2 Commonly Used Medications in Procedures
Local Anesthetics
Mechanism of Action
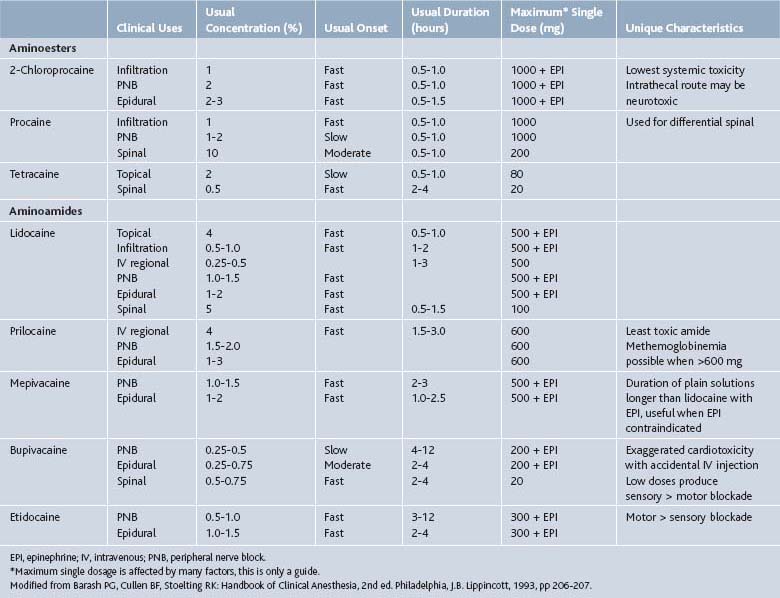
In addition to host factors, neural blockade by local anesthetics is affected by the volume and concentration of local anesthetic injected, the absence or presence of vasoconstrictor additives, the site of injection, the addition of bicarbonate, and temperature of the local anesthetic.1 Increasing the total milligrams of a local anesthetic dose shortens the onset and increases the duration of the local anesthetic. Epinephrine, norepinephrine, and phenylephrine are sometimes added to local anesthetics to reverse the intrinsic vasodilation effects of many of the local anesthetics and thereby reduce their systemic absorption. This increases the amount of local anesthetic available to block the nerve. More anesthetic means a quicker onset and longer duration. Application of the local anesthetic close to the nerve improves its ability to diffuse across the axon and block sodium channels. Highly vascular sites such as the intercostal nerve and caudal epidural space tend to result in slightly shorter duration of action. The addition of bicarbonate or CO2 (700 mm Hg) to local anesthetics hasten their onset. Bicarbonate raises the pH and the amount of uncharged local anesthetic for diffusion through the nerve membrane. CO2 will diffuse across the axonal membrane and lower the intracellular pH making more of the charged form of the local anesthetic available intracellularly to block the sodium channels. Temperature elevations decrease the pKa of the local anesthetic and hasten the onset of action.
Individual Agents
Local anesthetics are administered in the intradermal, subcutaneous, intraarticular, intramuscular, perineural, and epidural spaces during pain management procedures. Injections into vascular regions such as the oral mucosa and epidural space may result in rapid absorption and higher systemic concentrations. Local anesthetics administered into or near the epidural space should be preservative free. Methylparaben is a common preservative in multidose vials and is also a common allergen.2
Lidocaine
Lidocaine is the most versatile and widely used of the local anesthetics. It has a short onset of action, 0.5 to 15 minutes, and short duration of action, typically 0.5 to 3 hours. The difference between the effective dose and the toxic does is wide, resulting in a high therapeutic index compared to other common local anesthetics. Maximum doses are variably reported in the range of 400 to 500 mg of lidocaine. Typical concentrations are 0.5% to 2%. Final concentration is often diluted by the addition of a corticosteroid.1
Bupivicaine
Bupivacaine (Marcaine) is another widely used local anesthetic. Bupivacaine’s duration of action (2 to 5 hr) is longer than lidocaine’s as is its onset of action (5 to 20 min). Bupivacaine is commonly used in concentrations of 0.125% to 0.75%. Final concentrations are often diluted by 30% to 50% by the addition of a corticosteroid. The higher concentrations generally have a faster onset of action. Bupivacaine has more cardiotoxicity than lidocaine, especially if an injection is given intravenously inadvertently. The toxic dose of bupivacaine is only 80 mg (16 mL of a 0.5% solution) when given intravascularly, but may be up to 225 mg with an extravascular injection.1
Toxicity
Action of local anesthetics is affected by numerous factors reviewed above. Location of injection plays a primary role in determining the onset, duration, and toxic dose of these agents (Table 2-1). Vasoconstrictors such as epinephrine reduce local bleeding and thereby prolong the onset and duration, but are generally not employed in a pain management practice.
