CHAPTER 75 Clinical Overview of Movement Disorders
Movement disorders are a group of conditions that arise from functional aberrations in the motor and the nonmotor basal ganglia pathways.1 Movement disorders are common and affect all age groups. A list of the most common movement disorders and their reported incidences are presented in Table 75-1.2–12 The early signs and symptoms of movement disorders may be subtle and easily hidden by conscious or unconscious incorporation by the patient into common daily gestures. The key to diagnosing a movement disorder is careful study of its phenomenology, as well as its associated nonmotor features. In this chapter we provide an overview of movement disorders for practicing neurosurgeons, a topic that has also been covered by other authors.13–15
TABLE 75-1 Prevalence of Selected Movement Disorders in the United States
| SYNDROME | PREVALENCE (PER 100,000) | COMMON AGE GROUP |
|---|---|---|
| Parkinson’s disease | 295.6 | 60-70 |
| Progressive supranuclear palsy | 0.4-6.4 | 60-70 |
| Multiple system atrophy | 2.2-4.4 | 50-60 |
| Essential tremor | 415 | >40 |
| Huntington’s disease | 2-6.3 | <20 or >35 |
| Tourette’s syndrome | 1850-2990 | <18 |
| Cervical dystonia | 5.7-8.9 | 40 |
| Restless legs syndrome | 4200-9800 | >45 |
| Friedreich’s ataxia | 1.2-2 | 5-15 |
Data from references 2–12, 16–18.
Phenomenology: Defining Symptoms Through Observation
The patient should also be asked whether the movement is voluntary or involuntary. Movements may be referred to as “unvoluntary” when it is unclear which category applies.19 Triggers and relieving factors should be identified. Does the movement worsen with action or is it relieved? Do particular positions precipitate the abnormality? Is there specific sensory input that relieves the symptoms?
Finally, the presence of specific nonmotor symptoms can lead the clinician to the proper diagnosis. Table 75-2 lists common features of movement disorders and the specific diagnoses that they may suggest.
TABLE 75-2 Examples of Features, Categories, and Syndromes Helpful in Diagnosis
| FEATURES | CATEGORIES | EXAMPLES OF SPECIFIC SYNDROMES |
|---|---|---|
| Speed | Hyperkinetic | Tremor, chorea, myoclonus, tics, restless legs syndrome |
| Hypokinetic | Apraxia, blocking tics, parkinsonism: bradykinesia, primary progressive freezing of gait | |
| Region | Whole body | Hyperekplexia, generalized dystonia |
| Hemibody | Hemiparkinsonism, hemidystonia | |
| Segmental | Segmental myoclonus | |
| Multifocal | Polyminimyoclonus | |
| Focal | Writer’s cramp | |
| Proximal | Rubral tremor | |
| Distal | Painful legs when moving toes | |
| Oral | Tardive dyskinesia, neuroacanthocytosis | |
| Character | Rhythm |
Hyperkinesias
Tremor
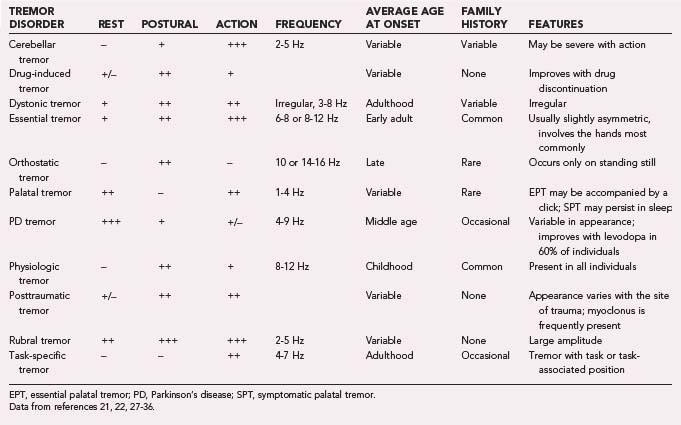
Tremor is classified according to its appearance or its cause.20,21 If the tremor occurs during movement, it is referred to as action or kinetic tremor. A tremor occurring in the absence of activity is classified as rest tremor. Postural tremor is manifested when a specific position is maintained (e.g., holding the arm extended). Finally, physiologic tremor is the term applied to nonpathologic postural tremor, which typically has a frequency of 8 to 12 Hz. Drug-induced tremors are usually due to an enhancement of physiologic tremor.
Tremor may be triggered by synchronized oscillatory signals arising from one of several locations. These signals may originate centrally, from circuits in either the basal ganglia or cerebellum that are involved in sensorimotor integration, motor timing, muscle coordination, or sympathetic control.21 One common example of centrally driven tremor is essential tremor (ET). ET has been ascribed to overactive central oscillators in the thalamus22,23 and to thalamocortical loop overactivity. In contrast, cerebellar and rubral tremors, which may occur after stroke or traumatic brain injury, are thought to result from motor dysregulation (i.e., from unbalanced feedforward or feedback systems, or from both).
Weighting a tremoring limb can help determine whether the tremor is physiologic or a pathologic tremor of central origin. Tremors predominantly of central origin will decrease in frequency when loaded, whereas the 8- to 12-Hz oscillation of physiologic tremor typically does not.22
Although it can be difficult to differentiate among subtypes of tremor solely on the basis of their frequency, it may be helpful to note that tremors of the hands greater than 11 Hz or less than 6 Hz are almost always pathologic.24 Pathologic tremors also seem to have a “floor” frequency. PD tremor and ET are among the lower frequency tremors and typically do not oscillate at less than 4 Hz.24 Tremors with frequencies in this range are usually due to malfunction of the brainstem or cerebellum. The frequency of a tremor may decrease slightly over time,25 in one series by approximately 2 to 3 Hz over a period of 4 to 8 years.26 This small degree of change does not usually lead to diagnostic confusion.
Amplitude cannot be used effectively to differentiate tremor types27 because it may vary widely within a particular tremor subtype. Generally, tremor subtypes with the lowest frequency can be expected to have the highest amplitude and vice versa, but this rule is not absolute. Emotional distress, exercise, and fatigue may exacerbate tremors of any subtype. Stressors tend to increase the amplitude of a tremor but have less effect on tremor frequency.
Common tremor conditions include ET, PD, dystonic tremor, cerebellar/outflow tremor, Holmes’ tremor, physiologic tremor, palatal tremor, neuropathic tremor, drug/toxin-induced tremors, task-specific tremor, primary writing tremor, and psychogenic tremor.21 The characteristics of these tremors are presented in Table 75-3.21,22,27–36
Specific Tremor Disorders
Physiologic Tremor
Physiologic tremor is a term applied to the 8- to 12-Hz tremor seen in any healthy person who is intentionally sustaining a posture. More proximal regions of the body oscillate at a lower frequency, more distal ones at a higher frequency. For example, physiologic tremor has a frequency of 3 to 5 Hz at the elbow, whereas metacarpophalangeal tremor usually ranges from 17 to 30 Hz.22 When this tremor impairs motor performance, it is referred to as enhanced physiologic tremor.30
Physiologic tremor is typically symmetrical. As with other tremor types, the amplitude is reported to decrease with age, particularly after the age of 50,20,27 although some authors have found otherwise.27 Age has not been shown to affect the frequency of physiologic tremor.27,37
It is unclear whether mild forms of the syndrome can be distinguished from ET. Both ET and physiologic tremor can be elicited by posture, both are fairly symmetrical, and both occur predominantly in the arms.27 Observing the progression of a tremor over time will eventually reveal whether a given patient has ET or physiologic tremor.
Essential Tremor
ET is the most common tremor disorder. It is generally manifested as a low-amplitude, bilateral action and postural tremor with a frequency of 6 to 8 Hz. The tremor usually has its onset in adulthood and worsens over time, but it may begin in childhood and can coexist with other movement disorders.38 The overall prevalence of ET is similar between genders,39 although women with ET seem to be more prone to head tremor than men.40
ET involves the upper limbs in more than 90% of patients.41 It less commonly involves the head, legs, or voice. It rarely affects the face or trunk. ET often has a postural component that may be reported as a rest tremor by patients. Patients commonly first complain of difficulty with tasks requiring fine coordination, such as threading a needle, tying knots, or writing. Later, more gross activities are also affected. In severe cases, basic activities of daily living may become impossible to perform.
Cognitive dysfunction42 and gait abnormalities43 may also be features of ET. Set shifting, verbal fluency, and other frontal cortex functions are impaired in patients with ET relative to age-matched controls. This cognitive impairment has been reported to not correlate with tremor severity.42
Several features of ET point to an underlying cerebellar or brainstem pathology. Patients with ET frequently have an end point tremor and difficulty with tandem gait. There are case reports of ipsilateral improvement in symptoms after cerebellar infarction,44 and inducing lesions of the cerebellothalamic receiving area (the ventral intermediate nucleus of the thalamus) is an effective treatment of ET. Although positron emission tomography has shown increased olivary glucose utilization and cerebellar blood flow,45 the brains of ET patients appear to be structurally normal.46
A family history of tremor is common in patients in whom ET is diagnosed. A positive family history has been reported in as many as 96% of patients47 and as few as 17%,48 depending on the sample. A survey of New York City residents showed a 5- to 10-fold increase in risk for ET in first-degree relatives, as well as an increase in the likelihood of ET developing in family members with earlier onset of symptoms in the patient.49
Several inherited forms of ET have been identified, including the gene loci EMT1 (on chromosome 3q13), EMT2 (on 2p24), and an unnamed gene locus on 6p23.48,50 ET has been reported in fragile X syndrome, Kennedy’s syndrome,51 XXYY syndrome,52 and Klinefelter’s syndrome.53 Sex chromosome–related tremors often have associated ataxia and may represent a separate tremor type.
The question of whether ET predisposes patients to the later development of PD is also a perplexing one. There are some cases in which families appear to be prone to both PD and ET. Jankovic’s group reported that the same locus yielded pure ET and ET-PD-dystonia in different families.54 As of this writing, the exact association between ET and PD remains a topic of discussion.
Parkinsonian Tremor
The tremor of PD was described by James Parkinson in 1817 in his historic Essay on the Shaking Palsy55 and further characterized by Charcot in the 1860s in his lectures at the Salpetriere.56 PD tremor is a 4- to 9-Hz low-amplitude rest tremor. The tremor often has a prominent proximal thumb component that gives it a “pill-rolling” quality. Nevertheless, the presence of a pill-rolling tremor is not diagnostic.
Although there is some thought that PD tremor may dampen or “burn out” over time, others have observed the opposite. Parkinson himself wrote that “as the debility increases … the tremulous agitation becomes more vehement [and] the motion becomes so violent as not only to shake the bed-hangings, but even the floor and sashes of the room.”55
Re-emergent tremor occurs while sustaining a prolonged position and most likely represents a rest tremor that has been reset by the relative stasis of a persistent position.57 Postural tremor that begins immediately on adopting a position is seen in as many as 93% of patients with PD and correlates with the degree of functional disability.
The pathogenesis of PD tremor is not well understood.58 In monkeys with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced parkinsonism, basal ganglia neurons begin to fire synchronously. Some authors have suggested that PD tremor originates from loss of segregation of these information channels and subsequent synchronization of adjacent circuits.59 Loss of dopamine in the basal ganglia may unmask pacemaker-like properties of the basal ganglia.60 It should be noted that the severity of PD tremor does not correlate with the severity of dopamine neuronal loss21 and that treatment with levodopa improves bradykinesia and rigidity more reliably than it does tremor.
Cerebellar Tremor
Cerebellar tremor is characterized as a jerky, low-frequency (2 to 5 Hz), high-amplitude action tremor. This tremor may be accompanied by other cerebellar signs such as ataxia, dysdiadochokinesia, dysarthria, dysmetria, and telegraphic speech. The normal pattern of cerebellar ballistic control, as described by Hallett and associates, consists of sequential agonist-antagonist–second agonist activation.61 Research into the tremor-generating mechanism is ongoing.
Rubral Tremor (Holmes’ Tremor)
Patients with lesions in the region of the red nucleus may be disposed to the development of what is referred to as a rubral tremor, first described by Holmes in 1904.61a Although predominantly an action tremor, rubral tremor frequently has a significant resting component. The amplitude of movement tends to be large and it can sometimes adopt a “wing-beating” appearance. Rubral tremors are among the slowest tremors, with frequencies often less than 4 Hz.24
Posttraumatic Tremor
The motor coordination control centers and their connections are situated deep in the brain, and to damage them generally requires substantial injury. Consequently, posttraumatic tremor is rarely an isolated finding. The character of the tremor depends on the region of the brain that is damaged. Damage to the brainstem may produce rest tremor if it affects the substantia nigra and related pathways. Damage to the cerebellum may result in a low-frequency action tremor. Because multiple regions are usually damaged, posttraumatic tremors are generally mixed in character. In one series of severe posttraumatic tremors, all patients displayed both action and postural tremors, whereas rest tremor was seen in just 56% of cases.62 Posttraumatic tremor is often accompanied by myoclonus. As noted by Obeso and Narbona, this apparent myoclonus appears in some cases to be an exaggeration of a beat of the ongoing tremor rather than true myoclonus.63
Drug-Induced Tremor
Drug-induced tremors are united by a common cause rather than a common appearance. The onset of tremor should be temporally related to drug ingestion.20,64
Drugs most commonly associated with tremor include alcohol, amiodarone, antidepressants, antiepileptic medications, beta-agonist bronchodilators, caffeine, immunosuppressive agents, lithium, neuroleptics, nicotine, steroids, and sympathomimetics.65 Alcohol intoxication (acute or chronic) and immunosuppressive agents may produce cerebellar tremors.66 Sympathomimetics, serotonin reuptake inhibitors, nicotine, and other centrally acting agents typically produce an enhanced physiologic tremor. Because the physiologic effects of an offending drug are rarely limited to tremor, the causative agent may also be recognized by associated non-neurological symptoms.
