Chapter 89 Colloids and blood products
Colloids are plasma expanders used to expand the blood volume. Hydrostatic and osmotic forces dictate movement of fluid between the different compartments of the body across semipermeable membranes (Starling’s forces).
Colloid osmotic pressure (COP) is the osmotic pressure exerted by the macromolecules (the colloid molecules).1,2 This can be measured using a membrane transducer system in which the membrane is freely permeable to small ions and water but largely impermeable to the colloid molecules.3 The membrane pore size and the molecular size distribution of the colloid being studied will dictate the measured value (see Figures 89.2 and 89.3).2,3 Capillary walls are made up of endothelial cells, which are freely permeable to small ions such as Na+ and Cl−, but are relatively impermeable to larger molecules such as colloids (Figure 89.3). A colloid is a homogeneous non-crystalline substance consisting of large molecules or ultramicroscopic particles of one substance dispersed through a second substance – the particles do not settle and cannot be separated out by ordinary filtering or centrifuging like those of a suspension such as blood.2
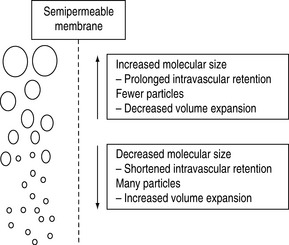
Figure 89.2 Polydispersity of colloid molecular size.
(Reproduced with permission from Grocott MPW, Mythen MG. Fluid therapy. In: Goldhill DR, Strunin L (eds) Clinical Anaesthesiology. London: Baillière Tindall; 1999: 363–81.)
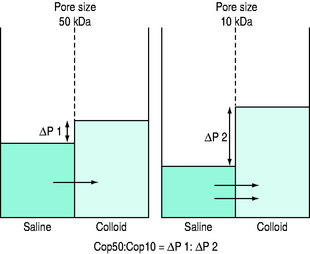
Figure 89.3 Schematic of technique for measurement of the COP50:COP10 ratio.
(Reproduced with permission from Grocott MPW, Mythen MG. Fluid therapy. In: Goldhill DR, Strunin L (eds) Clinical Anaesthesiology. London: Baillière Tindall; 1999: 363–81.)
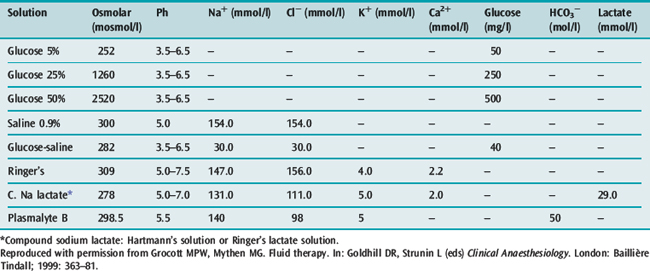
In practical terms, it is a fluid that when infused into the intravascular space should expand the blood volume by the volume infused.3,4 Crystalloids have larger volumes of distribution dependent on their composition (Figure 89.1 and Table 89.1). Colloids or plasma substitutes have molecular weights (MW) > 35 kDa, and infusion results in an initial increase in blood osmotic pressure. The ideal properties of a colloid are:
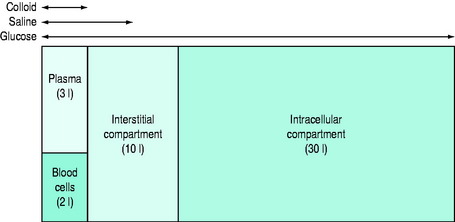
Figure 89.1 Simplified theoretical volumes of distribution of infused isotonic solutions of an ideal colloid, saline and glucose.
(Reproduced with permission from Grocott MPW, Mythen MG. Fluid therapy. In: Goldhill DR, Strunin L (eds) Clinical Anaesthesiology. London: Baillière Tindall; 1999: 363–81.)
