Chapter 32 Confirmation of Endotracheal Intubation
I Overview
A American Society of Anesthesiologists Closed Claims Studies
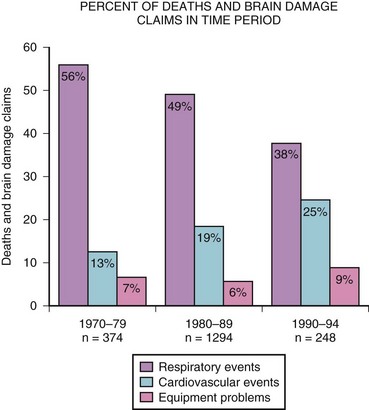
For the past several decades, the Committee on Professional Liability of the American Society of Anesthesiologists (ASA) has studied adverse anesthetic outcomes based on closed claims files of nationwide insurance carriers.1 From the beginning, it was evident that adverse outcomes involving the respiratory system constitute the single largest class of injury, representing one third of the overall claims. Generally involving healthy adults undergoing nonemergency surgery with general anesthesia, three mechanisms of injury accounted for approximately three quarters of the adverse respiratory events in the 1970s and early 1980s: inadequate ventilation (38%), esophageal intubation (18%), and difficult endotracheal intubation (DI; 17%). The remaining adverse respiratory events were produced by a variety of low-frequency (≤2%) mechanisms, including airway obstruction, bronchospasm, aspiration, premature and unintentional extubation, inadequate inspired oxygen delivery, and endobronchial intubation.1
Care was judged to be substandard in more than 80% of the cases of inadequate ventilation and esophageal intubation. Almost all (>90%) of the claims for inadequate ventilation and esophageal intubation were considered preventable with better monitoring, compared with only 36% of claims for DI. Death and permanent brain damage were more frequent in claims for inadequate ventilation and esophageal intubation (>90%) than in claims for DI (56%). Median payments were $240,000 for inadequate ventilation, $217,000 for esophageal intubation, and $76,000 for DI.1
In 23% of the claims for esophageal intubation, there was documentation of DI; in 73%, there was sufficient information to reconstruct the time to detection of esophageal intubation. Within this latter subset, 3% of esophageal intubations were detected within 5 minutes, 61% in 5 to 10 minutes, and 36% after 10 minutes. Auscultation of breath sounds (presumed) was documented in 63% of the claims for esophageal intubation. In 48% of cases, auscultation led to the erroneous conclusion that the tube was in the trachea. This diagnostic error was eventually recognized in a variety of ways, including reexamination with direct laryngoscopy, absence of the endotracheal tube (ETT) in the trachea at the time of an emergency tracheostomy, resolution of cyanosis after reintubation, and discovery of esophageal intubation at autopsy. Cyanosis was documented in 52% of the claims and preceded the recognition of esophageal intubation in only 34% of the cases.1
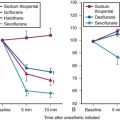
Because pulse oximetry and capnography have been used since the mid-1980s, data from the closed claims project were analyzed in 1999 to determine whether these monitoring modalities correlated with improvement in patients’ safety. After monitoring was instituted, respiratory events decreased, primarily in claims for injuries caused by inadequate ventilation and, to a lesser extent, esophageal intubation (Fig. 32-1).2
1 Pediatric Versus Adult Anesthesia Closed Malpractice Claims
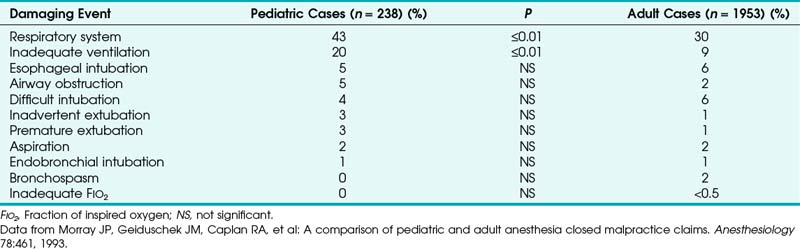
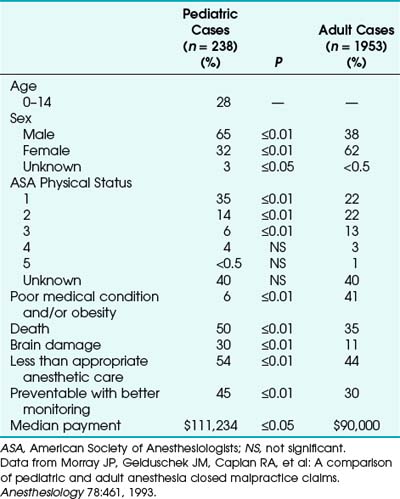
Outcome studies in pediatric patients revealed that adverse respiratory events constitute a leading cause of morbidity and mortality.3–5 The ASA closed claims study provided a database to compare pediatric and adult cases in which an adverse outcome occurred.5 The pediatric claims presented a different distribution of damaging events compared with claims of adults (Tables 32-1 and 32-2). Respiratory events and mortality rate were greater in pediatric claims.5 Anesthetic care was more often judged “less than appropriate,” and the complications more frequently were considered preventable with better monitoring. The median payment to the plaintiff was greater for pediatric claims than for adult claims. Cyanosis (49%), bradycardia (64%), or both often preceded cardiac arrest, resulting in death (50%) or brain damage (30%) in previously healthy children.5
B The Magnitude of the Problem of Endotracheal Tube Misplacement
Outcome studies have repeatedly identified adverse respiratory events including unrecognized esophageal intubation as a leading and recurring cause of injury in anesthetic practice.6–10 The report on confidential enquiries into maternal deaths in England and Wales in 1979-1981 revealed that 8 of 22 deaths attributable to anesthesia were related to difficulty in endotracheal intubation.9 In 4 patients, the ETT proved to be misplaced in the esophagus.9 An investigation of anesthesia deaths in Australia revealed that 69% were related to airway management, with esophageal intubation once again identified as an important contributing factor.8
All anesthesiologists experience esophageal intubations sometime in their career, especially during training and when difficulty in visualizing the larynx is encountered. Most esophageal intubations are immediately and easily recognized. What is intriguing is the rare situation in which misplacement of the ETT in the esophagus is not recognized, resulting in grave consequences. Failure to recognize esophageal intubation is not limited to junior residents or inexperienced personnel; it has occurred with experienced anesthesiologists. A case was reported in which three “consultant anaesthetists” failed to recognize esophageal intubation.6 There are also many case reports describing anesthetic catastrophes and near-disasters in patients whose esophagus had been unintentionally intubated and in whom some or many of the common signs indicative of proper ETT placement were misleading.8,11–17
Unintentional esophageal intubation occurs more frequently in emergency airway management, in critically ill patients, and in patients who suffer cardiac arrest during out-of-hospital paramedic intubation.18–20 In one report, esophageal intubation occurred in 8% of emergency intubation attempts in critically ill patients.20 In a study of paramedics trained in direct laryngoscopic endotracheal intubation, there were 14 esophageal intubations in 779 patients, an incidence of 1.8%.18 Esophageal tube placement was recognized and corrected in 11 of the 14 patients; three esophageal intubations (0.4% of the total) were not recognized and remained uncorrected. In another study, 25% of tracheas intubated in the prehospital setting had improperly placed ETTs detected on arrival at the hospital.21 Contributing factors to this high incidence of esophageal intubation include intubation under less than optimal conditions; unavailability of monitoring equipment, protocols, or algorithms; violation of the standard technique of auscultation of lung fields and the epigastrium; and intubation attempts by nonexpert personnel.18–22
II Confirmation of Endotracheal Tube Placement
A Is There an Ideal Test for Confirmation of Endotracheal Tube Placement?
Unfortunately, such a perfect test does not yet exist. Many clinical signs and technical aids have been described to confirm endotracheal intubation. A number of these reports relied on the placement of two ETTs, one in the trachea and the other in the esophagus.23–25 This scenario makes the observer’s decision as to which tube is in the trachea or esophagus much easier and helps in the recognition of esophageal intubation. However, the clinician does not have the luxury of choice between two ETTs in a given clinical situation.24,25
In the assessment of these tests, the reader must understand what is meant by false-negative and false-positive results. Although not all reports are in agreement, most use the term false-negative when the ETT is in the trachea but the test fails and false-positive when the ETT is in the esophagus but the result mimics that of endotracheal intubation.24,25 For capnography, a false-negative result occurs when the ETT is in the trachea but the waveform is absent, and a false-positive when the ETT is in the esophagus but the waveform is present.24,25 To avoid confusion and to maintain conformity, these definitions are retained throughout the discussion that follows.
B Methods of Verification of Endotracheal Tube Placement
1 Non-Failsafe Methods
a Observation and Palpation of Chest Movements
Commonly used maneuvers to confirm endotracheal intubation are observation of symmetrical bilateral chest movements and palpation of upper chest excursions during compression of the reservoir bag. These signs can easily distinguish tracheal from esophageal intubation in most patients. However, in obese patients, women with large breasts, and patients with a rigid chest wall, barrel chest, or less compliant lungs, these signs can be misinterpreted to indicate that the ETT is in the esophagus.26 More important, upper chest wall movements simulating ventilation of the lungs can be seen and felt with an esophageally placed ETT. This phenomenon has been described in many instances of what ultimately proved to be esophageal intubation, and it has been reported in patients with intrathoracic hiatal hernia and after gastric pull-up operations.11–1726
Pollard and Junius studied chest movements during esophageal ventilation in a male cadaver after a cuffed ETT was placed into the esophagus and attached to an inflating bag.16 As they reported, “Chest and epigastric movements observed when the bag was compressed were indistinguishable from those normally seen in ventilation of the lungs even in the upper chest area.” When the body was dissected starting with the epigastrium, it was noted that “the stomach was being inflated and was spontaneously deflating via the esophagus, the feel of the inflating bag being indistinguishable from normal pulmonary ventilation.” Even when the lower end of the esophagus was occluded, “chest movements still appeared identical to those seen when the lungs are inflated.” After the chest was entered, it was observed that “chest movements were caused by the flat esophagus distending into a firm tube that lifted the heart and upper mediastinal structures forward, elevating the sternum and ribs.” The lifting of the chest wall by the distended esophagus is the most plausible explanation for the presumed chest movements seen during esophageal ventilation. Another possible mechanism is that gastric insufflation causes upward displacement of the diaphragm and outward movement of the lower chest. With release of bag compression, gas escapes from the stomach up the esophagus, allowing the diaphragm to move downward and the lower chest to move inward.16
b Auscultation of Breath Sounds
There are several reasons why sounds heard with esophageal intubation may mimic breath sounds from the lungs. The combination of esophageal wall oscillations with gas movement and acoustic filtering can produce inspiratory or expiratory wheezes indistinguishable from sounds arising from gas movement in the airway.13,27,28 The high flow rate, distribution, and volume of gas delivered through the esophagus may lead to auscultation of predominantly bronchial breath sounds.13 In infants and children, esophageal sounds can be easily transmitted to wide areas of the chest wall.29 The quality of breath sounds may also differ depending on whether the chest is auscultated near the middle line or laterally near the axilla and may vary with the presence of pulmonary disease and from patient to patient.
Sounds retrieved by an esophageal stethoscope are different from those heard with a precordial stethoscope and should not be used to differentiate endotracheal from esophageal intuation.16,25 In patients with a thoracic stomach or hiatal hernia, many of the clinical signs of esophageal intubation may be obscured. Because of the intrathoracic location of a large distensible viscus, bilateral breath sounds may be heard during manual ventilation.12 For these reasons, whenever abnormal breath sounds are heard, they should not be relied on to confirm endotracheal intubations.30
Proper verification of ETT placement by auscultation is dependent on the clinician’s experience.31 In one study in the critical care setting, experienced clinicians identified ETT location correctly, whereas inexperienced examiners were correct in only 68% of cases.31
c Endobronchial Intubation
Intentional endobronchial intubation has been used to discriminate between endotracheal and esophageal intubation when doubt exists.32 The ETT is advanced until breath sounds are lost on one side and unilateral breath sounds are heard on the other side. That should not happen if the tube is in the esophagus. The ETT is then gradually withdrawn 1 to 2 cm beyond the point at which bilateral breath sounds are heard. This technique has been used in infants and children, particularly in infants with tracheoesophageal fistulas.33 However, it is not recommended for routine use because it can precipitate carinal irritation and bronchospasm.34
d Epigastric Auscultation and Observation for Abdominal Distention
Auscultation of the epigastric area to elicit air movement in the stomach has been suggested as a routine maneuver after endotracheal intubation, even before auscultation of the chest.14,15,25,26 However, normal vesicular breath sounds from the lungs can be transmitted to the epigastric area in tracheally intubated patients who are thin or small.26 On rare occasions, esophageal intubation may not be easily distinguishable from endotracheal intubation if epigastric auscultation alone is used. Furthermore, there are circumstances, such as obstetric emergencies, in which prepping of the abdomen before induction of anesthesia precludes epigastric auscultation after intubation.25
Abdominal distention caused by gastric insufflation after compression of the breathing bag in cases of esophageal intubation can be readily observed in most patients. Occasionally, this sign may not be a reliable indicator of esophageal intubation for the following reasons16,26,35:
1. Gastric insufflation and abdominal distention might have occurred during prior mask ventilation.
2. Gastric distention may not be apparent in obese patients.
3. A previously placed nasogastric tube (NGT) can cause intermittent decompression of the stomach.
4. Gradual gastric filling can be difficult to distinguish from normal abdominal movements because of the esophageal reflux of gases.
Conversely, gastric distention can occur in patients with congenital or acquired tracheoesophageal fistula despite placement of the tube in the trachea.33
e Combined Auscultation of Epigastrium and Both Axillae
In a study of 40 adult patients intubated in both the trachea and the esophagus, “blinded” observers auscultating both axillae failed to diagnose esophageal intubation in 15% of cases.36 When movement of the abdominal wall alone was used to assess ETT position, false results were obtained in 90% of cases. In contrast, the combined auscultation of the epigastrium and both axillae was found to be totally reliable in diagnosing esophageal intubation.36 These findings emphasize the importance of combining tests to achieve a high degree of reliability in assessing ETT placement.
