Chapter 135 Complications of Peripheral Nerve Surgery
Preoperative Assessment for Complication Avoidance
The goals of preoperative assessment include recognition of the pathology, identification of the disease etiology, and selection of an appropriate treatment plan. A number of considerations must be ruled out before any surgical intervention. These complicating factors include immunologic disorders, toxic polyneuropathies, metabolic processes, inflammatory diseases, nutritional deficiencies, drug-induced neuropathies, vasculitides, hormonal etiologies, and connective tissue disorders.1,2
Planning a successful surgery requires a thorough knowledge of normal and variant peripheral nerve anatomy, of the adjacent structures, and of the basic concepts of nerve regeneration and intraneural anatomy.1,3–12 Thus, an inadequate or misinterpreted preoperative assessment can lead to improper surgical interventions and subsequent complications.1,10,13–15
Clinical Examination
Many variants of peripheral nerve anatomy exist, and misinterpretation of nonspecific sensory findings can lead to an inaccurate assessment of the nerve injury. For example, in the Riche-Cannieu anomaly, branches of both the median and ulnar nerves supply sensation to the thumb, instead of the normal anatomic splitting.10,16 In the Martin-Gruber anastomosis there are median-to-ulnar crossover communications in the forearm.10,16,17
The clinical examination should begin with a visual and tactile examination for evidence of irregularities, tenderness, involuntary or voluntary movement, or atrophy. This examination should be followed by specific motor tasks to reveal a measurable neurologic deficit, and care is needed to differentiate between incongruous movements and legitimate pathologic processes. For example, muscle loss can be masked by compensatory adjacent muscle contractions.1
Electrodiagnostic Studies
Electrodiagnostic studies help determine the severity of the nerve injury and establish the baseline of the nerve’s physiologic status and functional integrity.18 In addition, sensory testing with electrophysiologic studies can confirm suspect findings, delineate the problem, or predict the possibility of spontaneous recovery.10,14,19,20 These data can be influenced by a patient’s adaptive response, temperature changes in the pain receptor area, thickness of the myelin sheath, or autonomic conditions.21–27
Nerve conduction velocity studies measure the velocity, intensity, and time that it takes an electrical signal to travel the length of the involved nerve. These studies are influenced by the type of instrument being used, the duration and intensity of the stimulation, and the relative distance of the internodes.28–30 Conduction velocity normally decreases with age from myelin degeneration and changes in the internodal distances.31 In addition, abnormal conduction test results do not necessarily correlate with a complete loss of sensation because a patient who has a sutured nerve may not fully display nerve conduction but can appreciate light touch.32
Nerve recordings are best measured directly proximal to a nerve lesion, and other interventions may hinder accurate recordings. For example, fluctuations in nerve waveforms can occur if a tourniquet is used or if a nerve is being dissected. Other confounding factors include extensive injuries, neurologic deficits, and the patient’s age and associated medical conditions. Nerve conduction velocity is inconclusive and may demonstrate slow to below-normal values or fibrillations in neuropathies with wallerian degeneration or “dying back” in a portion of an axon.33
Many authors advise using nerve stimulation to ascertain if distal segments are innervated before surgical exploration.34–37 However, in the absence of electrical conduction studies, surgical intervention within 3 months of the injury is warranted when there is loss of function in one or more neural elements.10
Imaging Studies
Frequently, peripheral nerve surgery is possible without any diagnostic imaging. A combination of a thorough history and physical examination with supplementary electrodiagnostic studies is often adequate. However, in cases where a mass lesion or tumor is suspected, MRI is necessary for a preoperative anatomic definition and for a differential diagnosis. Other imaging studies such as plain-film radiographs and CT scans play a role in diagnosing associated injuries that may complicate peripheral nerve surgery. When attempting to diagnose root avulsion, a CT scan with myelography is often helpful to visualize the pseudomeningocele.16 However, even in the presence of a pseudomeningocele, the nerve rootlets may not be compromised.38,39
Timing of Surgery
Proper timing of surgery is crucial to restore neuronal function, to reverse end-organ dysfunction, and to facilitate recovery. Because neural regeneration occurs at the rate of approximately 1 inch per month, prompt reestablishment of neuronal connections is essential. In nonpenetrating, stretch, and compression injuries without evidence of transection, conservative management should be used for 3 months to allow for maximum recovery.40
In trauma or postsurgery, evidence of vascular injuries such as diminished extremity pulses, auscultation of bruits or thrills, or large pulsatile hemorrhage warrants immediate intervention.41,42 A detailed clinical evaluation with careful documentation is needed, and further angiographic investigation may be used preoperatively or intraoperatively to outline the vascular anatomy and to rule out pseudoaneurysm. If available, MRI scans may be obtained to assess adjacent neurovascular structures, and a vascular surgeon should be consulted.
Immediate surgical intervention is also warranted when the patient presents with an acute neurologic deficit. If the nerve is nonviable and transected, nerve repair should be performed. Surgical exploration of gunshot wounds and other blunt transections can be delayed for 3 weeks when there is no evidence of neurovascular compromise.40 However, if the neurologic function deteriorates, surgery should proceed without delay.
Techniques of Nerve Repair
Functional outcomes are significantly affected by proper procedure selection, and an understanding of axonal regeneration and neurotropic factors has led to advances in the treatment of these diseases.43–47 Tube repair13,48 (with silicone, collagen, and polyglycolic acid) and allograft nerve transplantation49,50 are under investigation for restoring nerve continuity. The neurotropic factors produced by regenerating nerves are also being investigated for use in surgery or for implantation within the tubes.
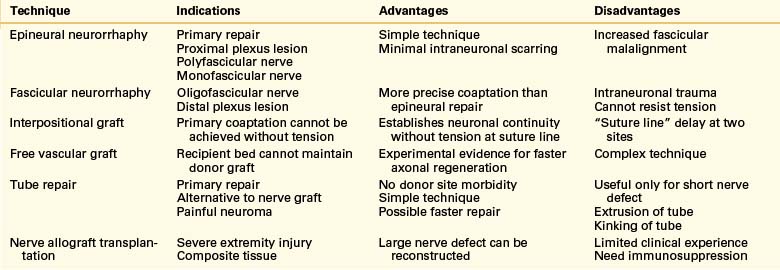
Nerve allograft transplantation may be an alternative to autograft repair, but this technique requires immunosuppression to avoid graft rejection. The indications for and relative advantages and disadvantages of commonly used techniques of nerve repair are summarized in Table 135-1.
Postoperative Management
Postoperative care is essential for improving outcomes and avoiding complications, and monitoring for wound infections and neurologic improvement is important. Postoperative complications mainly arise in the surgical area and its adjacent structures. Routine wound cleansing with hydrogen peroxide followed by an application of an antibiotic ointment can reduce bacterial infection. Early mobilization is recommended because this measure improves circulation and prevents soft tissue adhesions, and physical and occupational therapy consultations are needed. Aggressive pain management is also important to recovery. Finally, prophylactic use of anticoagulants, such as intravenous dextran, intravenous or subcutaneous heparin, or oral aspirin, may prevent thrombosis at the microsurgical region.51
Rehabilitation
After a peripheral nerve injury or surgery, physical therapy and range-of-motion exercises will optimize the recovery of motor function and minimize tethering adhesion formation. The need for long-term rehabilitation is determined on an individual basis because functional recovery depends on the type and extent of injury, the capability of the nerve to regenerate, associated medical conditions, and the patient’s motivation. The recovery process is slow and variable and may take as long as 5 years to complete. However, if functional recovery is not apparent after an appropriate rehabilitation period, other options such as arthrodesis and tendon transfers should be considered. Clearly, extensive injuries with multiple nerve lesions require a longer time to improve than isolated nerve injuries.