Orthostatic Tremor
Orthostatic tremor is a syndrome characterized by trembling of the legs and an intense feeling of unsteadiness that occurs on standing upright. The symptoms are relieved by walking or sitting down.32,67 Orthostatic tremor was first described by Heilman in 1984.32 The tremor is typically of low amplitude and is present predominantly in the legs. It is less frequently observed in the face, arms, or trunk. Surface electromyography (EMG) reveals a pathognomonic pattern of a 13- to 18-Hz tremor when the patient stands upright.24 When other body parts are affected, the tremor occurs at the same frequency as in the legs. Orthostatic tremor in the arms may be become evident if the patient is examined while on all fours.68 Given the high frequency and low amplitude of the tremor, it may be difficult to appreciate and may appear as little more than a subtle quivering.68
Impairment of balance and increased swaying are well-described features of the syndrome. They may result from disrupted sensory feedback69 or from the disruption of motor regulation at the muscular level.68
Orthostatic tremor’s underlying pathology remains unknown. The tremor is not solely brought on by load bearing—even when the load on the legs is reduced by suspending patients in the upright position, the tremor pattern remains stable.21
Palatal Tremor
Palatal tremor consists of a steady and constant-amplitude oscillation of either the tensor veli palatini or the levator veli palatini muscles. Palatal tremor is also referred to as palatal myoclonus; however, most authors now classify it as a tremor syndrome.15
Patients with SPT have the same slow and rhythmic movement of the soft palate as do patients with EPT. Unlike EPT, however, in SPT the movement is thought to be due to contraction of the levator veli palatini muscle and thus is not typically associated with an ear click. In 30% of patients with SPT the tremor is accompanied by rotatory or vertical pendular nystagmus.70 SPT is thought to arise from damage to the area bounded by the red nucleus, the inferior olive, and the dentate nucleus (the Guillain-Mollaret triangle). This results in autonomous firing of the inferior olive.70 Postmortem analysis of the ipsilateral inferior olive has provided further evidence of an olivary origin for SPT: pathologic examination reveals hypertrophic degeneration and enlarged neurons with cytoplasmic vacuolization.71 SPT is difficult to modify and is unusual among the hyperkinesias in that it persists even in sleep.
EPT is distinguished from SPT by the presence of an ear click, relief during sleep, and an absence of structural brainstem pathology. Some authors report that EPT predominantly involves the roof of the palate whereas in SPT the contraction is more notable at the palatal verge.65
Psychogenic Tremor
Although psychogenic tremors may affect any part of the body, they most frequently involve the head, arms, and legs. The tremor tends to shift from region to region even as it alters in its other characteristics. Most patients display rest, postural, and action tremor to a varying degree.13 Other clues to a psychogenic origin include entrainment and active resistance to passive range of motion. Entrainment occurs when a tremor’s frequency shifts to match that of a voluntary repetitive movement of another body part. Entrainment may be enhanced by distracting the patient. Psychogenic tremor may also respond paradoxically to loading, which increases rather than decreases the tremor in frequency and amplitude.
Psychogenic tremor can be difficult to distinguish from tremor of other causes. Hallett and colleagues reported a case of psychogenic palatal tremor in which the patient duplicated the typical clicking movements of that disorder by repetitively clapping his soft palate against his pharynx.61
A psychogenic origin of a movement disorder should be considered whenever there are obvious incongruities in a patient’s signs and symptoms. Resolution after suggestion or administration of placebo, coexisting psychiatric disorders, or inconsistency of symptoms over time should all raise clinicians’ suspicion.72
Deuschl and coworkers proposed that co-contraction of antagonist muscle groups is a necessary condition for psychogenic tremor. Patients with hand tremor of psychogenic origin tend to show EMG coactivation in the finger flexors and extensors shortly before onset of the tremor.21
Task-Specific Tremor
Primary writing tremor (PWT) is the stereotypical task-specific tremor: a unilateral action tremor of 4 to 7 Hz that occurs during the act of writing or while adopting the posture associated with that act. The task specificity of PWT suggests a central cause, as does report of successful treatment by deep brain stimulation.73
Some authors have classified task-specific tremor as a form of ET74 and some as being related to task-specific dystonia,75 whereas others believe it to be a distinct nosologic entity.76 Like dystonia, task-specific tremor is asymmetrical and triggered by a task. Both sometimes respond to anticholinergic therapy. Generalized dystonia and PWT have been found to cluster within certain kindreds.77
PWT and writer’s cramp may be distinguished on the basis of differential reciprocal muscle inhibition. Patients with writer’s cramp usually display forearm reciprocal muscle inhibition on EMG, whereas patients with PWT do not.78
Regardless of whether PWT is truly a form of dystonia, the two occur together often enough that patients with task-specific tremor should also be examined for signs of dystonia. Actions other than writing have also been associated with task-specific tremor. For example, task-related chin tremor has been reported.34
Dystonic Tremor
Dystonic tremor is a jerky postural and action tremor that is abolished by complete rest and occurs in a body part affected by dystonia. Its amplitude tends to be irregular, and it tends to have a variable frequency. Dystonic tremors usually oscillate at frequencies of 7 Hz or less.21 The amplitude of the movements can often be reduced if patients touch a particular part of their body (i.e., a geste antagoniste or “sensory trick”).
Other features of dystonia are discussed more fully in the section on dystonia.
Chorea
Chorea consists of random and complex involuntary movements that flit from body part to body part. Chorea may resemble exaggerated fidgetiness. The movements can be focal or generalized and are usually absent during sleep. The word chorea is derived from the Greek khoreia or “to dance.” Chorea may be among the first defined movement disorders. Chorea Sancti Viti (St. Vitus’ dance) was described in the Middle Ages. It was one term among several (St. John’s dance, tarantism) used to refer to the independent outbreaks of “dancing mania” that occurred in central Europe, most notably around the time of the plague.79 “St. Vitus’ dance” is now used predominantly to refer to Sydenham’s chorea. Choreas can be further classified by their appearance. Athetosis refers to a slow, sinuous, undulating movement, usually of the hands or feet. Sudden and large-amplitude movements are referred to as ballistic, derived from the Greek word meaning “to throw.”
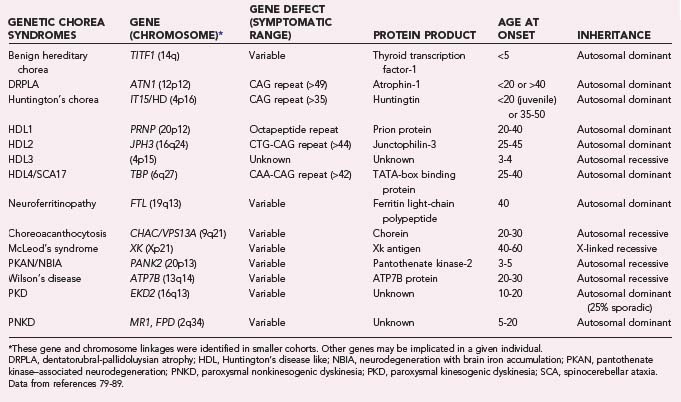
Multiple chorea syndromes have been described (Table 75-4), including Huntington’s chorea, Sydenham’s chorea, Wilson’s disease, neuroacanthocytosis, Friedreich’s ataxia, dentatorubral-pallidoluysian atrophy (DRPLA), McLeod’s syndrome, benign hereditary chorea (BHC), spinocerebellar ataxia (SCA types 2, 3, or 17), chorea gravidarum, drug-induced chorea, metabolic chorea (i.e., secondary to accumulation of toxins or liver, kidney, or endocrine disease), tardive dyskinesia, paraneoplastic syndromes, polycythemia vera, and psychogenic chorea.80–89
Chorea Syndromes
Huntington’s Disease
Huntington’s disease (HD) is the most common form of inherited chorea. Symptoms usually begin during the third to fifth decades of life. Although chorea is the most common initial symptom,90 unsteadiness of gait, dystonia, myoclonus, loss of bulbar control, and cognitive changes also occur and may appear before chorea does. Bradykinesia usually develops as the disease progresses, but it may be underappreciated in the presence of more obvious symptoms.
The chorea of HD is typically symmetrical and tends to increases in amplitude over time. The first manifestation of chorea may be a slight flicking of the fingers seen while walking.80 Patients are frequently unaware of their movements and may continue to treat their gyrations with indifference, even when made aware of them. Early symptoms include an impairment of rapid saccades,91,92 psychiatric and mood changes, and tics.93 Ataxia is unusual and should raise concern for another syndrome, such as neuroacanthocytosis, SCA, or Friedreich’s ataxia.80
Impersistence of movement is a classic feature of HD. Patients typically have difficulty maintaining tongue protrusion. They also tend to have difficulty keeping their gaze fixed on an object. Paradoxically, they may have trouble switching their attention from the examiner’s face. This has been referred to as a visual grasp reflex and is not specific for HD.94
HD is defined as being of juvenile onset if symptoms occur by the age of 20. It is more often associated with stiffness, eye movement difficulties, and bradykinesia than adult-onset HD is. Seizures are also more frequent in juvenile-onset HD.95 Adult-onset HD is occasionally manifested as this phenotype.96
The cognitive and behavioral features of HD are both prominent and disabling. They are similar to those seen after frontal lobe damage. Grasp, snout, and other primitive reflexes may be prominent. Scores on psychomotor tests such as the Trail Making B and Stroop Interference Test show declines earlier in the course of HD than do tests of memory. Worsening scores correlate with the degree of striatal atrophy present.97 Dementia occurs in the majority of patients, although exceptions may occur when the chorea is of late onset.98 Other psychiatric symptoms include apathy, depression, lability, impulsivity, outbursts of anger, mania, and paranoia.79,80 Physicians should always inquire about substance abuse and suicidality.65
The genetic defect responsible for HD is a CAG repeat on chromosome 4 in a region that encodes the protein huntingtin, whose function is unknown.99 The number of copies of this repeat determines the presence or absence of clinical HD; patients with 29 to 35 repeats are expected to be asymptomatic.79,100 The number of CAG repeats may increase in transmission and result in anticipation: earlier onset and increasing severity in successive generations. Paternal inheritance of HD has been correlated with a higher number of triplet repeats in the next generation,101,102 probably because of gene expansion during spermatogenesis.103 An increased number of triplet repeats correlates with both earlier disease onset and the degree of functional decline.104
The diagnosis of HD is based on clinical features and confirmed by genetic testing for the huntingtin gene. Striatal atrophy is the classic finding on imaging studies, but frontal lobe atrophy is also seen. Physicians may encounter the HD phenotype in the absence of the HD genotype. In one large series, approximately 7% of patients displaying the HD phenotype proved not to have a mutation in the huntingtin gene.105
Four Huntington’s disease–like (HDL) syndromes have been identified. All are rare. HDL1 is an inherited prion disorder. HDL2 is caused by a CAG/CTG expansion in the junctophilin-3 protein and is more common in patients of African, Mexican, Spanish, or Portuguese descent. HDL2 is the most HD-like of the HDLs in its symptomatology. It may be accompanied by erythrocyte acanthocytosis.88 An early childhood–onset HDL variant, HDL3, has been identified in isolated cohorts.88 Its genetic basis remains unknown. HDL4 is synonymous with SCA type 17 (SCA17). SCA17 has a variety of phenotypes, one of which closely mimics the symptoms of HD. HDL4 arises from a CAA-CAG repeat in chromosome 6.
Sydenham’s Chorea
Sydenham’s chorea is a delayed complication of infection with group A β-hemolytic streptococci that usually develops 4 to 8 weeks after the infection,79 but it may develop as long as 6 months afterward. Sydenham’s chorea may be the sole manifestation of rheumatic fever in as many as 20% of patients106,107 and remains the most common cause of acute childhood chorea in the world.
