Coating of tablets and multiparticulates
Stuart C. Porter
Chapter contents
Description of the film-coating process
Types of film-coating polymers: immediate-release coatings
Types of film-coating polymers: modified-release coatings
Ideal characteristics of film-coated products
Ideal characteristics of sugar-coated tablets
Description of the sugar-coating process
Description of the compression-coating process
Overview of coating of tablets
Mechanisms of drug release from multiparticulates
Key points
Introduction
Coatings may be applied to a wide range of oral solid dosage forms, including tablets, capsules, multiparticulates and drug crystals. While tablets represent the class of dosage form that is most commonly coated, coated multiparticulates are also popular.
Definition of coating
Coating is a process by which an essentially dry, outer layer of coating material is applied to the surface of a dosage form in order to confer specific benefits that broadly range from facilitating product identification to modifying drug release from the dosage form.
Reasons for coating
The reasons for coating pharmaceutical oral solid dosage forms are quite varied. The more common reasons include (no order of importance implied):
Types of coating processes
Three main types of process are used in the pharmaceutical industry today:
Film coating is the most popular technique and virtually all new coated products introduced to the market are film coated. Film coating involves the deposition, usually by spraying a liquid coating system, of a thin film of a polymer-based formulation onto the surface of a tablet, capsule or multiparticulate core.
Sugar coating is a more traditional process closely resembling that used for coating confectionery products. It has been used in the pharmaceutical industry since the late 19th century. It involves the successive application of sucrose-based coating formulations, usually to tablet cores, in suitable coating equipment. The water evaporates from the syrup, leaving a thick sugar layer around each tablet. Sugar coats are often shiny and highly coloured.
Compression coating, although traditionally a less popular process, has gained increased interest in recent years as a means of creating specialized modified-release products. It involves the compaction of granular material around a preformed tablet core using specially designed tableting equipment. Compression coating is essentially a dry process.
Each of these processes will now be considered in turn and an overview of relevant coating processes and materials will be given.
Film coating
Film coating is the more contemporary and thus commonly used process for coating oral solid dosage forms. As described above, it involves the application of a thin film to the surface of a tablet, capsule or multiparticulate core. Now all newly launched coated products are film coated rather than sugar coated, often for many of the reasons given in Table 32.1.
Table 32.1
| Features | Sugar coating | Film coating |
| Tablets | ||
| Appearance | Rounded with high degree of polish | Retains contour of original core Usually not as shiny as sugar coat types |
| Weight increase due to coating materials | 30–50% | 2–3% |
| Logo or ‘breaklines’ | Not possible | Possible |
| Other solid dosage forms | Coating possible but little industrial importance | Coating of multiparticulates very important in modified-release forms |
| Process | ||
| Stages | Multistage process | Usually single stage |
| Typical batch coating time | 8 hours, but easily longer | 1.5–2 hours |
| Functional coatings | Not usually possible apart from gastro-resistant (enteric) coating | Easily adaptable for controlled release |
Types of film coatings
Film coatings may be classified in a number of ways but it is common practice to do so in terms of the intended effect of the applied coating on drug release characteristics. Hence film coatings may be designated as either:
Immediate-release coatings are usually readily soluble in water, while gastro-resistant coatings are only soluble in water at pH values in excess of 5–6 and are intended to either protect the drug while the dosage form is in the stomach (in the case of acid-labile drugs) or prevent release of the drug in the stomach (in the case of drugs that are gastric irritants). More recently, gastro-resistant coatings have been employed as an integral part of colonic drug delivery systems (Alvarez-Fuentes et al 2004). For the most part, extended-release coatings are insoluble in water. They are designed to ensure that the drug is released in a consistent manner over a relatively long period of time (typically 6–12 hours) and thus reduce the number of doses that a patient needs to take in each 24-hour period. Additionally, extended-release film coatings are used to modify drug release in such a way that desired therapeutic benefits can more easily be achieved and thus drug efficacy improved.
Description of the film-coating process
Film coating involves the application of liquid, polymer-based formulations to the surface of the tablets (for the sake of brevity, the term ‘tablet’ will be used here to represent any form of dosage unit that is to be coated). It is possible to use conventional panning equipment but more usually specialized equipment is employed to take advantage of the fast coating times and high degree of automation possible.
The coating liquid (solution or suspension) contains a polymer in a suitable liquid medium, together with other ingredients such as pigments and plasticizers. This solution is sprayed on to a rotating, or fluidized, mass of tablets. The drying conditions employed in the process result in the removal of the solvent, leaving a thin deposit of coating material around each tablet core.
Process equipment
The vast majority of film-coated tablets are produced by a process which involves the atomization (spraying) of the coating solution or suspension on to the surface of tablets.