Detection of misplaced ETTs by auscultation of the chest and epigastrium is probably more difficult in infants than in adults. Uejima reported on two children,29 aged 7 months and 5 years, in whom esophageal intubation occurred. In both, bilateral chest movements and breath sounds were heard over four areas of the chest and over the epigastrium. However, these “breath sounds” were not vesicular in nature. The ease of transmission of sounds from the esophagus to the chest and epigastrium may mimic breath sounds, especially in infants, emphasizing again that whenever any but normal vesicular breath sounds are heard, they should not be relied on to confirm endotracheal intubation.25
Attempts have been made to quantify and assess breath sound characteristics using electronic stethoscopes placed over each hemithorax and the epigastrium. With the use of computerized analysis, researchers digitized and filtered breath sounds to remove selected frequencies. Preliminary results suggested that this technique, when incorporated into a three-component, electronic stethoscope-type device, provides an accurate, portable mechanism to reliably detect ETT position in adults.37 However, before this method gains wide acceptance, further evaluation is necessary in a broader population of patients with a variety of pathologic states and with different conditions and environments.
f Reservoir Bag Compliance and Refilling
Manual compression of the reservoir bag after endotracheal intubation and cuff inflation yields a characteristic feel of compliance of the lungs and chest wall, whereas passive exhalation on release of bag compression is accompanied by rapid bag refilling. In almost all esophageal intubations, compressing the reservoir bag does not inflate the chest and is not followed by appreciable refilling. However, exceptions to this rule do occur, as has been shown in reports of accidental esophageal intubations. In these cases, repeated filling and emptying of the stomach resulted in concomitant emptying and refilling of the reservoir bag, leading to the erroneous conclusion that the ETT was in the trachea.13,14,16,17,35
It is possible that high fresh gas flow might have contributed to reservoir bag refilling in these cases. To enhance the reliability of this test, it has been suggested that the fresh gas flow should be shut off temporarily.38 If this is done, chest inflation and rapid bag refilling can be repeatedly done (three to five times) in tracheally intubated patients but not in esophageally intubated patients. Because changes in lung or chest wall compliance can be misinterpreted by the clinician compressing the reservoir bag and high airway resistance can lead to slow refilling, this test, used alone, should not be relied on to distinguish esophageal from endotracheal intubation.26
g Reservoir Bag Movements with Spontaneous Breathing
Movement of the reservoir bag during spontaneous breathing has been considered one of the signs indicative of endotracheal intubation. However, it has been demonstrated that this sign can be unreliable. In a group of anesthetized patients, the trachea and esophagus were intubated and the patients were allowed to breathe spontaneously.39 With the ETT intentionally occluded, the high negative intrapleural pressures generated with spontaneous breathing efforts were transmitted to the esophagus, resulting in reservoir bag movements and measurable tidal volumes (VT) up to 180 mL. Therefore, slight reservoir bag movements or measurements of small VT during spontaneous breathing are not reliable indicators of endotracheal intubation.39
h Cuff Maneuvers and Neck Palpation
The higher-pitched sound produced by leakage around a tube placed in the trachea with the cuff deflated during compression of the reservoir bag, compared with the “flatus-like” sound of leakage around a tube placed in the esophagus, has been used as a distinguishing test.26 As has been shown in case reports, when the cuff of an esophageally placed ETT is inflated close to the cricoid cartilage, the characteristic guttural sound may be absent or may become higher pitched, resembling leakage around a tracheally placed ETT.16
Palpation of the cuff on each side of the trachea between the cricoid cartilage and the suprasternal notch while moving the tube has been proposed to confirm endotracheal intubation.26 After intubation and cuff inflation, 2 or 3 fingers are placed above the suprasternal notch. Several rapid inflations of the cuff are performed (up to 10 mL of air). An outward force is felt by the palpating fingers if the ETT is in correct position. Intermittent squeezing of the pilot balloon of a slightly overinflated cuff with the thumb and index finger while sensing the transmitted pulsations in the neck has also been suggested as a sign for confirming ETT placement.40 Likewise, a maneuver using the pilot balloon as a sensor has been proposed.34 A high-volume, low-pressure, prestretched cuff may not be palpable despite correct placement.34 Conversely, an inflated cuff can be palpated in the neck in cases of esophageal intubations.17 Therefore, these maneuvers should not be relied on in verifying endotracheal intubation.25,26,30
If an assistant gently palpates the trachea in the suprasternal notch or applies cricoid compression during endotracheal intubation, an “old washboard-like” vibration is appreciated as the ETT rubs against the tracheal rings.41 It has been suggested that there should be no false-positive results of this sign if the ETT is misplaced in the esophagus, because the esophagus is soft and lies posterior to the trachea. Thus far, there have been no controlled studies to confirm the validity of this sign.
i Sound of Expelled Gases during Sternal Compression
Pressing sharply on the sternum while listening over the proximal end of the ETT to detect a characteristic feel and sound of expelled gases from the airway is occasionally used to distinguish esophageal from endotracheal intubation.16 This test is mistrusted by many because of (1) inability to distinguish gases expelled passing through the ETT from gases passing through or around a tube misplaced in the esophagus, (2) inability to distinguish gases being expelled through the nose, and (3) inability to distinguish esophageal and stomach gases present from prior mask ventilation.16
j Tube Condensation of Water Vapor
The basic principle of tube condensation as a sign of ETT placement is that water vapor seen in clear plastic ETTs is more likely to be present with gases exhaled through a tracheally placed tube than with gases emanating from the stomach through a tube in the esophagus (Fig. 32-2). Two studies have demonstrated water condensation in all cases in which ETTs were placed in the trachea.36,42 However, water condensation can and does occur when ETTs are placed in the esophagus. The fact that condensation was noticed in 85% of esophageal intubations in one study and in 28% in another study should strongly discourage its presence from being interpreted as a reliable indicator of a successful intubation.36,42
k Nasogastric Tubes, Gastric Aspirates, Introducers, and Other Devices
A test devised to distinguish between tracheal and esophageal placement of an ETT involves threading a lubricated NGT through the tube in question, applying continuous suction, and attempting to withdraw the NGT.43 This test exploits the distinguishing feature that the esophagus will collapse around the NGT when suction is applied, whereas free suction applied in the trachea will continue.
In a study of 20 patients in whom both trachea and esophagus were intubated, the ability to maintain suction and the ease of withdrawal during continuous suction clearly distinguished between the two positions.43 When the NGT was in the trachea, suction applied to it could be maintained easily because the trachea remained patent and air was entrained through the open end of the tube. This allowed the NGT to be withdrawn easily despite suctioning. In contrast, when suction was applied to the NGT in the esophagus, the esophageal wall collapsed around it, thereby obstructing suction and interfering with easy withdrawal of the NGT. Although the length of the NGT that could be easily inserted before an impediment or resistance was felt (5 to 15 cm distal to the tip of the tube) identified correct tracheal placement, it was less useful in identifying esophageal intubation. Similarly, the nature of the aspirate (mucus versus bile or gastric juice) was found to be of limited value in most patients. Because the total time spent to make these observations was 20 to 30 seconds, the authors concluded that the test is reliable.43 Despite their enthusiasm, this test is very rarely used because of the availability of better methods.
The Eschmann introducer is a 60-cm-long device (10 cm shorter than other similar devices) composed of two layers: a core of tube woven from Dacron polyester threads and an outer resin layer to provide stiffness, flexibility, and a slippery, water-impervious surface. Frequently, the device is referred to as a “gum elastic bougie”—despite the fact that it is not gum, not elastic, and not a bougie.44 The introducer has a 35-degree kink located 2.5 cm from its distal end. Because its outer diameter (OD) is 5 mm, it can be used with ETTs with an inner diameter (ID) of 6 mm or greater. The device has been used for many years to facilitate intubation and has been introduced to differentiate tracheal from esophageal intubation in emergencies when there is doubt about the ETT location.26 When inserted through the lumen of the ETT, the curved tip of the lubricated introducer may be felt rubbing over the tracheal rings, and resistance is encountered as the tip of the introducer meets the carina or a main stem bronchus at approximately 28 to 32 cm in the adult. If the ETT is in the esophagus, the introducer passes without resistance to the distal end of the esophagus or stomach.26 Forceful insertion of an excessive length of the introducer could conceivably result in bronchial rupture or other injuries. This has led the manufacturer to discourage its use as an ETT changer, because other devices specifically designed for this purpose are now available.45
l Transtracheal Illumination
The success of transillumination of the soft tissues of the neck anterior to the trachea in accomplishing guided oral or nasal intubation has culminated in the development of improved lighted stylets and introducers (see Chapter 21).46–48 It has also prompted investigators to use transtracheal illumination to differentiate esophageal from tracheal ETT placement and to position the ETT accurately inside the trachea. Transmission of light through tissues depends on thickness, compactness, color, density, and light absorption characteristics of the tissue; wavelength, quality, and intensity of the light; proximity of the tissue to the light source; and the ambient lighting conditions in the room.49 Typically, endotracheal intubation with the lighted stylet or introducer inside the ETT or placement of the stylet in the lumen of the ETT after intubation gives off an intense, circumscribed, midline glow in the region of the laryngeal prominence and sternal notch (Fig. 32-3). The illumination is mostly seen opposite the thyrohyoid, cricothyroid, and cricotracheal membranes. In the event of esophageal intubation, the light is either absent or perceived as dull and diffuse.
To enhance transillumination, darkening the room, dimming the overhead lights, and applying cricoid pressure (to approximate the tracheal wall to the light source and stretch the soft tissues anterior to the light source) have been recommended.46–49 Newer lighted stylets or introducers, battery operated or incorporating a fiberoptic light source, have brighter lights than earlier prototypes. Consequently, dimming of the overhead lights or application of cricoid pressure may not be necessary. The reliability of transtracheal illumination in distinguishing esophageal from endotracheal intubation in adults has been demonstrated.49,50 In one study conducted on five cadavers, only 1 of 56 intratracheal placements was misidentified as esophageal, whereas of 112 extratracheal placements (esophageal or pyriform fossa), 1 was misidentified as intratracheal.50 In another study of 420 adult patients, tracheal transillumination was graded as excellent in 81% of patients and as good in the remaining 19%.49 In contrast, transesophageal illumination could not be demonstrated in any patient. Despite these reports, false-negative results (ETT in trachea but no transillumination) with the use of the newer lighted stylets and introducers in patients with neck swelling or dark skin and in obese patients have been noticed.31 Similarly, occasional false-positive results have been observed in thin patients. Nonetheless, the use of transillumination could reduce unrecognized esophageal intubations, especially outside the operating room where other technical aids are not available.49,50
Reports have delineated several complications with these transillumination devices.51–53 Loss of the bulb into the lung necessitating bronchoscopic removal has been reported.51 A change in design involving encasement of the bulb in a plastic retaining cover has virtually eliminated the possibility of this complication in future.47 Other reports have described arytenoid subluxation.52,53 However, this complication can occur after conventional intubation, and there is no evidence of an increased incidence with the use of lighted stylets.
m Pulse Oximetry and Detection of Cyanosis
Although unrecognized esophageal intubation ultimately leads to a severe fall in O2 saturation (and detectable cyanosis), minutes may elapse before this happens. A disturbing finding that emerged from the ASA closed claims study is that detection of esophageal intubation required longer than 5 minutes in 97% of cases.1 Furthermore, detectable cyanosis preceded the recognition of esophageal intubation in only one third of cases. Several factors contribute to the delay in diagnosing esophageal intubation by pulse oximetry or in detecting cyanosis, or both.
Reliance on the appearance of cyanosis as a clue to esophageal intubation can contribute to such a delay. Recognition of cyanosis usually necessitates the presence of more than 5 g/dL of reduced hemoglobin, which corresponds to 75% to 85% O2 saturation.54 The detection may also depend on the concentration and type of hemoglobin. With severe anemia, cyanosis may not be apparent until the arterial oxygen saturation (Sao2) falls to 60%. An infant with a high proportion of fetal hemoglobin may still look “pink” at an arterial partial pressure of oxygen (PaO2) near 40 mm Hg because of the increased blood affinity of O2 and leftward shifting of the fetal oxyhemoglobin dissociation curve; for this reason, a serious reduction in PaO2 may develop before cyanosis is apparent.55 Recognition of cyanosis is influenced by the limited exposure of the patient’s body and the insensitivity of the human eye to changes in skin color or even the color of the blood during arterial desaturation. It could also be affected by variations in room lighting and the color of the surgical drapes.
Noninvasive monitoring of oxygen saturation by pulse oximetry (SpO2) has proved to be a reliable indicator of Sao2.56,57 It is undoubtedly quicker to detect changes in SpO2 than to rely on clinical detection of cyanosis. The main limitation of pulse oximetry is that it does not measure PaO2, which may fall long before SpO2 is affected.
Preoxygenation of the patient’s lungs before anesthetic induction extends the period of time before a decrease in SaO2 occurs in case of esophageal intubation.58,59 In general, O2 deprivation or apnea after air breathing results in a substantial fall in PaO2 within 90 seconds, whereas PaO2, after O2 breathing, remains higher than 100 mm Hg for at least 3 minutes of apnea, and SaO2 does not change during this period.54,58 Consequently, pulse oximetry after preoxygenation does not immediately indicate that the esophagus has been intubated.59 Because of this prolonged interval with normal SpO2 being recorded initially after the intubation attempt, misplacement of the ETT may not be suspected later when SpO2 begins to decrease.1,59 Furthermore, the risk of misinterpretation of clinical findings suggestive of esophageal intubation may be greater when other clues such as decrease in SpO2 or cyanosis are not yet manifest.1 This “hazard” of preoxygenation has led a few clinicians to abandon such a practice so that esophageal intubation can be more readily appreciated.60 One does not need to go to such extremes as to abandon a practice that has definite merits. However, the presence of normal pulse oximetry readings after intubation should not be taken as evidence of successful endotracheal intubation, and after preoxygenation, oxyhemoglobin desaturation, as indicated by pulse oximetry, is a relatively late manifestation of esophageal intubation.1,13,59,61–63
Both O2 consumption and cardiac output can influence the Sao2 through their effects on mixed venous oxygen tension (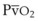 ) and mixed venous oxygen content (
) and mixed venous oxygen content (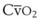 ).54 A decrease in O2 consumption associated with anesthetic induction or an increase in cardiac output or both can lead to an increase in
).54 A decrease in O2 consumption associated with anesthetic induction or an increase in cardiac output or both can lead to an increase in 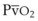 and
and 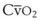 . The high
. The high 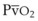 retards the decrease in PaO2 in the event of O2 deprivation resulting from esophageal intubation and consequently may delay its recognition. Conversely, in patients with low SaO2 and in those who have high O2 consumption (e.g., children, women in labor, morbidly obese patients) or decreased functional residual capacity (FRC), oxyhemoglobin desaturation may occur faster.
retards the decrease in PaO2 in the event of O2 deprivation resulting from esophageal intubation and consequently may delay its recognition. Conversely, in patients with low SaO2 and in those who have high O2 consumption (e.g., children, women in labor, morbidly obese patients) or decreased functional residual capacity (FRC), oxyhemoglobin desaturation may occur faster.
With the vocal cords open, manual ventilation into the esophagus can result in alveolar gas exchange. In 18 of 20 patients studied after intentional intubation of both the esophagus and the trachea, ventilation into the esophagus caused cyclic compression of the lungs by the distending stomach and esophagus, leading to some gas exchange evidenced by recording of carbon dioxide at the proximal end of the ETT.35 Although in this situation esophageal ventilation causes ventilation of the lungs with room air, it considerably delays the onset of cyanosis and oxyhemoglobin desaturation. Similarly, respiratory efforts by a spontaneously breathing patient through the unintubated trachea may delay the recognition of esophageal intubation. Esophageal ventilation may also be effective in yielding apneic oxygenation if the cords are open and there is a leak around the cuff of the misplaced esophageal tube.
n the Beck Airway Airflow Monitor
The Beck Airway Airflow Monitor (BAAM; Great Plains Ballistics, Lubbock, TX) consists of a cylindrical plastic whistle with a 2-mm-diameter aperture (Fig. 32-4). When connected to the proximal end of an ETT in a spontaneously breathing patient, the airflow is forced through the small lumen, producing a loud whistle.64 The pitch during inspiration and exhalation differs because of varying airflow velocities. The BAAM has been used to assist blind intubation and guide the ETT into the trachea. The loudness of the whistle indicates proximity to the tracheal air column, whereas cessation of the sound implies esophageal intubation, obstruction of the ETT, or excessive leak around the ETT. In patients who are not breathing spontaneously, a gentle squeeze of the chest produces whistling if the ETT is in the trachea. Because the whistle is produced by the airflow, an ETT in the pharynx can produce a whistle. Reports on this device have been limited to case reports and one study in neonates.64 More research is needed to determine its validity.
