trials have shown nesiritide to be more effective than placebo in improving hemodynamic parameters in patients with ADHF, but it is less clear what, if any, clinically important outcomes are improved. In the VMAC trial, pulmonary capillary wedge pressure was lower in patients receiving nesiritide than in those receiving nitroglycerin, but dyspnea scores at 3 and 24 hours were not significantly different between the two randomized groups.17 Studies have yet to show differences in more durable outcomes, such as of length of hospital stay and hospital costs.
Pooled data from several trials have suggested an association between nesiritide and adverse events, specifically, worsening renal function and death.18,19 In contrast, in a more recent multicenter trial, nesiritide demonstrated no excess adverse effects on either renal function or mortality in patients with ADHF. However, in this same trial, nesiritide failed to meet primary efficacy end points with respect to improvement in dyspnea or 30-day outcomes when compared with placebo.20
Inotropic Therapy
Outside the setting of cardiogenic shock (see later), inotropic therapy is not recommended for the routine management of ADHF. Although short-term inotropic therapy may improve hemodynamic performance and acute symptoms, the impact on outcomes is considerably less sanguine. One of the largest randomized placebo-controlled trials ever conducted in patients with ADHF, the Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF), showed no difference in mortality or readmission rate in patients receiving inotropic therapy but significantly higher rates of adverse events, particularly sustained hypotension and new atrial arrhythmias.21
Investigational Drugs
In the EVEREST trial, tolvaptan, a vasopressin antagonist, improved short-term signs and symptoms in patients hospitalized with ADHF and receiving standard therapy, but this was not a primary end point of the long-term study.22 Tolvaptan was, however, approved by the FDA in 2009 for the treatment of hyponatremia associated with heart failure, among other hypervolemic states.
Ultrafiltration
A novel approach to the problem of volume overload involves ultrafiltration of peripheral blood to remove excess fluid and electrolytes. Though typically reserved for patients with significant renal failure or volume overload unresponsive to diuretics, evidence from the Ultrafiltration vs IV Diuretics for Patients Hospitalized for Acute Decompensated CHF (UNLOAD) trial demonstrated that as an alternative to diuretic therapy, ultrafiltration results in greater fluid loss and lower rates of rehospitalization.23 In the ED, a major limitation of this approach is the feasibility of securing the necessary equipment and intravenous access.
Follow-Up and Next Steps in Care
The vast majority of patients with ADHF evaluated in the ED are admitted to the hospital.5 Discharge from the ED without adequate treatment may be associated with recurrent visits and short-term morbidity and mortality. ADHF is often a dynamic entity: one patient may appear dramatically ill at initial evaluation but respond rapidly to treatment, whereas another patient may experience serious complications after a period of apparent stability. For any individual patient, identifying and addressing the precipitant of the decompensation is critical to making the correct disposition.
The Heart Failure Society of America has established criteria for discharging patients with heart failure from the ED (Box 57.3). However, these guidelines have not been prospectively studied. It should be noted that previously published criteria from the U.S. Agency for Health Care Policy and Research failed to account for more than 30% of 30-day mortality.24 Thus, although published guidelines can assist with triage, the significant rate of morbidity mandates that clinical judgment be incorporated into the decision-making process.
Box 57.3 Heart Failure Society of America Recommendations for Discharge of Emergency Department Patients with Heart Failure
For patients with ADHF admitted to the hospital, inpatient mortality is approximately 4%, and the median length of stay exceeds 4 days.5 In those admitted with advanced stages of heart failure, inpatient mortality approaches 10%. Clinical correlates of major complications or death during hospitalization include hypotension; tachypnea; ECG abnormalities; hyponatremia; renal insufficiency; elevations in troponin, BNP, and NT-proBNP; and poor initial diuresis. However, even patients without any of these risk factors have measurable rates of in-hospital morbidity and mortality. A risk stratification tool derived from the ADHERE registry has been developed to help clinicians determine the risk for mortality in patients with ADHF (Fig. 57.4).25
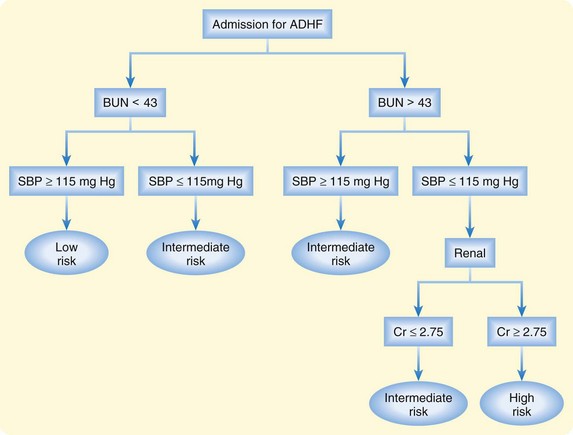
Fig. 57.4 Risk stratification of patients hospitalized for acute decompensated heart failure (ADHF).
BUN, Blood urea nitrogen; Cr, [serum] creatinine; SBP, systolic blood pressure.