Patients should be monitored for signs of toxicity including restlessness, anxiety, incoherent speech, lightheadedness, numbness, and tingling of the mouth and lips, blurred vision, tremors, twitching, depression or drowsiness. Injections into the head and neck area require the utmost care.3 Even small doses of local anesthetic may produce adverse reactions similar to systemic toxicity seen with unintentional intravascular injections of larger doses. Deaths have been reported.4
Corticosteroids
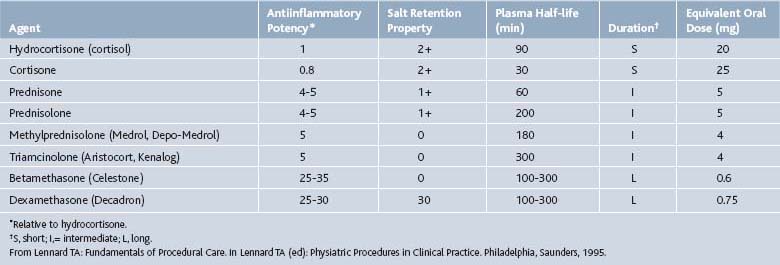
Corticosteroids can be helpful in a variety of conditions including rheumatoid arthritis, bursitis, tenosynovitis, entrapment neuropathies, crystal-induced arthropathies in patients who cannot tolerate systemic treatment well, radiculopathies, and at times, osteoarthritis (OA). Corticosteroids should never be injected directly into a tendon or nerve, subcutaneous fat, or an infected joint, bursa, or tendon (Table 2-2).
Mechanism of Action
Corticosteroids produce both antiinflammatory and immunosuppressive effects in humans. The primary mechanism of action may be their ability to inhibit the release of cytokines by immune cells.5 The effects of corticosteroids are species specific.6 Lymphocytes in humans are much less sensitive to the effects of corticosteroids than lymphocytes in common laboratory animals including the mouse, rat, and rabbit. In humans, corticosteroids reduce the accumulation of lymphocytes at inflammatory sites by a migratory effect.7 In contrast to this lymphopenia, is the neutrophilia seen by demargination of neutrocytes from the endothelium and an accelerated rate of release from the bone marrow.8 A temporary rise in white blood cell count is commonly observed for this reason after a corticosteroid dose and in isolation does not mark a post injection infection.
The antiinflammatory effects of corticosteroid also occur at the microvascular level. They block the passage of immune complexes across the basement membrane, suppress superoxide radicals, and reduce capillary permeability and blood flow.9 Corticosteroids inhibit prostaglandin synthesis,10 decrease collagenase formation, and inhibit granulation tissue formation.
The immunosuppressant effects of corticosteroids are generally via effects on T cells. These effects are not the desired effect of corticosteroid used in pain management procedures and are not observed following epidural injections.11 A review of these immunosuppressant effects can be found in other texts.11–14
Individual Agents
Betamethasone
An equal mixture of two betamethasone salts, Celestone Soluspan, allows for both immediate and delayed corticosteroid responses. Betamethasone sodium phosphate acts within hours, whereas betamethasone acetate is a suspension that is slowly absorbed over approximately 2 weeks. Betamethasone (Celestone Soluspan) is approved for intraarticular or soft tissue injection to provide short-term adjuvant therapy in osteoarthritis, tenosynovitis, gouty arthritis, bursitis, epicondylitis, and rheumatoid arthritis.15 It is also commonly employed in epidural injections. Typical intraarticular doses vary with the size of the joint and range from 0.25 to 2 mL (1.5 mg to 12 mg). Typically epidural injections range from 1 to 3 mL (6 to 18 mg). Betamethasone should not be mixed with local anesthetics that contain preservatives such as methylparaben as these may cause flocculation of the steroid.