Table 89.1 Comparison of contents, osmolarity and pH of crystalloid solutions for intravenous administration
COLLOID SOLUTIONS
There are two major groups of colloids – plasma derivatives or semisynthetics.5 Plasma derivatives include human albumin solutions, plasma protein fraction, fresh frozen plasma and immunoglobulin solutions. Three principal types of semisynthetic colloid molecule are commonly used in intravenous solutions – gelatins, dextrans and hydroxyethyl starches. All colloids are presented dissolved in a crystalloid solution. Isotonic saline is still the most commonly used carrier solution but isotonic glucose, hypertonic saline and isotonic balanced electrolyte solutions are also used.
Colloids often do not consist of uniform sized molecules. Human albumin solution contains more than 95% albumin with a uniform molecular size. This is described as monodisperse. Conversely, all the semisynthetic colloids have a distribution of molecular sizes and are described as polydisperse.1,2 The size–weight relationship is relatively constant although some colloids can have equivalent MW with different molecular size – succinylated and urea-linked gelatins have almost identical molecular weights but the succinylated product undergoes conformational change due to an increase in negative charge and is physically larger.2,3
HUMAN ALBUMIN SOLUTION
Human albumin is thought of as an ideal colloid and is commonly the reference solution against which other colloids are judged. Human albumin is a naturally occurring monodisperse colloid. Solutions (5% and 20%) are prepared from human plasma and heat treated to ensure that neither hepatitis nor HIV can be transmitted. It has a relatively short (∼1 year) shelf life at room temperature, but a 5-year shelf life at 2–8 °C. Human albumin 5% is used for the treatment of hypovolaemia in a wide variety of clinical conditions. Concentrated salt-poor 20% human albumin is used for the treatment of hypoalbuminaemia in the presence of salt and water overload (e.g. hepatic failure with ascites). Albumin has putative advantages that include limiting free radical-mediated damage but the importance of a normal serum albumin level remains uncertain.6–8
Human-derived colloid has a number of significant disadvantages. These are expensive products and concerns have been raised about transmission of infectious agents, such as new variant Creutzfeldt–Jakob disease (nvCJD) associated with bovine spongiform encephalopathy (BSE), that are not removed by currently available techniques. Concerns have also been raised by the conclusions of a highly controversial meta-analysis suggesting that the use of human albumin solution may be associated with increased mortality in the critically ill. These conclusions were probably unjustified,8,9 but there is certainly no evidence that the use of human albumin has any advantages over less expensive semisynthetic alternatives10,11 or indeed crystalloids. However, the latter applies to all colloids.12–14
GELATINS
Gelatins are prepared by hydrolysis of bovine collagen.15 The commonly available preparations are succinylated gelatin (Gelofusine) and urea-linked gelatin–polygeline (Haemaccel).1,2 Because of the significant calcium content of Haemaccel, citrated blood should not be infused through a giving set that has been previously used for this product; however, this does not apply to SAGM (saline–adenine–glucose–mannitol) blood. Gelatins are relatively inexpensive and stable, with long shelf lives. The gelatins’ plasma volume expanding effect only lasts about 90–120 minutes.3,4 They are mainly eliminated by renal excretion.
DEXTRANS
Dextrans are polysaccharides biosynthesised commercially from sucrose by the bacterium Leuconostoc mesenteroides using the enzyme dextran sucrase.1,2 This produces a high molecular weight dextran that is then cleaved by acid hydrolysis and separated by repeated ethanol fractionation to produce a final product with a relatively narrow molecular weight range. The products in current clinical use are described by their MW, Dextran 40 and Dextran 70 having MWs of 40 000 and 70 000 Da, respectively.