Observation Units
ED observation units have been advanced as a safe and cost-effective means of treating a subset of ADHF patients, thereby avoiding the need for hospital admission. Admission to an observation unit allows the ED physician to assess a patient’s response to diuretic (or other) therapy over time. Although interest in this field is growing, no randomized studies have been performed to date to substantiate the use of such units.26
1 Roger VL, Turner MB, on behalf of the American Heart Association Heart Disease and Stroke Statistics Writing Group. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209.
2 Heart Failure Society of America. Executive summary: HFSA 2010 comprehensive heart failure practice guideline. J Card Fail. 2010;16:475–539.
3 The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008. Eur Heart J. 2008;29:2388–2442.
4 Weintraub NL, Collins SP, Pang PS, et al. Acute heart failure syndromes: emergency department presentation, treatment, and disposition: current approaches and future aims: a scientific statement from the American Heart Association. Circulation. 2010;122:1975–1996.
5 Adams KF, Jr., Fonarrow GC, Emerman CL, et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J. 2005;149:209–216.
6 Follath F, Yilmaz MB, Delgado JF, et al. Clinical presentation, management and outcomes in the Acute Heart Failure Global Survey of Standard Treatment (ALARM-HF). Intensive Care Med. 2011;37:619–626.
7 Wang CS, FitzGerald JM, Schulzer M, et al. Does this dyspneic patient in the emergency department have congestive heart failure? JAMA. 2005;294:1944–1956.
8 Hubble MW, Richards ME, Jarvis R, et al. Effectiveness of prehospital continuous positive airway pressure in the management of acute pulmonary edema. Prehosp Emerg Care. 2006;10:430–439.
9 Hoffman JR, Reynolds S. Comparison of nitroglycerin, morphine and furosemide in treatment of presumed pre-hospital pulmonary edema. Chest. 1987;92:586–593.
10 Maisel AS, Krishnaswamy P, Nowak RM, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347:161–167.
11 Januzzi JL, Jr., Camargo CA, Anwaruddin S, et al. The N-terminal Pro-BNP Investigation of Dyspnea in the Emergency department (PRIDE) study. Am J Cardiol. 2005;95:948–954.
12 McCullough PA, Nowak RM, McCord J, et al. B-type natriuretic peptide and clinical judgment in emergency diagnosis of heart failure: analysis from Breathing Not Properly (BNP) Multinational Study. Circulation. 2002;106:416–422.
13 Maisel A, Hollander JE, Guss D, et al. Primary results of the Rapid Emergency Department Heart Failure Outpatient Trial (REDHOT): a multicenter study of B-type natriuretic peptide levels, emergency department decision making, and outcomes in patients presenting with shortness of breath. J Am Coll Cardiol. 2004;44:1328–1333.
14 Vital FM, Saconato H, Ladeira MT, et al. Non-invasive positive pressure ventilation (CPAP or bilevel NPPV) for cardiogenic pulmonary edema. Cochrane Database Syst Rev. 3, 2008. CD005351
15 Felker GM, Lee KL, Bull DA, et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011;364:797–805.
16 Cotter G, Metzkor E, Kaluski E, et al. Randomised trial of high-dose isosorbide dinitrate plus low-dose furosemide versus high dose furosemide plus low-dose isosorbide dinitrate in severe pulmonary edema. Lancet. 1998;351:389–393.
17 Publication Committee for the VMAC Investigators (Vasodilatation in the Management of Acute CHF). Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial. JAMA. 2002;287:1531–1540.
18 Sackner-Bernstein JD, Kowalski M, Fox M, et al. Short-term risk of death after treatment with nesiritide for decompensated heart failure: a pooled analysis of randomized controlled trials. JAMA. 2005;293:1900–1905.
19 Sackner-Bernstein JD, Skopicki HA, Aaronson KD. Risk of worsening renal function with nesiritide in patients with acutely decompensated heart failure. Circulation. 2005;111:1487–1491.
20 Hernandez AF. Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure Trial (ASCEND-HF)—nesiritide or placebo for improved symptoms and outcomes in acute decompensated HF. Chicago: American Heart Association 2010 Scientific Sessions; 2010.
21 Cuffe MS, Califf RM, Adams KF, et al. Short-term intravenous milrinone for acute exacerbation of chronic heart failure: a randomized controlled trial. JAMA. 2002;287:1541–1547.
22 Gheorghiade M, Konstam MA, Burnett JC, Jr., et al. Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST Clinical Status Trials. JAMA. 2007;297:1332–1343.
23 Costanzo MR, Guglin ME, Saltzberg MT, et al. UNLOAD Trial Investigators: ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol. 2007;49:675–683.
24 Graff L, Orledge J, Radford MJ, et al. Correlation of the Agency for Health Care Policy and Research congestive heart failure admission guideline with mortality: peer review organization voluntary hospital association initiative to decrease events (PROVIDE) for congestive heart failure. Ann Emerg Med. 1999;34:429–437.
25 Fonarow GC, Adams KF, Jr., Abraham WT, et al. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA. 2005;293:572–580.
26 Fermann GJ, Collins SP. Observation units in the management of acute heart failure syndromes. Curr Heart Fail Rep. 2010;7:125–133.