Dexamethasone
Dexamethasone sodium phosphate (Decadron Phosphate) is a rapid onset, short duration formulation of dexamethasone. It is available in a variety of strengths ranging from 4 mg/mL to 24 mg/mL. Large joints are often injected with 2 to 4 mg, small joints 0.8 to 1 mg, bursae 2 to 3mg, tendon sheaths 0.4 to 1mg, soft tissue infiltration 2 to 6 mg.15 Sulfites are common in the preparations of this salt also. Dexamethasone is approved for the treatment of osteoarthritis, bursitis, tendonitis, rheumatoid arthritis flares, epicondylitis, tenosynovitis, and gouty arthritis.15 Because it is considered to be a nonparticulate steroid it is also used off-label for epidural steroid injections as discussed subsequently.
Methylprednisolone
Methylprednisolone acetate (Depo-Medrol) has 1/5 to 1/6 the glucocorticoid potency of betamethasone but similar antiinflammatory effects to prednisolone. It has an intermediate duration of action. It, like the other corticosteroids, is approved for intraarticular and soft tissue injections for short-term adjuvant therapy of osteoarthritis, bursitis, tenosynovitis, gouty arthritis, epicondylitis, and rheumatoid arthritis.15 Depo-Medrol has been used for epidural administration also. Preparations of methylprednisolone acetate include polyethylene glycol as a suspending agent. Concerns developed as to whether the polyethylene glycol can cause arachnoiditis with (inadvertent) intrathecal injections.16 Animal studies have not demonstrated any adverse effects on neural tissues from the application of glucocorticoid.17 Methylprednisolone is now available without polyethylene glycol, PEG free. Typical doses range from 4 to 80 mg. Small joints are typically injected with 4 to 10 mg, medium joints 10 to 40mg, large joints 20 to 80 mg, bursae and peritendon 4 to 30 mg.15
Triamcinolone
Triamcinolone is available as three different salts: triamcinolone diacetate (Aristocort Forte), triamcinolone hexacetonide (Aristospan), and triamcinolone acetonide (Kenalog). Duration of action is shortest with the diacetate and longest with the acetonide formulations. Triamcinolone has similar glucocorticoid activity to methylprednisolone with a long half-life. The approved uses are the same as for the agents listed earlier and it, too, is used in epidural injections. Unfortunately, it has a higher incidence of adverse reactions including fat atrophy and hypopigmentation.15
Spinal Injections
Unique considerations are taken into account when considering corticosteroids for spinal injections. In particular, cervical transforaminal injections have lead to rare but significant neurologic complications such as spinal cord injury, stroke, and even death.18–22
The postulated cause of the majority of these complications is undetected vascular injections in the vertebral or spinal radicular arteries with particulate steroids causing embolic infarctions.22,23
Thoracic and lumbar transforaminal injections have similarly been implicated in neurologic complications with particulate steroids. Major complications are thought to arise from embolic events associated with injections into radicular arteries or the reinforcing radicular artery known as the artery of Adamkiewicz.24 This artery typically arises at thoracic levels but it can occur as low as L2 or L3 in about 1% of patients and more rarely at lower levels.25
Anatomic studies show that the size of particles in commonly used steroid preparations such as triamcinolone, methylprednisolone, and betamethasone equals or exceeds the caliber of many radicular arteries.26,27 These particulate steroids either are larger in diameter than a red blood cell or tend to aggregate and/or pack together to be larger than a red blood cell. This is not the case with dexamethasone sodium phosphate, which is a nonparticulate steroid.27 Thus, dexamethasone sodium phosphate should reduce the risk of embolic infarcts following intravascular injections.
Consistent with this, a study looked at vertebral artery injection of particulate and nonparticulate steroids in pigs while under general anesthesia. The animals that were injected with particulate steroids never regained consciousness. Subsequent magnetic resonance images (MRIs) revealed upper cervical cord and brain stem edema and histologic analysis showed ischemic changes. The animals injected with nonparticulate steroids did not have ischemic events and recovered without apparent adverse effects. The MRIs and subsequent histologic analysis were also normal in this group of animals.28
The risk with particulate steroids in cervical and thoracic transforaminal injections has led to the common use of dexamethasone sodium phosphate in these procedures. Thoracic and lumbar transforaminal injections may also lead to embolic events29–31 and this must be taken into consideration. The choice corticosteroids in lumbosacral transforaminal injections is debatable, especially if appropriate safety measures are used, such as contrast administration under live fluoroscopy and use of digital subtraction angiography. If vascular uptake is noted, the needle should be repositioned or the procedure aborted. Other spinal procedures such as interlaminar epidural injections or intraarticular injections have not been associated with embolic events with particulate steroids.
Both particulate and nonparticulate steroids appear to be effective but studies suggest that particulate steroids may be slightly more efficacious than nonparticulate steroids.32,33 Further studies are needed to clarify this.