The typical age at onset of Sydenham’s chorea is 8 to 9 years; it is rarely seen in children younger than 5 years.79,108 The chorea usually generalizes but there are exceptions, and 20% of patients remain hemichoreic. Sydenham’s chorea may be accompanied by tics and psychiatric symptoms. Obsessive-compulsive disorder (OCD) and attention-deficit/hyperactivity disorder (ADHD) occur in 20% to 30% of patients and may precede or follow the onset of chorea.109 The disease is self-limited and spontaneously remits after 8 to 9 months in a large percentage of patients, but up to 50% may still have chorea 2 years after infection.110
Antineuronal antibodies are present in a majority of patients with Sydenham’s chorea.109 Antistreptolysin (ASO) titers are typically elevated but are nonspecific for infection with group A streptococci; this test is not useful in diagnosing Sydenham’s chorea. However, elevated ASO titers may be of help in distinguishing a recurrence of Sydenham’s chorea from a chorea from some other cause.111,112 Magnetic resonance imaging (MRI) in patients with Sydenham’s chorea has been reported to show transient swelling in the striatum and globus pallidus and increased signal on T2-weighted images.113,114
Tardive Chorea/Dyskinesia
Tardive dyskinesia results from treatment with dopamine receptor blocking agents. Tardive syndromes are less frequently caused by atypical than by typical neuroleptics. Dopamine-depleting medications have not been definitively associated with tardive dyskinesia.65 Some common antiemetics (e.g., metoclopramide) and some antitussives (e.g., Phenergan) are dopaminergic blockers whose use may lead to the development of tardive movements.
The most common pattern of tardive dyskinesia is stereotyped and repetitive movement of the face. Tongue-thrusting and involuntary chewing movements reminiscent of those seen in choreoacanthocytosis may be seen. Tardive dyskinesia is often accompanied by a feeling of restlessness. This akathisia may be localized and reported as a “burning” sensation, often of the genitals or mouth.115
Although tardive chorea has been reported after treatment with atypical antipsychotics, it occurs infrequently in this setting. Of the neuroleptics, clozapine appears least likely to induce tardive disorders.![]() 116 Large clinical trials have recently suggested that although atypical antipsychotics produce tardive dyskinesia less often than first-generation antipsychotics do, the difference may not be as great as was thought.116,117
116 Large clinical trials have recently suggested that although atypical antipsychotics produce tardive dyskinesia less often than first-generation antipsychotics do, the difference may not be as great as was thought.116,117
Benign Hereditary Chorea
BHC is a slowly progressive childhood-onset chorea that is not associated with worsening dementia. The lack of cognitive worsening and early chorea differentiate it from juvenile HD. Onset most commonly occurs at 1 year of age, and symptoms may improve during adolescence.86
BHC is heterogeneous in its manifestations and may be associated with myoclonus, dystonia, dysarthria, and gait difficulties.82 This led to some doubt whether it truly represented a separate disorder118 until the discovery that a number of families with BHC possessed mutations in the gene encoding thyroid transcription factor-1 (TITF1).82 Defects in TITF1 have also been tied to a disorder consisting of chorea, congenital hypothyroidism, and pulmonary dysfunction,119 or “brain-thyroid-lung syndrome” (BTLS). Although BTLS has been differentiated from “classic” BHC, BHC and BTLS may represent two points on the same clinical spectrum.86 It can also be difficult to distinguish BHC and essential myoclonus. Both syndromes have comparable ages at onset and similar appearances. Features that vary between the two include gait involvement and improvement with alcohol. Gait involvement is common in BHC but rare in essential myoclonus.86 Improvement with alcohol ingestion is common in essential myoclonus but rare in BHC.
Neuroacanthocytosis
Two diseases fall under the rubric of neuroacanthocytosis: choreoacanthocytosis and McLeod’s syndrome. Both are characterized by acanthocytes on peripheral smear, peripheral neuropathy, psychiatric symptoms, and seizures.120 Serum creatinine kinase may be mildly elevated in either syndrome.
Choreoacanthocytosis is an autosomal recessive disease with an age at onset of 20 to 40 years. Orofacial dystonia is one of the characteristic features of the disease, and it is typically manifested as lip and tongue biting.79 Patients may involuntarily push food out of their mouths with their tongue when eating (“eating dystonia”). Generalized chorea, dystonia, and tics can also occur. Abnormalities of saccadic eye movement may develop, similar to those in HD.121 Unlike HD, peripheral neuropathy is typically present. A finding of areflexia with orolingual dystonia is highly suggestive of this syndrome. Choreoacanthocytosis is associated with mutations in the chorea acanthocytosis (CHAC) gene, which encodes a protein designated chorein.
McLeod’s syndrome is a rare X-linked acanthocytic disease caused by defects in a gene responsible for erythrocyte antigens. The mean age at the onset of CNS symptoms is 40 years,84 and they may lag behind the hematologic diagnosis by decades. Symptoms of McLeod’s syndrome include chorea, cognitive decline, paranoia, schizophrenia, and limb weakness. Atrophy is neurogenic and seen predominantly in the distal ends of the lower limbs. Case series suggest that unlike autosomal recessive choreoacanthocytosis, involuntary lip and tongue biting occurs in only a minority of patients.84 In addition to CNS symptoms, cardiomyopathy, arrhythmias, and hemolytic anemia may develop.
Dentatorubral-Pallidoluysian Atrophy
DRPLA produces a combination of chorea and ataxia and is named for the pattern of atrophy of the dentatofugal and pallidofugal pathways often seen in this syndrome.85 It occurs most commonly in Japan, although variant forms have been reported in the United States.122,123
DRPLA, like HD, is an autosomal dominant triplet repeat disease. Also as in HD, successive generations of DRPLA sufferers tend to have an earlier age at onset. This acceleration is more rapid with paternal transmission.85 There are five cardinal features of the syndrome: cerebellar ataxia, dementia or mental retardation, chorea, seizures, and myoclonus.
DRPLA may be divided primarily into juvenile- and adult-onset variants. The symptoms of juvenile-onset DRPLA often lead physicians to initially diagnose progressive myoclonic epilepsy because myoclonus and seizures are the predominant symptoms. Adult-onset DRPLA is characterized by chorea, ataxia, psychosis, and dementia. The dementia in adult-onset DRPLA is usually milder than that in juvenile-onset cases.85
An adult-onset variant of DRPLA has been found in an African American family living near North Carolina’s Haw River. The Haw River variant displays a number of differences from other DRPLA variants: microcalcification of the basal ganglia, demyelination of the centrum semiovale, atrophy of the posterior column, and prominent paranoia, delusions, and hallucinations. Although generalized tonic seizures are common in this cohort, they are not myoclonic.123
Other Causes of Chorea
Thyrotoxicosis can acutely result in chorea. Both generalized chorea and hemichorea have been reported. In most reports the chorea resolves once the patient is euthyroid, but cases of persistent chorea have occurred. Animal experiments suggest that the mechanism may be related to increased dopamine receptor sensitivity during thyrotoxicosis.124
Neuroferritinopathy is an autosomal dominant choreic syndrome first identified in a North West England family.124a The mean age at onset is 39 years, and 50% of patients have chorea as their first symptom. The chorea is typically symmetric. Eye movements are normal and there is an associated late-onset dementia of the frontal/subcortical type. The disease arises from deficits in the ferritin light polypeptide (FTL) gene. T2-weighted MRI aids greatly in distinguishing this condition from other choreas; the hallmark of this disease is hypointensity of the striatum and globus pallidus. Serum ferritin levels may be low, but this finding is insufficiently reliable for it to be diagnostic.124b
Polycythemia vera is a disease of red blood cell hyperproliferation that has been associated with numerous neurological symptoms, including migraine, vertigo, and chorea. Chorea may be this disease’s initial symptom.125 Most cases of polycythemia-associated chorea have occurred acutely in elderly women and in the setting of hematologic deterioration. Chorea is not typically seen in patients with secondary polycythemia.126
Chorea gravidarum is a choreic syndrome of pregnancy, usually with onset in the first or second trimester.127 The severity of the chorea tends to decrease as the pregnancy progresses. The syndrome appears to be associated with autoimmune disease. One series of patients with the syndrome reported rheumatic heart disease in five of five patients,127 but others have not been as definitive. Chorea gravidarum has been associated with systemic lupus erythematosus128 and with elevated antiphospholipid antibody titers.129 Approximately a third of patients see their symptoms resolve after delivery.130
Painful leg and moving toes syndrome (PLMTS) is a condition characterized by involuntary movement of the toes in the presence of chronic pain. This movement typically takes the form of low-amplitude flexion-abduction movements that may persist during sleep. The movements have been associated with lesions in the spinal cord, nerve root, or peripheral nerves. Soft tissue injury has also been associated with PLMTS.131 The pathophysiology of these movements remains unclear. PLMTS should always be distinguished from pseudoathetosis by checking joint position sense. PLMTS is not always manifested as chorea and may instead appear as a persistent jerking movement.
A painless variant has also been reported131 and has been referred to as painless legs–moving toes syndrome.
Jumpy stump refers to the involuntary twitching of an amputation stump. It is usually accompanied by severe pain.132 As with PLMTS, this syndrome may resemble myoclonus more than chorea.
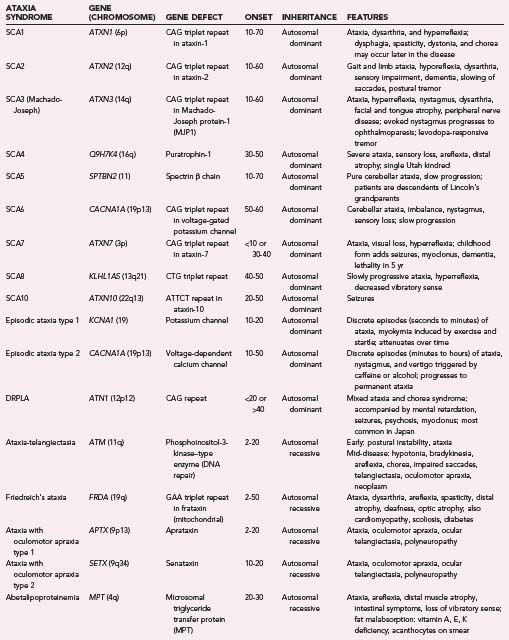
Chorea may be an unusual manifestation of Wilson’s disease (15%)80 or Friedreich’s ataxia.133 It is also an uncommon finding in Lesch-Nyhan syndrome (23%).134 Paroxysmal nonkinesigenic dyskinesia and paroxysmal kinesigenic dyskinesia may be manifested as chorea-like movements, as may SCA2, SCA3, and SCA17. These syndromes are dealt with more extensively in subsequent sections.
Myoclonus
Myoclonus is a sudden, arrhythmic, involuntary movement that is “shock-like” in its rapidity. When multiple, these movements do not flow into one another, which distinguishes them from chorea. True myoclonus is due to brief synchronous firing of agonist and antagonist muscles that typically lasts 10 to 50 msec and rarely more than 100 msec.135,136
Myoclonus can be classified by either phenomenology, extent, or trigger. Positive myoclonus occurs with active muscle contraction, of which hypnic jerks, a sudden body-wide contraction that occurs as a person drifts between sleep and wakefulness, are a commonly experienced example. Negative myoclonus is manifested as brief inhibition of a given muscle group. Asterixis is an example of negative myoclonus and consists of sudden and involuntary relaxation of a dorsiflexed hand or other body part. The EMG pattern of negative myoclonus is distinctive, with aperiodic electrophysiologic silences ranging from 0.05 to 0.5 second in the antagonist muscle groups.15,24 When frequent, these signs can be mistaken for postural tremor.
Myoclonus is most often encountered as one of a collection of symptoms rather than as a pathology![]() ’s primary manifestation. Symptomatic myoclonus may be a feature of any process involving cortical, basal ganglionic, or cerebellar degeneration, such as Creutzfeldt-Jakob disease or PD. Hepatic, renal, endocrine, and other metabolic derangements may variably be manifested as myoclonus. Primary myoclonic syndromes include the myoclonic epilepsies, essential hereditary myoclonus, palatal myoclonus, nocturnal myoclonus (also referred to as periodic leg movements of sleep), minipolymyoclonus, and physiologic myoclonus.137
’s primary manifestation. Symptomatic myoclonus may be a feature of any process involving cortical, basal ganglionic, or cerebellar degeneration, such as Creutzfeldt-Jakob disease or PD. Hepatic, renal, endocrine, and other metabolic derangements may variably be manifested as myoclonus. Primary myoclonic syndromes include the myoclonic epilepsies, essential hereditary myoclonus, palatal myoclonus, nocturnal myoclonus (also referred to as periodic leg movements of sleep), minipolymyoclonus, and physiologic myoclonus.137
Myoclonus Syndromes
Spinal segmental myoclonus is characterized by isolated contraction of muscles controlled by a particular spinal segment. It may follow spinal cord trauma, a mass lesion (tumor, vascular, or infectious), or inflammatory disease (such as multiple sclerosis). The symptoms may occur immediately after a spinal lesion or follow the insult by decades. One series reported an average of 3 years between spinal damage and the onset of symptoms.138
Epilepsia partialis continua (EPC) is defined as a localized muscular twitching of long duration without impairment of consciousness139 and arises from cortical epileptiform discharges that do not spread.140 EPC may develop as a result of intracranial neoplasms, encephalitides, mitochondrial disorders, and metabolic disorders or be idiopathic in origin.141 The most common cause of EPC in childhood is Rasmussen’s encephalitis.141
Multifocal and Generalized Myoclonus
Multifocal myoclonus of cortical origin is caused by a general hyperexcitability of the cortex. The same is true of generalized myoclonus. For this reason most pathology that gives rise to multifocal myoclonus can also give rise to generalized myoclonus. The two differ in appearance. Multifocal myoclonus may affect the body bilaterally, but not synchronously. Generalized myoclonus involves the contraction of large groups of muscles simultaneously. When the amplitude of these movements is low enough for them to be considered twitches rather than jerks, the syndrome may be referred to as minipolymyoclonus.142
Essential myoclonus is a nonprogressive multifocal myoclonus in which twitches or jerks are the predominant feature. It is first manifested in childhood or early adulthood.137 The syndrome appears in both a sporadic and an inherited form. When inherited, it is transmitted in an autosomal dominant fashion. There is no associated dementia, and electroencephalographic (EEG) testing produces normal results.