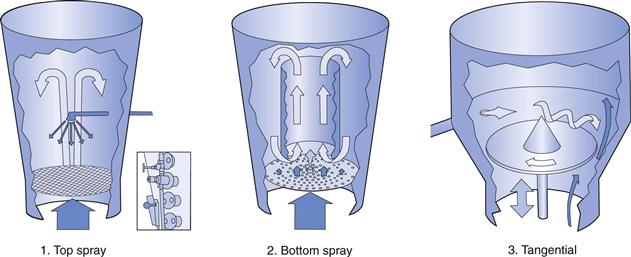
Modern pan-coating equipment is of the side-vented type (as shown in Fig. 32.1) and some typical examples include:
• Accela Cota – Thomas Engineering (USA)
• Premier Coater – Bosch-Manesty (UK)
• Hi-Coater – Freund-Vector (Japan and USA)
Manufacturers of units that operate on a fluidized-bed principle include:
Depending on the particular film-coating application involved, fluidized-bed equipment may be further divided into one of the three operating principles shown in Figure 32.2.
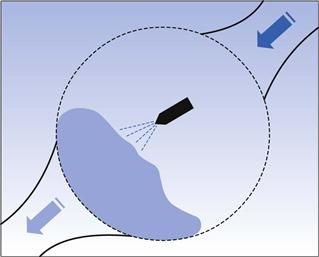
Fig. 32.1 Schematic of side-vented coating pan.
The coating formulation is invariably added as a solution or suspension via a spray gun. The design and operation of film-coating spray guns are similar in both perforated-pan and fluidized-bed coaters, although differences in the performance of spray guns provided by different vendors, especially in perforated-pan processes, have been noted (Macleod & Fell 2002). A typical example of spray coating in a pan coater is shown in Figure 32.3.
Basic process requirements for film coating
The fundamental requirements of a film-coating process are more or less independent of the actual type of equipment being used and include:
Film-coating formulations
Currently, the majority of film-coating processes involve the application of a coating liquid where a significant proportion of the main component (the solvent/vehicle) is removed by means of a drying process that is concurrent with the application of that coating liquid. Film-coating formulations typically comprise:
There are certain types of coating process that differ from this common approach. For example, some processes involve the application of hot-melt coatings that congeal on cooling (Jozwiakowski et al 1990), while others take advantage of recent developments in powder application technologies (Porter 1999).
It should be mentioned that all ingredients used in film-coating formulations must comply with the relevant regulatory and pharmacopoeial requirements current in the intended marketing area.
Film-coating polymers
The ideal characteristics of a film-coating polymer are discussed below.
Solubility
Polymer solubility is important for two reasons:
Film coatings that are used on immediate-release products should utilize polymers that have good solubility in aqueous fluids to facilitate the rapid dissolution of the active ingredient from the finished dosage form following ingestion. Such coatings are usually applied as solutions in an appropriate solvent system (with a strong preference being shown for water). However, film coatings used to modify the rate or onset of drug release from the dosage form tend to have limited or no solubility in aqueous media; such coatings are usually applied either as polymer solutions in organic solvents or as aqueous polymer dispersions (discussed later in this chapter).
Viscosity
Viscosity is very much a limiting factor with regard to the ease with which a film coating can be applied. High viscosity (typically that exceeding about 500 mPa s) complicates transfer of the coating liquid from the storage vessel to the spray guns, and subsequent atomization of that coating liquid into fine droplets. Ideally, therefore, polymers applied as solutions in a selected solvent should exhibit relatively low viscosities at the preferred concentration. This will help to facilitate easy, trouble-free spray application of the coating solution, especially in production-scale film-coating equipment.
Permeability
Appropriate permeability (to which the chosen polymer makes a significant contribution) is a key attribute when considering the various functional properties that film coatings are expected to possess. For example, coating permeability is of significant importance when the film coating is intended to:
• mask the unpleasant taste of the active ingredient in the dosage form
• modify the rate at which the active ingredient will be released from the dosage form.
These properties vary widely between the various polymers that might be considered for film-coating formulations.
Mechanical properties
In order to perform effectively for the purpose intended, a film coating must exist as a discrete, continuous coating around the surface of the product to be coated, and must be free from defects typically caused by the stresses to which the coating is likely to be exposed during the coating process, during packaging and during the subsequent distribution of the final product.
Consequently, film-coating polymers should possess suitable characteristics with respect to:
The generation and minimization of film-coating defects are discussed more fully later in this chapter.
Types of film-coating polymers: immediate-release coatings
Cellulose derivatives
Most cellulosic polymers used in film-coating formulations are substituted ethers of cellulose.