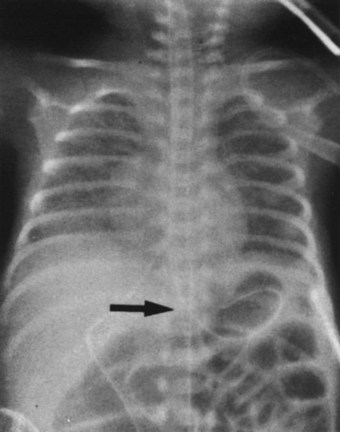
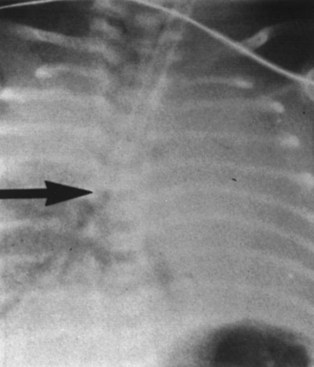
o Chest Radiography
A chest radiograph can diagnose esophageal intubation if the ETT is located in the lower esophagus distal to the carina (Fig. 32-5).65 Because a tube in the esophagus is often projected over the tracheal air column on anteroposterior chest radiographs, the radiologic features of esophageal intubation are usually difficult to assess.63 However, it should be suspected in the following situations63,65:
• If any part of the border of the ETT is seen outside or lateral to the air column of the tracheobronchial tree (in the absence of a pneumomediastinum)
• In the presence of esophageal air and gastric distention, particularly if an NGT is in place
• If there is a noticeable deviation of the trachea caused by an overinflated cuff.
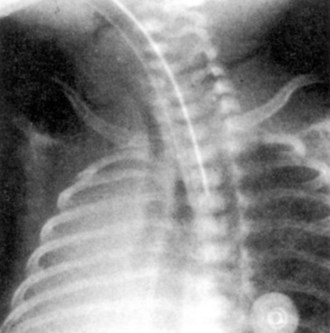
Although a lateral view of the chest could precisely reveal esophageal intubation, such views are often difficult to obtain. However, in one study, ETT location was identified correctly in 92% of the films taken with the patient in a 25-degree right posterior oblique position with the head turned to the right side.63 Because the esophagus is located slightly to the left as well as behind the trachea, this projection presents the relationship en face with respect to the radiologic beam, resulting in avoidance of superimposition of the trachea over the esophagus (Fig. 32-6). Because radiography is time-consuming and not failsafe, it should never be relied on for diagnosis of esophageal intubation, even in the critical care setting.
p Impedance Respirometry
Impedance respirometry, which measures electrical conductivity of the thorax, has been used to monitor respiratory rate in the critical care setting and to monitor apnea in infants. The ability of impedance respirometry to distinguish esophageal from endotracheal intubation has been explored.66,67 A high-frequency alternating current is passed between two electrodes placed on the anterior chest wall. With the increase in lung volume during inspiration, there is an associated decrease in lung electrical conductivity and therefore increased impedance.66,67 These changes can be measured and displayed as a waveform. If the esophagus is intubated, such an effect on thoracic impedance should be absent. Although assessment by thoracic impedance plethysmography was found to have 100% sensitivity in the detection of esophageal ETT placement in a study in adult patients,66 it correctly identified only 76 of 80 ETTs in children.67 Of those incorrectly identified, one was in the trachea and three were in the esophagus. Obviously, more studies are needed before any conclusions can be made. Although it is not a perfect test, use of impedance respirometry could decrease the time taken to identify incorrect placement when combined with other methods of ETT verification.67
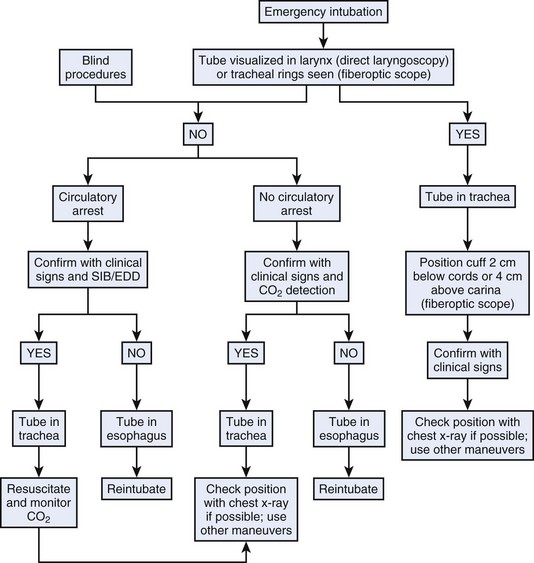
2 Almost Failsafe Methods
a Identification of Carbon Dioxide in Exhaled Gas
The availability of capnometry (measurement of CO2 in expired gas) and capnography (instantaneous display of the CO2 waveform during the respiratory cycle) for intraoperative monitoring has prompted clinicians to extend their use to facilitate detection of esophageal intubation.35,68–71 The principle of use stems from the fact that exhaled CO2 can be reliably detected during controlled or spontaneous ventilation in patients with adequate pulmonary flow whose trachea is intubated, whereas no CO2 can be detected in gases emanating from an ETT in the esophagus.
Studies have revealed that the combined use of capnography and pulse oximetry could avert more than 80% of mishaps considered preventable.72 In an amendment to its original basic intraoperative monitoring standards, the ASA stated the following: “When an ETT is inserted, its correct positioning in the trachea must be verified by clinical assessment and by identification of CO2 in the expired gas. End-tidal CO2 (Etco2) analysis, in use from the time of ETT placement, is strongly encouraged.” (Standards for Basic Anesthetic Monitoring, 2003 Directory of Members, American Society of Anesthesiologists, Park Ridge, IL, p 493.) Identification of CO2 in the exhaled gas has emerged as the standard for verification of proper ETT placement. Furthermore, interruption of CO2 sampling in the exhaled gas due to disconnection, obstruction, accidental tracheal extubation, or total loss of ventilation is immediately detected. Two main methods are currently used: capnography and colorimetric detection of CO2.
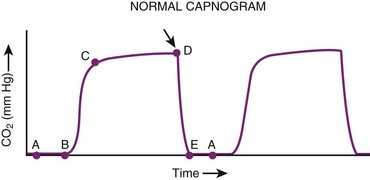
Capnography
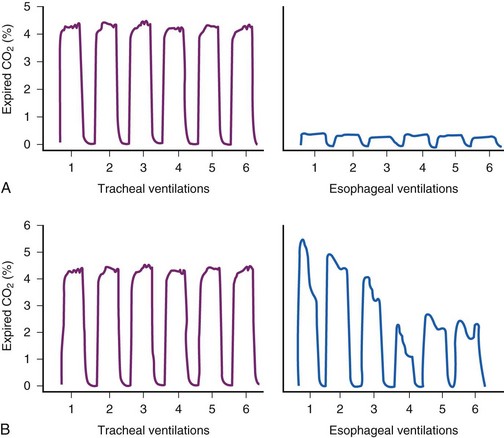
Currently available CO2 analyzers use various principles to measure CO2 in the inspired and exhaled gases on a breath-to-breath basis and display the CO2 waveform by mass spectrometry, infrared absorption spectrometry, or Raman scattering. A normal capnographic waveform in relation to the respiratory cycle is shown in Figure 32-7.73 A mass spectrometric tracing showing CO2 waveforms after esophageal intubation and after correct placement of the ETT in the trachea is shown in Figure 32-8. Although capnography typically distinguishes tracheal from esophageal intubation, false-negative results (i.e., ETT in trachea, absent waveform) and false-positive results (i.e., ETT in esophagus or pharynx, present waveform) have been reported.
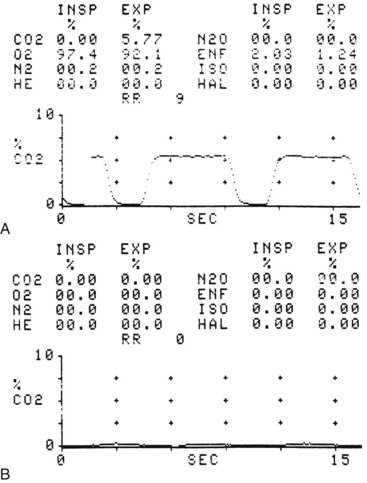
Figure 32-8 A, Normal capnograph, endotracheal tube (ETT) in trachea. B, Absent capnograph, ETT in esophagus.
Since the advent of capnography to confirm ETT placement, there have been reports of markedly different problems yielding unexpected false-negative results. For example, disconnection of the ETT from the breathing apparatus, apnea, and equipment failure may be misinterpreted as absent waveform caused by esophageal intubation.74 A kinked or obstructed ETT interferes with sampling of exhaled gas and may lead to an absent or distorted waveform. Lack of a CO2 waveform caused by severe bronchospasm has also been reported.74 Unintentional application of positive end-expiratory pressure (PEEP) to a loosely fitted or uncuffed ETT can cause exhaled gases to escape around the distal lumen of the tube and may result in a sampling error and absent waveform.75 In addition, dilution of exhaled gases by high fresh gas flow in a Mapleson D system when proximal sidestream sampling is used in infants weighing less than 10 kg leads to erroneous sampling.76,77 This problem can be corrected by distal sampling from the ETT, up to the 12 cm mark; by use of a mainstream sampling device; or by use of special ventilators.77 Lower sampling flow rates and gas sampling line leaks can result in artifactually low exhaled CO2 values and an abnormal waveform.78,79
Marked diminution of pulmonary blood flow increases the alveolar component of the dead space.54,70 Low cardiac output, hypotension, pulmonary embolism, pulmonary stenosis, tetralogy of Fallot, and kinking or clamping of the pulmonary artery during pulmonary surgery all cause a decrease in end-tidal carbon dioxide partial pressure (PETCO2), reflecting an increase in alveolar dead space.54,80,81 Other factors contributing to the increased alveolar dead space during anesthesia include patient age, large VT, use of negative phase during exhalation, short inspiratory phase, and presence of pulmonary disease.54,81 As a result of the widespread destruction of alveolar capillaries in patients with chronic obstructive pulmonary disease, an increased alveolar dead space ensues. An increase in alveolar dead space is manifested as a decrease in PETCO2 and an increase in the PaCO2 to PETCO2 difference (normally 0 to 5 mm Hg).54,81 If capnography alone is relied on to confirm ETT placement in patients who have a very large alveolar dead space, the low PETCO2 values might mislead the clinician into thinking that the ETT was not placed in the trachea.
An abrupt reduction in cardiac output reduces PETCO2 by two mechanisms: First, a reduction in venous return causes a decrease in CO2 delivered to the lungs, and second, the increase in alveolar dead space dilutes the CO2 from normally perfused alveoli, thus decreasing PETCO2.82–84 When cardiac arrest ensues, CO2 is no longer delivered to or eliminated through the lungs even if ventilation is adequate, and consequentially PETCO2 values exponentially decrease to remarkably low values (<0.5%). Ninety seconds after experimentally induced ventricular fibrillation, PETCO2 is usually decreased by 90%.85 Accordingly, a decision regarding the location of the ETT based on capnographic findings alone during cardiac arrest may lead to a misdiagnosis.
Experimental cardiac arrest demonstrated a remarkably high correlation between PETCO2, cardiac output, and coronary perfusion pressure during closed-chest precordial compression, during open-chest cardiac massage, and after resuscitation.86,87 Similar observations were reported during episodes of cardiac arrest in critically ill patients.86,87 The restoration of spontaneous circulation is heralded by a rapid increase in PETCO2 within 30 seconds. This overshoot in PETCO2, which is characteristic of successful resuscitation, may exceed the prearrest value and reflects washout of CO2 that has accumulated in venous blood and in tissues during circulatory arrest.86,88 In patients in whom resuscitative efforts fail to restore spontaneous circulation, PETCO2 remains low or even declines, whereas in those who eventually regain spontaneous circulation, PETCO2 reaches 19 mm Hg or higher. These observations suggest that capnography can be used for monitoring the adequacy of blood flow generated by precordial compression during cardiopulmonary resuscitation and can serve as a prognostic indicator of successful resuscitation.86,88
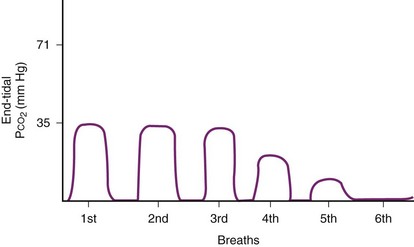
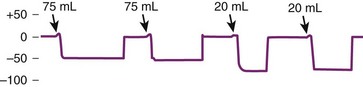
False-positive results can occur when the ETT is misplaced in the esophagus after exhaled gases are forced into the stomach during bag-mask ventilation preceding intubation attempts.85,89 If enough alveolar gas reaches the stomach, a CO2 concentration greater than 2% may be initially detected, and the CO2 waveform may be indistinguishable from that observed with endotracheal intubation.35,89,90 In fact, CO2 waveforms have been observed in one third of esophageal intubations,90 but repeated ventilation results in rapidly diminishing CO2 levels while the waveform becomes rather flat and irregular (Fig. 32-9).35,89 As a result of the dilution with successive ventilation, it is very unlikely that any CO2 would be detected after the sixth breath or after 1 minute in cases of esophageal intubation, making it easy to distinguish esophageal from endotracheal intubation.35 It has also been observed that compression of the chest caused clear, peaked CO2 elevation in 18 of 20 tracheally intubated patients but no changes in esophageal intubation.35 This simple, quick maneuver together with other signs can confirm proper ETT placement.
False-positive results can potentially occur in cases of esophageal intubation after ingestion of carbonated beverages or antacids.91–93 High CO2 levels (20%) are present in all carbonated beverages.92 Sodium bicarbonate, an ingredient found in most antacids (except sodium citrate), reacts with hydrochloric acid in the stomach, releasing CO2 levels comparable to those found in alveolar gas. With carbonated beverages, CO2 levels as high as 5.3% can be measured initially with esophageal ventilation, rendering correct assessment of ETT placement rather difficult.92 However, rapid decline in CO2 levels occurs with successive ventilations (Fig. 32-10). The abnormal CO2 waveform may give an important clue in the early detection of esophageal intubation.92 Because a normal-looking CO2 waveform during the first few ventilations is no guarantee of correct ETT placement, the waveform must be watched closely for at least 1 minute after placement of the tube.
It should be emphasized that a normal waveform is not synonymous with presence of the tube in the trachea.89 A normal waveform may be observed without a tube present in the trachea during spontaneous or controlled ventilation in cases of face mask anesthesia, use of a laryngeal mask airway, and use of the esophageal-tracheal Combitube (ETC) (Tyco-Healthcare-Kendall-Sheridan Corporation, Mansfield, MA), with which ventilation is carried out through the pharyngeal perforations.94,95 A normal waveform may also be present and sustained if the distal end of the ETT is in the pharynx but not necessarily in the trachea.94 This situation should be suspected by the unusual volume of cuff air required to stop the leak and an increased peak inspiratory pressure.