Adverse Reactions
Corticosteroid use should be carefully considered and avoided if possible in patients at increased risk for adverse reactions, including patients with active ulcer disease, ulcerative colitis with impending perforation or abscess, poorly controlled hypertension, congestive heart failure, renal disease, psychiatric illness or history of steroid psychosis, or a history of severe or multiple allergies.15,34 Intraarticular injections have been associated with osteonecrosis, infection, tendon rupture, postinjection flare, hypersensitivities, and systemic reactions.15 Intraspinal injections have been associated with adhesive arachnoiditis, meningitis, and conus medullaris syndrome.16
Adverse reactions to injected corticosteroids include a transient flare of pain for 24 to 48 hours in up to 10% of patients. Diabetics and those individuals with a predisposition to diabetes may become hyperglycemic and appropriate monitoring and corrective measures should be instituted. Adrenal cortical insufficiency is generally not seen associated with intermittent injections of corticosteroids, but remains a serious adverse reaction that could be precipitated by indiscriminate, frequent high-dose corticosteroid injections. Allergic reactions to systemic glucocorticoids have been reported and if slow release formulations are used, the allergic response may not occur until a week after the injection.35 Even with local injections of corticosteroids, some systemic response may occur.
Generally less serious side effects of corticosteroids include facial flushing, injection site hypopigmentation, subcutaneous fat atrophy, increased appetite, peripheral edema or fluid retention, dyspepsia, malaise, and insomnia.15 Prolonged or repeated doses can result in cushingoid changes.
Drug Interactions
A number of drug-drug interactions for corticosteroids have been reported. Some of the more common ones encountered in a pain management practice are mentioned here. Estrogens and oral contraceptives may potentiate the effect of the corticosteroid. Macrolide antibiotics (e.g., erythromycin, azithromycin) may greatly increase the effect of methylprednisolone by decreasing its clearance. In contrast, the hydantoins (e.g., phenytoin), rifampin, phenobarbital, and carbamazepine may increase corticosteroid clearance and decrease the antiinflammatory therapeutic effect. Theophylline and oral anticoagulants can interact variably with corticosteroids.15
Neurolytic Agents
Neurolytic drugs such as phenol are employed in pain management practice primarily to treat spasticity. Neurolytic agents also have been used for treating chronic pain including intractable cancer pain and facet denervation procedures. The use of neurolytic agents for facet joint neurotomies is being replaced by radiofrequency lesioning.36,37 Neurolytic agents are nonspecific in destroying all nerve fiber types. Phenol, ethyl alcohol, propylene glycol, chlorocresol, glycerol, cold saline, and hypertonic and hypotonic solutions have been employed as neurolytics. Of these, phenol is the most studied and widely used neurolytic.
Phenol
Phenol is the most widely instilled agent to treat severe spasticity. Phenol can be injected around a motor nerve to selectively reduce hypertonicity.38,39 Intrathecal injections of phenol have been used to treat spasticity of spinal cord origin and intractable pain disorders. Sympathectomies for peripheral vascular disease have also been accomplished by injection of phenol along the paravertebral and perivascular sympathetic fibers.40,41
Mechanism of Action
Phenol (carbolic acid) denatures protein and thereby causes denervation. Histologic sections show nonselective nerve destruction, muscle atrophy, and necrosis at the site of phenol injections.42–44 Higher concentrations of phenol are associated with greater tissue destruction. Optimal concentration has not been determined and long-term difference between injection of 2% and 3% solution have not been noted.44 Denervation potentials are seen as early as 3 weeks following phenol blocks.45 Clinical response of decreased pain or spasticity last between 2 months and 2 years irrespective of underlying disorder.43,44 Endoneural fibrosis is seen following phenol injections and is believed to impede reinnervation of the muscle by slow wallerian regeneration.
Adverse Reactions
Local reactions to phenol injection include delayed soreness from the associated necrosis and inflammation.42 This discomfort can be relieved with ice packs and analgesics and typically resolves within 24 hours. If the needle is withdrawn without flushing it with saline, phenol may come in contact with the skin and cause erythema, sloughing, and skin necrosis. Protective eyewear can minimize the chance of eye irritation—conjunctivitis from any phenol splashing into the patient’s or physician’s eyes.
Paresthesias or dysesthesias from mixed somatic nerve blocks are probably due to an incomplete block. Paresthesias/dysesthesias occur in up to 25% of nerve blocks and resolve within 3 months.38,46–55 Repeat blocks often alleviate these symptoms indicating the dysesthesias may stem more from an incomplete block than from phenol-induced dysesthesias.