Dextran preparations are stable at room temperature and have long shelf lives. Dextran 70 is used as a plasma substitute for the treatment of hypovolaemia and has an intravascular volume-expanding effect that lasts at least 6 hours. Dextran 40 is used for its effects on microcirculatory flow and blood coagulation in some types of surgery (e.g. vascular, neuro and plastic surgery). The beneficial effects on outcome when Dextran 40 is used as an ‘anti-sludging agent’ remain controversial. Dextran 40 should not be used as a plasma substitute for the treatment of hypovolaemia as although it produces an immediate plasma volume expansion as a result of its small molecular weight it may obstruct renal tubules and produce renal failure.5
Red cell aggregation is also reduced with the lower molecular weight dextrans. In patients whose haemostatic function is normal prior to infusion, a maximum dose of 1.5 g/kg is often recommended to avoid risk of bleeding complications. The anticoagulant effects of dextrans can be utilised perioperatively as a prophylaxis against thromboembolism.1,2,5
HYDROXYETHYL STARCHES
Hydroxyethyl starches (HES) are produced by hydroxyethyl substitution of amylopectin, a D-glucose polymer, obtained from sorghum or maize.1,2 The pattern of hydroxyethyl substitution on glucose moieties influences the susceptibility to hydrolysis by non-specific amylases in the blood. A high C2 to C6 substitution ratio and a high overall degree of substitution (the proportion of glucose moieties that have been substituted) both protect against enzymatic breakdown and prolong the effective activity of HES as a plasma volume expander. The different HES products are commonly described by their weight-averaged molecular weight (MWw, number of molecules at each weight times the particle weight divided by the total weight of all the molecules). They are also described by their degree of substitution.1,2–5 Hetastarches (C2/C6 ratio 0.6–0.7), pentastarches (C2/C6 ratio ≈0.5) and tetrastarches (C2/C6 ratio ≈0.4). Starch usage varies between countries.
In the USA, the only starches available for clinical use are high molecular weight (> 450 kDa) hetastarches presented in either normal saline or a lactate-buffered, glucose-containing, balanced electrolyte solution (Hextend).16,17 There is a move towards the use of the non-saline based starch as the administration of high volumes of normal saline to humans has been associated with clinically significant acid–base disturbances.18–21 In particular, normal saline infusion has been shown to cause a hyperchloraemic metabolic acidosis; however, the pathogenesis and significance of this phenomenon remains controversial.18–22 Colloids presented in balanced electrolyte solutions are becoming available outside the USA. In Europe, the trend over the past 5 years has been towards the use of lower molecular weight (130 kDa) tetrastarches presented in saline and more recently in balanced electrolyte solutions.23 Starch preparations are stable at room temperature and have long shelf lives. The duration of intravascular retention is proportional to molecular weight3,4,24 but is > 6 hours even for the 130 kDa tetrastarches.
HES products are associated with an acceptable incidence of adverse events including anaphylactoid reactions. Tissue deposition may result in intractable itching if large volumes of HES are infused over several days.25 Whether the administration of large volumes of HES may cause some renal dysfunction remains controversial.26 HES products can cause a coagulopathy.27,28 In particular, HES products cause a reduction in factor VIII (FVIII) levels and impair platelet function causing a von Willebrand-like syndrome. These effects are greater with high molecular weight HES molecules infused at larger volumes. The effects of the high molecular weight starches on coagulation seemed to be lessened in the reformulated balanced electrolyte alternative cited above.16
THE ROLE OF HYPERTONIC SOLUTIONS
In recent years hypertonic (600–1800 mosmol) crystalloid and colloid solutions have been introduced for certain clinical indications.29–33 The theoretical advantage of these solutions is that a small volume of administered fluid will provide a significant plasma volume expansion. The high osmolarity of these solutions draws tissue fluid into the intravascular space and thus should minimise tissue oedema for a given plasma volume increment. Hypertonic crystalloids and colloids presented in a hypertonic saline carrier have been shown to achieve adequate resuscitation in a number of clinical settings. A smaller volume of hypertonic solution is normally required to achieve similar plasma volume expansion. In particular, these solutions are thought to result in reduced cerebral oedema in those patients at risk of this complication and indeed these solutions may have a place in treating refractory cerebral oedema.31–33 Outside the perioperative arena they are finding use in the management of burns patients and in prehospital resuscitation of trauma victims. They are limited at present to single dose administrations.
BLOOD SUBSTITUTES – PERFLUROCARBON AND HAEMOGLOBIN THERAPEUTICS
In future, the use of artificial or semisynthetic oxygen-carrying solutions will become increasingly common.34 A number of products have been and are currently under evaluation: perflurocarbon, modified human haemoglobin (Hb), modified bovine Hb and recombinant Hb solutions. These products not only function as oxygen carriers but are also capable of sustained plasma volume expansion. Unlike Hb present in intact red cells, these products often have a linear O2Hb dissociation profile and the effects of this on O2 uptake in the lungs and release in the tissues are not yet clearly defined. Additionally, they may have specific pharmacological effects – for example, with stroma-free haemoglobin nitric oxide scavenging resulting in vasoconstriction is well recognised. A modified bovine haemoglobin solution (Hemopure) has been licensed for clinical use in South Africa since 2001 but is still not approved for use elsewhere in the world.