Hydroxypropyl methylcellulose (HPMC) is the most widely used of the cellulosic polymers. Its molecular structure is shown in Figure 32.4. It is readily soluble in aqueous media and forms films that have suitable mechanical properties, and coatings that are relatively easy to apply. Coatings that utilize this polymer may be clear or coloured with permitted pigments. The polymer is the subject of monographs in the major international pharmacopoeias.
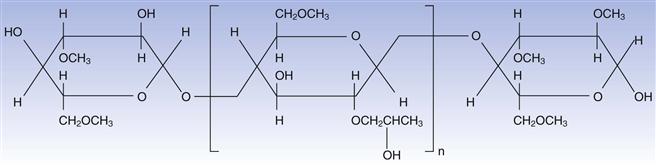
Fig. 32.4 Hydroxypropyl methylcellulose.
Other cellulosic derivatives used in film coatings, which have similar properties to HPMC, include methyl cellulose (MC) and hydroxypropyl cellulose (HPC).
Vinyl derivatives
The most commonly used vinyl polymer in pharmaceutical applications is polyvinyl pyrrolidone (PVP). Unfortunately, this polymer has limited use in film-coating formulations because of its inherent tackiness. A copolymer of vinyl pyrrolidone and vinyl acetate, copovidone, is considered a better film former than PVP. Another useful vinyl polymer is polyvinyl alcohol (PVA), a partial hydrolysate of polyvinyl acetate, which can be used to produce film coatings that have suitable mechanical properties and are highly adherent to pharmaceutical tablets. In addition, PVA exhibits good barrier properties to environmental gases (Okhamafe & York 1983) and water vapour (Jordan et al 1995).
Film coatings that use PVA as the primary polymer have mainly been exploited as special barrier coatings, helping to improve the stability of drug substances that are either sensitive to moisture (especially in countries that have humid climates) or are readily oxidized when exposed to atmospheric oxygen.
Recently, film coatings based on a copolymer of vinyl alcohol and ethylene glycol (Ziegler & Koller 2003) have become available. These coatings are less tacky than traditional PVA coatings and have the additional benefit of being extremely flexible, thus improving film robustness and allowing greater expansion capabilities should the tablet cores expand slightly during the coating process.
Aminoalkyl methacrylate copolymers
These acrylic copolymers are readily soluble in aqueous media at low pH only, and thus are of prime importance in coating dosage forms where the need to achieve effective taste masking is a critical attribute (Dittgen et al 1997). These polymers are typically applied as solutions in organic solvents, although special forms may also be used to prepare aqueous polymer dispersions. An example of the molecular structure of this type of acrylic polymer is shown in Figure 32.5.
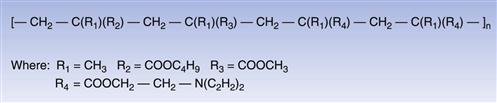
Fig. 32.5 Aminoalkyl methacrylate copolymer.
Types of film-coating polymers: modified-release coatings
Cellulose derivatives
As is the case with cellulosic polymers used in immediate-release applications, cellulosic polymers used for modified-release purposes are typically substituted ethers of cellulose. However, the level of substitution in this case is usually much higher, thus rendering the polymer insoluble in water. A typical example of such a cellulosic polymer is ethyl cellulose (EC), which is preferred for many extended-release applications (Porter 1989). Historically, ethyl cellulose has been applied as solutions in organic solvents, although aqueous polymer dispersions are commercially available.
Other cellulose derivatives used for modified-release applications include cellulose esters such as cellulose acetate (CA).
Methylmethacrylate copolymers
Acrylic ester polymers are typically insoluble in water but can be prepared with varying degrees of permeability to render them suitable for a variety of extended-release applications (Dittgen et al 1997). Originally intended for use as solutions in organic solvents, these polymers are commonly used today as aqueous polymer dispersions.
Methacrylic acid copolymers
The special functionality conferred by the presence of carboxylic acid groups enables this class of polymer to function as gastro-resistant coatings (Dittgen et al 1997). This is because the polymer is insoluble in water at the low pH that typifies conditions in the stomach but gradually becomes soluble as the pH rises towards neutrality, a condition that is more typical of the upper part of the small intestine. Currently, methacrylic acid copolymers are also commonly used as aqueous polymer dispersions. An example of the molecular structure of this type of acrylic polymer is shown in Figure 32.6.
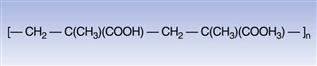
Fig. 32.6 Methacrylic acid copolymer.
Phthalate esters
In terms of functionality, phthalate ester polymers exhibit similar properties to methacrylic acid copolymers (Chang 1990), in that they are most suited to delayed-release applications. Chemically, they are formed by the substitution of phthalic acid (or similar) groups into polymers that have been commonly used in other film-coating applications. Thus common examples of phthalate ester polymers are hydroxypropylmethylcellulose phthalate (HPMCP), cellulose acetate phthalate (CAP), and polyvinyl acetate phthalate (PVAP). Phthalate ester polymers may be applied as solutions in organic solvents or as aqueous polymer dispersions.