Unverricht-Lundborg disease (progressive myoclonic epilepsy type 1 or EPM1) is characterized by an initial onset of stimulus-sensitive myoclonus and generalized seizures between the ages of 6 and 16 years.143 It is followed several years later by progressive ataxia, tremor, and mild cognitive decline.143 In several kindreds the gene responsible appears to be an unstable repeat in the cystatin B gene on chromosome 21q22. The EEG pattern is typified by paroxysms of generalized spike and wave discharges and photosensitivity.144 Treatment with phenytoin may worsen the symptoms. Valproate is the current antiepileptic of choice.144
Lafora’s disease (progressive myoclonus epilepsy type 2 or EPM2) is a childhood-onset disease manifested as myoclonus, seizures, and severe dementia. Death usually follows within 10 years of onset. A rare late-onset form exists and has a more benign course.145 The hallmark of the disease is the presence of polyglucosan periodic acid–Schiff–positive inclusions in the brain, liver, muscle, and sweat glands.
Neuronal ceroid lipofuscinoses are a group of lysosomal storage diseases manifested in childhood as myoclonus, seizures, and dementia. Adult forms also exist. The late infantile and juvenile forms may be accompanied by blindness. The adult forms may be dominated by cognitive and psychiatric impairment.146 Pathologic examination reveals an accumulation of lipopigment, “fingerprint profiles,” and curvilinear bodies in the brain, eccrine glands, muscle, and gut. The diagnosis may be made by demonstrating eccrine curvilinear inclusion bodies in axillary sweat gland biopsy samples.
Sialidosis (cherry-red spot myoclonus syndrome) is a lysosomal storage disease of adolescent onset associated with a deficiency of α-N-acetylneuraminidase or α-galactosidase.147 Myoclonic jerks may be stimulus sensitive or insensitive and may persist during sleep.147 The syndrome is typically inherited in an autosomal recessive fashion.
Opsoclonus-myoclonus syndrome (OMS or “dancing eyes and dancing feet syndrome”) is characterized by continuous, multidirectional, saccadic movement of the eyes accompanied by multifocal myoclonus and encephalopathy. The typical age at onset is 6 to 18 months, but adult onset does occur.148 OMS is predominantly a syndrome. In children, OMS is due to an autoimmune reaction to a neuroblastoma in 50% of cases. In adults, the most commonly involved neoplasms are ovarian, breast, and small cell lung cancer.149 In addition to following neoplasms, OMS has followed hyperosmolar coma, drug administration (amitriptyline, lithium, phenytoin, and diazepam), intracranial hemorrhage, acquired immunodeficiency syndrome, sarcoid, and celiac disease.149
Posthypoxic myoclonus syndrome (Lance-Adams syndrome) was first described by Lance and Adams in 1963 as an action myoclonus with associated asterixis, seizures, and gait problems.150 The myoclonus may show features of exaggerated startle, multifocal myoclonus, generalized myoclonus, or a mix of these symptoms.151 Negative myoclonus is a prominent feature of posthypoxic myoclonus, and postural lapses may follow the myoclonic jerks and lead to falls. Posthypoxic myoclonus has been reported to respond well to treatment with 5-hydroxytryptophan in some case reports.152,153 Because the underlying deficit in Lance-Adams syndrome is static, symptoms tend to be nonprogressive.
Myoclonus-dystonia syndrome is a genetically diverse disorder with onset in the first or second decade of life. The phenotype can vary considerably even within a given kindred. The myoclonus typically consists of proximal bilateral jerks involving mainly the arms.65 This syndrome is discussed in the section on dystonia (as DYT11).
Startle Syndromes
Hyperekplexia consists of an exaggerated myoclonic response to a startling stimulus and is encountered in both hereditary and acquired (symptomatic) varieties. Hereditary hyperekplexia (also called stiff baby syndrome) is usually manifested in infancy as violent body-wide jerks in response to a sudden noise or touch, followed by minutes of stiffness and trembling.154 Although the exaggerated startle persists throughout life, the episodes of stiffness subside. Mental development is usually normal.155 Patients may adopt an odd “toddling” gait. EEG findings are normal, and myoclonus is absent during sleep. Symptomatic hyperekplexia is a sign of supraspinal disorders but is not specific for the site of the disorder. Some researchers have suggested a final common brainstem pathway.156 Many patients with either hereditary or sporadic hyperekplexia display a mutation in the glycine receptor α subunit encoded on chromosome 5q32.
There are several reports of startle-induced bizarre behavior that is observed only in particular cultural groups. The aptly named “jumping Frenchmen of Maine” were first described by Beard in 1878. He observed a series of French Canadian patients who displayed an exaggerated startle response, sometimes accompanied by echolalia, echopraxia, or compulsive obedience.157 Latah is a disorder with the same features in Indonesia, observed predominantly in women of low social status.158 Other startle syndromes with similar symptoms have been reported, including the Acadian “ragin’ Cajuns” of the southern United States159 and Imu in the Ainu people of Japan.160
Startle-induced epileptic seizures also exist161 and can be distinguished from startle syndromes by the accompaniment of features typical of seizures: an ictal period, underlying structural brain abnormality, or the presence of a provoking illness.
Dyskinesia
The designation “dyskinesia” is most commonly used to describe the movements observed in patients receiving chronic dopaminergic therapy for PD; however, dyskinesia may also be tardive (i.e., a delayed side effect of dopamine-blocking medications). Although usually secondary to prolonged medication use, there have been reports of tardive dyskinesia developing after only a month’s exposure to neuroleptic medications.162,163
Dyskinesia syndromes include abdominal (belly dancer![]() ’s) dyskinesia, levodopa-induced dyskinesia, tardive dyskinesia, and the paroxysmal dyskinesias.164
’s) dyskinesia, levodopa-induced dyskinesia, tardive dyskinesia, and the paroxysmal dyskinesias.164
Dyskinesia Syndromes
Paroxysmal Dyskinesias
The paroxysmal dyskinesias are typified by episodes of involuntary hyperkinesis without loss of consciousness.165 They may be divided into four categories based on inciting events: paroxysmal exercise-induced dystonia (PED), paroxysmal hypnogenic dyskinesia (PHD), paroxysmal kinesigenic dyskinesia (PKD), and paroxysmal nonkinesigenic dyskinesia (PNKD).83
PED is brought on by prolonged exercise,166 and individual attacks last from 5 to 30 minutes.164 PHD, also called paroxysmal nocturnal dystonia, is characterized by brief attacks of dystonia that arise out of sleep. Onset is usually in childhood. Since originally described, PHD has been reclassified as a form of frontal lobe epilepsy.167
PNKD (dystonia-torsion type 8) is usually first manifested as involuntary movement of a single limb83,164 and may be dystonic or choreic, or both. Age at onset ranges from childhood to early adulthood.164 Attacks typically last longer than those of PKD and persist for several minutes to an hour. They may involve the limbs, face, or trunk.164
PNKD may be idiopathic or secondary to a defect in the myofibrillogenesis regulator-1 (MR1) gene.165 Patients with the MR1 mutation tend to have an earlier age at onset (infancy or childhood), have attacks that are precipitated by alcohol or caffeine, and improve with benzodiazepine therapy.83 Precipitation by emotional stress and improvement with sleep are features seen in both idiopathic and MR1-induced PNKD. A large proportion of MR1-negative PNKD patients will also display PED.165 Secondary cases of PNKD have been reported at a rate of 18% in one series of paroxysmal dyskinesias.81 Causes included infarction, trauma, and CNS infection.
PKD (dystonia-torsion type 10) is typified by frequent attacks lasting seconds to minutes. These involuntary movements are usually dystonic but may be choreic. They are precipitated by sudden movements (such as jumping up from a chair) or by being startled.81 PKD may affect a single body side or a single limb.164 Several PKD kindreds have been identified, within which a high proportion of family members have had infantile seizures. Analysis of these families suggests that PKD is related to an unknown defect in the pericentromeric region of chromosome 16.164,168 PKD may also be idiopathic or secondary. Secondary PKD may result from hypoparathyroidism169 or sources of focal CNS lesions such as trauma or infection.81
Belly dancer’s dyskinesia consists of slow rhythmic contractions of the abdominal wall that produce a movement resembling the undulations of a dancer. It has been ascribed to a variation of spinal myoclonus,170,171 although authors have disagreed on the exact cause.172
Tics
Tics are brief movements that are commonly preceded by a feeling of discomfort that builds until the tic appears, followed by a temporary feeling of relief. These preceding “premonitory urges” may consist of a feeling of itching or tension in the affected body part.173 These sensations are also referred to as sensory tics.174 One of the hallmarks of tics is that they are temporarily suppressible, although they typically rebound with increased frequency and severity after conscious suppression. Tics occupy a middle ground between voluntary and involuntary movements. They are usually described by those who have them as being purposefully executed but performed out of a feeling of need.175 This mix of volition and compulsion has led some to refer to these movements as “semivoluntary” or “unvoluntary.”19
Tics can be clonic (i.e., brief), dystonic (i.e., sustained), or phonic (vocal). These subtypes can in turn be simple or complex. Simple tics consist of isolated actions, such as throat clearing or winking. Complex tics consist of speech or coordinated actions. They sometimes include obscene gestures, in which case they are termed copropraxia. When the obscenity is verbal, the complex phonic tic is referred to as coprolalia. Tics can also be manifested as interruptions in or slowing of ongoing motion. Jankovic has also described blocking tics, or abrupt interruptions of activity preceded by a premonitory urge.14
Tic disorders include transient tourettism, Tourette’s syndrome, chronic tic disorder, tardive tourettism, and drug-induced tourettism. Adult onset of a primary tic disorder is highly unusual. Any adult with a first manifestation of tics should be carefully examined for secondary causes such as infection, neuroleptic exposure, cocaine use, or trauma.176
Tic Disorders
Tourette’s Syndrome
Tourette’s syndrome is defined by the onset of motor and vocal tics before adulthood (<18 years) that cannot be ascribed to another medical condition. The full definition, as set out by the Tourette Syndrome Classification Study Group, adds that the tics must occur multiple times throughout a period of at least a year and that the tics must evolve over time.177 Findings on neurological examination in a patient with Tourette’s syndrome are generally normal.
The first tics are usually observed around the age of 5 or 6, and tic severity peaks 4 to 5 years later.178 Only 4% of Tourette patients fail to manifest tics by the age of 11.179 Tic frequency is lowest in patients’ early 20s, coincident with frontal lobe maturation.178 In addition to this long-term variation, tics also wax and wane on a day-to-day basis. Tics are worsened by heightened emotional states, stress, and fatigue.
The tics of Tourette’s syndrome are commonly accompanied by ADHD and OCD.180 ADHD has been reported to precede tic onset by a mean of 2 years,181 whereas OCD is reported to be manifested in adolescence.182 The phenotype of Tourette-related OCD differs from that of primary OCD. In primary OCD, patients’ obsessions often focus on fears of contamination or a need for checking. In Tourette’s syndrome, obsessions center on concerns with symmetry, fear of violent thoughts, and a need to perform activities in a particular manner.183 These obsessions may lead to self-injurious behavior. Patients have been reported to hit themselves in the eyes or throat or bite and scratch themselves.184,185 Such behavior may be seen in as many as 53% of patients with Tourette’s syndrome.179
A growing body of research suggests that Tourette’s syndrome has a strong hereditary component.186–188 The syndrome appears to follow a sex-influenced but autosomal dominant mode of transmission.189 In Tourette kindreds, men appear more likely to manifest a typical tic-predominant syndrome and women are more likely to have OCD without tics.190 When obtaining a family history, it should be recalled that mild symptoms may be ascribed to idiosyncrasies of personality by family members.
The pathology of the disorder has been attributed to dysfunction of the corticostriatal-thalamocortical pathway,182 and further localization remains speculative. The striatum has been a past focus of research. Evidence of frontal cortex involvement has also been increasing.191,192
The Diagnostic and Statistical Manual of Mental Disorders, fourth edition, lists chronic tic disorder and transient tourettism (transient tic disorder of childhood) alongside Tourette’s syndrome as the primary tic disorders. Chronic tic disorder differs from Tourette’s syndrome in that the patient need not have both phonic and motor tics. Transient tourettism differs from Tourette’ syndrome in that symptoms last less than 1 year. This syndrome is the mildest and most common tic disorder. Tics can be seen transiently in 20% of children younger than 10 years.193 Both transient tourettism and chronic tic disorder probably represent points on a continuum of tic-causing pathology, of which Tourette’s syndrome is the most severe expression.