PLASMA DERIVATIVES
FRESH FROZEN PLASMA
Fresh frozen plasma (FFP) contains normal plasma levels of all the clotting factors, albumin and immunoglobulin. A unit is typically 200–250 ml and has a FVIII level of at least 0.7 IU/ml (i.e. 70% of normal levels) and about 0.5 g of fibrinogen. It is kept at a temperature of −50 °C in order to preserve coagulant levels. FFP is indicated for the replacement of multiple clotting factor deficiencies (e.g. liver disease, coumarin anticoagulant overdose and coagulopathy associated with massive blood transfusion).35–37 An initial dose of 12–15 ml/kg (four packs for a 70-kg adult) is appropriate. Although it is effective, FFP should not be used as a plasma volume expander solely for the treatment of hypovolaemia.
CRYOPRECIPITATE
Cryoprecipitate is usually supplied as a pool of six or so single donor units in one pack. It is prepared from FFP by using a freeze thaw method, collection of the precipitate and then resuspending it in about 20 ml of plasma. It contains about 50% of the coagulant factor activity of the original unit (e.g. fibrinogen 250 mg, FVIII 100 IU). Cryoprecipitate is stored at –30 °C and remains stable for up to a year.35 It is indicated in the treatment of coagulation defects, including massive haemorrhage and DIC if there is microvascular bleeding associated with a fibrinogen level < 0.5 g/l and as an alternative to FVIII concentrate in the treatment of inherited deficiencies of von Willebrand factor, FVIII and FXIII.36,37 For an adult, transfusion of 3–4 g of fibrinogen (about 16 packs) should raise the fibrinogen level by 1 g/l.
FACTOR VIII
Factor VIII concentrate is prepared from large pools of donor plasma. One freeze-dried vial contains about 300 FVIII units.35 The concentrates are heat or chemically treated to inactivate HIV-1. It is stable for 2 years when stored at 4 °C. Further purification of the concentrate by chromatography results in high-purity FVIII and the relative merits of high- and intermediate-purity products are being investigated.
Recombinant FVIII is being used increasingly commonly in many parts of the world. Indications are the treatment of haemophilia A and von Willebrand’s disease.38 A dose of about 10–15 U/kg may be given, depending on the severity of bleeding and repeated 12 hourly, as required.
FACTOR IX
Factor IX concentrate (prothrombin concentrate) contains factors II, VII, IX and X in varying amounts, and is prepared from pools of plasma.35 It is available as a freeze-dried preparation, and is reconstituted with water immediately before use. Factor IX concentrate of intermediate purity is used to treat haemophilia B and to correct bleeding disorders due to an overdose of coumarin anticoagulants in patients who cannot tolerate large volumes of FFP.35 Purified factor IX concentrates are now available and are 100 times purer than the traditional concentrate, with 75% of total protein being factor IX.
OTHER PLASMA CONCENTRATES
Activated factor VII concentrate can be used in patients with inhibitors to factor VIII or IX, as it bypasses the requirement for these factors.35 Activated factor VII has also been used in the treatment of severe life-threatening haemorrhage but this remains controversial.39 Other concentrates, such as antithrombin III, C1 esterase inhibitor, and proteins C and S, are available for treating deficient patients at times of risk, such as surgery. Most of these concentrates have been used experimentally with varying success for the treatment of DIC and septic shock.
Recombinant versions of most plasma factors are either clinically available or in development and will probably become the standard of care. Recombinant activated protein C has been shown to be beneficial in a large human study for the treatment of septic shock40 (see Chapter 11).