Complications of Peripheral Nerve Surgery
General Complications
Postoperative Wound Infections
To prevent infection, the dissection should be carried along intramuscular planes and along the nerve course, and wound closure should eliminate the dead spaces that can later become infected.52 Postoperative wound care with an antibiotic ointment and daily inspections and dressing changes is needed. Postoperative antibiotic prophylaxis is used for 24 hours but should not be necessary beyond this point unless gross contamination was present.
Postoperative Hematoma
Clinically significant postoperative hematoma is a rare but serious entity. This lesion may act as a nidus for infection, a source of postoperative scarring, and a compressive mass. Extensive and repetitive muscle dissection often creates fistulae, fascial planes, and bleeding points that can contribute to hematoma formation. Bipolar electrocautery is recommended for hemostasis because this modality minimizes thermal injury to the adjacent neurovascular structures and soft tissues.10 Infected hematomas and adjacent tissues should be surgically excised and appropriate cultures and stains obtained.
Pulmonary Complications
Pulmonary complications can occur, especially if general anesthesia and lengthy operative time are necessary. These complications include atelectasis, pneumonia, pulmonary embolus, and acute respiratory distress syndrome.53 Preoperative assessment of pulmonary risk factors aids in prevention, and postoperative pulmonary care assists in avoiding unnecessary complications. Specifically, pleural effusion, pneumothorax, hemothorax, and diaphragmatic paralysis can result from brachial plexus or thoracic outlet procedures. Each complication should be appropriately identified based on imaging and clinical judgment and should subsequently be treated appropriately.
Anatomic Variants
Anatomic variants of neural elements and non-neural structures are common and can be a source of iatrogenic injury (Table 135-2). These variations are usually encountered in certain areas, and a thorough knowledge of normal and aberrant anatomy is paramount.1 For example, the nerve to the triceps commonly can arise off the dorsal spinal cord of the brachial plexus in the axilla. The subscapular nerve may arise from the posterior cord as part of a common trunk with the nerve to the subscapularis, or from the axillary nerve itself. The musculocutaneous nerve may arise from the median nerve or can be associated with the tendon of the pectoralis major muscle. Thus, when retracting, dividing, or reapproximating this tendon, the surgeon should pay close attention to preserving this nerve.
| Affected Nerve | Procedure |
|---|---|
| Spinal accessory | Lymph node biopsy |
| Transverse cervical or greater auricular | Lymph node biopsy |
| Brachial plexus | Radical neck dissection or mastectomy |
| Median | Carpal tunnel release or removal of ganglion |
| Radial | Arterial puncture or osteosynthesis |
| Ulnar | Removal of ganglion or wrist osteotomy |
| Superficial radial | Kirschner wire placement or removal of ganglion |
| Anterior interosseous | Internal fixation of forearm |
| Posterior interosseous | Osteosynthesis or cast |
| Sciatic | Hip arthroplasty |
| Femoral | Hip arthroplasty or femoral arterial graft |
| Genitofemoral or ilioinguinal | Hernia repair |
| Tibial | Injection or orthopedic procedure |
| Common peroneal | Surgery of the knee or removal of Baker cyst |
| Sural | Vein extirpation |
| Saphenous | Vein stripping |
Complications of Surgery for Peripheral Nerve Tumors
Both benign and malignant tumors affect peripheral nerves.54–57 These tumors cause motor and sensory symptoms through local mass effect with entrapment as well as compression of adjacent neurovascular structures. Before surgical excision, it is prudent to consider all tumors malignant until confirmed by pathologic examination.
Patients with peripheral nerve tumors should be referred to specialized centers that have experience with peripheral nerve lesions. In general, nerve tumors should be removed when small to decrease the incidence of complications. Biopsies are not recommended in lesions likely to be benign because this may lead to nerve damage or hemorrhage.58
Benign Peripheral Nerve Tumors
Neurofibromas can be multiple and fusiform, especially ones that arise from the nerve trunks, and surgery is needed for neurologic deterioration or intractable pain.56,59 Resection typically does not result in loss of neurologic function or permanent disability. However, superficial neurofibroma resection may result in a temporary decrease in cutaneous sensation.57
Malignant Peripheral Nerve Sheath Tumors
The appropriate diagnosis of malignant peripheral nerve sheath tumors is essential, and reliable clinical findings suggesting the presence of malignancy include a large tumor mass at initial presentation and rapid increase in size over a period of weeks to months.56,60 Initial management should include a percutaneous nerve biopsy to provide histologic evidence of malignancy, because intraoperative frozen sections are frequently unreliable.57 Once the diagnosis is established, radical resection of these lesions is recommended, and this resection needs to extend 5 to 10 cm beyond the tumor margin in a contiguous nerve if possible.
Complications of Surgery for Entrapment Neuropathies
Diagnostic Pitfalls
The preoperative assessment includes a thorough history and physical and electrophysiologic studies; misinterpretation of these findings may lead to delay in therapeutic or surgical interventions.1,13,15 Associated medical conditions may include diabetes, hormonal diseases, connective tissue disorders, rheumatoid disease, arthritis, and metabolic deficiency. In addition, central nervous system pathologies such as syringomyelia, spinal cord lesions, and intracranial masses should be excluded before any surgical intervention.
Electrodiagnostic studies are often used to confirm peripheral nerve entrapment neuropathies.10,15 Inaccurate results may be due to anatomic variations, associated medical conditions, overlapping sensory innervation, errors in technical skill, and misinterpretation of the results. Examples of overlapping sensory innervation include the Riche-Cannieu anomaly (both branches of the median and ulnar nerves communicate) and the Martin-Gruber anastomosis (the median nerve or the anterior interosseous branch communicates with the ulnar nerve). Awareness of these pitfalls allows for correct interpretation and management.