Plasticizers
Plasticizers are generally added to film-coating formulations to modify physical properties of the polymer. This is necessary because most acceptable film-coating polymers can be brittle and inflexible in nature. It is generally accepted that the mechanism by which plasticizers exert their effect is for plasticizer molecules to interpose themselves between the polymer molecules, thus increasing free volume and facilitating increased polymer chain motion within the structure of the coating. The positive benefits of this interaction include:
Examples of commonly used plasticizers are:
• polyols, such as polyethylene glycols and propylene glycol
• organic esters, such as diethyl phthalate and triethyl citrate
For a given application, it is generally desirable to use plasticizers that are soluble in the solvent system being used.
Colourants
Pharmaceutically acceptable colourants are available in both water-soluble form (known as dyes) and water-insoluble form (known as pigments). The insoluble form is preferred in film-coating formulations, based on the fact that pigments tend to be more chemically stable towards light, provide better opacity and covering power, and provide a means of optimizing the permeability properties of the applied film coating. In addition, water-insoluble pigments will not suffer from the disadvantageous phenomenon of mottling (caused by solute migration, as discussed in Chapter 29) that can be observed with water-soluble dyes.
Examples of colourants are:
While the selection of a colourant is typically based on the need to achieve a certain visual effect and, to a lesser extent, the potential influence on film mechanical properties, an underlying selection criterion is that of regulatory acceptance. While there are many colourants that can be used, few have the full global regulatory acceptance required to facilitate the world-wide use of the same coating formulation.
Solvents
Initially, film-coating processes were very much dependent on the use of organic solvents (such as methanol/dichloromethane combinations or acetone) in order to achieve the rapid drying characteristics demanded by the process. Unfortunately, organic solvents possess many disadvantages (Hogan 1982) that are related to the following factors:
Such disadvantages have provided the momentum for the current utilization of aqueous coating formulations as the preferred option. However, as a result of improved efficiency and decreased costs associated with solvent recovery, there has been an upsurge in interest in solvent coating but almost exclusively in modified-release coating applications.
Aqueous polymer dispersions
Essentially, all polymers used in modified-release film-coating applications are, in order to achieve their intended functionality, generally insoluble in water. Hence, they have been applied as solutions in organic solvents. The growing demand for aqueous formulations in modern film-coating processes, thus, initially caused a dilemma for pharmaceutical formulators, a dilemma that has ultimately been resolved by creating aqueous polymer dispersions of many of these polymers. The term ‘aqueous polymer dispersion’ is broadly used to describe polymer systems that are supplied as lattices, as well as those that have been formed into aqueous suspensions, and their use has been comprehensively reviewed by McGinity (2008).
Ideal characteristics of film-coated products
A film-coated product and its associated manufacturing process should be designed and controlled to ensure that the following characteristics are evident:
When designing film-coating formulations, it is often useful to use specialized assessment techniques that allow the properties of the individual ingredients being considered (including their potential beneficial interactions), of the coating formulations, and of the final coated product, to be evaluated. A review of many of these useful assessment techniques has been provided by Porter and Felton (2010).
Film-coating defects
Film coating is not a perfect process. Defects and imperfections in the coat can occur if the formulation of the coat and the core, or the coating process, is not adequately designed and controlled. The defects that are commonly attributed to film coating are usually:
By far the most common defects are those in the former category and these have been described in detail by Rowe (1992), with suggestions for their resolution. Visual defects can be categorized as those relating to:
Sugar coating
Sugar coating has long been the traditional method for coating pharmaceutical products (usually tablets). It involves the successive application of sucrose-based coating formulations to tablet cores in suitable coating equipment. Conventional panning equipment with manual application of syrup has been extensively used, although more specialized equipment and automated methods are now making an impact on the process. A comparison between sugar coating and film coating is tabulated in Table 32.1 and illustrated in Figure 32.7.
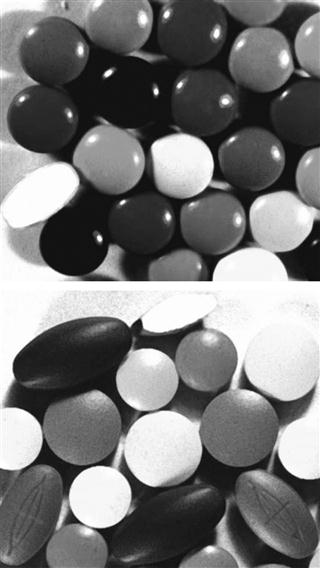
Fig. 32.7 Sugar- and film-coated tablets.