IMMUNOGLOBULINS (GAMMAGLOBULINS)
Normal human immunoglobulin preparation is made from plasma pools obtained from normal blood donors. It contains antibodies to hepatitis A, measles, mumps, varicella, polio and prevalent bacteria.35 Immunoglobulins are indicated in the prevention or treatment of patients with hypogammaglobulinaemia, and may have a role in the treatment of autoimmune diseases, such as thrombocytopenic purpura and myasthenia gravis. Specific immunoglobulins are available for a range of infectious agents including tetanus, hepatitis B, rubella, measles, rabies and varicella zoster. They are made from donor plasma known to contain high levels of the specific IgG antibodies and are used for prophylaxis and treatment in patients who have not been actively immunised. Rhesus-D immunoglobulin is prepared from plasma containing high levels of anti Rh-D antibodies and it prevents sensitisation of Rh-negative mothers to Rh-D positive cells that may enter their circulation. The principal use of Rh-D immunoglobulin is in the prevention of haemolytic disease of the newborn.41
1 Grocott MPW, Mythen MG. Fluid therapy. In: Goldhill DR, Strunin L, editors. Clinical Anaesthesiology. London: Baillière Tindall; 1999:363-381.
2 Vercueil A, Grocott MP, Mythen MG. Physiology, pharmacology, and rationale for colloid administration for the maintenance of effective hemodynamic stability in critically ill patients. Transfus Med Rev. 2005;19:93-109.
3 Webb AR, Barclay SA, Bennett ED. In vitro colloid osmotic pressure of commonly used plasma expanders and substitutes: a study of the diffusibility of colloid molecules. Intensive Care Med. 1989;15:116-120.
4 Lamke LO, Liljedahl SO. Plasma volume changes after infusion of various plasma expanders. Resuscitation. 1976;5:93-102.
5 Boldt J, Suttner S. Plasma substitutes. Minerva Anestesiol. 2005;71:741-758.
6 Halliwell B. Albumin – an important extracellular antioxidant? Biochem Pharmacol. 1988;37:569-571.
7 Vincent JL, Navickis RJ, Wilkes MM. Morbidity in hospitalized patients receiving human albumin: a meta-analysis of randomized, controlled trials. Crit Care Med. 2004;32:2029-2038.
8 Cochrane Injuries Group Albumin Reviewers. Human albumin administration in critically ill patients: systematic review of randomised controlled trials. BMJ. 1998;317:235-240.
9 Bunn F, Alderson P, Hawkins V. Colloid solutions for fluid resuscitation (Cochrane Review). The Cochrane Library. 4, 2000.
10 Stockwell M, Soni N, Riley B. Colloid solutions in the critically ill. A randomized comparison of albumin and polygeline. 1. Outcome and duration of stay in the intensive care unit. Anaesthesia. 1992;47:3-6.
11 Stockwell M, Scott A, Riley B, et al. Colloid solutions in the critically ill. A randomised comparison of albumin and polygeline. 2. Serum albumin concentration and incidences of pulmonary oedema and acute renal failure. Anaesthesia. 1992;47:7-9.
12 Finfer S, Bellomo R, Boyce N, et al. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350:2247-2256.
13 Schierhout G, Roberts I. Fluid resuscitation with colloid or crystalloid solutions in critically ill patients: a systematic review of randomised trials. BMJ. 1998;316:961-964.
14 Choi P, Yip G, Quinonez L, et al. Crystalloids vs colloids in fluid resuscitation: a systematic review. Crit Care Med. 1999;27:200-210.
15 Davies MJ. Polygeline. Dev Biol Stand. 1987;67:129-131.
16 Gan TJ, Bennett-Guerrero E, Phillips-Bute B, et al. Hextend, a physiological balanced plasma expander for large volume use in major surgery: a randomized phase III clinical trial. Anesth Analg. 1999;88:992-998.
17 Waters JH, Gottlieb A, Schoenwald P, et al. Normal saline versus lactated Ringer’s solution for intraoperative fluid management in patients undergoing abdominal aortic aneurysm repair: an outcome study. Anesth Analg. 2001;93:817-822.
18 Reid F, Lobo DN, Williams RN, et al. (Ab)normal saline and physiological Hartmann’s solution: a randomized double-blind crossover study. Clin Sci (Lond). 2003;104:17-24.