Other Causes of Tics
Drug-induced tourettism is also well described. Although antiepileptic drugs and dopamine-blocking medications have been used to treat tics, both classes of drug have also been reported to lead to Tourette-like symptoms.194,195 Of the drugs of abuse, cocaine has been implicated most frequently in tic production.176 Secondary tourettism may also be seen with HD,93 autism spectrum disorders,196 and choreoacanthocytosis197 and sometimes after trauma.198
Akathisia
Akathisia refers either to an uncomfortable sensation of inner restlessness or to the voluntary activity performed to relieve that restlessness. It is often manifested by an inability to remain seated, crossing and uncrossing the legs, or pacing.199 Akathisia usually occurs after the administration of neuroleptic medications. It may occur shortly after exposure (acute akathisia) or as a late complication of treatment (tardive akathisia).
Akathisia can be difficult to distinguish from tics200 and restless legs syndrome (RLS).201 All three of these disorders are characterized by movements that are performed to relieve an unpleasant internal sensation. Akathetic patients do not typically report the feeling of building tension that tic sufferers do. Akathisia also differs from both tic disorders and RLS in that the movements that akathetic patients perform feel neither compelled nor involuntary.
Akathetic Disorders
Restless Legs Syndrome
RLS may be defined as a feeling of unease or dysesthesia that is referred specifically to the lower limbs and is improved by movement.202 The symptoms of RLS are usually bilateral and diurnal. Symptoms may be worse during the nighttime hours, even when patients remain awake.203
RLS is the most common of the movement disorders. Large population surveys have found that it affects 3% to 19% of adults, depending on their age.12,204 RLS is typically a disease of patients’ middle years, although it should be noted that in one patient series, up to a third of patients experienced their first symptoms before the age of 10.205
Drug-Induced Akathisia
Dopamine-blocking agents may lead to either acute or delayed feelings of restlessness, and it may be included among the tardive syndromes as tardive akathisia.35 Less commonly, serotonin reuptake inhibitors,206 calcium channel blockers,207 and tricyclic antidepressants208 have also been associated with akathisia.
Stereotypies
Stereotypies are repetitive movements or vocalizations that mimic a purposeful action, are performed outside that action’s normal context, and are involuntary or “semivoluntary.”210 The hand wringing of Rett’s syndrome is one example of stereotyped behavior.210 Stereotypies should be differentiated from automatisms. Automatisms, such as the odd behavior that can occur during partial complex seizures, are sudden in onset, occur in the background of a clouded sensorium, are time limited, do not reliably occur after periods of stress, may take place during sleep, may be followed by a postictal behavioral change, and occur randomly.
Stereotypies should also be distinguished from repetitive perseverative behavior, or behavior that represents “a restriction of behavioral possibilities without excessive production.”211 A mannerism is “a bizarre way of carrying out a purposeful act which usually occurs as the result of the incorporation of a stereotypy into a goal directed behavior.”14,212
Stereotypies may be triggered by an inability to adopt competing motor patterns when faced with an environmental cue,211 as opposed to compulsive behaviors and tics, which are thought to be triggered by internal cues.
As outlined by Jankovic, the most common stereotypies are facial grimacing, staring at lights, waving objects before the eyes, repetitive sounds, arm flapping, body rocking, repetitive touching, feeling and smelling objects, jumping, toe walking, and hand and body gesturing.![]() 213 Stereotypies can be seen in patients with autism, Asperger’s syndrome,214 schizophrenia, and mental retardation and after exposure to neuroleptic medications (tardive stereotypy).215 Although it is common to find stereotypies in any of these syndromes, stereotypies are most typical of autism.216,217
213 Stereotypies can be seen in patients with autism, Asperger’s syndrome,214 schizophrenia, and mental retardation and after exposure to neuroleptic medications (tardive stereotypy).215 Although it is common to find stereotypies in any of these syndromes, stereotypies are most typical of autism.216,217
Stereotypy Syndromes
Stereotypies of Autism
Although autism spectrum disorders are defined by emotional detachment, movement disorders are also common in this patient group.218 Stereotypies are among the most common of such aberrant behavior. Tics, self-injury, catatonia, cerebellar dysfunction, and other disorders have also been reported.214,219
Rett’s Syndrome
Rett’s syndrome is typically a childhood disease characterized by mental regression and stereotyped hand wringing. The overall prevalence of the disorder has been estimated to be 0.44 cases per 100,000.220
In addition to hand-wringing stereotypies, these patients can display a number of other movement disorders. In one series, bruxism was seen in 97% of patients, oculogyric crises in 63%, and parkinsonism and dystonia in 59%.210 Scoliosis has been reported in as many as 75% of patients by the age of 13.221
The phenotype of Rett’s syndrome varies with age. Younger patients tend to have a greater degree of hyperkinesis. Older patients have more evident bradykinesia. Rett’s syndrome is reported almost exclusively in women. Survival rates in the United States match those of the general population until the age of 10 years but then decrease to 70% at age 35.222
The underlying defect in Rett’s syndrome appears to be aberrant brain protein methylation by the methyl-CpG binding protein-2 (MECP2) gene. In individuals with “typical” Rett’s syndrome, 92% to 95% of patients possess an MECP2 abnormality, depending on the series.222 MECP2 mutations may be present, if rarely, in individuals without Rett’s syndrome.223 Neuroimaging of patients with Rett’s disorder shows global hypoplasia and cerebellar atrophy that worsens with age.224
Hypokinesias
Bradykinesia
Bradykinesia Syndromes
Stiff Person Syndrome
Stiff person syndrome is a rare disorder consisting of progressive proximal muscle rigidity that was first described by Moersch and Woltman.225
The syndrome begins with pain and clumsiness of the trunk and legs, usually between 40 and 50 years of age. As time passes, tonic contraction of the muscles of the back, hips, and abdomen develops. This leads to the typical picture of prominent lordosis, stiff gait, and a “board-like” abdomen. The face, distal ends of the limbs, and sphincter muscle are spared. In addition to this persistent contraction, patients experience superimposed spasms that are triggered by sudden stimuli (emotional excitement, startle). Sudden spasms of the legs may lead to falls. These spasms may be so strong that they result in dislocations or fractures.226
Stiff person syndrome appears to have an autoimmune etiology—60% or more of patients have detectable serum anti–glutamic acid decarboxylase antibodies.227 Glutamic acid is necessary for the synthesis of γ-aminobutyric acid. Identification of typical versus atypical stiff person syndrome is important for determining prognosis. Typical stiff person syndrome was defined by Marsden and Brown as consisting of stiffness and rigidity in the axial muscles, abnormal axial posture, superimposed spasms, continuous motor activity in at least one axial muscle, and the absence of other neurological signs.226 If all these criteria are present, a good response to muscle relaxants and benzodiazepines is considered the norm. The fraction of “typical” patients with anti–glutamic acid decarboxylase antibodies is approximately 90%.228
Patients who do not meet these criteria may be considered to have “stiff person plus syndrome.”225 This may be seen after polioencephalomyelitis and is usually accompanied by myoclonus, brainstem signs, cerebrospinal fluid pleocytosis, and dysautonomia. This myoclonus may come in paroxysms, which may impede respiration and require intubation, and is called “jerking stiff man syndrome.”225,229 “Stiff person plus” in which limb rigidity is the predominant feature may be due to autoantibodies to presynaptic vesicles and may be associated with small cell lung or breast cancer. Stiff person plus syndrome frequently preferentially affects the distal ends of limbs and must be differentiated from neuromyotonia. Unlike neuromyotonia, stiff person and stiff person plus syndrome are CNS disorders and disappear during sleep. They may respond to centrally acting agents.
Diabetes mellitus occurs in most patients with classic stiff person syndrome, along with other autoimmune endocrinopathies.230 EMG may show continuous muscle contraction despite a patient’s efforts to relax. Nerve conduction velocities should be normal.
Neuromyotonia
Neuromyotonia is a disease consisting of peripheral nerve hyperexcitability in which bilateral and multifocal muscular contraction restricts movement.231 It is also referred to as Isaac’s syndrome232 or myotonia with impaired motor relaxation.
The most common symptom of neuromyotonia is muscular twitching, which is seen in 90% of patients in whom the disorder is diagnosed.231 These twitches take the form of a continuous rippling of muscles under the skin, or myokymia. Myokymia is typically seen in the limbs but may involve the trunk or face. EMG or palpation may reveal myokymia when it is not otherwise apparent.
Cramps and stiffness may occur and impair gait and manual dexterity. Stiffness has been observed to cause persistent finger flexion, restriction of respiration, and tonic calf contraction.231 These symptoms may be worsened by exercise or changes in temperature. Continuous muscle activation can also lead to hyperhidrosis and hypertrophy. Muscle hypertrophy is most commonly seen in the calves.
Pseudomyotonia, or difficulty relaxing after muscle contraction, is variably present (a third of patients). It may be the first symptom of the syndrome231 and is typically manifested as difficulty releasing one’s grip or opening a closed eye.
The pathology of neuromyotonia is generally autoimmune and caused by antibodies against voltage-gated potassium channels.233 Secondary causes of neuromyotonia include chronic inflammatory demyelinating polyneuropathy,234 neoplasm,235 staphylococcal infection,236,237 or toxin-related peripheral nerve damage.238,239
Disorders with Mixed Hypokinesia and Hyperkinesia
Parkinsonian Disorders
James Parkinson eloquently described what we now know as PD in his Essay on the Shaking Palsy in 1817,55 in which he identified rest tremor, stooped posture, excessive salivation, and festination as features of the disease. Little was added to this description until Charcot’s lectures of the 1860s,240 in which he added rigidity and akathisia to the list of PD symptoms. By 1893, physicians were distinguishing patients’ “fixity of feature and of limb” from their “slowness of movement”241 as they realized that bradykinesia and rigidity were separable symptoms.
Parkinson’s Disease
PD is defined as a parkinsonism characterized by the aforementioned cardinal features. PD is not always responsive to dopaminergic medications. Although improvement after the administration of levodopa or dopamine agonists is suggestive, it is neither necessary nor sufficient for the diagnosis of PD.242
The first symptoms of PD usually manifest in the fifth decade of life.243 Rest tremor is the first symptom in 70% of PD patients.244 Young-onset PD is defined as beginning in the second through fourth decades of life. Patients with this type of PD are more likely to have dystonia and levodopa-induced dyskinesias early in their disease course.245,246 Juvenile-onset PD (first symptom before the age of 20) is rare and suggests genetic or secondary parkinsonism.246 Individuals with young-onset PD constitute approximately 5% of referred patients in western countries.245
PD tremor has a frequency of approximately 5 Hz and variable amplitude. The tremor is typically more distal than proximal. It may be intermittent and is almost always asymmetric.60 Like most tremors, it is worsened by distraction—either cognitive tasks (e.g., performing arithmetic) or motor tasks (e.g., walking). Strong emotion also tends to exacerbate PD tremor.
Postural and gait instability is the least specific of the cardinal features of PD. The parkinsonian gait is characterized by shuffling of the feet, decreased arm swing on one or both sides of the body, and flexion of the neck and spine. Patients are typically unable to turn in a single step and instead break their turns into multiple small increments. The body characteristically remains aligned with the feet during these maneuvers (“en bloc turns”). Patients are unstable and may be unable to recover from a backward tug. Both festination and retropulsion may be seen. Festination arises from an inability to return to an erect posture once leaning forward. Patients appear to chase their center of gravity as a result and are “thrown on the toes and forepart of the feet; being … irresistibly impelled to take much quicker and shorter steps, and thereby to adopt unwillingly a running pace.”55 Retropulsion is an analogous behavior that occurs as a result of patients’ inability to recover from a backward-leaning posture.
The pathogenesis of PD probably involves both environmental and genetic factors.247 Environmental factors that have been linked to PD include pesticide exposure, living in a rural area, and drinking well water.248,249 Interestingly, tobacco use is inversely associated with risk for PD.250 MPTP, a by-product of meperidine synthesis, caused parkinsonism in drug users exposed to it in the in the late 1970s and early 1980s.251 Nonhuman primates injected with MPTP display levodopa-responsive akinesia, rigidity, and tremor, as well as levodopa-induced dyskinesias.252 MPTP exposure results in selective death of dopaminergic cells of the substantia nigra, the same abnormality observed in the autopsied brains of patients with PD. Based on cadaveric studies, PD is believed to be clinically manifested only after approximately 80% of striatal dopamine and 50% of nigral neurons have been lost.253
Approximately 10% to 15% of patients with PD also report PD in a first-degree relative.254–256 Common environmental exposure probably accounts for a proportion of these cases,257 but the fact that individuals with similar exposure vary in their expression of parkinsonian symptoms, coupled with the identification of multiple susceptibility genes,258,259 supports the idea that the pathogenesis of PD involves both genetic predisposition and environmental exposure. Several genetic defects have been implicated in inherited PD, including alterations in the parkin,260 LRRK2,261 phosphatase and tensin homolog (PTEN)-induced kinase (PINK1),262 and α-synuclein (PARK1 or SNCA) genes.263 Although the list of familial forms of PD continues to lengthen, PARK1, LRRK2, and PINK1 are the most common forms currently identified and together account for 3% of all patients with parkinsonism.264 Genetic forms of PD tend to have a younger onset than sporadic PD does.