Types of sugar coatings
Sugar coatings are composed of ingredients that are readily soluble, or disintegrate rapidly, in water. Although it is technically feasible to apply sugar coatings to a wide range of pharmaceutical core materials, it is almost exclusively reserved for coating tablets. In general, sugar-coated tablets are intended to exhibit immediate-release attributes. However, one of the stages of the sugar-coating process, the sealing step (discussed below), involves the deposition of a polymer-based coating to the surface of the uncoated tablets. At this stage, it is possible to use some of the speciality polymers (described earlier under film coating) that are either partially or completely insoluble in water, thus enabling the sugar-coated product to exhibit delayed (gastro-resistant)-release or extended-release characteristics.
Ideal characteristics of sugar-coated tablets
Sugar-coated tablets should possess a smooth, rounded contour, with even colour coverage and a glossy finish. Those tablets that have been imprinted should exhibit high print quality (clear distinct print with no smudging or missing print). As is the case with film-coated tablets, sugar-coated tablets must be compliant with finished product specifications and any relevant compendial requirements.
Process equipment
Typically, tablets are sugar coated by a panning technique, using a traditional rotating sugar-coating pan with a supply of drying air (preferably capable of being thermostatically controlled), and an extraction system to remove dust- and moisture-laden air (illustrated in Fig. 32.8).

Fig. 32.8 Traditional sugar-coating pan.
Traditional sugar-coating processes involve manual application techniques, whereby the coating liquid is ladled directly onto the surface of the cascading bed of tablets. In efforts to improve control and to speed up the sugar-coating process, many of the process equipment improvements discussed under film coating are used. Automated dosing techniques and control procedures can also now be employed to great effect.
Description of the sugar-coating process
Sugar coating is a multistage process and can be divided into the following steps:
At each stage of the coating process, multiple applications of the specified coating formulation are made using a sequential approach that involves:
• applying a predetermined amount of coating liquid
• drying by directing air of the desired temperature onto the surface of the rolling mass of tablets
• repeating each of these steps the required number of times.
Each of these substeps of a typical sugar coating process is now discussed in turn.
Sealing
Sugar coatings are aqueous formulations that are, quite literally, poured directly onto the tumbling tablets. Hence, water has an opportunity to penetrate directly into the tablet cores, potentially affecting product stability and possibly causing premature tablet disintegration. To prevent these problems, the cores are usually sealed initially with a water-proofing or sealing coat. Traditionally, alcoholic solutions of shellac were used for this purpose although the use of synthetic polymers, such as cellulose acetate phthalate or polyvinyl acetate phthalate, is now favoured. Unless a modified-release feature needs to be introduced, the amount of sealing coat applied has to be carefully calculated so that there is no negative influence on drug release characteristics for what should otherwise be an immediate-release product.
Subcoating
Sugar coatings are usually applied in quite substantial quantities to the tablet core (typically increasing the weight by as much as 50–100%) in order to round off the tablet edges. Much of this material build-up occurs during the subcoating stage and is achieved by adding bulking agents such as calcium carbonate to the sucrose solutions. In addition, antiadherents such as talc may be used to prevent tablets sticking together, and polysaccharide gums, such as gum acacia, may also be added as a binder in order to reduce brittleness.
Smoothing
The subcoating stage is notorious for producing a surface finish that is somewhat rough. To facilitate the application of the colouring layer (which requires a smooth surface), subcoated tablets are usually smoothed out by applying a sucrose coating that is often coloured with titanium dioxide to achieve the desired level of whiteness.
Colouring
Nearly all sugar-coated tablets are coloured, since visual elegance is usually considered to be of great importance with this type of coated dosage form. Colour coatings usually consist of sucrose syrups containing the requisite colouring materials. As with film coating colours, sugar-coating colourants may be subdivided into either water-soluble dyes or water-insoluble pigments. Traditionally, water-soluble dyes have been used but in order to speed up the coating process and minimize colour migration problems, dyes have gradually been replaced with pigments. The actual colourants used must comply with regulations promulgated by the national legislation of the country where the products are to be marketed.
Polishing
Once the colour coating layers have been applied and dried, the tablet surface tends to be smooth but somewhat dull in appearance. To achieve the glossy finish that typifies sugar-coated products, a final stage involving the application of waxes is employed. Suitable waxes include beeswax, carnauba wax or candelilla wax applied as finely ground powders or as suspensions/solutions in an appropriate organic solvent.
Printing
It is common practice to identify all oral solid dosage forms with a manufacturer’s logo, product name, dosage strength or other appropriate code. For sugar-coated products such identification can only be achieved by means of a printing process, which is typically an offset gravure process using special edible inks. However, alternative printing processes, such as ink-jet and pad-printing processes, have also gained acceptance.