Surgical Pitfalls
Some lesions are unrecognizable or cannot be accurately localized with electrodiagnostic studies but will be detected intraoperatively. Aggressive radical decompression may carry more risks than benefits, and in many cases simple external or internal neurolysis is more beneficial to the patient.61,62
Cubital Tunnel Release
Iatrogenic ulnar neuropathy may be caused by direct pressure on the nerve at the medial aspect of the elbow while positioning the patient, and thus appropriate cushioning is vital. Surgery on the ulnar nerve also can have significant complications. For example, nerve transposition procedures may exacerbate cubital tunnel syndrome because the recently transposed ulnar nerve can be compressed at the entry of the cubital tunnel, at the intermuscular septum, or by fascial slings.10,15,63–65 This complication may be avoided by dissecting the nerve beyond the distal and proximal ends to permit relaxation of the nerve and to prevent kinking. Simple ulnar decompression is often preferred over transposition because it is relatively easy to perform and has fewer complications.59,66,67
Thoracic Outlet Syndrome
A dorsal subscapular approach is used for patients who have a large neck and large cervical ribs. The advantage of this technique is that it allows the surgeon to visualize the spinal nerves at their intervertebral foramina; however, this approach can cause damage to the long thoracic nerve, resulting in scapular winging. This approach may be used as a salvage procedure after failure of the transaxillary rib approach.68
Standard Open Carpal Tunnel Release
Improper interpretation of the history and clinical examination is a common cause of inappropriate carpal tunnel release, and surgical intervention is difficult to justify without confirmation from electrodiagnostic studies. The Phalen maneuver and Tinel sign are consistent with carpal tunnel disorder, but abnormal nerve conduction velocity recordings and electromyography will confirm it.10,15,69
Iatrogenic injuries that result from anatomic variations are common in carpal tunnel surgeries. Caution should be taken not to injure the ulnar neurovascular bundle when retracting the retinaculum, or to damage the ulnar nerve and artery, which are located radial to the hook of the hamate. Injury to the superficial palmar arch during carpal tunnel release can also occur.70,71 Beris et al. reported anatomic variations in the median nerve in 11 of 110 patients who underwent carpal tunnel release. The anomalous groups reported by these authors include variations of the thenar branch, accessory branches in the distal or proximal tunnel, and proximal division of the nerve.72
Persistent wrist pain after a carpal tunnel release indicates an incomplete resection of the transverse carpal ligament or an injury to the terminal branches of the anterior interosseous nerve.13,71,73,74 These complications can be avoided by visualizing and inspecting the extended course of the transverse carpal ligament to ensure a complete ligamental transection. A small incision may be inadequate because the transverse carpal ligament may be located distally and is embedded in the palmar fascia. In addition, the distal edge of the ligament contains a ramus communicans located between the median and ulnar nerves that should be preserved.75 The motor division of the median nerve also may course along the ligament and may come from a superficial or ulnar origin.1,76 Thus, maintaining an extended incision along the ulnar aspect of the carpal ligament and an awareness of the motor branch during dissection are needed.10 In rare cases, an anomalous ulnar nerve course can result in nerve transection as well.77
Minor or debilitating postoperative pain may be caused by the formation of a neuroma. Neuromas can occur with incisions at the thenar crease or transverse at the wrist.71,76,78 When a neuroma is suspected, reexploration and resection of this lesion is indicated. Other causes of postoperative pain include injury to the dorsal sensory branch of the radial nerve, to the palmar branch of the digital nerve, or to the palmar cutaneous branch of the median nerve.79 The palmar cutaneous branch is particularly at risk because of its inconsistent course.1
Postoperative carpal arch widening or flexor tendon bowstringing can occur, which may decrease grip strength.71,74,80 Bony instability from ligamental transection also may compress the small cutaneous or periosteal nerves.70,73,78 Although the free margins of the flexor tendon could be resutured, the risk of nerve injury is high.10 The best preventive measure is early wrist splinting after surgery.73
Anterior interosseous nerve injury may result from retractor pressure beneath the distal antebrachial fascia, so careful placement is necessary.13 In addition, a Colles fracture or forearm crush injury may significantly compress the distal antebrachial fascia.1
Keloid formation is a concern for both cosmetic reasons and its potential for nerve entrapment, and no particular incision reduces the risk of this complication. 73,74,81 Because keloid resection will likely lead to further keloid formation, other therapies such as cortisone cream, local anesthesia, analgesics, and reassurance are recommended.
Endoscopic Carpal Tunnel Release
Ulnar neuropraxia due to pressure or retraction may result from the transbursal approach of the dual-portal Chow technique. In addition, a complete transection of the ulnar nerve may occur if the trocar is accidentally placed into the Guyon canal.82 Thus, the endoscopic trocar should be inserted anterior to the superficial palmar arch and midpalmar digital nerve. Other complications of the Chow technique include tissue scarring, pseudoaneurysm and injury to the superficial palmar arch, median nerve injury, flexor digitorum superficialis laceration, and reflex sympathetic dystrophy (RSD).83
In the Brown dual-portal technique, common postoperative complications are superficial palmar arch injury, transient paresthesias, and RSD.84–86 In the extrabursal approach (subligamentous dual-portal technique) developed by Resnick and Miller, the flexor tendon is dissected beneath the transverse ligament.87 To avoid injuring the median nerve branches, an incision can be made at the ulnar margin and a Freer elevator used to delineate the median nerve from the carpal tunnel ligament before inserting the endoscopic trocar.
The single-portal Agee technique carries the risk of injury from blind sharp dissection through the carpal tunnel. The subsequent modified single-portal Agee endoscopic system allows blade visualization to reduce iatrogenic injury. The reported complication rate of this procedure is 1.83%, whereas the failure rate is 1.44%.32,85,88–95 Other possible complications associated with the Agee technique include postoperative infection, hematoma, pillar causalgia, laceration of the flexor digitorum sublimis, and RSD.93 The incidence of RSD can be as high as 5% and may be even higher with coexistent Dupuytren disease.73,74,79,96
A comprehensive review of 8068 endoscopic carpal tunnel release (ECTR) procedures by Jimenez et al.93 demonstrated that complication, failure, and success rates are similar for both endoscopic and standard open procedures. The retrospective questionnaire revealed an overall complication rate of 2.6%.93 Postoperative complications associated with artery, nerve, and tendon injuries were relatively higher in ECTR (1.6%) than in open carpal tunnel release (OCTR; 0.8%), and no statistically significant differences in persistent and recurrent symptoms were found in these two groups (ECTR 7.5% vs. OCTR 7.7%).
A successful outcome in ECTR depends on appropriate patient selection, proper identification of anatomic landmarks, and adequate endoscopic visualization. Contraindications to ECTR include previous fracture, cysts (ganglion or synovial), neuromas, aberrant anatomy, sepsis, and previously failed carpal tunnel release.93 In addition, experience with these techniques plays a role in outcome. The option exists for conversion from ECTR to OCTR when encountering technical difficulty or poor visualization.
Brachial Plexus Surgery
Vascular injuries include both venous and arterial sources. Most small veins may be sacrificed unless the patient had previous radiation or lymph node dissections, which may predispose the patient to lymphedema. The larger veins should be preserved to prevent upper extremity swelling. Any significant arterial injuries should be immediately repaired by a vascular surgeon, and these injuries are more likely with scarring from previous explorations.40
Other Peripheral Nerve Surgeries
Any peripheral nerve surgery carries the risk of nerve damage. Other such procedures include decompression of the sciatic, peroneal, tibial, femoral, and inguinal nerves. In sciatic nerve surgery, postoperative muscular atrophy, vascular injury, and nerve damage may occur. In particular, the superior and inferior gluteal arteries must be preserved because transection may lead to life-threatening hemorrhage from vessel retraction. Patients with peroneal and tibial nerve surgery also may have complicated postoperative courses because of pain and swelling for months after surgery. Procedures directed at the femoral, inguinal, and other associated groin nerves may also have surgery-related complications. In particular, the complex anatomy and frequent overlapping distributions in this region may lead to improper decompression, and the anatomic location is more prone to infection as well as lymphoceles.58
Pain of Peripheral Nerve Origin
Pain prevention is essential to the treatment of peripheral nerve problems.15,36 Neuropathic pain results from neural injury in the central or peripheral nervous system and is characterized by paresthesia or numbness in a specific distribution. This pain may also lack an identifiable source.97,98 Postoperative pain is a possible complication of peripheral nerve surgery and, as with other complications, the best method of treatment is prevention. Careful dissection and an anatomic awareness will prevent transection of the nerve, and electrocoagulation should be avoided to prevent neuroma formation.99
When a complete or partial cutaneous nerve laceration is the source of pain, immediate repair is necessary. When an intact nerve is the source of pain, neurolysis can successfully relieve symptoms.100 Severe postoperative pain should be investigated immediately because it may be the source or sign of significant postoperative complications, and precise localization is necessary. For example, a stocking distribution of pain may be a sign of vascular injury.
Sympathetic involvement or “posttraumatic pain events” include paresthesia and intolerance.101 These symptoms may progress to the pain syndrome of RSD, which is hypothesized to be related to impaired macrocirculatory and microcirculatory hemodynamics (nutritional deficiency).101 Thus, the diagnosis is aided by evaluating the patient’s hemodynamic and thermoregulatory components.102–104 Nutritional homeostasis is reestablished by addressing the vascular injury.