Of the cardinal features of PD, bradykinesia has the best correlation with disease severity. Bradykinesia and rigidity are also the symptoms best explained by current models. According to the Alexander, DeLong, and Strick model, bradykinesia arises from excessive inhibition of the thalamus by the globus pallidus (pars) interna (GPi), either directly by GPi overactivity or indirectly by overactivation of the GPi by an overactive subthalamic nucleus.1 This model does not perfectly account for all observed PD phenomena (for example, lesions in the GPi reduce dyskinesias rather than increasing them).265 Models that emphasize the role of aberrant motor plan selection, neuronal oscillation, and neuronal synchrony have been proposed and continue to evolve.266–268
Parkinsonian rigidity is a function of enhanced static or postural reflexes and should be differentiated from bradykinesia,269 in the same way that stiffness and slowing are not identical. The rigidity may be of either a “lead pipe” or “cogwheel” quality and is typically asymmetric.
Care should be taken to exclude cases of parkinsonism caused by the administration of phenothiazine antiemetics or dopamine-depleting neuroleptics.60 Suspicion of a Parkinson’s plus syndrome should be raised by the presence of cerebellar deficits, corticospinal tract signs, or vertical gaze restriction. Autonomic dysfunction or postural instability early in the disease course are also atypical for idiopathic PD.270
Dementia with Lewy Bodies
Dementia with Lewy bodies (DLB) is the second most common cause of dementia after Alzheimer’s disease.271,272 It is characterized by progressive parkinsonism, hallucinosis, and dementia.273 In the parkinsonism of DLB, tremor is commonly minimal or absent. Hallucinations and delusions may occur spontaneously or be provoked by dopaminergic medications, even at low doses. Patients’ hallucinations tend to be visual, vivid, complex, and well formed. Interestingly, patients usually have good insight into the unreality of their visions.274 A fluctuating level of attention and alertness is also typical of DLB, and patients may vary dramatically in their alertness from hour to hour or day to day.60 Unlike PD, myoclonus frequently appears early in the course of DLB. Supranuclear ophthalmoparesis has also been reported.275
Differentiating DLB from Alzheimer’s disease can be difficult, but the presence of hallucinosis, gait impairment, rigidity, or tremor early in the disease course should strongly suggest the diagnosis of DLB.276
Progressive Supranuclear Palsy
PSP was first described by Steele, Richardson, and Olszewski in 1964.277 It is characterized by progressive and symmetric parkinsonism, gait instability, and gaze palsies.277 Frequent falls are the most common initial symptom and arise from a combination of gait freezing and loss of postural reflexes. Patients typically lack insight into this impairment, thereby compounding their difficulties.279 As with DLB, tremor is not a prominent part of the clinical picture of PSP.
Downgaze paralysis is the classic manifestation of PSP, but it is usually preceded by a slowing of vertical saccades.280 Convergence failure and square-wave jerks may be seen. Patients may progress to complete ophthalmoparesis. Initially, doll’s eye maneuvers can overcome this problem, but the vestibulo-ocular reflex is often lost over time.281
Bulbar symptoms are prominent in PSP. The increased muscular tone of the face and throat produces a characteristic “startled face” and a low-pitched dysarthria. Eyelid-opening apraxia is a frequent complicating factor, as is dysphagia. Dysphagia is the most frequent cause of death; most PSP patients die of aspiration-related complications within a decade of the diagnosis.282
Several pathologic series have suggested that the syndrome of pure akinesia with gait freezing, a disorder of gait interruption and akinesis on gait initiation, is sufficiently similar in its pathology to PSP to be considered a variant.283,284
Corticobasal Degeneration
Corticobasal degeneration (CBD) is manifested as asymmetric parkinsonism with cortical symptoms. Asymmetric hand “clumsiness” is the most common initial complaint and was seen in 50% of patients in one series.285 Other motor symptoms include asymmetric rest tremor, limb dystonia, rigidity, and cortical myoclonus. The alien hand phenomenon (AHP), in which the patient’s limb performs uncontrolled movements and is thought of as “other,” is also seen in CBD.286 Although it aids in the diagnosis, AHP is not specific. AHP may also be seen after vascular lesions,287 gunshot wounds,288 seizures,289,290 and Alzheimer’s disease.291
The dementia of CBD is characterized by progressive aphasia, frontal lobe symptoms, dyscalculia, mild memory difficulty, and apraxia. The apraxia is most often of an ideomotor type and is detected during neurological examination by patients’ inability to pantomime tool-using actions. Although aphasia was once thought to be rare in CBD, it is common and may be the initial feature.292
Multiple System Atrophy
Multiple system atrophy (MSA) is subdivided into two syndromes based on whether parkinsonism (MSA-P) or cerebellar ataxia (MSA-C) predominates.293,294
MSA-P, or striatonigral degeneration, most resembles PD. The combination of rapid disease progression, symmetrical symptoms, absence of rest tremor, and a paucity of levodopa response suggests a diagnosis of MSA.295,296 Patients with MSA-C, or olivopontocerebellar atrophy, display progressive cerebellar ataxia and parkinsonism. Gaze-evoked or positional nystagmus occurs more frequently in MSA-C than in MSA-P. Square-wave jerks may be seen in both subtypes.297
Dysautonomia eventually develops in most patients with MSA. Many authors no longer consider dysautonomia-predominant MSA (Shy-Drager syndrome) to be a distinct category. The autonomic symptoms of MSA include impotence, diaphoresis, orthostatic hypotension, and incontinence. Incipient orthostatic hypotension may be unmasked by treatment with dopaminergic medications.298
Normal sphincter EMG findings are rare in patients with MSA of any subtype, as long as the symptoms have been present for at least 5 years.299 Whether sphincter EMG is useful in differentiating MSA from other parkinsonisms earlier in the course of the disease remains a topic for research. MSA of any subtype can be difficult to distinguish from either PD or PSP on a patient’s first visit. Litvan and coauthors300 reported that the presence of at least six of the following eight features at the patient’s first visit—sporadic adult onset, lack of levodopa response, cerebellar signs, dysautonomia, parkinsonism, pyramidal signs, no downward gaze palsy, and no cognitive dysfunction—was predictive of MSA with a median sensitivity of 59% and positive predictive value of 67%. MRI abnormalities such as the “hot cross bun sign” (cross-shaped T2-weighted hyperintensity of the pons because of the degeneration of transverse pontine fibers) and putaminal enhancement may be seen in as many as 65% of patients with MSA.301,302
Dystonia
Dystonias may be classified according to their anatomic distribution (focal, general, or segmental), their underlying cause (primary, secondary, or genetic), or young (i.e., <26 years) versus older age at the onset of symptoms. Focal dystonias are most common, but many dystonias with a focal onset generalize later (Table 75-5).303–315319 The younger the age at the onset of symptoms, the more likely it is for the dystonia to generalize.
Dystonic Syndromes
Primary Dystonia
Primary dystonias are “pure” dystonias that cannot be ascribed to another disease process. They may be either sporadic or genetic. Most sporadic primary dystonias are focal (e.g., torticollis, blepharospasm, lingual dystonia, spasmodic dysphonia, or task-specific dystonia).316 The numerous heritable dystonias are subclassified into dystonia-torsion (DYT) categories and are summarized in Table 75-5.
The most common cause of early-onset generalized dystonia is DYT1 dystonia (Oppenheim’s dystonia). It occurs relatively frequently in the Ashkenazi Jewish population, with a prevalence of 1 in 2000. DYT1 is inherited in an autosomal dominant fashion with a penetrance of 30% to 40%.317 The onset of symptoms is usually in late childhood/early adolescence, and they generally begin in one leg and later generalize.
DYT7, or adult-onset focal dystonia, is another autosomal dominant dystonia.311 Onset is typically in middle age. Symptoms are focal or multifocal and involve predominantly the upper part of the body.
Dystonia Plus Syndromes
A dystonia-parkinsonism syndrome can be seen in either DYT3 or DYT12. DYT3 (also known as Lubag dystonia) is an X-linked disease found primarily in the denizens of Panay Island in the Philippines. The majority of patients have dystonic symptoms (94% in one series). The dystonia typically begins focally and subsequently generalizes over the course of 5 to 7 years.318 Parkinsonism appears later in the course of the disease and levodopa may improve the symptoms. DYT12 is typified by the onset of parkinsonism and dystonia over a period of several hours or weeks and is not usually levodopa responsive.319
DYT5, or Segawa’s disease, is a childhood-onset levodopa-responsive dystonia. The initial symptom is generally foot dystonia, with a marked diurnal fluctuation that attenuates with age. A postural tremor typically develops in adulthood, followed later by bradykinesia. Rest tremor is absent. Response to levodopa is marked and sustained. Both autosomal dominant and autosomal recessive subtypes have been identified.320
DYT9 is associated with paroxysmal dystonia triggered by alcohol, exercise, fatigue, or stress. It is also inherited in an autosomal dominant fashion and has its onset in childhood to adolescence. Spastic paraplegia may be present either during or between attacks. Patients may also experience episodic ataxia, headaches, or perioral and leg paresthesias. Attacks range in frequency from twice daily to twice yearly. Like the episodic ataxias, DYT9 appears to be due to a defect in a gene encoding a channel protein (KCNA1).304
DYT11 has its onset in childhood or adolescence and is manifested as dystonia with alcohol-responsive proximal myoclonic jerks. The dystonia is usually mild and present in 50% of patients. Cervical dystonia and writer’s cramp are the most common.65 Many adults with myoclonus-dystonia syndrome report dramatic improvement in their symptoms with alcohol ingestion. It is caused primarily by mutations in the ε-sarcoglycan gene (i.e., torsion dystonia type 11—DYT11) localized to chromosome 7q21.321 Other chromosomal abnormalities have been identified in isolated kindreds.310,322 Inheritance is typically autosomal dominant. It is slowly progressive and tends to plateau in severity. It may be associated with substance abuse, anxiety, or psychosis.65
Secondary Dystonias
Any insult to the sensorimotor circuitry can lead to dystonia, and thus secondary dystonias have a variety of causes. Dystonia has been reported after ischemia, hypoxia, infection, neoplasm, drug or toxin exposure, metabolic derangement, inflammatory disease, and trauma. In evaluating a patient with dystonia, it is important to identify any treatable conditions, such as Wilson’s disease or drug exposure. Tardive dystonia results from exposure to neuroleptics. The many causes of secondary dystonia are summarized in Table 75-6.
| Infection |
AVM, arteriovenous malformation; HIV, human immunodeficiency virus; PKAN, pantothenate kinase–associated neurodegeneration.
Used with permission from Fernandez HH, Rodriguez RL, Skidmore FS, et al. A Practical Approach to Movement Disorders: Diagnosis and Medical and Surgical Management. New York: Demos Medical; 2007.
Disorders of Coordination
Ataxia
![]() The inherited ataxia syndromes are most easily classified by their pattern of inheritance: autosomal dominant, autosomal recessive, and X-linked. These disorders are summarized in Table 75-7.122,323–327
The inherited ataxia syndromes are most easily classified by their pattern of inheritance: autosomal dominant, autosomal recessive, and X-linked. These disorders are summarized in Table 75-7.122,323–327
Ataxia Syndromes
Autosomal Dominant Ataxias
Many of the autosomal dominant ataxias (SCA types 1, 2, 3, 6, 7, and 8 and DRPLA) are trinucleotide repeat disorders. They demonstrate the anticipation typically seen in these disorders: subsequent generations of affected individuals tend to have a larger number of repeats and earlier and more severe symptoms. This effect is more readily seen with paternal transmission. There are two exceptions to this rule: SCA6 does not demonstrate anticipation, and in SCA8, anticipation occurs more often with transmission along the maternal line. The phenotypes of the SCAs overlap and a definitive diagnosis is typically made by genetic assay. Worldwide, SCA types 1, 2, 3, 6, and 7 account for up to 65% of all autosomal dominant ataxias, of which SCA3 is the most common.323 In the majority of the remaining families, although an autosomal dominant pattern can be established, the genotype remains unknown.
As one of the most common of the SCAs, SCA3, or Machado-Joseph disease, deserves special mention. Ataxia, hyperreflexia, nystagmus, and dysarthria develop early in the disease. Patients may also show gaze-evoked nystagmus that progresses to ophthalmoparesis. Symptoms of bulbar and peripheral nerve compromise include facial and tongue wasting and general sensory loss.122
The episodic ataxias are also worth separate consideration. Eight forms of episodic ataxia have been thus far been described (EA1 to EA7 and DYT9), all caused by ion channel mutations. EA1 and EA2 are of particular interest because they arise from defects in the same chromosome (chromosome 19—a locus shared by SCA6 and familial hemiplegic migraine) but have different phenotypes. EA1 is caused by mutations in a potassium channel gene324 and EA2 by mutations in a voltage-dependent calcium channel.325 EA1 is characterized by kinesigenic attacks of myokymia and ataxia that last seconds to minutes. EA2 is characterized by nonkinesigenic episodes of ataxia and vertigo that can last days. Although the symptoms of EA1 abate over time, EA2 is a lifetime affliction.