Systemic reactions to phenol are usually the result of inadvertent intravascular or central blockade.56–59 Adverse systemic reactions most commonly affect the cardiovascular and central nervous systems.58 Cardiac dysrhythmias, hypotension, venous thrombosis, spinal cord infarcts, cortical infarcts, meningitis, and arachnoiditis have been reported.58,60,61
Contrast Agents
Contrast agents are administered to help visualize the location of the needle tip, confirm the flow of injectant or visualize the involved structure (e.g., joint, bladder, bursa). Inadvertent vascular uptake despite negative aspiration is not uncommon. The toxicity of local anesthetics and corticosteroids increases with intravascular injection and contrast-enhanced fluoroscopic guidance helps minimize these toxicities. Contrast agents are all iodinated compounds that allow opacification of structures for visualization. Contrast media is divided into ionic and nonionic agents. The nonionic contrast agents are low osmolality and may decrease the potential for adverse reactions. Although these nonionic agents decrease minor reactions such as nausea and urticaria, they have not been shown to decrease the incidence of more severe reactions.62,63 They do not eliminate the possibility of severe or fatal anaphylactic reactions. Potential for adverse reaction can be minimized by limiting the quantity of the contrast media injected and adequately screening patients.
Spinal procedures including epidural steroid injections, facet joint injections, sympathetic blocks, discography, spinal nerve blocks, and sacroiliac joint injections are all ideally performed with the aid of fluoroscopy and contrast enhancement.64,65 The nonionic contrast agents are used for these injections because the potential for subarachnoid spread exists with any of these procedures. The two most common nonionic agents are iopamidol (Isovue) and iohexol (Omnipaque). Both agents are nonionic, readily available as an injectable liquid, water soluble and quickly cleared. The first of the nonionic contrast agents, metrizamide (Amipaque), is a powder which must be reconstituted. Metrizamide also is associated with a higher incidence of seizures than either iohexol or iopamidol and is rarely used now for procedures. Generally, 0.2 to 2 mL of nonionic contrast is sufficient for the experienced injectionist to confirm location and spread of the contrast. These agents are 90% eliminated through the kidneys within 24 hours. Side effects are uncommon but include nausea, headaches, and CNS disturbances.66
Premedication for Allergic Reactions
The risk of anaphylactoid reactions is 1% to 2% when radiopaque agents are used. This risk increases to 17% to 35% when repeat exposure to radiopaque agents occurs in individuals with known sensitivities.54,66–68 If premedication with diphenhydramine and methylprednisolone is given, the risk of anaphylactoid reactions is reduced to approximately 3.1%.66 The current recommended prophylactic protocol is methylprednisolone 32 mg by mouth 12 and 2 hours prior to contrast use.69 Concurrent use of specific H1 and H2 blockers is also recommended.70,71
Viscosupplementation
Several randomized controlled trials have demonstrated that viscosupplementation is superior to placebo but the clinical efficacy is likely modest.72 A 2003 meta-analysis in JAMA looking at 22 trials concluded that HA was superior to placebo injections but had a relatively small effect. The effect was probably similar to NSAIDs. It also raised concern about a possible publication bias with 17 of 22 trials being industry sponsored, which may overestimate effects of viscosupplementation.73
Another meta-analysis in 2004 looked at 13 randomized controlled trials and found that it is an effective treatment for patients with knee OA who have ongoing pain or are unable to tolerate conservative treatment or joint replacement. HA appears to have a slower onset than intraarticular steroid injections and may last longer.74 A more recent review of viscosupplementation suggested that clinical improvement attributable to viscosupplementation is likely small.75
Adverse reactions with HA injections are generally mild but reports vary regarding frequency. Mild side effects include pain at injection site (1% to 33%), local joint pain and swelling (<1% to 30%) and local skin reactions (3% to 21%). A pseudoseptic reaction can occur but is uncommon (1% to 3%).75
Treatment of Medication Adverse Reactions
Medication adverse reactions can be minimized by careful patient selection and vigilance during the procedure. However, it is impossible to completely eliminate the possibility of allergic or other reactions and the practitioner must be prepared to deal with these emergency situations. Immediate access to and familiarity with emergency medications and protocols is critical.