Although capnography has been widely used in operating rooms, its use in emergency rooms, intensive care units, and hospital floors has been rather limited because of the need for warm-up and careful calibration time; the requirement for an external power source, which limits use in emergency situations; and the fact that the technology is not easily transferable to the patient’s side. Portable apnea monitors that use infrared spectrometry for sensing CO2 have been used for confirmation of ETT placement.96 Although they do not accurately quantify CO2 concentration, a moving-bar indicator with seven light-emitting diodes does provide an estimate of 1.5 percentage points per illuminated diode. Gas is aspirated through plastic tubing attached to the elbow connector proximal to the ETT, allowing the monitor to sense CO2 in the first exhalation after intubation. The device is cheap and portable, operates for 90 minutes on batteries, and requires neither warm-up time nor calibration. This electronic instrument can also function as a breathing circuit disconnection alarm.96
Colorimetric End-Tidal Carbon Dioxide Detection
Early studies attempted to quantitate the amount of CO2 in exhaled gas and relied on the reaction of CO2 with another substance such as barium hydroxide to produce a color change.97 Thereafter, chemical indicators that change colors in the presence of increased hydrogen ion concentrations were used. The Einstein CO2 detector was constructed by attaching a 15-mm adapter to one end of a DeLee mucus trap containing a mixture of 3 mL of phenolphthalein and 3 mL of cresol. When the exhaled gas is bubbled through the chamber, carbonic acid formation causes a dramatic color change from red to yellow within seconds. Failure of the solution to change from red to yellow should suggest esophageal intubation.98
Several disposable colorimetric CO2 detectors are currently available. The Easy Cap II CO2 Detector (Nellcor Puritan Bennett, New York, NY; Fig. 32-11) can be connected between an ETT and a breathing circuit or bag-mask assembly.99–104 The detector contains filter paper impregnated with a colorless liquid base and a pH-sensitive indicator (metacresol purple) that reversibly changes from purple to yellow as a result of pH change when exposed to CO2 and reverts to purple when CO2 is no longer present. The color changes are made visible through a transparent dome in the plastic housing of the device. In general, a purple color (A range) indicates a CO2 level of 0.5% or less. The B range is a dusty tan color, reflecting levels of 0.5% to 2%. When the detector is exposed to CO2 levels greater than 2%, the color brightens to a yellow-tan color, the C range.

Figure 32-11 The colorimetric carbon dioxide detector.
(Easy Cap II CO2 Detector, Nellcor Puritan Bennett, New York, NY).
Studies have shown that colorimetric PETCO2 monitoring is reliable in verifying proper ETT placement in nonarrested patients.99–104 In one study,103 the mean minimum CO2 concentration required for detection of the perceivable color change was 0.54% (4.1 mm Hg) and ranged from 0.25% to 0.60% (1.9 to 4.6 mm Hg). When an ETT is correctly placed in a patient with adequate pulmonary blood flow, the detector should register C. The color change occurs immediately with the first breath in almost all patients. In the nonarrested patient when the device registers low or absent CO2 (A reading), esophageal intubation should be strongly suspected. If a C reading is obtained in an arrested patient, proper ETT placement is confirmed. In contrast, an A reading (absence of color change) during manual ventilation in a patient with cardiac arrest is consistent with either an esophageal or an endotracheal intubation in patients with profound low-flow state resulting from prolonged arrest or inadequate resuscitation. In another study, for 28 of 106 endotracheal intubations in “pulseless” patients the detector did not show any color change, indicating a PaCO2 of less than 4 mm Hg.104 A multicenter trial of a colorimetric CO2 detection device found that all cardiac arrest patients who survived to admission had a value of C registered on the monitor.105 No patient in whom the detector failed to register color change survived. Therefore, the device may be useful as a prognostic indicator of successful resuscitation.104
Although the availability of such a device is considered a great leap forward, it has its own pitfalls and is not a substitute for assessment skills. Because the device has a dead space of 38 mL, the manufacturer does not recommend its use in children weighing less than 15 kg, although its efficacy in verifying endotracheal intubation has been confirmed in infants as young as 6 months.106 The color change may be difficult to discern under low-light conditions and may be misinterpreted by color-blind individuals. The detector is not sensitive to temperature, but it is eventually affected by humidity. Water vapor interferes with the chemical reaction and inactivates the device; it can be rendered ineffective within 15 minutes if the patient is receiving humidified gases. It has been suggested that trapping the humidity with a passive moisture exchanger may extend the useful life of the device.106 The manufacturer cautions against use of the device in conjunction with a heated humidifier or nebulizer and emphasizes that it is not intended to be used for longer than 10 minutes after intubation. Because the indicator permanently changes color if exposed for a prolonged period to low CO2 or other acids in the air, the device is packaged in a gas-impermeable metallic foil and is marketed as a single-use item. The packaged detector has a shelf life of 15 months if left unopened but may not function properly if the package is accidentally opened and the device is exposed to room air for several hours. For this reason, it is essential to verify that the indicator color is purple before use. Clinicians should familiarize themselves with the EtCO2 detectors in their institutions and the appropriate color changes before use of these detectors.
Widespread use of colorimetric CO2 detectors has been hampered by limitations in their performance characteristics. Newer colorimetric CO2 indicators seem to have overcome some of these limitations.107 With this improved technology, it is expected that detectors will be more durable during prolonged exposure to heat, humidity, and CO2. Furthermore, they will be cheaper and will have a longer shelf life. However, there are potential sources of rare errors with the use of colorimetric CO2 monitoring, even in the nonarrested patient. After manual bag-mask ventilation before intubation, CO2 from the exhaled air may be blown into the stomach and may result in detectable CO2 levels, yielding a false-positive yellow color if the ETT is misplaced in the esophagus. Ventilating the patient with six breaths results in a washout of CO2 to near-zero if the ETT is misplaced in the esophagus. Close observation of the color of the detector is essential during and after the delivery of six quick breaths so that false-positive results are avoided.
Concerns about inadvertent detection of gastric CO2 from ingested beverages after esophageal intubation, which can potentially vitiate colorimetric CO2 detection, have been raised.101 In a study of carbonated beverage ingestion and esophageal intubation in the cardiac arrest porcine model, the amount of CO2 released did not result in spurious color change of the detector in the four animals studied and did not cause difficulty in interpretation of the readings.108 Therefore, concern that false-positive results might be caused by esophageal placement in a patient who has recently ingested carbonated beverages appears to be unwarranted.104,108
b Esophageal Detector Device/Self-Inflating Bulb
Use of the esophageal detector device (EDD) is based on anatomic differences between the trachea and the esophagus.109 The trachea in the adult is 10 to 12 cm long, and its diameter can vary from 13 to 22 mm. The trachea remains constantly patent because of C-shaped rigid, cartilaginous rings that are joined vertically by fibroelastic tissue and closed posteriorly by unstriped trachealis muscle. The esophagus is a fibromuscular tube, 25 cm long in adults, which extends from the cricopharyngeal sphincter to the gastroesophageal junction; there is no intrinsic structure to maintain its patency.
Wee and O’Leary and their colleagues introduced a new method using a simple device to distinguish esophageal from endotracheal intubation.109,110 The principle underlying use of the EDD is that the esophagus collapses when a negative pressure is applied to its lumen, whereas the trachea does not. The device consists of a 60-mL syringe fitted by an adapter that can be attached to an ETT connector. When the syringe is attached to an ETT placed in the trachea, withdrawal of the plunger of the syringe aspirates gas freely from the patient’s lungs without any resistance apart from that inherent in the device (Fig. 32-12). If the ETT is in the esophagus, withdrawal of the plunger causes apposition of the walls of the esophagus, occluding its lumen around the ETT, and a negative pressure or resistance is felt when the plunger is pulled back. Wee conducted a study in 100 patients in whom placement of ETTs and esophageal tubes was assessed using the EDD.109 There were 99 first-time correct identifications of ETT placement, and the mean time required to diagnose tube placement was 6.9 seconds. Application of constant but slow aspiration has been recommended to avoid the suction effect and prevent mucosal damage. Endotracheal intubation is confirmed if 30 to 40 mL of gas is aspirated without resistance in adults or 5 to 10 mL in children older than 2 years of age.109–111
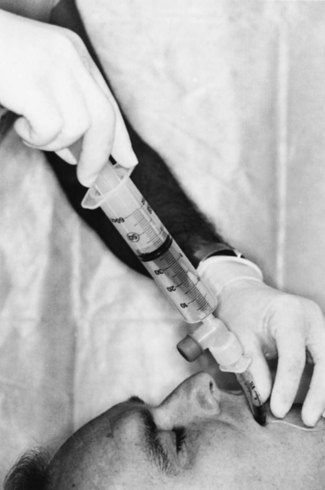
Figure 32-12 The esophageal detector device consists of a 60-mL syringe fitted by an adapter to an endotracheal tube connector.
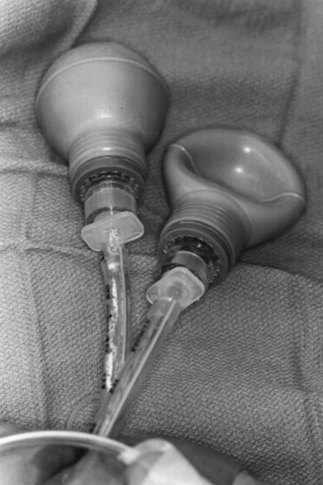
Nunn simplified the EDD by replacing the syringe with an Ellik evacuator, which is a self-inflating bulb (SIB) with a capacity of 75 to 90 mL.112 After intubation, the device is connected to the ETT, and the bulb is compressed. Compression is silent, and refill is instantaneous if the ETT is in the trachea. In contrast, if the ETT is in the esophagus, compression of the SIB is accompanied by a characteristic flatus-like noise, and the SIB remains collapsed on release. The test can be accomplished easily within 3 seconds, and the outcome is unmistakable.113 This technique has been further modified by compressing the SIB before, rather than after, connection to the ETT connector.114 Investigations that used the latter technique confirmed earlier studies and demonstrated that the sensitivity, specificity, and predictive value of the SIB are 100% (Figs. 32-13 and 32-14).115–118

Figure 32-13 The self-inflating bulb is fitted with a standard 15-mm adapter.
(From Salem MR, Wafai Y, Joseph NJ, et al: Efficacy of the self-inflating bulb in detecting esophageal intubation: Does the presence of a nasogastric tube or cuff deflation make a difference? Anesthesiology 80:42–48, 1994.)
Despite the efficacy of both the EDD and the SIB in differentiating esophageal from endotracheal intubation in essentially healthy patients, there have been reports of false-negative results (i.e., the ETT is in the trachea, but gas cannot be aspirated by the EDD or the SIB does not reinflate). The EDD or the SIB may fail to confirm ETT placement in infants, in whom the tracheal wall is not held open by rigid cartilaginous rings; if the ETT is obstructed by kinking or by the presence of material in the ETT; and in patients with severe bronchospasm.119–122 Slow or no reinflation of the SIB may be encountered if the tube bevel is at the carina or in the right main stem bronchus.24 In such cases, slight retraction or rotation of the ETT usually corrects the position of the tube and orientation of the bevel, resulting in instantaneous reinflation of the SIB.121,123 The SIB may also fail to reinflate or may reinflate slowly when connected to a properly placed ETT in patients with morbid obesity (MO)123,124 and in patients who have a marked reduction in expiratory reserve volume, such as in those with pulmonary edema or adult respiratory distress syndrome, parturients undergoing cesarean section, and patients undergoing chest compression during resuscitation. The presence of secretions causing airway obstruction may also lead to a false-negative result.123,125
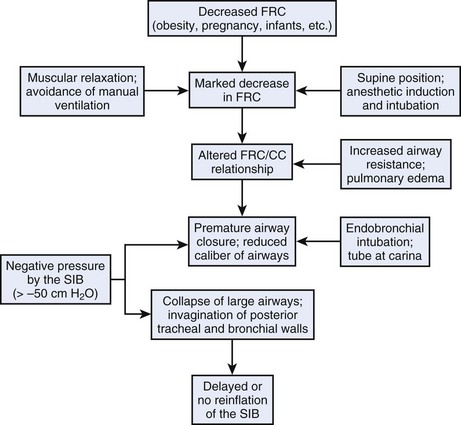
In a study involving 2140 consecutive anesthetized adult patients,123 the overall incidence of false-negative results with the SIB (i.e., no reinflation or reinflation delayed for longer than 4 seconds) was 3.6%. It was apparent that the choice of technique used can contribute to false-negative results. When the SIB was fully compressed before connection to the ETT in 1117 patients, the incidence was 4.6%, whereas false-negatives were reduced to 2.4% in 1023 patients when the SIB was compressed after connection to the ETT. Most of the patients (85.5%) in whom false-negative results were obtained had MO (body mass index >35 kg/m2) (Table 32-3). We surmise that this phenomenon seen in patients with MO and in pregnant women is related to marked reduction in FRC after anesthetic induction and muscular paralysis in the supine position leading to reduced caliber of intrathoracic airways and collapsibility of the trachea on application of subatmospheric pressure by the SIB.123–125 It is also possible that the negative pressure generated (greater than −50 cm H2O) may cause collapse and invagination of the posterior tracheal wall. Further compression can occur as a result of mediastinal compression.124,125 The chain of events that may lead to failure of the SIB to confirm endotracheal intubation is presented in Figure 32-15. If the SIB is compressed after connection to the ETT rather than before, a volume of gas is first introduced into the airway before subatmospheric pressure is generated by the SIB; this would limit the collapse of the SIB that is observed when it is compressed before connection to the ETT.123–125
TABLE 32-3 Demographics of False-Negative Results in Determining Correct ETT Placement with the Self-Inflating Bulb
| Patient Condition | T1 | T2 |
|---|---|---|
| Morbid obesity | 45 | 20 |
| Severe bronchospasm | 2 | 2 |
| Elderly with chronic obstructive pulmonary disease | 2 | 2 |
| Main stem intubation | 1 | 0 |
| Pulmonary secretions | 1 | 0 |
| Pulmonary edema | 0 | 1 |
| Total | 51 | 25 |
In T1, the self-inflating bulb (SIB) is compressed before it is connected to the endotracheal tube (ETT). In T2, the SIB is first connected to the ETT and is then compressed. See text for details.
From Wafai Y, Salem MR, Joseph NJ, Baraka A: The self-inflating bulb for confirmation of endotracheal intubation: Incidence and demography of false negatives. Anesthesiology 81:A1303, 1994.
Advantages of the SIB include low cost, unlimited shelf life, no power source, usability with one hand, and usability in areas of low light. It is quick to use (<10 seconds) and is unaffected by ingested carbonated beverages, antacids, or exhaled CO2 in the event of esophageal intubation. Unlike capnography, it can confirm endotracheal intubation before manual ventilation is initiated, thus avoiding the undesirable effects of esophageal ventilation.116,118 The performance of the SIB should not be affected by a cardiac arrest, the presence of an NGT, or cuff deflation.118 Studies found the EDD/SIB to be more accurate in verifying proper ETT placement in patients with out-of-hospital cardiac arrest and during emergency intubations than CO2 detection methods.126,128 Indeed, it has been the method preferred by emergency medical personnel.126–128 Verification of ETT placement with the SIB enables physicians and emergency personnel to proceed with other resuscitation duties. Other studies found the SIB to be complementary to CO2 detection.31,129,130
Some studies have found a high incidence of false-negative results with the use of the SIB in patients with out-of-hospital cardiac arrest.130,131 Although the SIB correctly identified all esophageal intubations, it identified only 72% of ETTs placed in the trachea, whereas 60% of the latter were identified by CO2 detection. Combining the SIB with CO2 detection enhanced the sensitivity to 90.8%.130 Possible reasons for incomplete or no reinflation of the SIB were similar to those presented in Table 32-3.