Sugar-coating defects
Sugar coating is technically and practically a difficult process that requires a great deal of skill and experience. Defects in the coating can occur without adequate process control. Although they may well have drug release or stability implications, defects associated with sugar-coated tablets are likely to be visual in nature. Common sugar-coat defects include:
Compression coating
Compression coating differs radically from film coating and sugar coating. The process involves the compaction of granular material around preformed tablet cores (Fig. 32.9) using specially designed tableting equipment. Compression coating is essentially a dry process (although the coating formulation may have been produced by a wet-granulation process).

Fig. 32.9 Diagram of compression-coated tablet.
Description of the compression-coating process
Compression coating is based on a modification of the traditional tableting process. Tablet cores are first prepared and then mechanically transferred, on the same machine, to another, slightly larger die that has partially been filled with the coating powder. The tablet core is positioned centrally into this partially filled die, more coating powder is filled on top of the core and the whole composite mass undergoes a second compaction event. A detailed description of the compression-coating process has been given by Gunsel & Dusel (1990).
Traditionally, compression coating has been used to separate incompatible materials (one contained in the tablet core and the second in the coating). With the traditional compression-coating process, there is still an interface layer (between the two layers) that may potentially compromise product stability. Thus, it is also possible to apply two coating layers where the first coating layer is an inert, placebo formulation that effectively separates the core and the final coating layer, each of which contains a drug substance that is incompatible with the other.
Compression coating is a mechanically complex process that requires careful formulation and processing of the coating layer. Large or irregularly shaped granules will cause the core to tilt in the second die used for compression of the coating, increasing the likelihood of compressing an uneven or incomplete coating, with the core occasionally being visible at the tablet surface.
Types of compression coatings
Compression coatings are generally made from powdered ingredients that either dissolve, or readily disintegrate, in aqueous media and thus have commonly been used for immediate-release tablet products. There has been increased use of compression coatings for the purpose of creating modified-release products (Chopra 2003, Waterman & Fergione 2003).
Coating of tablets
Overview of coating of tablets
Tablets are by far the most common type of oral solid dosage form that is coated. They may be coated with both immediate-release and modified-release coatings. Of the techniques available for applying coatings to tablets, the following are the most likely to be used:
Standards for coated tablets
In general, pharmacopoeias have similar requirements for coated and uncoated tablets, the differences being that:
Coating of multiparticulates
Coated multiparticulates, often referred to as ‘pellets’ or ‘beads’, commonly form the basis for a wide range of modified-release dosage forms (as described by Tang et al 2005 and in Chapter 31). Typically used for extended-release products, interest has grown in this type of dosage form for delayed-release (gastro-resistant) applications as well. Coated multiparticulates possess many benefits compared to conventional non-disintegrating tablets (coated or otherwise) for a broad range of modified-release applications. These benefits are discussed more fully in Chapters 22 and 31 but are summarized here:
Coated multiparticulates are most commonly filled into two-piece, hard gelatin capsules to form the final dosage unit, although they may also be compacted (as long as appropriate formulation and processing strategies are used to avoid rupturing of the coating) into tablets.
Types of multiparticulates
A number of different types of multiparticulates are illustrated in Figure 32.10. They are commonly film coated in order to create modified-release products.
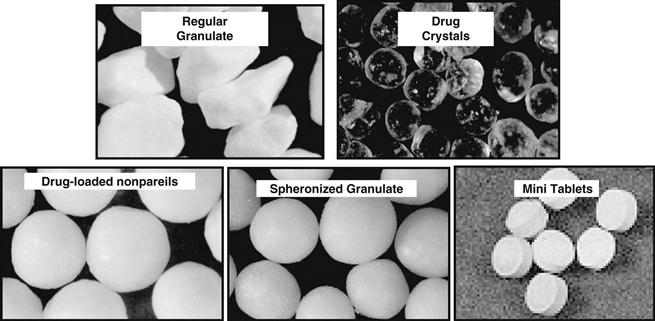
Fig. 32.10 Examples of film-coated multiparticulates.
Drug crystals
Drug crystals, as long as they are of the appropriate size and shape (elongated or acicular crystals should be avoided), can be directly coated with a modified-release film coating.
Irregular granules
Granulates, such as those regularly used to prepare tablets, can be film coated but variation in particle size distribution (from batch to batch), as well as the angular nature of such particles, can make it difficult to achieve uniform coating thickness around each particle.
Spheronized granules
Spheroidal particles simplify the coating process. The production of the spheroidal cores is detailed in Chapter 28.