Pain in the form of claudication may be a sign of vascular injury, and repair within 24 to 72 hours is necessary to prevent edema. Thrombosis also may produce segmental ischemia with subsequent neuropathic pain. However, these pathologies can be masked if adequate collateral circulation is present.105
Treatment of Peripheral Nerve Pain
The stellate ganglion is found in the neck region and supplies sympathetic innervation to the head, neck, and upper extremities. A nerve block in this area aids diagnosis and treatment for neuropathic pain in the head, neck, and upper extremities. However, complications of stellate ganglion block include respiratory depression and hypotension due to local anesthetic injection into a dural cuff. Furthermore, if local anesthetic is injected into a vertebral artery, it precipitates seizure and in some instances can cause loss of consciousness.98
Peripheral Nerve Biopsy
Nerve biopsy is used to diagnose a variety of peripheral nerve pathologies. The sural and popliteal nerves are commonly used for this purpose. Postoperative wound complications include infection, hematoma, and neuroma formation. Hilton et al. compiled their results with those of previous studies and found postoperative rates of 30% for pain, 40% for paresthesia, 33% for dysesthesia, and 8% for wound issues. These data highlight the importance of appropriate patient selection and indications for biopsy.106
Complications of Other Surgeries and Injuries Involving Peripheral Nerves
Many medical and surgical interventions place peripheral nerves at risk, and injury to these nerves can be a common complication. Common procedures that can injure the nerves include patient positioning, retraction during surgery, medication injection, local nerve blocks, and direct sectioning of the nerve during surgery. Postoperative hematomas are a potential source of peripheral nerve injury. Medical conditions such as diabetes, tobacco use, and hypertension can also contribute to poor outcomes. One study showed that the incidence of perioperative peripheral nerve injuries was 0.03%.107
Kömürcü et al. reported 82 cases of treatment of iatrogenic nerve injury and showed that nearly any nerve can be at risk. They treated their patients with a variety of conservative and surgical methods and emphasized the importance of timely diagnosis and an individualized, multidisciplinary approach.108
Complications of Surgery for Traumatic Nerve Injury
Traumatic peripheral nerve injuries can cause temporary or permanent nerve dysfunction as well as pain, and a prompt and accurate assessment is necessary. In many cases, immediate surgical intervention may be the most important strategy because nerve fiber damage can be irreversible when ischemia lasts more than 6 hours.7,109 Until confirmed by intraoperative findings, it is prudent to diagnose traumatic peripheral nerve injuries as neurotmesis.
Most traumatic injuries fall into three categories: (1) laceration of the nerve, caused by knife wounds, motor vehicle accidents, or work-related injuries; (2) projectile injuries, such as gunshot wounds; and (3) dislocation, fracture, and traction injuries as can be found with sports injuries. Functional outcomes vary depending on the injury. For example, digital nerves are capable of regenerating and may recover sensory function to near-normal status.27
Laceration
In sharp nerve transections with avulsion, the current recommendation is to secure the nerve stumps to the adjacent soft tissues and return at 2 months for primary nerve repair. At that time, the normal and aberrant neural anatomy will be demarcated and the appropriate therapy may be selected. Laceration of the nerve (Seldon neurotmesis or Sunderland lesion V) can cause complete loss of nerve function and typically does not result in spontaneous recovery. Intraoperative nerve action potential recordings provide data on the functional status of the injured nerve and aid the surgeon in deciding whether to resect the injured nerve.
Complications after nerve repair may have several sources. For example, applying too much tension to the nerve ends may alter intracellular nutrition, change endoneural fluid pressurization, and cause scarring at the suture line.9,110–112 Repair can be further compromised if the cross-sectional area of the nerve is diminished by high tension. Unsuccessful surgery may result from failure to resect the nerve stumps to normal, viable fascicular neural tissue or failure to recognize and resect a neuroma in continuity.
Projectile Injuries
Ballistic missile wounds cause crushing and laceration, shock waves, and cavitation.113 Handguns typically create low-velocity shock waves, creating neurapraxia and axonotmesis, but shotgun wounds cause a higher percentage of peripheral nerve injuries because of the damage to neurovascular structures. These patients typically have a poor prognosis, but preventing further complications involves the four Do Nots:
1. Do not cut down both saphenous veins, because one saphenous vein may be needed for vascular grafting.
2. Do not insert intravenous lines into the injured limb.
3. Do not use a tourniquet on the injured limb.
4. Do not attempt to clamp the bleeding wound blindly, because of the risk of severing or lacerating the functioning neurovascular structures.
With all appropriate interventions, spontaneous recovery has been observed in 90% of nerves 3 to 9 months after an injury, and in some cases has occurred as long as 11 months after an injury.114 The functional recovery rate is faster in low-distal extremity injuries than it is in high-proximal extremity injuries, and the prognosis in low-velocity gunshot wounds is similar to that for high-velocity injuries, which is a 1- to 4-month recovery period for neurapraxia and 4 to 9 months for axonotmesis.115
Fracture and Dislocation
Patients presenting with an upper extremity fracture must be evaluated for nerve damage because 95% of upper extremity fractures have associated nerve injuries.116 Humeral fractures frequently cause radial nerve damage, and complete medial nerve resection can rarely result from a Colles fracture.117 The median nerve is also susceptible to pressure as it courses the carpal tunnel and may be entrapped by bony fractures, edema, or hemorrhage. This knowledge is important because surgical reduction can be complicated by further damage to the nerves.
The time frame for spontaneous recovery of clinical function after a fracture is 1 to 4 months, and 3 to 6 months after a traction injury.21 The chance for spontaneous recovery is inversely related to the severity of the nerve injury.25
Iatrogenic Nerve Injuries
Tourniquet Injury
Tourniquets are commonly used for hemostasis in severe extremity injuries and to improve visualization. However, tourniquet-induced peripheral nerve complications can result even with proper use because this device applies direct pressure on a nerve.10,13,118 The most commonly injured nerve in the upper extremity is the radial nerve.1,119
The most important factor is tourniquet time because prolonged pressure creates considerable compression of the peripheral nerves. Although some surgeons advise releasing tourniquet pressure after 1 to 3 hours of surgery,1,112,120 30 minutes of high cuff pressure can produce palsies.96,112 A tourniquet pressure not exceeding 70 mm Hg above the patient’s systolic blood pressure should be sufficient to maintain hemostasis, and the risk of damage is significantly increased when the tourniquet is placed below the elbow or knee.112 Electrophysiologic studies show changes in nerve action potentials and frequent fibrillations 5 to 10 minutes after pressure is applied, so deflation of the cuff for 10 minutes each hour is recommended to prevent nerve injury.
Tourniquet-induced peripheral nerve injuries are usually mild and spontaneously resolve within 3 to 6 months. Prognosis varies because these injuries are difficult to assess accurately and qualitatively.1,13,82,112 Conservative management includes limb rest and appropriate physical therapy. Surgical exploration is necessary in cases of serious and irreversible damage or no evidence of functional improvement.