Based on the findings in a porcine cardiac arrest model, it has been suggested that the decreased muscle tone of the posterior tracheal wall that occurs during cardiac arrest could result in mucosal aspiration, airway blockage, and increased false-negative results associated with the use of EDDs.132 These findings, however, could be explained by the excessive negative pressure generated with the use of certain SIBs.133 Because no single test for verifying ETT position is reliable, all available modalities should be tested and used in conjunction with proper clinical judgment in cases of out-of-hospital cardiac arrest.130,131
Concern has been raised that false-positive results (i.e., ETT in esophagus, but SIB reinflates) may occur as a result of gastric insufflation after bag-mask ventilation before intubation. In one study, it was demonstrated that even after the intentional delivery of three small breaths (300 to 350 mL each), the SIB was effective in detecting esophageal intubation in all 72 patients.116 In another study, despite the use of mask ventilation before intubation, the authors did not observe a single instance of instantaneous reinflation of the SIB from the esophagus.113 In a study of one pig, Foutch and associates134 used a syringe similar to that of Wee109 and found that the device was effective in detecting esophageal intubation even after 1 minute of bag-mask ventilation.132 Although these studies imply that the SIB is effective in detecting esophageal intubation after mask ventilation (and modest gastric insufflation), it may fail occasionally in cases of massive gastric insufflation or if the lower esophageal tone is decreased.125,135 It is recommended that, when the SIB is used to confirm endotracheal intubation, the test should be undertaken before ventilation is initiated through the ETT.
It is essential that the SIB be of an appropriate size.118,119 Although a smaller SIB (capacity 20 mL) has a smaller radius and therefore generates a higher negative pressure (Fig. 32-16), it is unreliable in detecting esophageal intubation if the SIB is compressed after connection to the ETT.136 The larger SIB (capacity 75 mL) is recommended because it does not yield false-positive results when either technique is used.137 Although SIBs can be constructed by fitting a bulb with a standard 15-mm adapter, they are commercially available for single use. The devices are checked before use by connecting the compressed SIB to a clamped ETT; absence of reinflation is an indication of airtightness (Fig. 32-17). We have found that proper function of the device is not affected when a bacterial or viral filter is placed between the device and the ETT connector.
An SIB that incorporates a colorimetric CO2 detector is now available (Fig. 32-18). This new device (Salem SIB; Mercury Medical, Clearwater, FL) should enable clinicians to assess reinflation of the SIB after connection to the ETT and simultaneously to observe the color change indicative of the presence or absence of CO2 in the expired gas during reinflation of the SIB. The use of this device should eliminate most of the false results and lead to accurate verification of the location of the ETT before manual ventilation is initiated.
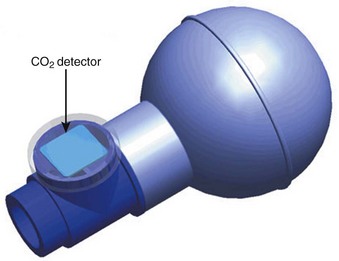
Figure 32-18 Salem self-inflating bulb. See text for details.
(Courtesy of Mercury Medical, Clearwater, FL.)
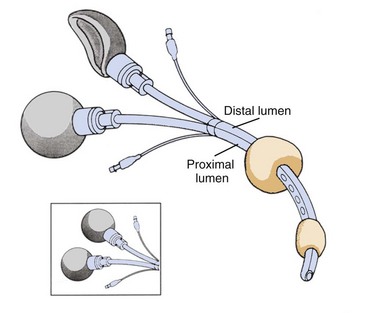
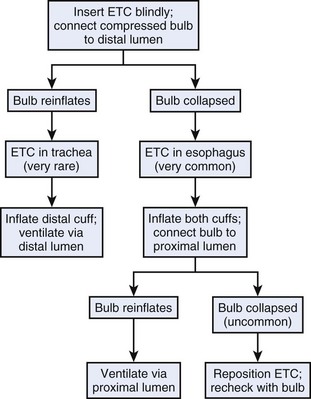
Use of the SIB has been extended to identify the location of the ETC and facilitate its proper positioning using a simple algorithm (Figs. 32-19 and 32-20).95 This may be of importance if the ETC is used in patients whose lungs cannot be ventilated by mask and whose trachea cannot be intubated. After blind placement, the compressed SIB is connected to the distal lumen. Instantaneous reinflation implies that the ETC is in the trachea and ventilation is carried out through the distal lumen; absence of reinflation is indicative of esophageal placement, which is very common. In this position, the pharyngeal balloon and the distal cuff are inflated and the compressed SIB is connected to the proximal lumen; instantaneous reinflation is expected to occur because the compressed SIB aspirates gas from the lungs through the pharyngeal perforations. If slow or no reinflation of the SIB occurs, repositioning the ETC (pulling it back 1 to 2 cm) may be indicated, and rechecking with the SIB confirms proper placement. Controlled ventilation is then carried out through the proximal lumen. Proper positioning and adequacy of ventilation can be further confirmed by the presence of breath sounds, absence of gastric insufflation, capnography or colorimetric CO2 detection, and pulse oximetry.95
c Acoustic Devices and Reflectometry
Devices that use sonic techniques as the basis for distinguishing tracheal from esophageal intubation have been developed. The mechanism of action of the Sonomatic Confirmation of Tracheal Intubation (SCOTI) device (Penlon; Abingdon, Oxfordshire, UK) is recognition of the resonating frequencies, which vary according to whether the tube is in an open structure (trachea) or a closed structure (esophagus).138 Although the concept is intriguing, the inability to configure the device correctly with all types and lengths of ETTs has limited its usefulness as an indicator of endotracheal intubation.139–142 Disappointing sales led to withdrawal of the device from the market in 1996.143
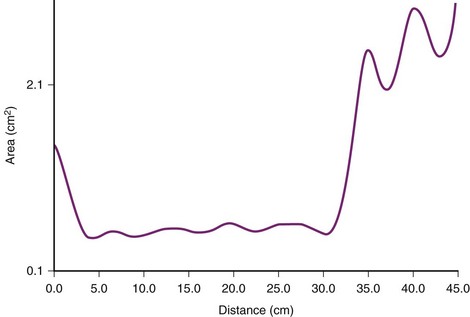
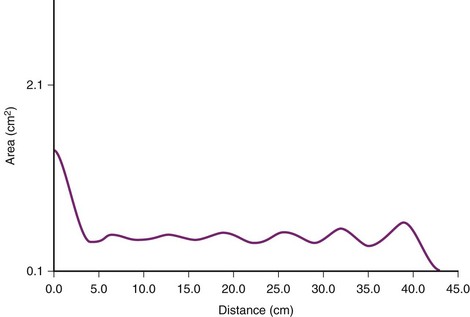
Another approach employs the principle of acoustic reflectometry by projecting a series of sonic impulses into the airway and placing a miniature microphone in the ETT wall to monitor sound pressure.144 The presence of deflection at the ETT tip allows discrimination between esophageal and endotracheal intubation. With acoustic reflectometry, one-dimensional image of a cavity, such as the airway or the esophagus, is constructed. The reflectometric area-distance profile consists of a constant cross-sectional area segment (length of the ETT), followed either by a rapid increase in the area beyond the tube in case of endotracheal intubation or by an immediate decrease in the area in case of esophageal intubation.145 In a study of 200 endotracheal intubations confirmed by capnography, acoustic reflectometry correctly identified 198 (Figs. 32-21 through 32-23). In 2 patients, endotracheal intubations were interpreted as an esophageal intubation. However, all 14 esophageal intubations were correctly identified.146 Because it is noninvasive and rapid (<3 seconds), acoustic imaging may become popular in future in the determination of the location of ETT placement in patients with cardiac arrest, particularly when visualization of the glottis is not possible. With technical improvement in the computer-based system, acoustic reflectometry may have value as an imaging adjunct device that can be used in the diagnosis and treatment of airway emergencies.146
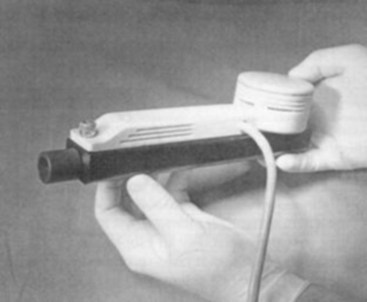
Figure 32-21 A Hood Labs (Pembroke, MA) two-microphone acoustic reflectometer.
(From Raphael DT, Benbassat M, Arnaudov D, et al: Validation study of two-microphone acoustic reflectometry for determination of breathing tube placement in 200 adult patients. Anesthesiology 97:1371–1377, 2002.)
3 Failsafe Methods
a Direct Visualization of the Endotracheal Tube Between the Cords
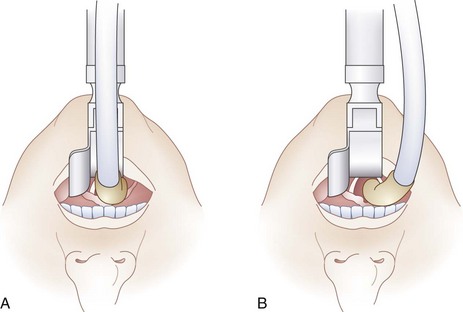
If the ETT is introduced directly posterior to the laryngoscope blade, as shown in Figure 32-24A, the laryngoscopist’s view of the cords may be obscured and the ETT may inadvertently enter the esophagus. This can be avoided by directing the ETT from the right corner of the mouth toward the larynx. As seen in Figure 32-24B, this maneuver can allow visualization of the ETT entering the larynx, thus confirming endotracheal intubation. The other maneuver that can be performed after intubation but before removing the laryngoscope from the mouth involves gentle posterior displacement of the ETT toward the palate (Fig. 32-25).147 The backward push on the tube against the forward traction of the laryngoscope blade exposes the cords by altering the direction of the ETT as it enters the larynx.147 This maneuver can be helpful in cases in which the cords are obscured by the ETT as it enters the larynx.
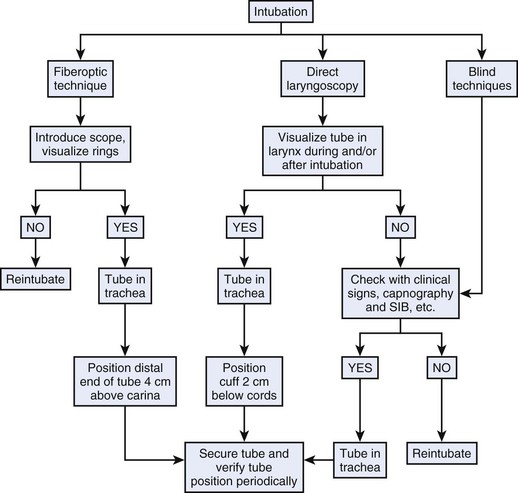
b Flexible Fiberoptic Bronchoscopy
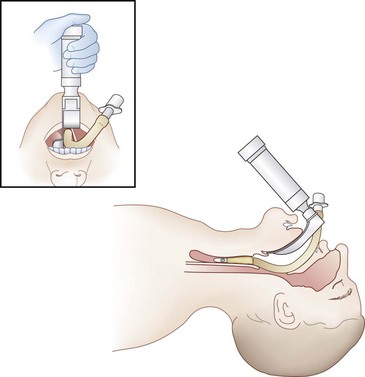
A sure method for confirmation of endotracheal intubation is visualization of the tracheal rings and carina with a flexible fiberoptic bronchoscope (FFB) after intubation.147–149 This is convenient only when an FFB is readily available or when the instrument is used to aid intubation (see Chapter 19). It should be emphasized that visualization of the vocal cords and tracheal rings through the FFB before the ETT is threaded over it does not guarantee endotracheal intubation. There are three reasons why the ETT may not follow the path of the FFB into the trachea.148,150–152 First, a stiff, large ETT may carry a relatively thin FFB into the esophagus even if the tip of the scope was originally placed in the larynx and trachea.148,150 Second, the tip of the tube and its Murphy eye are at 90 degrees to the right when the concavity of the ETT is facing anteriorly.148,152 Consequently, the tip of the tube may be blocked from entering the larynx by the right arytenoid cartilage, the vocal cord, or both. If excessive force is used, the tube may slip into the esophagus. This problem can be corrected by a 90-degree counterclockwise rotation.148,152 Third, if the scope is inserted through a tube placed nasally, the FFB may exit the tube through the Murphy eye and may actually enter the trachea, but in this case it will be impossible to thread the tube over the scope.148,151 To ensure that endotracheal intubation has been accomplished, the FFB should be withdrawn after placement of the tube and then reintroduced to visualize the tracheal rings and carina and to determine the distance from the distal end of the tube to the carina.148
III Verification of Endotracheal Tube Insertion Depth
According to the ASA closed claims analysis, the combination of inadvertent extubation and main stem intubation accounts for 2% of all adverse respiratory events in adults and 4% in pediatric patients.1,5 Analysis of the Australian Incident Monitoring Study (AIMS) found that “accidental” bronchial intubation accounted for 3.7% of the total incidents reported. Most of the bronchial intubations were detected in the operating room (93.5%), during maintenance of anesthesia (77.9%), and by unexplained O2 desaturation alone (63.6%). Capnography remained normal or unremarkable during 88.5% of episodes.153 The incidence of main stem intubation in the critical care setting, not detected by clinical examination but discovered on chest radiography after intubation, is 4%.20 Applying a strict definition of “acceptable ETT placement” as more than 2 cm but no more than 6 cm above the carina (with the head in a neutral position) in adult patients after emergent intubation, Schwartz and colleagues found an incidence of 15.5% of inappropriately placed ETTs according to radiologic assessment, with a higher incidence in women than in men.154
Fortunately, endobronchial intubation is not a common cause of death, but if it remains unrecognized, it can lead to hypoxemia secondary to collapse of the contralateral lung and hyperinflation of the intubated lung with resultant tension pneumothorax. The ensuing hypoxemia depends on the degree of venous admixture (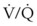 > 0), the magnitude of intrapulmonary shunting where
> 0), the magnitude of intrapulmonary shunting where 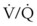 = 0, the fraction of inspired oxygen (FIO2), the degree of inhibition of hypoxic pulmonary vasoconstriction, and the level of
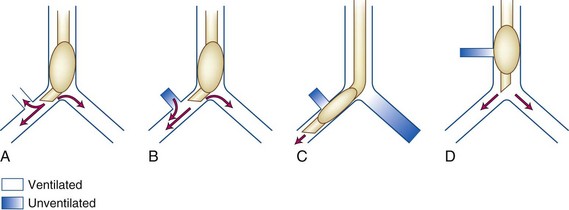
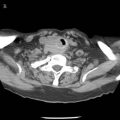
= 0, the fraction of inspired oxygen (FIO2), the degree of inhibition of hypoxic pulmonary vasoconstriction, and the level of 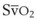 . Unintentional main stem intubation can result in one of several scenarios depending on the location of the distal end of the tube (Fig. 32-26).155 Placement of the ETT high in the right main bronchus (or even at the carina) may result in preferential ventilation of the right lung. Retrograde gas flow may lead to partial ventilation of the left lung if the ETT cuff does not provide a tight seal (see Fig. 32-26A).153 A biphasic CO2 waveform may be noticed (when the two lungs have different time constants).156 Ventilation with high flow rates through an ETT whose tip is placed just proximal to the orifice of the right upper lobe bronchus promotes negative pressure (Bernoulli effect) and atelectasis of the right upper lobe (see Fig. 32-26B). Placement of the ETT further down the right main bronchus prevents ventilation of the left lung and occludes the right upper lobe bronchus (see Fig. 32-26C). An ETT placed in the lower portion of the trachea may obstruct a congenital tracheal bronchus, causing upper right lobe atelectasis (Fig. 32-27; see Fig. 32-26D).157,158 In this rare anomaly, the bronchus to the right apical lung segment or the bronchus to the right upper lobe arises directly from the trachea, usually less than 2 cm from the carina.