Drug-loaded nonpareils
Another process for producing spheroidal particles involves the application of drug to the surface of placebo pellets, often called nonpareils. These are preformed spherical particles about 1 mm in diameter consisting primarily of sucrose and starch, although some such particles may also be prepared using microcrystalline cellulose. Application of the drug uses either:
Mini tablets
Many of the other types of multiparticulates described so far suffer from two potential batchwise drawbacks, namely:
Such variability can result in variable coating thickness and thus product performance. This problem can be overcome by using mini compressed tablets (typically in the size range of 1–2 mm) produced using a modification of traditional tableting processes.
Mechanisms of drug release from multiparticulates
There are many factors that influence drug release from coated multiparticulates, some of which are related to the formulations used and others to the various manufacturing processes employed. Irrespective of the actual number of factors involved, it is generally accepted that the mechanisms of drug release (Ozturk et al 1990, Zhang et al 1991) can generally be ascribed to specific conditions.
Diffusion
Diffusion is primarily a process whereby drug will partition into the film coat membrane and permeate through it. The rate at which the drug is released by this mechanism is primarily influenced by the drug concentration gradient across the membrane, the thickness of the membrane, the solubility of the drug in the membrane and the permeability coefficient governing passage of the drug through the membrane.
Osmosis
Once water has passed through the film coating, dissolution of soluble components (excipients and drug) within the core can allow an osmotic pressure to be generated inside the coated particle that will influence the rate at which the drug will be pushed out through pores or a preformed aperture in the membrane.
Dialysis
Dialytic effects describe conditions where water-filled channels are formed in a microporous membrane (often created by the imperfections common to many applied film coatings) through which drug in solution can pass. The key factors influencing drug release by this mechanism include the length and tortuosity of these channels, as well as the solubility of the drug in water.
Erosion
Some coatings are designed to erode gradually with time, thereby releasing the drug contained within the pellet in a controlled manner. Examples of these types of coating are usually those that consist of natural materials such as shellac (the solubility of which in water increases with increasing pH) or waxes and fats that become soft enough to facilitate erosion as the coated multiparticulates are subjected to intense agitation as they pass through the gastrointestinal tract.
Processes for coating multiparticulates
Traditionally, multiparticulates were coated using pan-coating processes, often employing techniques very similar to those used in sugar coating. As coating processes evolved, spray application techniques became more prevalent and today, fluidized-bed processes are preferred because of their ability to:
• enable discrete coatings to be applied to small particles while minimizing the risk of agglomeration
• ensure that coatings are uniformly deposited on the surface of each multiparticulate in the batch.
All the polymeric film-coating procedures described above can be used for the coating of multiparticulates. The fluidized bed is used in preference to the perforated pan. Another coating technique that is finding favour for the coating of modified-release pellets is hot-melt coating.
Hot-melt coating
Hot-melt coatings are usually applied to multiparticulates in order to mask taste and modify drug release. They consist of waxy materials (such as beeswax, synthetic spermaceti and other synthetic mono/diglycerides) that have melting points in the range of 55–65 °C and exhibit melt viscosities that are typically less than 100 mPa s (in order to allow the formation of smooth coatings on the surfaces of particles). Hot-melt coatings are preferably applied to multiparticulates using fluidized-bed coating processes, as described by Kennedy & Niebergall (1996).
References
1. Alvarez-Fuentes J, Fernandez-Arevalo M, Gonzalez-Rodriguez ML, Cirri M, Mura P. Development of enteric-coated timed-release matrix tablets for colon targeting. Journal of Drug Targeting. 2004;12:607–612.
2. Chang R-K. A comparison of rheological and enteric properties among organic solutions, ammonium salt aqueous solutions, and latex systems of some enteric polymers. Pharmaceutical Technology. 1990;14:62–70.
3. Chopra SK. Procise: drug delivery systems based on geometric configuration. In: Rathbone MJ, Hadgraft J, Roberts MS, eds. Modified-Release Drug Delivery Technology (Drugs and the Pharmaceutical Sciences). New York: Marcel Dekker; 2003.
4. Dittgen M, Durrani M, Lehmann K. Acrylic polymers: a review of pharmaceutical applications. S.T.P Pharma Sciences. 1997;7:403–437.
5. Gunsel WC, Dusel RG. Compression-coated and layer tablets. In: Pharmaceutical Dosage Forms: Tablets. 2nd edn New York: Marcel Dekker; 1990;Lieberman HA, Lachman L, Schwartz JB, eds. Pharmaceutical Dosage Forms: Tablets vol. 1.
6. Hogan JE. Aqueous versus organic solvent film coating. International Journal of Pharmaceutical Technology and Product Manufacture. 1982;3:17–20.