1. Williams M.J. Pharmacology for regional anesthetic techniques. In: Hahn M.B., McQuillan P.M., Sheplock G.J., editors. Regional anesthesia: An atlas of anatomy and techniques. St. Louis: Mosby-Year Book; 1996:3-17.
2. SchorrParaben Allergy W.F. Jama. 1968;204:107-110.
3. Covino B.G. Clinical pharmacology of local anesthetic agents. In: Cousins M.J., Bridenbaugh P.O., editors. Neural blockade in clinical anesthesia and pain management. Philadelphia: J.B.Lippincott; 1996:111-144.
4. Olin B.R. Miscellaneous products: Local anesthetics, injectable. B.R. Olin. St. Louis: Wolters Kluwer. 1993:2654-2665.
5. Crabtree G.R., Gillis S., Smith K.A., Munck A. Glucocorticoids and immune responses.[Review]. Arthritis Rheum. 1979;22(11):1246-1256.
6. Claman H.N. Corticosteroids and lymphoid cells. N Engl J Med. 1972;287(8):388-397.
7. Peters W.P., Holland J.F., Senn H., et al. Corticosteroid administration and localized leukocyte mobilization in man. N Engl J Med. 1972;286(7):342-345.
8. Fauci A.S., Dale D.C., Balow J.E. Glucocorticosteroid Therapy: Mechanism of action and clinical considerations. Ann Intern Med. 1976;84(3):304-315.
9. Schayer R.S. Synthesis of histamine, microcircularoty regulation and the mechanisms of action of the adrenal glucocorticoid hormones. Prog Allergy. 1963;7:187.
10. Robinson D.R. Prostaglandins and the mechanism of action of anti-inflammatory drugs.[Review]. Am J Med. 1983;75(4B):26-31.
11. Robinson J.P., Brown P.B. Medications in low back pain. Phys Med Rehabil Clin North Am. 1991;2:97-126.
12. Brown P.B. The use of steroidal agents in the oral route. In: Wilkens R.F., Dali S.L., editors. Therapeutic Controversies in the Rheumatic Diseases. Orlando: Grune and Stratton, 1987. p 71
13. Cupps R.R., Fauci A.S. Corticosteroid-mediated immunoregulation in man.[Review]. Immunol Rev. 1982;65:133-155.
14. Fauci A.S., Dale D.C., Balow J.E. Glucocorticosteroid therapy: mechanism of action and clinical considerations. Ann Intern Med. 1976;84(3):304-315.
15. Olin B.R., editor. Facts and Comparisons. St Louis: Wolters Kluwer. 1993:458-486.
16. Nelson D.A. Intraspinal therapy using methylprednisolone acetate. Spine. 1993;18:278-286.
17. Delaney T., Rowlingson R.C., Carron H. Epidural steroid effects on nerve and meninges. Anesth Analg. 1980;59:610-614.
18. McMillan M.R., Crumpton C. Cortical blindness and neurologic injury complicating cervical transforaminal injection for cervical radiculopathy. Anesthesiology. 2003;99:509-511.
19. Rozin L., Rozin R., Koehler S.A., et al. Death during a transforaminal epidural steroid nerve root block(C7) due to perforation of the left vertebral artery. Am J Forensic Med Path. 2003;24:351-355.
20. Tiso R.L., Cutler T., Catania J.A., Whalen K. Adverse central nervous system sequelae after selective transforaminal block: The role of corticosteroids. Spine J. 2004;4:468-474.
21. Brouwers P.J.A.M., Kottnik E.J.B.L., Simon M.A.M., Prevo R.L. A cervical anterior spinal artery syndrome after diagnostic blockade of the right C6-nerve root. Pain. 2001;91:397-399.
22. Baker R., Dreyfuss P., Mercer S., Bogduk N. Cervical transforaminal injection of corticosteroids into a radicular artery: A possible mechanism for spinal cord injury. Pain. 2003;103:211-215.
23. Rathmell J.R., Aprill C., Bogduk N. Cervical transforaminal injection of steroids. Anesthesiology. 2004;100:1595-1600.
24. Bogduk N., Dreyfuss P., Baker R., et al. Complications of spinal diagnostic and treatment procedures. Pain Med. 2008;9:S11-S34.
25. Lo D., Vallee J.N., Spell L., et al. Unusual origin of the artery of Adamkiewicz from the fourth lumbar artery. Neuroradiology. 44, 2002. 153–17
26. Tiso R.L., Cutler T., Catania J.A., Whalen K. Adverse central nervous system sequelae after selective transforaminal block: The role of corticosteroids. Spine J. 2004;4:468-474.