19 Brill SA, Stewart TR, Brundage SI, et al. Base deficit does not predict mortality when secondary to hyperchloremic acidosis. Shock. 1999;17:459-462.
20 Williams EL, Hildebrand KL, McCormick SA, et al. The effect of intravenous lactated Ringer’s solution versus 0.9% sodium chloride solution on serum osmolality in human volunteers. Anesth Analg. 1999;88:999-1003.
21 Wilkes NJ, Woolf R, Stephens R, et al. The effects of balanced versus saline based intravenous solutions on acid base status and gastric mucosal perfusion in elderly surgical patients. Anesth Analg. 2001;93:811-816.
22 Ho AM, Karmakar MK, Contardi LH, et al. Excessive use of normal saline in managing traumatized patients in shock: a preventable contributor to acidosis. J Trauma. 2001;51:173-177.
23 Boldt J, Wolf M, Mengistu A. A new plasma-adapted hydroxyethylstarch preparation: in vitro coagulation studies using thrombelastography and whole blood aggregometry. Anesth Analg. 2007;104:425-430.
24 James MF, Latoo MY, Mythen MG, et al. Plasma volume changes associated with two hydroxyethyl starch colloids following acute hypovolaemia in volunteers. Anaesthesia. 2004;59:738-742.
25 Morgan PW, Berridge JC. Giving long persistent starch as volume replacement can cause pruritus after cardiac surgery. Br J Anaesth. 2000;85:696-699.
26 Zander R, Boldt J, Engelmann L, et al. The design of the VISEP trial: Critical appraisal. Anaesthesist. 2007;56:71-77.
27 Treib J, Baron JF. Hydroxethyl starch: effects on hemostasis. Ann Fr Anesth Reanim. 1998;17:72-81.
28 Roche AM, James MF, Bennett-Guerrero E, et al. A head-to-head comparison of the in vitro coagulation effects of saline-based and balanced electrolyte crystalloid and colloid intravenous fluids. Anesth Analg. 2006;102:1274-1279.
29 Wade CE, Grady JJ, Kramer GC. Efficacy of hypertonic saline dextran fluid resuscitation for patients with hypotension from penetrating trauma. J Trauma. 2003;54(5 Suppl):S144-S148.
30 Oi Y, Aneman A, Svensson M, et al. Hypertonic saline-dextran improves intestinal perfusion and survival in porcine endotoxin shock. Crit Care Med. 2000;28:2843-2850.
31 Simma B, Burger R, Falk M, et al. A prospective, randomized, and controlled study of fluid management in children with severe head injury: lactated Ringer’s solution versus hypertonic saline. Crit Care Med. 1998;26:1265-1270.
32 Dubick MA, Bruttig SP, Wade CE. Issues of concern regarding the use of hypertonic/hyperoncotic fluid resuscitation of hemorrhagic hypotension. Shock. 2006;25:321-328.
33 Shackford SR, Bourguignon PR, Wald SL, et al. Hypertonic saline resuscitation of patients with head injury: a prospective, randomized clinical trial. J Trauma. 1998;44:50-58.
34 Farrar D, Grocott M. Intravenous artificial oxygen carriers. Hosp Med. 2003;64:352-356.
35 McClelland DBL, editor. Handbook of Transfusion Medicine. London: HMSO, 1989.
36 Mammen EF. Disseminated intravascular coagulation (DIC). Clin Lab Sci. 2000;13:239-245.
37 Mythen MG, Machin SJ. Derangements of blood coagulation. In: Hanson GC, editor. Critical Care of the Surgical Patient. London: Chapman and Hall; 1997:185-194.
38 Hambleton J. Advances in the treatment of von Willebrand disease. Semin Hematol. 2001;38:7-10.
39 Moscardo F, Perez F, de la Rubia J, et al. Successful treatment of severe intra-abdominal bleeding associated with disseminated intravascular coagulation using recombinant activated VII. Br J Haematol. 2001;114:174-176.
40 Bernard GR, Vincent JL, Laterre PF, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699-709.
41 Greenough A. The role of immunoglobulins in neonatal rhesus haemolytic disease. BioDrugs. 2001;15:533-541.