. Unintentional main stem intubation can result in one of several scenarios depending on the location of the distal end of the tube (Fig. 32-26).155 Placement of the ETT high in the right main bronchus (or even at the carina) may result in preferential ventilation of the right lung. Retrograde gas flow may lead to partial ventilation of the left lung if the ETT cuff does not provide a tight seal (see Fig. 32-26A).153 A biphasic CO2 waveform may be noticed (when the two lungs have different time constants).156 Ventilation with high flow rates through an ETT whose tip is placed just proximal to the orifice of the right upper lobe bronchus promotes negative pressure (Bernoulli effect) and atelectasis of the right upper lobe (see Fig. 32-26B). Placement of the ETT further down the right main bronchus prevents ventilation of the left lung and occludes the right upper lobe bronchus (see Fig. 32-26C). An ETT placed in the lower portion of the trachea may obstruct a congenital tracheal bronchus, causing upper right lobe atelectasis (Fig. 32-27; see Fig. 32-26D).157,158 In this rare anomaly, the bronchus to the right apical lung segment or the bronchus to the right upper lobe arises directly from the trachea, usually less than 2 cm from the carina.
A Methods of Verification of Endotracheal Tube Insertion Depth
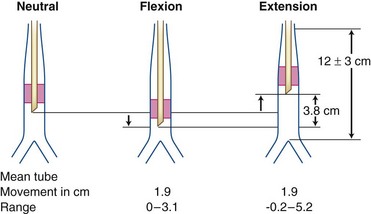
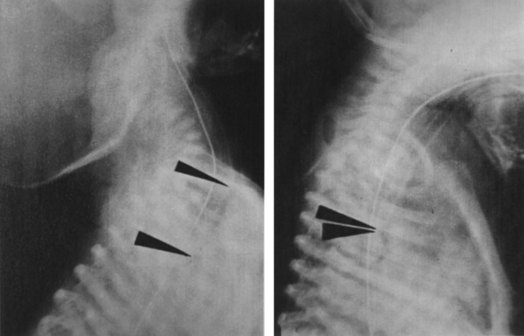
1 Referencing the Marks on the Tube Before and After Intubation
In one method, the ETT is placed alongside the patient’s face and neck with the tip of the tube lying at the suprasternal notch and the tube aligned to conform externally to the position of the nasal or oral ETT. The centimeter marking at which the tube intersects with the teeth or gums for oral intubation, or the naris for nasal intubation, is noted so that the ETT can be secured in that position after intubation. In another method, the orally placed ETT is secured at the upper incisor teeth (or gums) at the 23-cm mark in men and the 21-cm mark in women of average adult size.159 This method was found to be reliable in anesthetized patients but not in critically ill patients.20,159 We have observed that in tall men and in patients in whom excessive head extension is needed, the ETT may need to be secured at the 24- or 25-cm mark; in some shorter women, it may need to be secured at the 19-cm mark for optimal placement of the distal end of the ETT in the middle of the trachea. Formulas regarding the appropriate length of the ETT are available for infants and children. The reader is referred to pediatric anesthesia texts for this information.
2 Direct Visualization of the Tube and Its Cuff
Positioning of the distal end of the ETT in the middle of the trachea is a relatively easy maneuver when the ETT can be seen entering the larynx during direct laryngoscopy. Because the length of the trachea is 10 to 13 cm in an average adult, placing the upper end of the cuff of an ETT (7 or 8 mm ID in size) 2 cm below the vocal cords positions the distal end of the tube approximately 4 cm from the carina. In a simple study based on measurements, it was found that, except in cases of short tracheas, placing the upper end of the cuff 2 cm below the cords predictably positioned the distal end of the ETT in the middle of the trachea.160 This maneuver should be used whenever orotracheal intubation is performed under direct laryngoscopy.
3 Prevention of Endotracheal Tube Displacement After Intubation
Despite initial proper positioning, the ETT can still slip out of the larynx (or move closer to the carina) while the laryngoscope blade is being removed, during taping of the tube, after the position of the head is changed, during positioning of the patient, or during transportation. Several precautions should be undertaken to prevent ETT displacement (Box 32-1).
Box 32-1 Precautions to Prevent Endotracheal Tube Displacement
The laryngoscope blade should be gently removed after intubation while the endotracheal tube (ETT) is secured in position.
The ETT should be taped or anchored carefully in place.
An oropharyngeal airway (or a bite block) placed adjacent to the ETT can minimize movement of the ETT inside the mouth and prevent its dislodgement, especially during coughing.
The head should be kept in neutral position unless head extension or flexion is needed for the surgical procedure.
4 Influence of Positioning on Endotracheal Tube Insertion Depth
Excessive movement of the ETT can occur during extension and flexion of the head.161,162 In a radiologic study in adults, Conrardy and coworkers demonstrated an average 3.8-cm movement of the ETT toward the carina when the head was moved from full extension to full flexion (Fig. 32-28).161 In some patients, this movement reached as much as 6.4 cm. With lateral head rotation, the ETT moved an average of 0.7 cm away from the carina. Because the average movement of the ETT when the head is moved from the neutral position to full extension or full flexion is 1.9 cm in the adult, it is unlikely that extubation or main stem intubation would occur during this movement if the distal end of the tube is in the middle of the trachea, but it could occur if it is too high or too low in the trachea.
Malpositioning of the ETT can occur with changes in patient positioning,163 with displacement of the diaphragm, and during surgical manipulation of the trachea or esophagus, especially if the ETT is not initially placed in the middle of the trachea. A high incidence of main stem intubation has been reported after institution of the Trendelenburg position. The weight of the abdominal organs results in cephalad shift of the diaphragm and the carina, which may cause a taped ETT to relocate into a main stem bronchus. The opposite can happen when the reverse Trendelenburg position is used.
Many reports have confirmed tracheal tube migration on creation of a pneumoperitoneum during laparoscopic procedures, increasing the risk for endobronchial intubation.164–169 Although the Trendelenburg tilt can lead to further migration, movement can also occur during upper abdominal laparoscopic procedures performed in the reverse Trendelenburg position.165 The mechanism is similar to that for the Trendelenburg position, but the diaphragm is displaced cephalad by the insufflated gas rather than the weight of the abdominal contents. After positioning of the tip of the ETT at 30 to 40 mm from the carina, it was found that the distance significantly decreased to 26 mm (range, 17 to 35 mm) in patients undergoing laparoscopic cholecystectomy. The maximum distance of tube migration was 8 mm (range, 0 to 15 mm). One fifth of the patients would have been at risk of bronchial intubation if the ETT had not been carefully positioned.167 ETT migration occurs more often in obese patients undergoing laparoscopic procedures compared with open abdominal surgery. In 17% of obese patients, the tube advances into the right bronchus.168 ETT displacement also occurs in children undergoing laparoscopic procedures. In one study,169 maximal displacement was 0.5 + (0.05 × age in years) for 20-degree head-down tilt, 0.6 + (0.09 × age) after insufflations, and 1.2 + (0.11 × age) for 20-degree head-down tilt and insufflations. However, in no patient did endobronchial intubation occur when the ETT was placed according to the intubation depth marking as recommended by the manufacturer.
Obviously, the tip of the ETT should be placed in the middle of the trachea, or 3 to 4 cm above the carina in adults. If the ETT moves toward the carina, then carinal stimulation may cause tachycardia, hypertension, or bronchospasm and increase the risk of bronchial intubation.163 If the ETT moves outward, the cuff may cause injury to the vocal cords and increase the risk of accidental extubation. If the ETT is placed appropriately in the middle of the trachea, it is very unlikely (except in infants) that endobronchial intubation or extubation will ensue.
The ease with which main stem intubation can occur with head flexion and unintentional extubation with head extension is of particular concern in the infant, especially in the neonate, whose trachea is only 4.7 to 5.7 cm long (Fig. 32-29).170,171 When endotracheal intubation is performed in infants, precautions should be taken to ensure that the ETT is placed far enough in the trachea but not in a main stem bronchus. One precaution is to use an ETT that has circumferential marks at 2.2 cm from the distal end and to introduce the marker on the ETT as far as the cords in term infants, slightly above the cords in preterm infants, and slightly below the cords in older infants. Another alternative that has been recommended is to use ETTs with diameters of 2.5, 3.0, and 3.5 mm and marks at 2.2, 2.4, and 2.6 cm, respectively, from the distal end. The reader is referred to pediatric anesthesia texts for more details.
5 Observation and Palpation of Chest Movements and Auscultation of Breath Sounds
Observation or palpation of an asymmetrical chest movement and detection of unequal bilateral breath sounds should alert the clinician to the possibility of a main stem intubation (usually on the right side). In addition, absence of right apical movement or breath sounds, or both, implies that the ETT and its cuff are obstructing the right upper lobe bronchus. Gradual withdrawal of the tube should correct the problem. Although auscultation of bilateral breath sounds is the most common method used in detecting main stem intubation, investigations revealed that this may not be a reliable diagnostic modality.153,172,173 Brune and colleagues observed that 60% of endobronchial intubations occurred despite the presence of equal breath sounds on auscultation.172
The inaccuracy of the auscultation method may be related to the structure of the tip of the ETT when it is placed near the carina.174 The Murphy eye, which is present in most ETTs, is a 1.0-cm elliptical port located 0.8 cm from the tip on the right side of the tube, opposite the bevel. The eye was originally designed to allow ventilation of the lungs if the bevel becomes occluded and also to allow ventilation of the right upper lobe if the ETT is accidentally advanced into the right main stem bronchus.144 Studies confirmed that the Murphy eye can maintain gas flow through the space between the ETT cuff and the right main stem bronchus and that it permits ventilation (albeit inadequate) of the left lung, thus allowing breath sounds to be heard bilaterally, until the tip is inserted 2 cm beyond the carina. Unilateral breath sounds may not be heard until the tube tip is further advanced to 3.2 cm beyond the carina.174 Accordingly, the Murphy eye reduces the reliability of chest auscultation in detecting endobronchial intubation.174
Despite the limitations of auscultation of bilateral breath sounds, continuous or repeated auscultation to detect intraoperative complications such as bronchial intubation and obstructed airway has been emphasized.175 The limitations and difficulties encountered in continuously and simultaneously auscultating right and left breath sounds have led investigators to explore other methods to amplify breath sounds.37,176 In one method, already discussed, breath sounds are quantified using electronic stethoscopes placed over each hemithorax and epigastrium.37 In another, a system that makes it possible to “visualize” breath sounds is used. The visual stethoscope allows real-time fast transformation of the sound signal and three-dimensional color rendering of the results on a computer with simultaneous processing of two individual sound signals.176 With continuous bilateral breath sounds displayed, the clinician can monitor left and right breath sounds simultaneously and can rapidly detect any changes.176 This system has potential advantages in assessing breath sounds throughout the surgical procedure, not just to verify ETT placement. Furthermore, more than one individual can monitor breath sounds.
Preliminary studies of both the electronic and the visual stethoscope showed precise accuracy for detecting endobronchial intubation.37,176 During advancement of the ETT, alterations of the shape of the visualized breath sounds appeared before changes in breath sounds were detected by auscultation. However, these studies were conducted in nonobese patients without lung pathology in a quiet operating room. Further evaluation is needed in patients with a variety of pathologic conditions and under different ambient conditions.37,176
6 Cuff Maneuvers and Neck Palpation
Various maneuvers involving palpation of the ETT cuff on each side of the trachea above the suprasternal notch (e.g., rapid cuff inflations, intermittent squeezing of the pilot balloon of a slightly overinflated cuff, use of the pilot balloon as a sensor while palpating the cuff in the neck) have been proposed to ensure that the ETT is not in a main bronchus.177,178 Suprasternal palpation of the cuff provides a high degree of confidence that the distal tip of the tube is more than 2 cm from the carina, whereas if the cuff is not palpable, the tip is probably close to the carina.178
7 Use of Fiberoptic Bronchoscopes
During fiberoptic intubation, the distance from the distal end of the ETT to the carina should be determined and the position of the ETT can be easily corrected, if necessary.148,149 The distal end of the tube is usually positioned approximately 4 cm from the carina in adult patients. The use of FFB to evaluate the position of the ETT has been extended to the critical care setting to obviate the necessity of frequent chest radiographs.
8 Transtracheal Illumination
Transillumination techniques can help position the ETT tip at a reliable distance above the carina. Two methods have been suggested. In one, the flexible lighted stylet is placed inside the ETT so that the stylet bulb is positioned at the tube’s distal opening before intubation.49,50 By observing maximal illumination at the sternal notch (a consistent anatomic landmark) during intubation, the tip of the tube can be placed consistently 5.0 ± 1.0 cm from the carina.50 In the other method, the tip of the lighted stylet is placed inside the ETT, just proximal to the cuff, before intubation. Visualization of transillumination distal to the cricoid cartilage is indicative of proper cuff positioning. In this position, the distance between the tip of the ETT and the carina varies from 3.7 to 4 cm in adults. Use of either method can reduce the need for radiographic confirmation of ETT positioning.
9 Capnography
In cases of main stem intubation, the capnographic waveform usually shows a normal pattern. If a biphasic waveform is noticed, main stem intubation or impingement of the tube on the carina should be suspected.156 Other causes of biphasic waveforms should be excluded, such as lateral decubitus position, kyphoscoliosis causing compression of the lung, pulmonary disease, spontaneous breathing efforts, hiccups, and sampling line leak.30,79,156
10 Use of the Esophageal Detector Device/Self-Inflating Bulb
The EDD with SIB is of no value in diagnosing main stem intubation. However, main stem intubation should be suspected if there is slow reinflation of the SIB.137 Rotation or gradual pullback of the ETT, or both, may lead to instantaneous reinflation of the SIB.
11 Chest Radiography
In critically ill patients, a portable chest radiograph can easily detect main stem intubation and can determine the location of the distal end of the tube in relation to the carina (Fig. 32-30).20,65,154 Because malpositioned ETTs may not be detected by routine clinical assessment, some investigators are recommending that chest radiographs should remain the standard practice in the critical care setting.65,154
IV Conclusions
On the basis of available information, two algorithms are proposed: one for verification of ETT position in elective intubation (Fig. 32-31) and the other for emergency intubation (Fig. 32-32). These algorithms are designed to assist the clinician and should not be a substitute for clinical judgment. Under no circumstances should clinical signs be ignored in the presence of conflicting information from monitors and technical aids.
V Clinical Pearls
• The ideal test for confirmation of endotracheal tube (ETT) placement should be simple, quick, and repeatable; it should function in patients of different age groups, in various locations, and during difficult intubation (DI); and it should not yield false results even in patients with cardiac arrest.
• Contributing factors to the high incidence of esophageal intubation in patients who experience cardiac arrest during out-of-hospital paramedic intubation include intubation under less than optimal conditions; unavailability of monitoring equipment, protocols, or algorithms; violation of the standard technique of auscultation; and intubation attempts by nonexpert personnel.
• Whenever “abnormal” breath sounds are heard by auscultation, they should not be relied on to confirm endotracheal intubation.
• Tube condensation of water can and does occur when an ETT is placed in the esophagus, and its presence should not be interpreted as a reliable indicator of a successful intubation.
• Oxyhemoglobin desaturation is a relatively late manifestation of esophageal intubation.
• CO2 levels as high as 5% can be detected initially in cases of esophageal intubation after the ingestion of carbonated beverages or antacids. However, rapid decline in CO2 levels occurs with successive ventilation.
• Capnography and use of colorimetric end-tidal CO2 detectors can provide a prognostic indicator of successful resuscitation.
• Visualization of the tracheal rings through a flexible fiberoptic bronchoscope (FFB) before the ETT is threaded over it does not guarantee successful endotracheal intubation.
• Common causes of false-negative results with the use of the self-inflating bulb (SIB) include decreased FRC (e.g., obesity, pregnancy, infants), ETT at the carina or main stem bronchus intubation, bronchospasm, and secretions.