27. Derby R., Date E., Lee C.H., et al. Size and aggregation of corticosteroids used for epidural injections. Interventional Spine. ISIS Newsletter. 2005;5(4):30-37.
28. Okubadejo G.O., Talcott M.R., Schmidt R.E., et al. Perils of intravascular methylprednisolone injection into the vertebral artery. An animal study. J Bone Joint Surg Am. 2008;90:1932-1938.
29. Somyaji H.S., Saifuddin A., Casey A.T.H., Briggs T.W.R. Spinal cord infarction following therapeutic CT-guided left L2 nerve root injection. Spine. 2005;30:E106-E108.
30. Houten J.K., Errico T.J. Paraplegia after lumbosacral nerve root block: Report of three cases. Spine J. 2002;2:70-75.
31. Quintero N., Laffont I., Bouhmidi L., et al. Transforaminal epidural steroid injection and paraplegia: Case report and bibliographic review. Ann Readapt Med Phys. 2006;49:242-247.
32. Dreyfuss P., Baker R., Bogduk N. Comparative effectiveness of cervical transforaminal injections with particulate and nonparticulate corticosteroid preparations for cervical radicular pain. Pain Med. 2006;7:237-242.
33. O’Donnell C., Cano W., Eramo G. Comparison of triamcinolone to dexamethasone in the treatment of low back pain and leg pain via lumbar transforaminal epidural steroid injection. Spine J. 2008;8:S65.
34. Goebert H.W., Jallo S.J., Gardner W.J., Wasmuth C.E. Painful radiculopathy treated with epidural injections of procaine and hydrocortisone acetate: Results in 113 patients. Anesth Analg. 1961;140:130-134.
35. Preuss L. Allergic reactions to systemic glucocorticoids: A review. Ann Allergy. 1985;55:772-775.
36. Dreyfuss P., Halbrook B., Pauza K., et al. Lumbar percutaneous RF medial branch neurotomy for chronic zygapophysial joint pain—a pilot study. Denver: International Spinal Injection Society; 1997.
37. Lord S.M., Barnsley L., Wallis B.J., et al. Percutaneous radio-frequency neurotomy for chronic cervical zygapophyseal-joint pain. New Engl J Med. 1996;335(23):1721-1726.
38. Khalili A.A., Harmel M.H., Forster S., et al. Management of spasticity by selective peripheral nerve block with dilute phenol solutions in clinical rehabilitation. Arch Phys Med Rehabil. 1964;45:513-519.
39. Wood K.M. The use of phenol as a neurolytic agent: A review. Pain. 1978;5:205-229.
40. Hughes-Davies D.I., Redman L.R. Chemical lumbar sympathectomy. Anesthesia. 1976;31:1068-1075.
41. Reid W., Watt J.K., Gray T.G. Phenol injections of the sympathetic chain. Br J Surg. 1970;57:45-50.
42. Garland D.E., Lucie R.S., Waters R.I. Current uses of open phenol nerve block for adult acquired spasticity. Clin Orthop Rel Res. 1982;165:217-222.
43. Halpern D. Histologic studies in animals after intramuscular neurolysis with phenol. Arch Phys Med Rehabil. 1977;58:438-443.
44. Mooney V., Frykman G., McLamb J. Current status of intraneural phenol injections. Clin Orthop Relat Res. 1969;63:122-131.
45. Brattstrom M., Moritz U., Svantesson G. Electromyographic studies of peripheral nerve block with phenol. Scand J Rehabil Med. 1970;2:17-22.
46. Copp E.P., Harris R., Keenan J. Peripheral nerve block and motor point block with phenol in the management of spasticity. Proc R Soc Med. 1970;63:937-938.
47. Copp E.P., Keenan J. Phenol nerve and motor point block in spasticity. Rheum Phys Med. 1972;11:287-292.
48. Katz J.K., Knott L.W., Feldman D.J. Peripheral nerve injections with phenol in the management of spastic patients. Arch Phys Med Rehabil. 1967;48:97-99.
49. Khalili A.A. Physiatric management of spasticity by phenol nerve and motor point block. In: Ruskin A.P., editor. Current Therapy in Physiatry. Philadelphia: Saunders, 1984. 464-474
50. Khalili A.A., Betts H.B. Peripheral nerve block with phenol in the management of spasticity. Indications and complications. JAMA. 1967;200:1155-1157.