Autosomal Recessive Ataxias
Friedreich’s Ataxia
Friedreich’s ataxia is the most common genetic ataxia, with a prevalence of 1 in 50,000 persons,326 and it occurs secondary to a GAA repeat located on chromosome 9. The syndrome is characterized by the childhood onset of gait ataxia, weakness, and dysarthria. Although areflexia may develop, this appears to depend on the age at onset. Reflexes may be maintained in late-onset Friedreich’s ataxia. Hearing loss, visual loss, and sphincter dysfunction occur late and are not universal.
Friedreich’s ataxia affects multiple systems not typically considered to be in the neuroscientist’s domain: cardiomyopathy and scoliosis are frequent features of the disease, and more than half of the patients may die of cardiac causes.328 Pathologic studies typically reveal spinal cord atrophy with relative preservation of the cerebellum.328
Ataxia with Isolated Vitamin E Deficiency
Ataxia with isolated vitamin E deficiency is a mimicker of Friedreich’s ataxia and is similarly characterized by the childhood onset of ataxia, dysarthria, areflexia, and bony deformities. It is important to keep this condition in the differential because timely treatment with vitamin E can slow or reverse the disease.65
Ataxia-Telangiectasia
Ataxia-telangiectasia is the second most common autosomal recessive ataxia, with a frequency of 1 in 100,000 persons.323 The disorder arises from a deficit in the DNA repair pathway, and patients are predisposed to the development of neoplasms. Onset is in early childhood, with postural instability and ataxia first becoming apparent as the child begins to walk.329 Later, hypotonia, bradykinesia, areflexia, and proprioceptive deficits develop. Chorea may occasionally be seen. The eponymous telangiectasias develop later in childhood.
Patients are often wheelchair bound by their second decade, and death usually occurs in the fourth to fifth decade as a result of either pulmonary infection or malignancy.328a Sensory neuropathy can be demonstrated on EMG and cerebellar atrophy on MRI.328b
X-Linked Ataxias
Fragile X–Associated Tremor Ataxia Syndrome
Fragile X–associated tremor ataxia syndrome (FXTAS) is characterized by the presence of a premutation for fragile X, that is, presence of the characteristic CGG triplet repeat in insufficient number (55 to 200 repeats) to cause the full fragile X syndrome. It is accompanied by intention tremor, gait ataxia, rigidity and bradykinesia, polyneuropathy, and autonomic manifestations.327 FXTAS causes a substantial proportion of sporadic ataxias in adults. It is seen in 30% of male FMR1 premutation carriers older than 50 years and in 75% of male carriers older than 80 years.323 T2-weighted MRI may reveal symmetrical hyperintensities in the middle cerebellar peduncles.330
Movement Disorders Caused by Metabolic or Systemic Disease
Toxic/Metabolic Syndromes
Wilson’s Disease
Wilson’s disease is an autosomal recessive disorder of copper metabolism that causes both liver and basal ganglia damage. For this reason, Wilson’s disease is also referred to as hepatolenticular degeneration. The disease is well known for manifesting a variety of symptoms. The initial symptom may be a tremor, dystonia, or chorea. The findings may differ substantially even within the same kindred.331 Given this fact and the potentially treatable nature of the syndrome, all patients with a movement disorder before the age of 50 should be checked for the presence of Wilson’s disease.
Wilson’s disease causes neurological symptoms in roughly 40% of patients, with liver disease in 40% and psychiatric disease in the remainder.332 The age at onset varies, but the first symptoms usually appear between the ages of 11 and 25. Patients with liver impairment tend to be affected at a younger age than those with neurological symptoms.333,334
Based on epidemiologic studies in Europe and the United States, the prevalence of Wilson’s disease is estimated to be about 15 to 30 cases per 1,000,000 people. Heterozygous carriers are present in the general population at a rate of 1 in 100 to 150 individuals.335,336
The underlying pathology is accumulation of copper in the liver and basal ganglia as a result of impaired copper excretion secondary to mutations in an adenosine triphosphatase (ATPase) that aids in transmembrane copper transportation and binding of copper to the carrier protein ceruloplasmin. This ATPase is encoded by the gene ATP7B, located on chromosome 13.87 ATP7B defects result in accumulation of copper within hepatocytes, which eventually spills over into other tissues, including the brain. Copper accumulation leads to increased, but insufficient urinary copper excretion and high serum levels of free copper.
Ceruloplasmin testing is a useful screening tool, but serum levels of this protein may be normal even in symptomatic individuals.337 Testing for elevated copper levels in a 24-hour urinary collection is the best single test for the syndrome. The classic Kaiser-Fleischer rings—flecks of copper visible in the cornea under slit-lamp examination—are almost universally present in patients with neurological symptoms. MRI findings are abnormal in symptomatic patients and typically show generalized atrophy and pallidal hypointensity on T2-weighted images.338 Central pontine myelinolysis may also be present.
Toxic/Metabolic Causes of Movement Disorders
Hereditary Aceruloplasminemia
Ceruloplasmin is responsible for the oxidation of ferrous to ferric iron, and consequently, aceruloplasminemia results in impaired iron transport and iron accumulation. It is a rare, autosomal recessive disease that is estimated to occur at a rate of 0.5 per million Japanese.339,340
Lesch-Nyhan Disease
Lesch-Nyhan disease is a syndrome of uric acid overproduction caused by a breakdown in the purine salvage pathway. Symptoms usually first appear in infancy as motor delay secondary to hypotonia. The clinical picture subsequently evolves to mental retardation and action-induced dystonia superimposed on generalized hypotonia.134 A minority of patients display spasticity. Oromandibular and lingual movements are typical, and self-injurious behavior is common. The syndrome is transmitted in an X-linked recessive fashion.
Pantothenate Kinase–Associated Neurodegeneration
Pantothenate kinase–associated neurodegeneration (PKAN), along with neuroferritinopathy, falls into the category of neurodegeneration with brain iron accumulation. PKAN is a disorder of basal ganglia iron deposition that arises from mutations in the pantothenate kinase-2 (PANK2) gene. PKAN usually has its onset before the age of 6, and patients generally lose their ability to walk in their second decade. The predominant symptoms are dystonia, rigidity, corticospinal signs, and pigmentary retinopathy.341 In patients with an older onset, the first manifestation is usually speech difficulty or a psychiatric disorder.
Iron deposition in the basal ganglia leads to partial hyperintensity of the globus pallidus on T2-weighted MRI against a hypointense pallidal background, which has been referred to as eye-of-the-tiger sign.341 Although originally thought to be pathognomonic, the eye-of-the-tiger sign has been also been reported in MSA and CBD.342,343
Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357-381.
, 1993 . A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. The Huntington’s Disease Collaborative Research Group. Cell. 1993;72;:971-983.
Ashizawa T, Wong LJ, Richards CS, et al. CAG repeat size and clinical presentation in Huntington’s disease. Neurology. 1994;44:1137-1143.
Benabid AL, Pollak P, Gao D, et al. Chronic electrical stimulation of the ventralis intermedius nucleus of the thalamus as a treatment of movement disorders. J Neurosurg. 1996;84:203-214.
Bergman H, Feingold A, Nini A, et al. Physiological aspects of information processing in the basal ganglia of normal and parkinsonian primates. Trends Neurosci. 1998;21:32-38.
Brown P, Marsden CD. The stiff man and stiff man plus syndromes. J Neurol. 1999;246:648-652.
Brown P, Rothwell JC, Thompson PD, et al. The hyperekplexias and their relationship to the normal startle reflex. Brain. 1991;114:1903-1928.
Bruno MK, Lee HY, Auburger GW, et al. Genotype-phenotype correlation of paroxysmal nonkinesigenic dyskinesia. Neurology. 2007;68:1782-1789.
Burke JR, Wingfield MS, Lewis KE, et al. The Haw River syndrome: dentatorubropallidoluysian atrophy (DRPLA) in an African-American family. Nat Genet. 1994;7:521-524.
Burns RS, Chiueh CC, Markey SP, et al. A primate model of parkinsonism: selective destruction of dopaminergic neurons in the pars compacta of the substantia nigra by N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Proc Natl Acad Sci U S A. 1983;80:4546-4550.
Cardoso F, Seppi K, Mair KJ, et al. Seminar on choreas. Lancet Neurol. 2006;5:589-602.
Cohen AJ, Leckman JF. Sensory phenomena associated with Gilles de la Tourette’s syndrome. J Clin Psychiatry. 1992;53:319-323.
Demirkiran M, Jankovic J. Paroxysmal dyskinesias: clinical features and classification. Ann Neurol. 1995;38:571-579.
Deng H, Le W, Jankovic J. Premutation alleles associated with Parkinson disease and essential tremor. JAMA. 2004;292:1685-1686.
Deuschl G, Raethjen J, Lindemann M, et al. The pathophysiology of tremor. Muscle Nerve. 2001;24:716-735.
Deuschl G, Wilms H. Clinical spectrum and physiology of palatal tremor. Mov Disord. 2002;17(suppl 2)):S63-S66.
Elble RJ. Central mechanisms of tremor. J Clin Neurophysiol. 1996;13:133-144.
Fahn S, Williams DT. Psychogenic dystonia. Adv Neurol. 1988;50:431-455.
Fernandez HH, Rodriguez RL, Skidmore FS, et al. A Practical Approach to Movement Disorders: Diagnosis and Medical and Surgical Management. New York: Demos Medical; 2007.
Footitt DR, Quinn N, Kocen RS, et al. Familial Lafora body disease of late onset: report of four cases in one family and a review of the literature. J Neurol. 1997;244:40-44.
Ford B, Greene P, Fahn S. Oral and genital tardive pain syndromes. Neurology. 1994;44:2115-2119.
Gibb WR, Lees AJ. The clinical phenomenon of akathisia. J Neurol Neurosurg Psychiatry. 1986;49:861-866.
Hallett M. Electrophysiologic evaluation of movement disorders. In: Aminoff M, editor. Electrodiagnosis in Clinical Neurology. New York: Churchill Livingstone; 1999:365-380.
Hallett M, Cloninger CR. Psychogenic Movement Disorders: Neurology and Neuropsychiatry. Philadelphia: Lippincott Williams & Wilkins; 2006.
Hallett M, Shahani BT, Young RR. EMG analysis of stereotyped voluntary movements in man. J Neurol Neurosurg Psychiatry. 1975;38:1154-1162.
Heilman KM. Orthostatic tremor. Arch Neurol. 1984;41:880-881.
Hewer R. The heart in Friedreich’s ataxia. Br Heart J. 1969;31:5-14.
Hohler A, Samii A. Approach to Movement Disorders. In: Winn HR, Youmans JR, editors. Youmans Neurological Surgery. 5th ed. Philadelphia: WB Saunders; 2004:2729-2744.
Jankovic J. Tourette syndrome. Phenomenology and classification of tics. Neurol Clin. 1997;15:267-275.
Jankovic J, Tolosa E. Parkinson’s Disease and Movement Disorders, 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2007.
Kanazawa I. Dentatorubral-pallidoluysian atrophy or Naito-Oyanagi disease. Neurogenetics. 1998;2:1-17.
Klein C, Brin MF, Kramer P, et al. Association of a missense change in the D2 dopamine receptor with myoclonus dystonia. Proc Natl Acad Sci U S A. 1999;96:5173-5176.
Kremer B, Almqvist E, Theilmann J, et al. Sex-dependent mechanisms for expansions and contractions of the CAG repeat on affected Huntington disease chromosomes. Am J Hum Genet. 1995;57:343-350.
Lang A. Patient perception of tics and other movement disorders. Neurology. 1991;41:223-228.
Lees AJ. Facial mannerisms and tics. Adv Neurol. 1988;49:255-261.
Leigh PN, Rothwell JC, Traub M, et al. A patient with reflex myoclonus and muscle rigidity: “jerking stiff-man syndrome. ”. J Neurol Neurosurg Psychiatry. 1980;43;:1125-1131.
Lombardi WJ, Woolston DJ, Roberts JW, et al. Cognitive deficits in patients with essential tremor. Neurology. 2001;57:785-790.
Manford M, Andermann F. Complex visual hallucinations. Clinical and neurobiological insights. Brain. 1998;121:1819-1840.
Marsalek M. Tardive drug-induced extrapyramidal syndromes. Pharmacopsychiatry. 2000;33(suppl 1)):14-33.
Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol. 1996;50:381-425.
Moersch FP, Woltman HW. Progressive fluctuating muscular rigidity and spasm (“stiff-man” syndrome); report of a case and some observations in 13 other cases. Mayo Clin Proc. 1956;31:421-427.
Parkinson J. An essay on the shaking palsy. 1817. J Neuropsychiatry Clin Neurosci. 2002;14:223-236.
Ringman JM, Jankovic J. Occurrence of tics in Asperger’s syndrome and autistic disorder. J Child Neurol. 2000;15:394-400.
Schols L, Bauer P, Schmidt T, et al. Autosomal dominant cerebellar ataxias: clinical features, genetics, and pathogenesis. Lancet Neurol. 2004;3:291-304.
Schrag A, Good CD, Miszkiel K, et al. Differentiation of atypical parkinsonian syndromes with routine MRI. Neurology. 2000;54:697-702.
Stoppelbein L, Greening L, Kakooza A. The importance of catatonia and stereotypies in autistic spectrum disorders. Int Rev Neurobiol. 2006;72:103-118.
Swartz MS, Stroup TS, McEvoy JP, et al. What CATIE found: results from the schizophrenia trial. Psychiatric Serv. 2008;59:500-506.