• The Murphy eye reduces the reliability of chest auscultation in detecting endobronchial intubation.
All references can be found online at expertconsult.com.
1 Caplan RA, Posner KL, Ward, RJ, et al. Adverse respiratory events in anesthesia: a closed claims analysis. Anesthesiology. 1990;73:828–833.
20 Schwartz DE, Matthay MA, Cohen NH. Death and other complications of emergency airway management in critically ill adults. Anesthesiology. 1995;82:367–376.
63 Smith GM, Reed JC, Choplin RH. Radiographic detection of esophageal malpositioning of endotracheal tubes. AJR Am J Roentgenol. 1990;154:23–26.
86 Falk JL, Rackow EC, Weil MH. End-tidal carbon dioxide concentration during cardiopulmonary resuscitation. N Engl J Med. 1988;318:607–611.
116 Salem MR, Wafai Y, Baraka A, et al. Use of self-inflating bulb for detecting esophageal intubation after “esophageal ventilation.”. Anesth Analg. 1993;77:1227–1231.
124 Lang DJ, Wafai Y, Salem MR, et al. Efficacy of the self-inflating bulb in confirming tracheal intubation in the morbidly obese. Anesthesiology. 1996;85:246–253.
146 Raphael DT, Benbassat M, Arnaudov D, et al. Validation study of two-microphone acoustic reflectometry for determination of breathing tube placement in 200 adult patients. Anesthesiology. 2002;97:1371–1377.
167 Inada T, Uesugi S, Kawachi S, Takubo K. Changes in tracheal tube position during laparoscopic cholecystectomy. Anaesthesia. 1996;51:823–826.
174 Sugiyama K, Yokoyama K, Satch K, et al. Does the Murphy eye reduce the reliability of chest ausculation in detecting endobronchial intubation? Anesth Analg. 1999;88:1380–1383.
178 Pollard R, Lobato E. Endotracheal tube location verified reliably by cuff palpation. Anesth Analg. 1995;81:135–138.
1 Caplan RA, Posner KL, Ward RJ, et al. Adverse respiratory events in anesthesia: A closed claims analysis. Anesthesiology. 1990;73:828–833.
2 Cheney FW. The American Society of Anesthesiologists Closed Claims Project: What have we learned, how has it affected practice, and how will it affect practice in the future? Anesthesiology. 1999;91:552–556.
3 Salem MR, Bennet EJ, Schweiss JF, et al. Cardiac arrest related to anesthesia: Contributing factors in infants and children. JAMA. 1975;233:238–241.
4 Keenan RL, Boyan CP. Cardiac arrest due to anesthesia: A study of incidence and causes. JAMA. 1985;253:2373–2377.
5 Morray JP, Geiduschek JM, Caplan RA, et al. A comparison of pediatric and adult anesthesia closed malpractice claims. Anesthesiology. 1993;78:461–467.
6 Gannon K. Mortality associated with anaesthesia: A case review study. Anaesthesia. 1991;46:962–966.
7 Green RA, Taylor TH. An analysis of anesthesia medical liability claims in the United Kingdom, 1977–1982. Int Anesthesiol Clin. 1984;22:73–89.
8 Holland R, Webb RK, Runciman WB. Oesophageal intubation: An analysis of 2000 incident reports. Anaesth Intensive Care. 1983;21:608–610.
9 Morgan M. The confidential enquiry into maternal deaths in England and Wales [editorial]. Anaesthesia. 1986;41:689–691.
10 Utting JE. Pitfalls in anaesthetic practice. Br J Anaesth. 1987;59:877–890.
11 Batra AK, Cohn MA. Uneventful prolonged misdiagnosis of esophageal intubation. Crit Care Med. 1983;11:763–765.
12 Heiselman D, Polacek DJ, Snyder JV, et al. Detection of esophageal intubation in patients with intrathoracic stomach. Crit Care Med. 1985;13:1069–1070.
13 Howells TH, Reithmuller RJ. Signs of endotracheal intubation. Anaesthesia. 1980;35:984–986.
14 Ogden PN. Endotracheal tube misplacement [letter]. Anaesth Intensive Care. 1983;11:273–274.
15 Peterson AW, Jacker LM. Death following inadvertent esophageal intubation: A case report. Anesth Analg. 1973;52:398–401.
16 Pollard BJ, Junius F. Accidental intubation of the oesophagus. Anaesth Intensive Care. 1980;8:183–186.
17 Stirt JA. Endotracheal tube misplacement. Anaesth Intensive Care. 1982;10:274–276.
18 Stewart RD, Paris PM, Winter PM, et al. Field endotracheal intubation by paramedical personnel: Success rates and complications. Chest. 1984;85:341–345.
19 Shea SR, MacDonald JR, Gruzinski G. Prehospital endotracheal tube airway or esophageal gastric tube airway: A critical comparison. Ann Emerg Med. 1985;14:102–112.
20 Schwartz DE, Matthay MA, Cohen NH. Death and other complications of emergency airway management in critically ill adults. Anesthesiology. 1995;82:367–376.
21 Katz SH, Falk JL. Misplaced endotracheal tubes by paramedics in an urban emergency medical services system. Ann Emerg Med. 2001;37:32–37.
22 MacKenzie CF, Martin P, Xiao Y. Video analysis of prolonged uncorrected esophageal intubation. Anesthesiology. 1996;84:1494–1503.
23 Charters P, Wilkinson K. Confirmation of tracheal tube placement [reply]. Anaesthesia. 1988;43:72.
24 Wee MY. Comments on the oesophageal detector device [letter]. Anaesthesia. 1989;44:930–931.
25 Salem MR, Baraka A. Confirmation of tracheal intubation. In: Hagberg CA, ed. Benumof’s airway management. ed 2. Philadelphia: Mosby Elsevier; 2007:697–727.
26 Birmingham PK, Cheney FW, Ward RJ. Esophageal intubation: A review of detection techniques. Anesth Analg. 1986;65:886–891.
27 Banaszak EF, Kory RC, Snider GL. Phonopneumography. Am Rev Respir Dis. 1973;107:449–455.
28 Forgacs P. The functional basis of pulmonary sounds. Chest. 1978;73:399–405.
29 Uejima T. Esophageal intubation [letter]. Anesth Analg. 1987;66:481–482.
30 Salem MR. Verification of endotracheal tube position. Anesthesiol Clin North Am. 2001;19:813–839.
31 Knapp S, Kofler J, Stoiser B, et al. The assessment of four different methods to verify tracheal tube placement in the critical care setting. Anesth Analg. 1999;88:766–770.
32 Wallace CT, Cooke JE. A new method of positioning endotracheal tubes [letter]. Anesthesiology. 1976;44:272.
33 Salem MR, Wong AY, Lin YH, et al. Prevention of gastric distention during anesthesia for newborns with tracheoesophageal fistula. Anesthesiology. 1973;38:82–83.
34 Triner L. A simple maneuver to verify proper positioning of an endotracheal tube [letter]. Anesthesiology. 1982;57:548–549.
35 Linko K, Paloheimo M, Tammisto T. Capnography for detection of accidental oesophageal intubation. Acta Anaesthesiol Scand. 1983;27:199–202.
36 Andersen KH, Hald A. Assessing the position of the tracheal tube: The reliability of different methods. Anaesthesia. 1989;44:984–985.
37 O’Connor CJ, Mansy H, Balk RA, et al. Identification of endotracheal tube positions using computerized analysis of breath sounds via electronic stethoscopes. Anesth Analg. 2005;101:735–739.
38 Baraka A, Tabakian H, Idriss A, et al. Breathing bag refilling [letter]. Anaesthesia. 1989;44:81–82.
39 Robinson JS. Respiratory recording from the esophagus [letter]. BMJ. 1974;26:225.
40 Munro TN. Oesophageal misplacement of a tracheal tube [letter]. Anaesthesia. 1985;40:919.
41 Roy RC. Esophageal intubation [letter]. Anesth Analg. 1987;66:482.
42 Gillespie JH, Knight RG, Middaugh RE, et al. Efficacy of endotracheal tube cuff palpation and humidity in distinguishing endotracheal from esophageal intubation [abstract]. Anesthesiology. 1988;69:A265.
43 Kalpokas M, Russell WJ. A simple technique for diagnosing oesophageal intubation. Anesth Intensive Care. 1989;17:39–43.
44 El-Orbany MI, Salem MR, Joseph NJ. The Eschmann tracheal tube introducer is not gum, elastic, or a bougie [letter]. Anesthesiology. 2004;101:1240.
45 El–Orbany M, Salem MR. The Eschmann tracheal tube introducer is not an airway exchange device [letter]. Anesth Analg. 2004;99:1269–1270.
46 Williams RT, Stewart RD. Transillumination of the trachea with a lighted stylette. Anesth Analg. 1986;65:542–543.
47 Ellis DG, Jakymec A, Kaplan RM, et al. Guided orotracheal intubation in the operating room using a lighted stylet: A comparison with direct laryngoscopic technique. Anesthesiology. 1986;64:823–826.
48 Fox DJ, Castro T, Jr., Rastrelli AJ. Comparison of intubation techniques in the awake patient: The Flexi-lum surgical light (lightwand) versus blind nasal approach. Anesthesiology. 1987;66:69–71.
49 Mehta S. Transtracheal illumination for optimal tracheal tube placement: A clinical study. Anaesthesia. 1989;44:970–972.
50 Stewart RD, LaRosee A, Kaplan RM, Ilkhanipour K. Correct placement of an endotracheal tube using a flexible lighted stylet. Crit Care Med. 1990;18:97–99.
51 Stone DJ, Stirt JA, Kaplan MJ, et al. A complication of lightwand-guided nasotracheal intubation. Anesthesiology. 1984;61:780–781.
52 Debo RF, Colonna D, Dewerd G, et al. Cricoarytenoid subluxation: Complication of blind intubation with a lighted stylet. Ear Nose Throat J. 1989;68:517–519.
53 Szigeti CL, Baeuerie JJ, Mongan PD. Arytenoid dislocation with lighted stylet intubation: Case report and retrospective review. Anesth Analg. 1994;78:185–186.
54 Nunn JF. Nunn’s applied respiratory physiology, ed 4. Oxford: Butterworth-Heinemann; 1993. pp 219–305
55 Lees MH, King DH. Cyanosis in the newborn. Pediatr Rev. 1987;9:36–42.
56 Brodsky JB, Shulman MS, Swan M, et al. Pulse oximetry during one-lung ventilation. Anesthesiology. 1985;63:212–214.
57 Yelderman M, New W. Evaluation of pulse oximetry. Anesthesiology. 1983;59:349–352.
58 Baraka AS, Salem MR. Preoxygenation. In: Hagberg CA, ed. Benumof’s airway management: Principles and practice. ed 2. New York: Elsevier Science; 2007:303–318.
59 McShane AJ, Martin JL. Preoxygenation and pulse oximetry may delay detection of esophageal intubation. J Natl Med Assoc. 1987;79:987–992.
60 Howells TH. A hazard of pre-oxygenation [letter]. Anaesthesia. 1985;40:86.
61 Warden JC. Accidental intubation of the oesophagus and preoxygenation [letter]. Anaesth Intensive Care. 1980;8:377.
62 Guggenberger H, Lenz G, Federle R. Early detection of inadvertent oesophageal intubation: Pulse oximetry vs. capnography. Acta Anaesthesiol Scand. 1989;33:112–115.
63 Smith GM, Reed JC, Choplin RH. Radiographic detection of esophageal malpositioning of endotracheal tubes. AJR Am J Roentgenol. 1990;154:23–26.
64 Cook RT, Maglia BA. The Beck air flow monitor as an aid for evaluation of endotracheal tube placement in neonatal patients. J Pediatr. 1996;128:568–570.
65 Mandel GA. Neonatal intensive care radiology. In: Goodman L, Putman CE. Intensive care radiology: Imaging of the critically ill. Philadelphia: WB Saunders; 1983:290.
66 Mehta KH, Turley A, Peyrasse P, et al. An assessment of the ability of impedance respirometry to distinguish oesophageal from tracheal intubation. Anaesthesia. 2002;57:1090–1093.
67 Absolom M, Roberts R, Bahlmann UB, et al. The use of impedance respirometry to confirm tracheal intubation in children. Anaesthesia. 2006;61:1145–1148.
68 Murray IP, Modell JH. Early detection of endotracheal tube accidents by monitoring carbon dioxide concentration in respiratory gas. Anesthesiology. 1983;59:344–346.
69 Bashein G, Cheney FW. Carbon dioxide detection to verify intratracheal placement of a breathing tube. Anesthesiology. 1984;61:782–783.
70 Duberman SM, Bendixen HH. Concepts of fail-safe in anesthesia practice. Int Anesthesiol Clin. 1984;22:149–165.
71 Peters RM. Monitoring of ventilation in the anesthetized patient. In: Gravenstein JS, Newbower RS, Ream AK, et al. Monitoring surgical patients in the operating room. Springfield, IL: Charles C Thomas; 1979:142–149.
72 Tinker JH, Dull DL, Caplan RA, et al. Role of monitoring devices in prevention of anesthetic mishaps: A closed claims analysis. Anesthesiology. 1989;71:541–546.
73 May WS, Heavner JE, McWhorter D, Racz G. Capnography in the operating room: An introductory directory. New York: Raven Press; 1985. pp 1–58
74 Dunn SM, Mushlin PS, Lind LJ, et al. Tracheal intubation is not invariably confirmed by capnography. Anesthesiology. 1990;73:1285–1287.
75 Markovitz Bp, Silverberg M, Godinez RI. Unusual cause of an absent capnogram. Anesthesiology. 1989;71:992–993.
76 Badgwell JM, Heavner JE, May WS, et al. End-tidal PCO2 monitoring in infants and children ventilated with either a partial rebreathing or a nonrebreathing circuit. Anesthesiology. 1987;66:405–410.
77 Badgwell JM, McLoed ME, Lerman J, et al. End-tidal PCO2 measurements sampled at the distal and proximal ends of the endotracheal tube in infants and children. Anesth Analg. 1987;66:959–964.
78 Gravenstein N. Capnometry in infants should not be done at lower sampling flow rates [letter]. J Clin Monit. 1989;5:63–65.
79 Zupan J, Martin M, Benumof JL. End-tidal CO2 excretion waveform and error with gas sampling line leak. Anesth Analg. 1988;67:579–581.
80 Kern KB, Sanders AB, Voorhees WD, et al. Changes in expired end-tidal carbon dioxide during cardiopulmonary resuscitation in dogs: A prognostic guide for resuscitation. J Am Coll Cardiol. 1989;13:1184–1189.
81 Salem MR. Hypercapnia, hypocapnia, and hypoxemia. Semin Anesthesiol. 1987;6:202–215.
82 Isseries SA, Breen PH. Can changes in end-tidal PCO2 measure changes in cardiac output? Anesth Analg. 1991;73:808–814.
83 Kalenda Z. The capnogram as a guide to the efficacy of cardiac massage. Resuscitation. 1978;6:259–263.
84 Shibutani K, Whelan G, Zung N, Ferlazzo P. End-tidal CO2: A clinical noninvasive cardiac output monitor. Anesth Analg. 1991;72:S2251.
85 Trevino RP, Bisera J, Weil MH, et al. End-tidal CO2 as a guide to successful cardiopulmonary resuscitation: A preliminary report. Crit Care Med. 1985;13:910–911.
86 Falk JL, Rackow EC, Weil MH. End-tidal carbon dioxide concentration during cardiopulmonary resuscitation. N Engl J Med. 1988;318:607–611.