51. Petrillo C., Chu D., Davis S. Phenol block of the tibial nerve in the hemiplegic patient. Orthopedics. 1980;3:871-874.
52. Petrillo C., Knoploch S. Phenol block of the tibial nerve for spasticity: A long-term follow-up study. Int Disabil Stud. 1988;10:97-100.
53. Reeves K.D., Baker A. A mixed somatic peripheral phenol nerve block for painful or intractable spasticity: A review of 30 years of use. Am J Pain Manage. 1992;2:205-210.
54. Shehadi W.H. Contrast media adverse reactions: Occurrence, recurrence and distribution patterns. Radiology. 1982;143(1):11-17.
55. Spira R. Management of spasticity in cerebral palsied children by peripheral nerve block with phenol. Dev Med Child Neurol. 1971;13:164-173.
56. Benzon H.T. Convulsions secondary to intravascular pheno: A hazard of celiac plexus block. Anesth Analg. 1979;58:150-151.
57. Holland A.J.C., Yousseff M. A complicaton of subarachnoid phenol blockade. Anesthesia. 1979;34:260-262.
58. Swerdlow M. Complications of neurolytic neural blockade. In: Cousins M.J., Bridenbaugh P.O., editors. Neural blockade in clinical anesthesia and management of pain. New York: J.B. Lippincott; 1988:719-735.
59. Totoki T., Kato T., Nomoto Y., et al. Anterior spinal artery syndrome: A complication of cervical intrathecal phenol injections. Pain. 1979;6:99-104.
60. Macek C. Venous thrombosis results from some phenol injections. JAMA. 1983;249:1807.
61. Morrison J.E.Jr., Matthews D., Washington R., et al. Phenol motor point blocks in children: Plasma concentrations and cardiac dysrhythmias. Anesthesiology. 1991;75:359-362.
62. Lawrence V., Matthai W., Hartmaier S. Comparative safety of high-osmolality and low-osmolality radiographic contrast agents. Report of a multidisciplinary working group. Invest Radiol. 1992;27:2-28.
63. Steinberg E.P., Moore R.D., Powe N.R., et al. Safety and cost effectiveness of high-osmolality as compared with low-osmolality contrast material in patients undergoing cardiac angiography. N Engl J Med. 1992;326(7):425-430.
64. Renfrew D.L. Correct placement of epidural steroid injections: Fluoroscopic guidance and contrast administration. Am J Neuroradiol. 1991;12:1003-1007.
65. White A.H., Derby R., Wynne G. Epidural injections for diagnosis and treatment of low-back pain. Spine. 1980;5:78-86.
66. Olin B.R. Miscellaneous products: Radiopaque agents. In: Olin B.R., editor. Facts and Comparisions. St. Louis: Wolters Kluwer.; 1993:2824-2831.
67. Caro J.J., Trindade E., McGregor M. The risks of death and of severe nonfatal reactions with high- vs. low-osmolality contrast media: A meta-analysis. Am J Roentgenol. 1991;156:825-832.
68. Katayama H., Yamaguchi K., Kozuka T., et al. Adverse reactions to ionic and nonionic contrast media: A report from the Japanese Committee on the Safety of Contrast Media. Radiology. 1990;175:621-628.
69. Lasser E.C., Berry C.C., Talner L.B., et al. Pretreatment with corticosteroids to alleviate reactions to intravenous contrast material. N Engl J Med. 1987;317(14):845-849.
70. Cusmano J. Premedication regimen eases contrast reaction. Diagn Imaging. 1992. 181-182, 185-186
71. Greenberger P.A., Patterson R. The prevention of immediate generalized reactions to radiocontrast media in high-risk patients. J Allergy Clin Immunol. 1991;87:867-872.
72. Roberts W.N., Furst D.E., Romain P.L. Intraarticular and soft tissue injections: What agent(s) to inject and how frequently? Uptodate.com. 2009;17:1.
73. Lo G.H., LaValley M., McAlindon T., Felson D.T. Intra-articular hyaluronic acid in treatment of knee osteoarthritis: A meta-analysis. JAMA. 2003;290:3115.
74. Aggarwal A., Sempowski I.P. Hyaluronic acid injections for knee osteoarthritis. Systematic review of the literature. Can Fam Physician. 2004;50:249-256.
75. Woods S., Davis E., Schechtel M., et al. Three treatments for osteoarthritis of the knee: Evidence shows lack of benefit. Agency for Healthcare Research and Quality. U.S. Department of Health and Human Services. 2009.