Patient Malpositioning
Malpositioning may result in peripheral nerve injury and even permanent neurologic deficits, but this complication is entirely avoidable. Malpositioning leads to peripheral nerve compression against rigid surfaces like aberrant joints or osseoligamentous structures.112 However, no correlation has been found between the degree of compression damage and surgical duration; nerve compression injury has been reported in surgeries that lasted 1 hour, as well as those lasting up to 4 hours.1,121–124 Nerve compression may range from a low-grade stretching that interrupts the blood supply to high-grade lesions with associated hemorrhage and necrosis, and the results of electrographic studies have varied from slowed conduction to complete block.125
High-risk patients include those who are thin and of small stature.10 Several other factors can predispose a patient to nerve compression, including bony fractures, aberrant anatomy, compaction of the cervical ribs, associated medical conditions, a history of peripheral neuropathies, anticoagulant use, use of skeletal muscle relaxants during anesthesia, or physiologic hypothermia.1,121–124
Several sites and positions have an increased risk of nerve compression. For example, the superficial course of the radial and ulnar nerves in relationship to the shaft of the humerus is of concern, and when the arms are secured at the patient’s sides, they should be fully padded with foam or soft towels. Improper arm positioning can also occur when the patient is placed in a lateral rotation position, internal rotation, external rotation, abduction, arm-shoulder distraction, pronation, or supination. The patient’s arms should not be abducted further than the head for a long period and should never be crossed, because these positions can injure the upper brachial plexus.10 Such injuries usually present as painless motor weakness.125
When the patient is placed in a lateral decubitus position, there is significant risk to the peripheral nerves in both the upper and lower extremities. The patient’s legs should be placed on soft pillows and secured with a leg strap. The leg strap should be positioned away from the fibular head, where the peroneal nerve is superficially located. In addition, one must realize that the femoral cutaneous nerve is located adjacent to the pelvic protuberance and the sciatic nerve is confined within the pelvic outlet region. Injury to the saphenous and sciatic nerves has also been reported in patients who have been placed in a lithotomy position.15 Nearly all peripheral nerves are vulnerable to injury, and a review by Winfree and Kline extensively details the many different nerve compressions.125
If a compressive neuropathy develops, recovery depends on the severity and extent of the injury. Complete recovery can be expected within 6 months in 90% of cases.124 Mild forms of peripheral neuropathy, such as a first-degree conduction block, have better recovery rates than do severe forms. If the patient exhibits signs of compressive neuropathy, a careful history and physical examination in combination with electrodiagnostic studies are necessary, and if neurologic deficits fail to improve, surgical exploration is indicated.
Injection Injury
Various pharmacologic agents have been found to cause injection injury when inadvertently introduced into a nerve. A thorough knowledge of nerve anatomy and attention to needle trajectory are essential to avoid this complication. The highest risk of nerve damage comes from epineural, intraneural, and intrafascicular injections, and misdirected injections can have serious consequences.1,13,126
Injury from Regional Anesthesia
Local nerve blocks can have complications that cause injury to any peripheral nerve. In a review by Borgeat and Blumenthal, three types of injury were shown to occur: axonal sideration (neurapraxia), axonal interruption with sheath preservation (axonotmesis), and fascicular interruption (neurotmesis). The reported incidence of long-term complications was approximately 0.2%, but these complications may be a significant source of malpractice insurance claims. Any anesthetic agent is potentially neurotoxic if used without proper precautions.127
Newer studies have examined postoperative neurologic complications with the use of ultrasound. Fredrickson and Kilfoyle examined peripheral nerve complications in ultrasonography-guided local anesthetic nerve blocks for extremity orthopedic surgery in 911 patients. They found an immediate complication rate of 8.2% and a 6-month rate of 0.6%. The most common symptoms were numbness, tingling, and pain. Weakness was a rare complication.128
Misdirected Surgical Procedures
During surgical exploration, exposed nerves should be carefully protected to prevent iatrogenic stretching or tension injuries, nerve laceration, or intraneuronal physiologic damage. Most iatrogenic injuries are associated with orthopedic, trauma, or hand surgery.129 For example, during open reduction of a fractured humerus, the radial nerve may be injured, and common procedures such as knee and hip arthroplasties and knee arthroscopy can place many of the lower extremity nerves at risk.130 Minor surgical procedures such as lymph node biopsy in the dorsal triangle of the neck may transect or stretch the spinal accessory nerve.29 In addition, cutaneous sensory nerves are often damaged during surgeries on wrist ganglions, operations at the elbow (antebrachial cutaneous nerves), decompression of the median nerve (palmar cutaneous branches of the median nerve), surgeries for varicose veins (saphenous nerve and branches), and surgeries to treat Dupuytren disease (digital nerves).131
Retractor blades are also a common cause of nerve injury.123,132,133 For example, damage to the femoral nerve may be caused by the pelvic retractors used in gynecologic surgery.129 Inexperience with peripheral nerve tumor surgery also can lead to significant complications. This is especially true for malignant tumor resections because of the more intrinsic nature of the lesion and its adherence to adjacent neural structures and other soft tissues.
Medicolegal Aspects of Iatrogenic Injuries
Iatrogenic peripheral nerve injuries may have medicolegal implications, and although many of these injuries are unavoidable, a few can result from negligence. To avoid further damage, appropriate management after injury is essential. This management begins with timely recognition and referral for the injury because outcome improves with prompt interventions.134,135
Despite aggressive management, functional recovery may be difficult to predict, but general trends can be identified. First, spontaneous recovery is more likely with incomplete lesions, but the recovery process is slow. Second, if operative intervention is necessary, a peripheral nerve specialist is most appropriate. Finally, repair should take place within the first 3 or 4 months after injury to produce optimal results.134,135
Summary
To avoid complications in peripheral nerve surgery, the peripheral nerve surgeon must
• Have a thorough understanding of normal and variant anatomy
• Perform a complete clinical assessment
• Correctly identify and localize lesions and interpret electrodiagnostic studies
• Anticipate complications that may arise during surgery and know how to manage them
Kline D.G., Hudson A.R. Nerve injuries: operative results for major nerve injuries, entrapments, and tumors. Philadelphia: WB Saunders; 1995.
Kömürcü F., Zwolak P., Benditte-Klepetko H., et al. Management strategies for peripheral iatrogenic nerve lesions. Ann Plast Surg. 2005;54:135-142.
Reisner G.G., Tindall S.C. Complications of peripheral nerve surgery. In: Benzel E.C., editor. Spine surgery. Philadelphia: Churchill Livingstone; 1999:947-959.
Russell S.M., Kline D.G. Complication avoidance in peripheral nerve surgery: preoperative evaluation of nerve injuries and brachial plexus exploration—part 1. Neurosurgery. 2006;59(4 Suppl 2):441-448.
Russell S.M., Kline D.G. Complication avoidance in peripheral nerve surgery: injuries, entrapments, and tumors of the extremities—part 2. Neurosurgery. 2006;59(4 Suppl 2):449-457.
Wilbourn A.J. Iatrogenic nerve injuries. Neurol Clin. 1998;16:55-82.
Winfree C.J., Kline D.G. Intraoperative positioning nerve injuries. Surg Neurol. 2005;63:5-18.
1. Sunderland S. Nerves and nerve injuries, ed 2. Edinburgh: Churchill Livingstone; 1978.
2. Young H.A. Surgical management of peripheral entrapment neuropathy. In: Schmidek H.H., Sweet W.H., editors. Operative neurosurgical techniques. ed 2. Philadelphia: WB Saunders; 1988:1583.
3. Bunge R.P. Some observations on the role of Schwann cell in peripheral nerve regeneration. In: Jewett D.L., McCarroll H.R., editors. Nerve repair and regeneration: its clinical and experimental basis. St. Louis: Mosby; 1980:58.
4. Fawcett J.W., Keynes R.J. Peripheral nerve regeneration. Ann Rev Neurosci. 1990;13:43-60.
5. Jabaley M.E., Wallace W.H., Heckler F.R. Internal topography of major nerves of the forearm and hand: a current view. J Hand Surg [Am]. 1980;5:1-18.