87 Sanders AB, Atlas M, Ewy GA, et al. Expired PCO2 as an index of coronary perfusion pressure. Am J Emerg Med. 1985;3:147–149.
88 Garnett AR, Ornato JP, Gonzalez ER, et al. End-tidal carbon dioxide monitoring during cardiopulmonary resuscitation. JAMA. 1987;257:512–515.
89 Sum Ping ST. Esophageal intubation [letter]. Anesth Analg. 1987;66:481–483.
90 Sum Ping ST, Mehta MP, Anderton JM. A comparative study of methods of detection of esophageal intubation. Anesth Analg. 1989;69:627–632.
91 Rosenblatt WH, Kharatian A. Capnography: Never forget the false positives! [letter]. Anesth Analg. 1991;73:509–510.
92 Sum Ping ST, Mehta MP, Symreng T. Reliability of capnography in identifying esophageal intubation with carbonated beverage or antacid in the stomach. Anesth Analg. 1991;73:333–337.
93 Zbinden S, Schupfer G. Detection of esophageal intubation: The cola complication [letter]. Anaesthesia. 1989;44:81.
94 Deluty S, Turndof H. The failure of capnography to properly assess endotracheal tube location. Anesthesiology. 1993;78:783–784.
95 Wafai Y, Salem MR, Baraka A, et al. Effectiveness of the self-inflating bulb for verification of proper placement of the esophageal-tracheal Combitube. Anesth Analg. 1995;80:122–126.
96 Owen RL, Cheney FW. Use of an apnea monitor to verify endotracheal intubation. Respir Care. 1985;30:974–976.
97 Smith RH, Volpitto PP. Simple method of determining CO2 content in alveolar air [letter]. Anesthesiology. 1959;20:702–703.
98 Berman JA, Burgiuele JJ, Marx GF. The Einstein carbon dioxide detector [letter]. Anesthesiology. 1984;60:613–614.
99 Strunin L, Williams T. The FEF end-tidal carbon dioxide detector [letter]. Anesthesiology. 1989;71:621–622.
100 Denman WT, Hayes M, Higgins D, et al. The Fenem CO2 detector device: An apparatus to prevent unnoticed oesophageal intubation. Anaesthesia. 1990;45:465–467.
101 O-Flaherty D, Adams AP. The end-tidal carbon dioxide detector: Assessment of a new method to distinguish oesophageal from tracheal intubation. Anaesthesia. 1990;45:653–655.
102 Goldberg JS, Rawle PP, Zehnder IL, et al. Colorimetric end-tidal carbon dioxide monitoring for tracheal intubation. Anesth Analg. 1990;70:191–194.
103 Jones BR, Dorsey MJ. Sensitivity of a disposable end-tidal carbon dioxide detector. J Clin Monit. 1991;7:268–270.
104 MacLeod GJ, Heller MB, Gerard J, et al. Verification of endotracheal tube placement with colorimetric end-tidal CO2 detection. Ann Emerg Med. 1991;20:267–270.
105 Ornato JP, Shipley JB, Racht EM, et al. Multicenter study of a portable, hand-size, colorimetric end-tidal carbon dioxide detection device. Ann Emerg Med. 1992;21:518–523.
106 Kelly JS, Wilhoit RD, Brown RE, et al. Efficacy of the FEF colorimetric end-tidal carbondioxide detector in children. Anesth Analg. 1992;75:45–50.
107 Gedeon A, Krill P, Mebius C. A new colorimetric breath indicator (Colobri): A comparison of the performance of two carbon dioxide indicators. Anaesthesia. 1994;49:798–803.
108 Heller MB, Yealy DM, Seaberg DC, et al. End-tidal CO2 detection. Ann Emerg Med. 1989;18:1375.
109 Wee MYK. The oesophageal detector device: Assessment of a method to distinguish oesophageal from tracheal intubation. Anaesthesia. 1988;43:27–29.
110 O’Leary JJ, Pollard BJ, Ryan MJ. A method of detecting esophageal intubation or confirming tracheal intubation. Anaesth Intensive Care. 1988;16:299–301.
111 Wee MYK, Walker KY. The oesophageal detector device: An assessment with uncuffed tubes in children. Anaesthesia. 1991;46:869–871.
112 Nunn JS. The oesophageal detector device [letter]. Anaesthesia. 1998;43:804.
113 Williams KN, Nunn JF. The oesophageal detector device: A prospective trial in 100 patients. Anaesthesia. 1989;44:412–414.
114 Baraka A, Muallem M. Confirmation of correct tracheal intubation by a self-inflating bulb. Middle East J Anesthesiol. 1991;11:193–194.
115 Oberly D, Stein S, Hess D, et al. An evaluation of the esophageal detector device using a cadaver model. Am J Emerg Med. 1992;10:317–320.
116 Salem MR, Wafai Y, Baraka A, et al. Use of self-inflating bulb for detecting esophageal intubation after “esophageal ventilation.”. Anesth Analg. 1993;77:1227–1231.
117 Zaleski L, Abello D, Gold MI. The esophageal detector device: Does it work? Anesthesiology. 1993;79:244–247.
118 Salem MR, Wafai Y, Joseph NJ, et al. Efficacy of the self-inflating bulb in detecting esophageal intubation: Does the presence of a nasogastric tube or cuff deflation make a difference? Anesthesiology. 1994;80:42–48.
119 Baraka A. The esophageal detector device [letter]. Anaesthesia. 1991;45:697.
120 Haynes SR, Morten NS. Use of the oesophageal detector device in children under one year of age. Anaesthesia. 1991;45:1067–1069.
121 Smith I. Confirmation of correct endotracheal tube placement [letter]. Anesth Analg. 1991;72:263.
122 Morten NS, Stuart JC, Thompson MF, Wee MY. The esophageal detector device: Successful use in children. Anaesthesia. 1989;5:523–524.
123 Wafai Y, Salem MR, Joseph NJ, Baraka A. The self-inflating bulb for confirmation of tracheal intubation: Incidence and demography of false negatives [abstract]. Anesthesiology. 1994;81:A1303.
124 Lang DJ, Wafai Y, Salem MR, et al. Efficacy of the self-inflating bulb in confirming tracheal intubation in the morbidly obese. Anesthesiology. 1996;85:246–253.
125 Baraka A, Khoury PJ, Siddik SS, et al. Efficacy of the self-inflating bulb in differentiating esophageal from tracheal intubation in the parturient undergoing cesarean section. Anesth Analg. 1997;84:533–537.
126 Donahue PL. The esophageal detector device: An assessment of accuracy and ease of use by paramedics. Anaesthesia. 1994;49:863–865.
127 Bozeman WP, Hexter D, Liang HK, et al. Esophageal detector device versus detection of end-tidal carbon dioxide level in emergency intubation. Ann Emerg Med. 1996;27:595–599.
128 Schaller RJ, Huff JS, Zahn A. Comparison of colorimetric end-tidal CO2 detector and an esophageal aspiration device for verifying endotracheal tube placement in the pre-hospital setting: A six-month experience. Prehosp Disaster Med. 1997;12:57–63.
129 Kasper DL, Deem US. The self-inflating bulb to detect esophageal intubation during emergency airway management. Anesthesiology. 1998;88:898–902.
130 Tanigawa K, Takeda T, Goto E, Tanaka K. Accuracy and reliability of the self-inflating bulb to verify tracheal intubation in out-of-hospital cardiac arrest. Anesthesiology. 2000;93:1432–1436.
131 Tanigawa K, Takeda T, Goto E, Tanaka K. The efficacy of esophageal detector devices in verifying tracheal tube placement: A randomized cross-over study of out-of-hospital cardiac arrest patients. Anesth Analg. 2001;92:375–378.
132 Schuh JR, Bettendorf R, Shapiro D, et al. Negative intratracheal pressure produced by esophageal detector devices causes tracheal wall collapse in a porcine cardiac arrest model. Anesth Analg. 2010;111:468–472.
133 Lang DJ, Salem MR. Tracheal wall collapse produced by esophageal detector devices in a porcine cardiac arrest model. Anesth Analg. 2011;112:994–995.
134 Foutch RG, Magelssen MD, MacMillan JG. The esophageal detector device: A rapid and accurate method of assessing tracheal versus esophageal intubation in a porcine model. Ann Emerg Med. 1992;21:1073–1076.
135 Andres AH, Langenstein H. The esophageal detector device is unreliable when the stomach has been ventilated. Anesthesiology. 1999;91:566–568.
136 Wafai Y, Salem MR, Czinn EA, et al. The self-inflating bulb in detecting esophageal intubation: Effect of bulb size and technique used [abstract]. Anesthesiology. 1993;79:A496.
137 Salem MR, Wafai Y, Joseph NJ, et al. The esophageal detector device: Ellick’s evacuator versus syringe [reply]. Anesthesiology. 1995;82:313–316.
138 Murray D, Ward ME, Sear JW. SCOTI: A new device for identification of tracheal intubation. Anaesthesia. 1995;50:1062–1064.
139 Nandwani N, Caranza R, Lin ES, Raphael JH. Configuration of the SCOTI device with different tracheal tubes. Anaesthesia. 1996;51:932–934.
140 Lochey DJ, Woodward W. SCOTI vs. Wee: An assessment of two oesophageal intubation detection devices. Anaesthesia. 1997;52:242–243.
141 Haridas RP, Chesshire NJ, Rocke DA. Evaluation of the SCOTI device. Anaesthesia. 1997;52:453–456.
142 Trikha A, Singh C, Rewari V, Arora MK. Evaluation of the SCOTI device. Anaesthesia. 1999;54:347–349.
143 Maleck WH. Distinguishing endotracheal and esophageal intubation. Anesthesiology. 2001;94:539–540.
144 Mansfield JP, Lyle RP, Voorhees WD, Wodicka GR. An acoustical guidance and position monitoring system for endotracheal tubes. IEEE Trans Biomed Eng. 1993;40:1330–1335.
145 Raphael DT. Acoustic reflectometry profiles of endotracheal and esophageal intubation. Anesthesiology. 2000;92:1293–1299.
146 Raphael DT, Benbassat M, Arnaudov D, et al. Validation study of two-microphone acoustic reflectometry for determination of breathing tube placement in 200 adult patients. Anesthesiology. 2002;97:1371–1377.
147 Ford RWJ. Confirming tracheal intubation: A simple manoeuvre. Can Anaesth Soc J. 1983;30:191–192.
148 Benumof JL. Management of the difficult adult airway: With special emphasis on awake tracheal intubation. Anesthesiology. 1991;75:1087–1110.
149 Whitehouse AC, Klock LE. Evaluation of endotracheal tube position with the fiberoptic intubation laryngoscope [letter]. Chest. 1975;68:848.
150 Moorthy SS, Dierdorf SF. An unusual difficulty in fiberoptic intubation [letter]. Anesthesiology. 1985;63:229.
151 Nichols KP, Zornow MH. A potential complication of fiberoptic intubation. Anesthesiology. 1989;70:562–563.
152 Schwartz KD, Johnson C, Roberts J. A maneuver to facilitate flexible fiberoptic intubation. Anesthesiology. 1989;71:470–471.
153 McCoy EP, Russell WJ, Webb RK. Accidental bronchial intubation: An analysis of AIMS incident reports from 1988 to 1994 inclusive. Anaesthesia. 1997;52:24–31.
154 Schwartz DE, Lieberman JA, Cohen NJ. Women are at greater risk than men for malpositioning of the endotracheal tube after emergent intubation. Crit Care Med. 1994;22:1127–1131.
155 Mecca RS. Management of the difficult airway. In: Kirby RR, Gravenstein N. Clinical anesthesia practice. ed 2. Philadephia: WB Saunders; 2002:921–954.
156 Gilbert D, Benumof JL. Biphasic carbon dioxide elimination waveform with right mainstem bronchial intubation. Anesth Analg. 1989;69:829–832.
157 Venkateswarlu T, Turner CJ, Carter JD, Morrow DH. The tracheal bronchus: An unusual airway problem. Anesth Analg. 1976;55:746–747.
158 Vredevoe LA, Brechner T, Moy P. Obstruction of anomalous tracheal bronchus with endotracheal intubation. Anesthesiology. 1981;55:581–583.
159 Owen RL, Cheney F. Endobronchial intubation: A preventable complication. Anesthesiology. 1987;67:255–257.
160 Cristoloveanu C, Salem MR, Joseph NJ. Does positioning the upper end of the tracheal tube cuff 2 cm below the vocal cords assure proper tracheal tube positioning? [abstract]. Anesthesiology. 2003;99:A1239.
161 Conrardy PA, Goodman LR, Lainge F, et al. Alteration of endotracheal tube position: Flexion and extension of the neck. Crit Care Med. 1976;4:8–12.
162 Hartrey R, Kestin G. Movement of oral and nasal tracheal tubes as a result of changes in head and neck position. Anaesthesia. 1995;50:682–687.
163 Heinonen J, Takki S, Tamisto T. Effect of the Trendelenburg tilt and other procedures on the position of endotracheal tubes. Lancet. 1969;26:850–853.
164 Cunningham A, Brull SJ. The laparoscopic cholecystectomy: Anesthetic implications. Anesth Analg. 1993;76:1120–1130.
165 Brimacombe JR, Orland H, Graham D. Endobronchial intubation during upper abdominal laparoscopic surgery in the reverse Trendelenburg position. Anesth Analg. 1994;78:607.
166 Morimura N, Inouek MiwaT. Chest roentgenogram demonstrates cephalad movement of the carina during the laparoscopic cholecystectomy. Anesthesiology. 1994;81:1301–1302.
167 Inada T, Uesugi S, Kawachi S, Takubo K. Changes in tracheal tube position during laparoscopic cholecystectomy. Anaesthesia. 1996;51:823–826.
168 Ezri T, Hazin V, Warters D, et al. The endotracheal tube moves more often in obese patients undergoing laparoscopy compared with open abdominal surgery. Anesth Analg. 2003;96:278–282.
169 Bottcher-Haberzeth S, Dullenkopf A, Gitzelmann CA, Weiss M. Tracheal tube tip displacement during laparoscopy in children. Anaesthesia. 2007;62:131–134.
170 Boseman YK, Foster PA. Endotracheal intubation and head posture in infants. S Afr Med J. 1977;52:71–73.
171 Todres ID, deBros F, Kramer SS, et al. Endotracheal tube displacement in the newborn infant. J Pediatr. 1976;89:126–127.
172 Brunel W, Coleman DL, Schwartz DE, et al. Assessment of routine chest roentgenograms and the physical examination to confirm endotracheal tube position. Chest. 1989;96:1043–1045.
173 Szekely SM, Webb RK, Williamson JA, Russell WJ. Problems related to the endotracheal tube: An analysis of 2000 incident reports. Anaesth Intensive Care. 1993;21:611–616.
174 Sugiyama K, Yokoyama K, Satch K, et al. Does the Murphy eye reduce the reliability of chest ausculation in detecting endobronchial intubation? Anesth Analg. 1999;88:1380–1383.
175 Schwartz N. Monitoring bilateral breath sounds. [letter]. Anesthesiology. 1987;66:711–712.
176 Kato H, Susuki A, Nakajima Y, et al. A visual stethoscope to detect the position of the tracheal tube. Anesth Analg. 2009;109:1836–1842.
177 Bednarek FJ, Kuhns LR. Endotracheal tube placement in infants determined by suprasternal palpation: A new technique. Pediatrics. 1975;56:2–4.
178 Pollard R, Lobato E. Endotracheal tube location verified reliably by cuff palpation. Anesth Analg. 1995;81:135–138.