7. Jordan, M.P., Easterbrook, M.G., Hogan, J.E. (1995) Investigations into the moisture barrier properties of polyvinyl alcohol (PVA) tablet film coatings. Proceedings of 1st World Meeting, APGI.APV, Budapest, May 9–11.
8. Jozwiakowski MJ, Jones DM, Franz RM. Characterization of a holt-melt fluid bed coating process for fine granules. Pharmaceutical Research. 1990;7:1119–1126.
9. Kennedy JP, Niebergall PJ. Development and optimization of a solid dispersion hot-melt fluid bed coating method. Pharmaceutical Development and Technology. 1996;1(1):51–62.
10. Macleod GS, Fell JT. Comparison of atomization conditions between different spray guns used in pharmaceutical film coating. Pharmaceutical Technology Europe 2002; November Issue.
11. (Drugs and the Pharmaceutical Sciences 176) McGinity JW, ed. Aqueous Polymeric Coatings For Pharmaceutical Dosage Forms. 3rd edn New York: Marcel Dekker; 2008.
12. Okhamafe AO, York P. Analysis of the permeation and mechanical properties of some aqueous-based film coating systems. Journal of Pharmacy and Pharmacology. 1983;35:409–415.
13. Ozturk AG, Ozturk SS, Palsson BO, Wheatley TA, Dressman JB. Mechanism of release from pellets coated with an ethylcellulose-based film. Journal of Controlled Release. 1990;14:203–213.
14. Porter SC. Controlled-release film coatings based on ethylcellulose. Drug Development and Industrial Pharmacy. 1989;15:1495–1521.
15. Porter SC. A review of trends in film-coating technology. American Pharmaceutical Review. 1999;2(1):32.
16. Porter SC, Felton LA. Techniques to assess film coatings and evaluate film-coated products. Drug Development and Industrial Pharmacy. 2010;36 182–142.
17. Rowe RC. Defects in film-coated tablets: aetiology and solutions. In: Advances in Pharmaceutical Sciences. London: Academic Press; 1992.
18. Tang ESK, Chan LW, Heng PWS. Coating of multiparticulates for sustained release. American Journal of Drug Delivery. 2005;3:17–28.
19. Waterman KC, Fergione MB. Press-coating of immediate-release powders onto coated controlled-release tablets with adhesives. Journal of Controlled Release. 2003;89:387–395.
20. Zhang G, Schwartz JB, Schnaare RL. Bead coating I Change in release kinetics (and mechanism) due to coating levels. Pharmaceutical Research. 1991;8:331–335.
21. Ziegler, R., Koller, J. (2003) Protection of light-sensitive active ingredients by instant-release coatings based on Kollicoat IR. Poster presented at AAPS Annual Meeting and Exposition, Salt Lake City, Utah, USA, October 26–30.
Bibliography
1. Cole GC, Hogan JE, Aulton ME. Pharmaceutical Coating Technology. London: Taylor & Francis; 1995.
2. Chopra SK. Precise: drug delivery systems based on geometric configuration. In: Rathbone MJ, Hadgraft J, Roberts MS, eds. Modified-Release Drug Delivery Technology (Drugs and the Pharmaceutical Sciences). New York: Marcel Dekker; 2003.
3. am Ende DJ. Spray atomization modelling for tablet film coating. Chap 40 In: am Ende DJ, ed. Chemical Engineering in the Pharmaceutical Industry: R&D to manufacture. New Jersey, USA: John Wiley & Sons (in conjunction with AIChE); 2010.
4. Gunsel WC, Dusel RG. Compression-coated and layer tablets. In: Pharmaceutical Dosage Forms: Tablets. 2nd edn New York: Marcel Dekker; 1990;Lieberman HA, Lachman L, Schwartz JB, eds. Pharmaceutical Dosage Forms: Tablets vol 1.
5. Jones D. Development, optimization and scale-up of process parameters: Wurster coating. In: Qui Y, Chen Y, Zhang GGZ, eds. Developing Solid Oral Dosage Forms: Pharmaceutical Theory and Practice. MA, USA: Academic Press; 2009.
6. (Drugs and the Pharmaceutical Sciences 176) McGinity JW, Felton LA, eds. Aqueous Polymeric Coatings For Pharmaceutical Dosage Forms. 3rd edn New York: Marcel Dekker; 2008.
7. Porter SC, Sackett G, Liu L. Development, optimization and scale-up of process parameters: Pan coating. In: Qui Y, Chen Y, Zhang GGZ, eds. Developing Solid Oral Dosage Forms: Pharmaceutical Theory and Practice. MA, USA: Academic Press; 2009.
8. Rowe RC, Sheskey PJ, Cook WG, Fenton ME. Handbook of Pharmaceutical Excipients. 7th edn London: The Pharmaceutical Press; 2012.