6. Kuczynski K. Functional micro-anatomy of the peripheral nerve trunks. Hand. 1974;6:1-10.
7. Lundborg G. The intrinsic vascularization of human peripheral nerves: structural and functional aspects. J Hand Surg [Am]. 1979;4:34-41.
8. Lundborg G. Structure and function of the intraneural microvessels as related to trauma, edema formation, and nerve function. J Bone Joint Surg [Am]. 1975;57:938-948.
9. Millesi H., Meissl G. Consequences of tension at the suture line. In: Gorio A., Millesi H., Mingrino S., editors. Posttraumatic peripheral nerve regeneration: experimental basis and clinical implications. Philadelphia: Lippincott-Raven; 1981:277.
10. Reisner G.G., Tindall S.C. Complications of peripheral nerve surgery. In: Benzel E.C., editor. Spine surgery. Philadelphia: Churchill Livingstone; 1999:947-959.
11. Smith J.W. Factors influencing nerve repair: I. Blood supply of peripheral nerves. Arch Surg. 1966;93:335-341.
12. Smith J.W. Factors influencing nerve repair: II. Collateral circulation of peripheral nerves. Arch Surg. 1966;93:433-437.
13. Mackinnon S.E., Dellon A.L. Surgery of the peripheral nerve. New York: Thieme; 1988.
14. Smorto M.P., Basmajian J.V. Electrodiagnosis: a handbook for neurologists. Hagerstown, MD: Harper and Row; 1977.
15. Tindall S.C. Chronic injuries of peripheral nerves by entrapment. Youmans J.R., editor. Neurological surgery, ed 3, vol. 4. Philadelphia: WB Saunders, 1990;2511.
16. Russell R.C., Pribaz J.P., Zook E.G. Clinical motor function testing-upper extremity. In: Omer G.E.Jr., Spinner M., Van Beek A.L., editors. Management of peripheral nerve problems. ed 2. Philadelphia: WB Saunders; 1998:39-49.
17. Leibovic S.J., Hastings H.II. Martin-Gruber revisited. J Hand Surg [Am]. 1992;17:47-53.
18. Omer G.E.Jr. Continuous peripheral epineural infusion for the treatment of acute pain. In: Omer G.E.Jr., Spinner M., Voorhies R.M., editors. Management of peripheral nerve problems. ed 2. Philadelphia: WB Saunders; 1998:116-119.
19. Peterson G.W., Will A.D. Newer electrodiagnostic techniques in peripheral nerve injuries. Orthop Clin North Am. 1988;19:13-25.
20. Terzis J.K., Dykes R.W., Hakstian R.W. Electrophysiological recordings in peripheral nerve surgery: a review. J Hand Surg [Am]. 1976;1:52-66.
21. Omer G.E.Jr. Injuries to nerves of the upper extremity. J Bone Joint Surg [Am]. 1974;56:1615-1624.
22. Omer G.E.Jr. The management of pain. In: Lamb D.W., editor. The paralyzed hand. Edinburgh: Churchill Livingstone; 1987:213-216.
23. Omer G.E.Jr. Management of pain syndromes in upper extremity. In: Hunter J.M., Schneider L.H., Mackin E.H., et al, editors. Rehabilitation of the hand. St. Louis: Mosby; 1978:341-349.
24. Omer G.E.Jr. Management techniques for the painful upper extremity. In: Terzis J.K., editor. Microreconstruction of nerve injuries. Philadelphia: WB Saunders; 1987:145-159.
25. Omer G.E.Jr. Present thoughts on the management of pain in the upper extremity. Clin Plast Surg. 1984;11:85-93.
26. Omer G.E.Jr. Sensation and sensibility in the upper extremity. Clin Orthop Relat Res. 1974;104:30-36.
27. Omer G.E.Jr., Bell-Krotoski J. Sensibility testing. In: Omer G.E.Jr., Spinner M., Van Beek A.L., editors. Management of peripheral nerve problems. ed 2. Philadelphia: WB Saunders; 1998:11-28.
28. Berne R.M., Levy M.N. Physiology. St. Louis: Mosby; 1983.
29. Dorfman L.J., Cummins K.L., Leifer L.J. Conduction velocity distributions: a population approach to electrophysiology of nerve. New York: Liss; 1981.
30. Kimura J. Principles and pitfalls of nerve conduction studies. Ann Neurol. 1984;16:415-429.
31. Aminoff M.J. Electrodiagnosis in clinical neurology. New York: Churchill Livingstone; 1980.
32. Bande S., De Smet L., Fabry G. The results of carpal tunnel release: open versus endoscopic technique. J Hand Surg [Br]. 1994;19:14-17.
33. Adams R.D., Victor M., Ropper A.H. Principles of neurology. New York: McGraw-Hill; 1997.
34. Van Beek A.L. Electrodiagnostic evaluation of peripheral nerve injuries. Hand Clin. 1986;2:747-760.
35. Van Beek A.L. Intraoperative nerve stimulation and recording techniques. In: Omer G.E.Jr., Spinner M., Van Beek A.L., editors. Management of peripheral nerve problems. ed 2. Philadelphia: WB Saunders; 1998:251-259.
36. Van Beek A.L., Hubble B., Kinkead L., et al. Clinical use of nerve stimulation and recording techniques. Plast Reconstr Surg. 1983;71:225-240.
37. Van Beek A.L., Massac E.Jr., Smith D.O. The use of the signal averaging computer for evaluation of peripheral nerve problems. Clin Plast Surg. 1986;13:407-418.
38. Rorabeck C.H., Harris W.R. Factors affecting the prognosis of brachial plexus injuries. J Bone Joint Surg [Br]. 1981;63:404-407.
39. Sedel L. The results of surgical repair of brachial plexus injuries. J Bone Joint Surg [Br]. 1982;64:54-66.
40. Russell S.M., Kline D.G. Complication avoidance in peripheral nerve surgery: preoperative evaluation of nerve injuries and brachial plexus exploration—part 1. Neurosurgery. 2006;59(4 Suppl 2):441-448.
41. Ordog G.J., Balasubramanium S., Wasserberger J., et al. Extremity gunshot wounds. Part one: Identification and treatment of patients at high risk of vascular injury. J Trauma. 1994;36:358-368.
42. Wiss D.A., Gellman H. Gunshot wounds to the musculoskeletal system. In: Browner B.D., Jupiter J.B., Levine A.M., et al, editors. Skeletal trauma. Philadelphia: WB Saunders; 1992:367-400.
43. Breidenbach W.C. Vascularized nerve grafts: an experimental and clinical review. Ann Plast Surg. 1987;18:137-146.
44. Frykman G.K., Cally D. Interfascicular nerve grafting. Orthop Clin North Am. 1988;19:71-80.
45. Kline D.G. Surgical repair of peripheral nerve injury. Muscle Nerve. 1990;13:843-852.
46. Millesi H. Forty-two years of peripheral nerve surgery. Microsurgery. 1993;14:228-233.
47. Tupper J.W., Crick J.C., Matteck L.R. Fascicular nerve repairs: a comparative study of epineurial and fascicular (perineurial) techniques. Orthop Clin North Am. 1988;19:57-69.
48. Dahlin L.B., Lundborg G. Use of tubes in peripheral nerve repair. Neurosurg Clin North Am. 2001;12:341-352.
49. Evans P.J., MacKinnon S.E., Midha R., et al. Regeneration across cold preserved peripheral nerve allografts. Microsurgery. 1999;19:115-127.
50. Mackinnon S.E., Doolabh V.B., Novak C.B., et al. Clinical outcome following nerve allograft transplantation. Plast Reconstr Surg. 2001;107:1419-1429.
51. Jones N.F. Intraoperative and postoperative monitoring of microsurgical free tissue transfers. Clin Plast Surg. 1992;19:783-797.
52. Jones N.F., Lister G.D. Free skin and composite flaps. In: Green D.P., Hotchkiss R.N., Pederson W.C., editors. Green’s operative hand surgery. ed 4. Philadelphia: Churchill Livingstone; 1999:1164.
53. Leffert R.D. Surgery of the peripheral nerves and brachial plexus. In: Schmidek H.H., Sweet W.H., editors. Operative neurosurgical techniques. ed 2. Philadelphia: WB Saunders; 1988:1563.
54. Ariel I.M. Tumors of the peripheral nervous system. Semin Surg Oncol. 1988;4:7-12.
55. DasGupta T. Tumors of the peripheral nerves. Clin Neurosurg. 1978;25:574-590.
56. Donner T.R., Voorhies R.M., Kline D.G. Neural sheath tumors of major nerves. J Neurosurg. 1994;81:362-373.
57. Hudson A.R., Gentili F., Kline D.G. Peripheral nerve tumors. In: Schmidek H.H., Sweet W.H., editors. Operative neurosurgical techniques. ed 2. Philadelphia: WB Saunders; 1988:1599.
58. Russell S.M., Kline D.G. Complication avoidance in peripheral nerve surgery: injuries, entrapments, and tumors of the extremities—part 2. Neurosurgery. 2006;59(4 Suppl 2):449-457.
59. Kline D.G., Hudson A.R. Nerve injuries: operative results for major nerve injuries, entrapments, and tumors. Philadelphia: WB Saunders; 1995.
60. Lusk M.D., Kline D.G., Garcia C.A. Tumors of the brachial plexus. Neurosurgery. 1987;21:439-453.
61. Mackinnon S.E., Dellon A.L. Evaluation of microsurgical internal neurolysis in a primate median nerve model of chronic nerve compression. J Hand Surg [Am]. 1988;13:345-351.
62. Rhoades C.E., Mowery C.A., Gelberman R.H. Results of internal neurolysis of the median nerve for severe carpal-tunnel syndrome. J Bone Joint Surg [Am]. 1985;67:253-256.
63. Broudy A.S., Leffert R.D., Smith R.J. Technical problems with ulnar nerve transposition at the elbow: findings and results of reoperation. J Hand Surg [Am]. 1978;3:85-89.
64. Leffert R.D. Anterior submuscular transposition of the ulnar nerves by the Learmonth technique. J Hand Surg [Am]. 1982;7:147-155.
65. Macnicol M.F. The results of operation for ulnar neuritis. J Bone Joint Surg [Br]. 1979;61:159-164.
66. Chan R.C., Paine K.W., Varughese G. Ulnar neuropathy at the elbow: comparison of simple decompression and anterior transposition. Neurosurgery. 1980;7:545-550.
67. Wilson D.H., Krout R. Surgery of ulnar neuropathy at the elbow: 16 cases treated by decompression without transposition. Technical note. J Neurosurg. 1973;38:780-785.
68. Dubuisson A.S., Kline D.G., Weinshel S.S. Posterior subscapular approach to the brachial plexus. J Neurosurg. 1993;79:319-330.
69. Hope D.G., Mulvihill J.J. Malignancy in neurofibromatosis. Adv Neurol. 1981;29:33-56.
70. Louis D.S., Greene T.L., Noellert R.C. Complications of carpal tunnel surgery. J Neurosurg. 1985;62:352-356.
71. MacDonald R.I., Lichtman D.M., Hanlon J.J., et al. Complications of surgical release for carpal tunnel syndrome. J Hand Surg [Am]. 1978;3:70-76.
72. Beris A.E., Lykissas M.G., Kontogeorgakos V.A., et al. Anatomic variations of the median nerve in carpal tunnel release. Clin Anat. 2008;21:514-518.
73. Blair S.J. Avoiding complications of surgery for nerve compression syndromes. Orthop Clin North Am. 1988;19:125-130.
74. Gartsman G.M., Kovach J.C., Crouch C.C., et al. Carpal arch alteration after carpal tunnel release. J Hand Surg [Am]. 1986;11:372-374.
75. May J.W., Rosen H. Division of the sensory ramus communicans between the ulnar and median nerves: a complication following carpal tunnel release. A case report. J Bone Joint Surg [Am]. 1981;63:836-838.
76. Lilly C.J., Magnell T.D. Severance of the thenar branch of the median nerve as a complication of carpal tunnel release. J Hand Surg [Am]. 1985;10:399-402.
77. Favero K.J., Gropper P.T. Ulnar nerve laceration: a complication of carpal tunnel decompression: case report and review of the literature. J Hand Surg [Br]. 1987;12:239-241.
78. Kulick M.I., Gordillo G., Javidi T., et al. Long-term analysis of patients having surgical treatment for carpal tunnel syndrome. J Hand Surg [Am]. 1986;11:59-66.
79. Langloh N.D., Linscheid R.L. Recurrent and unrelieved carpal-tunnel syndrome. Clin Orthop Relat Res. 1972;83:41-47.
80. Das S.K., Brown H.G. In search of complications in carpal tunnel decompression. Hand. 1976;8:243-249.
81. Semple J.C., Cargill A.O. Carpal-tunnel syndrome: results of surgical decompression. Lancet. 1969;1:918-919.
82. Nath R.K., Mackinnon S.E., Weeks P.M. Ulnar nerve transection as a complication of two-portal endoscopic carpal tunnel release: a case report. J Hand Surg [Am]. 1993;18:896-898.
83. Luallin S.R., Toby E.B. Incidental Guyon’s canal release during attempted endoscopic carpal tunnel release: an anatomical study and report of two cases. Arthroscopy. 1993;9:382-386. discussion 381
84. Brown M.G., Keyser B., Rothenberg E.S. Endoscopic carpal tunnel release. J Hand Surg [Am]. 1992;17:1009-1011.
85. Brown M.G., Rothenberg E.S., Keyser B., et al. Results of 1236 endoscopic carpal tunnel release procedures using the Brown technique. Contemp Orthop. 1993;27:251-258.
86. Brown R.A., Gelberman R.H., Seiler J.G.3rd, et al. Carpal tunnel release: a prospective, randomized assessment of open and endoscopic methods. J Bone Joint Surg [Am]. 1993;75:1265-1275.
87. Resnick C.T., Miller B.W. Endoscopic carpal tunnel release using the subligamentous two-portal technique. Contemp Orthop. 1991;22:269-277.
88. Agee J.M., McCarroll H.R.Jr., North E.R. Endoscopic carpal tunnel release using the single proximal incisional technique. Hand Clin. 1994;10:647-659.
89. Agee J.M., McCarroll H.R.Jr., Tortosa R.D., et al. Endoscopic release of the carpal tunnel: a randomized prospective multicenter study. J Hand Surg [Am]. 1992;17:987-995.
90. Agee J.M., Peimer C.A., Pyrek J.D., Walsh W.E. Endoscopic carpal tunnel release: a prospective study of complications and surgical experience. J Hand Surg [Am]. 1995;20:165-171.
91. Elmaraghy M.W., Hurst L.N. Single-portal endoscopic carpal tunnel release: Agee carpal tunnel release system. Ann Plast Surg. 1996;36:286-291.
92. Feinstein P.A. Endoscopic carpal tunnel release in a community-based series. J Hand Surg [Am]. 1993;18:451-454.
93. Jimenez D.F., Gibbs S.R., Clapper A.T. Endoscopic treatment of carpal tunnel syndrome: a critical review. J Neurosurg. 1998;88:817-826.
94. McDonough J.W., Gruenloh T.J. A comparison of endoscopic and open carpal tunnel release. Wis Med J. 1993;92:675-677.
95. Palmer D.H., Paulson J.C., Lane-Larsen C.L., et al. Endoscopic carpal tunnel release: a comparison of two techniques with open release. Arthroscopy. 1993;9:498-508.
96. Nissenbaum M., Kleinert H.E. Treatment considerations in carpal tunnel syndrome with coexistent Dupuytren’s disease. J Hand Surg [Am]. 1980;5:544-547.
97. Cimino C. Painful neurological syndromes. In Aronoff G., editor: Evaluation and treatment of chronic pain, ed 2, Baltimore: Williams & Wilkins, 1992.
98. Schultz D. Indications for utilization of a pain clinic. In: Omer G.E.Jr., Spinner M., Van Beek A.L., editors. Management of peripheral nerve problems. ed 2. Philadelphia: WB Saunders; 1998:120-133.
99. Lewin-Kowalik J., Marcol W., Kotulska K., et al. Prevention and management of painful neuroma. Neurol Med Chir (Tokyo). 2006;46:62-68.
100. Vrettos B.C., Rochkind S., Boome R.S. Low velocity gun shot wounds of the brachial plexus. J Bone Joint Surg [Br]. 1995;20:212-214.
101. Koman L.A., Smith T.L., Poehling G.G. Reflex sympathetic dystrophy. Curr Opin Orthop. 1993;4:85-88.
102. Koman L.A., Nunley J.A. Thermoregulatory control after upper extremity replantation. J Hand Surg [Am]. 1986;11:548-552.
103. Koman L.A., Nunley J.A., Goldner J.L., et al. Isolated cold stress testing in the assessment of symptoms in the upper extremity: preliminary communication. J Hand Surg [Am]. 1984;9:305-313.
104. Nunley J.A., Penny W.H.III, Woodbury M.A., et al. Quantitative analysis of cold stress performance after digital replantation. J Orthop Res. 1990;8:94-100.
105. Koman L.A., Goldner J.L., Smith T.L. The effect of extremity blood flow on pain and cold tolerance. In: Omer G.E.Jr., Spinner M., Van Beek A.L., editors. Management of peripheral nerve problems. ed 2. Philadelphia: WB Saunders; 1988:107-115.
106. Hilton D.A., Jacob J., Househam L., et al. Complications following sural and peroneal nerve biopsies. J Neurol Neurosurg Psychiatry. 2007;78:1271-1272.
107. Welch M.B., Brummett C.M., Welch T.D., et al. Perioperative peripheral nerve injuries: a retrospective study of 380,680 cases during a 10-year period at a single institution. Anesthesiology. 2009;111:490-497.
108. Kömürcü F., Zwolak P., Benditte-Klepetko H., et al. Management strategies for peripheral iatrogenic nerve lesions. Ann Plast Surg. 2005;54:135-142.
109. Dahlin L.B., Shyu B.C., Danielsen N., et al. Effects of nerve compression or ischaemia on conduction properties of myelinated and non-myelinated nerve fibres: an experimental study in the rabbit common peroneal nerve. Acta Physiol Scand. 1989;136:97-105.
110. Millesi H., Meissl G., Berger A. Further experience with interfascicular grafting of the median, ulnar, and radial nerves. J Bone Joint Surg [Am]. 1976;58:209-218.
111. Ochoa J., Marotte L. The nature of the nerve lesion caused by chronic entrapment in the guinea pig. J Neurol Sci. 1973;19:491-495.
112. Millesi H., Meissl G., Berger A. The interfascicular nerve-grafting of the median and ulnar nerves. J Bone Joint Surg [Am]. 1972;54:727-750.
113. Rybeck B., Janzon B. Absorption of missile energy in soft tissue. Acta Chir Scand. 1976;142:201-207.
114. Rakolta G.G., Omer G.E.Jr. Combat-sustained femoral nerve injuries. Surg Gynecol Obstet. 1969;128:813-817.
115. Omer G.E.Jr. The prognosis for untreated traumatic injuries. In: Omer G.E.Jr., Spinner M., Van Beek A.L., editors. Management of peripheral nerve problems. ed 2. Philadelphia: WB Saunders; 1998:365-370.
116. Goodall R.J. Nerve injuries in fresh fractures. Tex Med. 1956;52:93-94.
117. Lusthaus S., Matan Y., Finsterbush A., et al. Traumatic section of the median nerve: an unusual complication of Colles’ fracture. Injury. 1993;24:339-340.
118. Gilliatt R.W., Ochoa J., Rudge P., et al. The cause of nerve damage in acute compression. Trans Am Neurol Assoc. 1974;99:71-74.
119. Sanders R. The tourniquet: instrument or weapon? Hand. 1973;5:119-123.
120. Neimkin R.J., Smith R.J. Double tourniquet with linked mercury manometers for hand surgery. J Hand Surg [Am]. 1983;8:938-941.
121. Alvine F.G., Schurrer M.E. Postoperative ulnar nerve palsy: are there predisposing factors? J Bone Joint Surg [Am]. 1987;69:255-259.
122. Bonney G. Iatrogenic injuries of nerves. J Bone Joint Surg [Br]. 1986;68:9-13.
123. Dawson D.M., Krarup C. Perioperative nerve lesions. Arch Neurol. 1989;46:1355-1360.
124. Parks B.J. Postoperative peripheral neuropathies. Surgery. 1973;74:348-357.
125. Winfree C.J., Kline D.G. Intraoperative positioning nerve injuries. Surg Neurol. 2005;63:5-18.
126. Gentili F., Hudson A., Kline D.G., et al. Peripheral nerve injection injury: an experimental study. Neurosurgery. 1979;4:244-253.
127. Borgeat A., Blumenthal S. Nerve injury and regional anaesthesia. Curr Opin Anaesthesiol. 2004;17:417-421.
128. Fredrickson M.J., Kilfoyle D.H. Neurological complication analysis of 1000 ultrasound guided peripheral nerve blocks for elective orthopaedic surgery: a prospective study. Anaesthesia. 2009;64:836-844.
129. Kretschmer T., Antoniadis G., Braun V., et al. Evaluation of iatrogenic lesions in 722 surgically treated cases of peripheral nerve trauma. J Neurosurg. 2001;94:905-912.
130. Yacub J.N., Rice J.B., Dillingham T.R. Nerve injury in patients after hip and knee arthroplasties and knee arthroscopy. Am J Phys Med Rehabil. 2009;88:635-641.
131. Birch R., Bonney G., Wynn Parry C.B. Surgical disorders of the peripheral nerves. Edinburgh: Churchill Livingstone; 1998.
132. Burkhart F.L., Daly J.W. Sciatic and peroneal nerve injury: a complication of vaginal operations. Obstet Gynecol. 1966;28:99-102.
133. Lusskin R., Battista A., Lenzo S., Price A. Surgical management of late post-traumatic and ischemic neuropathies involving the lower extremities: classification and results of therapy. Foot Ankle. 1986;7:95-104.
134. Kline D.G., Hudson A.R. Acute injuries of peripheral nerves. In: Youmans J.R., editor. Neurological surgery. ed 3. Philadelphia: WB Saunders; 1990:2511.
135. Wilbourn A.J. Iatrogenic nerve injuries. Neurol Clin. 1998;16:55-82.