Teitelbaum P, Teitelbaum O, Nye J, et al. Movement analysis in infancy may be useful for early diagnosis of autism. Proc Natl Acad Sci U S A. 1998;95:13982-13987.
Tezenas du Montcel S, Clot F, Vidailhet M, et al. Epsilon sarcoglycan mutations and phenotype in French patients with myoclonic syndromes. J Med Genet. 2006;43:394-400.
Williams DR, Holton JL, Strand K, et al. Pure akinesia with gait freezing: a third clinical phenotype of progressive supranuclear palsy. Mov Disord. 2007;22:2235-2241.
1 Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357-381.
2 Schrag A, Ben-Shlomo Y, Quinn NP. Prevalence of progressive supranuclear palsy and multiple system atrophy: a cross-sectional study. Lancet. 1999;354:1771-1775.
3 ESDE: A prevalence study of primary dystonia in eight European countries. J Neurol. 2000;247:787-792.
4 Hornse H, Banerjee S, Zeitlin H, et al. The prevalence of Tourette syndrome in 13-14-year-olds in mainstream schools. J Child Psychol Psychiatry. 2001;42:1035-1039.
5 Koenig M. Friedrich’s ataxia. In: Rubinsztein D, Hayden M, editors. Analysis of Triplet Repeat Disorders. Oxford: BIOS Science; 1998:219-238.
6 Leone M, Brignolio F, Rosso MG, et al. Friedreich’s ataxia: a descriptive epidemiological study in an Italian population. Clin Genet. 1990;38:161-169.
7 Nutt JG, Muenter MD, Aronson A, et al. Epidemiology of focal and generalized dystonia in Rochester, Minnesota. Mov Disord. 1988;3:188-194.
8 Manconi M, Ferini-Strambi L, Filippi M, et al. Multicenter case-control study on restless legs syndrome in multiple sclerosis: the REMS study. Sleep. 2008;31:944-952.
9 Mason A, Banerjee S, Eapen V, et al. The prevalence of Tourette syndrome in a mainstream school population. Dev Med Child Neurol. 1998;40:292-296.
10 Mastaglia FL, Grainger K, Kee F, et al. Progressive supranuclear palsy (the Steele-Richardson-Olszewski syndrome) clinical and electrophysiological observations in eleven cases. Proc Aust Assoc Neurol. 1973;10:35-44.
11 Rajput AH, Birdi S. Epidemiology of Parkinson’s disease. Parkinsonism Relat Disord. 1997;3:175-186.
12 Rothdach AJ, Trenkwalder C, Haberstock J, et al. Prevalence and risk factors of RLS in an elderly population: the MEMO study. Memory and Morbidity in Augsburg Elderly. Neurology. 2000;54:1064-1068.
13 Hallett M, Cloninger CR. Psychogenic Movement Disorders: Neurology and Neuropsychiatry. Philadelphia: Lippincott Williams & Wilkins; 2006.
14 Jankovic J, Tolosa E. Parkinson’s Disease and Movement Disorders, 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2007.
15 Watts RL, Koller WC. Movement Disorders: Neurologic Principles & Practice, 2nd ed. New York: McGraw-Hill; 2004.
16 Haerer AF, Anderson DW, Schoenberg BS. Prevalence of essential tremor. Results from the Copiah County study. Arch Neurol. 1982;39:750-751.
17 McCusker EA, Casse RF, Graham SJ, et al. Prevalence of Huntington disease in New South Wales in 1996. Med J Aust. 2000;173:187-190.
18 Kokmen E, Ozekmekci FS, Beard CM, et al. Incidence and prevalence of Huntington’s disease in Olmsted County, Minnesota (1950 through 1989). Arch Neurol. 1994;51:696-698.
19 Jankovic J. Tourette syndrome. Phenomenology and classification of tics. Neurol Clin. 1997;15:267-275.
20 Anouti A, Koller WC. Tremor disorders. Diagnosis and management. West J Med. 1995;162:510-513.
21 Deuschl G, Raethjen J, Lindemann M, et al. The pathophysiology of tremor. Muscle Nerve. 2001;24:716-735.
22 Elble RJ. Central mechanisms of tremor. J Clin Neurophysiol. 1996;13:133-144.
23 Benabid AL, Pollak P, Gao D, et al. Chronic electrical stimulation of the ventralis intermedius nucleus of the thalamus as a treatment of movement disorders. J Neurosurg. 1996;84:203-214.
24 Deuschl G, Krack P, Lauk M, et al. Clinical neurophysiology of tremor. J Clin Neurophysiol. 1996;13:110-121.
25 Findley LJ. Classification of tremors. J Clin Neurophysiol. 1996;13:122-132.
26 Elble RJ, Higgins C, Hughes L. Longitudinal study of essential tremor. Neurology. 1992;42:441-443.
27 Elble RJ. Characteristics of physiologic tremor in young and elderly adults. Clin Neurophysiol. 2003;114:624-635.
28 Biary N, Cleeves L, Findley L, et al. Post-traumatic tremor. Neurology. 1989;39:103-106.
29 Deuschl G, Mischke G, Schenck E, et al. Symptomatic and essential rhythmic palatal myoclonus. Brain. 1990;113:1645-1672.
30 Deuschl G, Bain P, Brin M. Consensus statement of the Movement Disorder Society on Tremor. Ad Hoc Scientific Committee. Mov Disord. 1998;13(suppl 3)):2-23.
31 Grosse P, Edwards M, Tijssen MA, et al. Patterns of EMG-EMG coherence in limb dystonia. Mov Disord. 2004;19:758-769.
32 Heilman KM. Orthostatic tremor. Arch Neurol. 1984;41:880-881.
33 Koller WC, Vetere-Overfield B, Barter R. Tremors in early Parkinson’s disease. Clin Neuropharmacol. 1989;12:293-297.
34 Miles TS, Findley LJ, Rothwell JC. Electrophysiological observations on an unusual, task specific jaw tremor. J Neurol Neurosurg Psychiatry. 1997;63:251-254.
35 Orti-Pareja M, Jimenez-Jimenez FJ, Vazquez A, et al. Drug-induced tardive syndromes. Parkinsonism Relat Disord. 1999;5:59-65.
36 Schneider SA, Edwards MJ, Mir P, et al. Patients with adult-onset dystonic tremor resembling parkinsonian tremor have scans without evidence of dopaminergic deficit (SWEDDs). Mov Disord. 2007;22:2210-2215.
37 Raethjen J, Pawlas F, Lindemann M, et al. Determinants of physiologic tremor in a large normal population. Clin Neurophysiol. 2000;111:1825-1837.
38 Jankovic J, Madisetty J, Vuong KD. Essential tremor among children. Pediatrics. 2004;114:1203-1205.
39 Louis ED, Ottman R, Hauser WA. How common is the most common adult movement disorder? Estimates of the prevalence of essential tremor throughout the world. Mov Disord. 1998;13:5-10.
40 Louis ED, Borden S, Moskowitz CB. Essential tremor centralized brain repository: diagnostic validity and clinical characteristics of a highly selected group of essential tremor cases. Mov Disord. 2005;20:1361-1365.
41 Elble RJ. Diagnostic criteria for essential tremor and differential diagnosis. Neurology. 2000;54:S2-S6.
42 Lombardi WJ, Woolston DJ, Roberts JW, et al. Cognitive deficits in patients with essential tremor. Neurology. 2001;57:785-790.
43 Singer C, Sanchez-Ramos J, Weiner WJ. Gait abnormality in essential tremor. Mov Disord. 1994;9:193-196.
44 Dupuis MJ, Delwaide PJ, Boucquey D, et al. Homolateral disappearance of essential tremor after cerebellar stroke. Mov Disord. 1989;4:183-187.
45 Deuschl G, Elble RJ. The pathophysiology of essential tremor. Neurology. 2000;54:S14-S20.
46 Rajput AH, Rozdilsky B, Ang L, et al. Clinicopathologic observations in essential tremor: report of six cases. Neurology. 1991;41:1422-1424.
47 Busenbark K, Barnes P, Lyons K, et al. Accuracy of reported family histories of essential tremor. Neurology. 1996;47:264-265.
48 Deng H, Le W, Jankovic J. Genetics of essential tremor. Brain. 2007;130:1456-1464.
49 Louis ED, Ford B, Frucht S, et al. Risk of tremor and impairment from tremor in relatives of patients with essential tremor: a community-based family study. Ann Neurol. 2001;49:761-769.
50 Shatunov A, Sambuughin N, Jankovic J, et al. Genomewide scans in North American families reveal genetic linkage of essential tremor to a region on chromosome 6p23. Brain. 2006;129:2318-2331.
51 Jankovic J. Essential tremor: a heterogenous disorder. Mov Disord. 2002;17:638-644.
52 Donati F, Gasser S, Mullis P, et al. [48,XXYY syndrome in a boy with essential tremor. Comparison with 120 cases from the literature.]. Monatsschr Kinderheilkd. 1992;140:216-219.
53 Leehey MA, Munhoz RP, Lang AE, et al. The fragile X premutation presenting as essential tremor. Arch Neurol. 2003;60:117-121.
54 Higgins JJ, Loveless JM, Jankovic J, et al. Evidence that a gene for essential tremor maps to chromosome 2p in four families. Mov Disord. 1998;13:972-977.
55 Parkinson J. An essay on the shaking palsy. 1817. J Neuropsychiatry Clin Neurosci. 2002;14:223-236.
56 Goetz CG. Charcot on Parkinson’s disease. Mov Disord. 1986;1:27-32.
57 Jankovic J, Schwartz KS, Ondo W. Re-emergent tremor of Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1999;67:646-650.
58 Deuschl G, Raethjen J, Baron R, et al. The pathophysiology of parkinsonian tremor: a review. J Neurol. 2000;247(suppl 5)):V33-V48.
59 Bergman H, Feingold A, Nini A, et al. Physiological aspects of information processing in the basal ganglia of normal and parkinsonian primates. Trends Neurosci. 1998;21:32-38.
60 Hohler A, Samii A. Approach to movement disorders. In: Winn HR, Youmans JR, editors. Youmans Neurological Surgery. 5th ed. Philadelphia: WB Saunders; 2004:2729-2744.
61 Hallett M, Shahani BT, Young RR. EMG analysis of stereotyped voluntary movements in man. J Neurol Neurosurg Psychiatry. 1975;38:1154-1162.
61a Holmes G. On certain tumors in organic cerebral lesions. Brain. 1904;27:327-375.
62 Krauss JK, Wakhloo AK, Nobbe F, et al. Lesion of dentatothalamic pathways in severe post-traumatic tremor. Neurol Res. 1995;17:409-416.
63 Obeso JA, Narbona J. Post-traumatic tremor and myoclonic jerking. J Neurol Neurosurg Psychiatry. 1983;46:788.
64 Alterman RL, Miravite J, Weisz D, et al. Sixty hertz pallidal deep brain stimulation for primary torsion dystonia. Neurology. 2007;69:681-688.
65 Fernandez HH, Rodriguez RL, Skidmore FS, et al. A Practical Approach to Movement Disorders: Diagnosis and Medical and Surgical Management. New York: Demos Medical; 2007.
66 Neuhaus P, McMaster P, Calne R, et al. Neurological complications in the European multicentre study of FK 506 and cyclosporin in primary liver transplantation. Transpl Int. 1994;7(suppl 1)):S27-S31.
67 McManis PG, Sharbrough FW. Orthostatic tremor: clinical and electrophysiologic characteristics. Muscle Nerve. 1993;16:1254-1260.
68 Britton TC, Thompson PD, van der Kamp W, et al. Primary orthostatic tremor: further observations in six cases. J Neurol. 1992;239:209-217.
69 Fung VS, Sauner D, Day BL. A dissociation between subjective and objective unsteadiness in primary orthostatic tremor. Brain. 2001;124:322-330.
70 Deuschl G, Wilms H. Clinical spectrum and physiology of palatal tremor. Mov Disord. 2002;17(suppl 2)):S63-S66.
71 Barron KD, Dentinger MP, Koeppen AH. Fine structure of neurons of the hypertrophied human inferior olive. J Neuropathol Exp Neurol. 1982;41:186-203.
72 Fahn S, Williams DT. Psychogenic dystonia. Adv Neurol. 1988;50:431-455.
73 Racette BA, Dowling J, Randle J, et al. Thalamic stimulation for primary writing tremor. J Neurol. 2001;248:380-382.
74 Koller WC, Glatt S, Biary N, et al. Essential tremor variants: effect of treatment. Clin Neuropharmacol. 1987;10:342-350.
75 Elble RJ, Moody C, Higgins C. Primary writing tremor. A form of focal dystonia? Mov Disord. 1990;5:118-126.
76 Modugno N, Nakamura Y, Bestmann S, et al. Neurophysiological investigations in patients with primary writing tremor. Mov Disord. 2002;17:1336-1340.
77 Hayashi M, Koide H. Idiopathic torsion dystonia and writing tremor within a family. Brain Dev. 1997;19:556-558.
78 Bain PG, Findley LJ, Britton TC, et al. Primary writing tremor. Brain. 1995;118:1461-1472.
79 Cardoso F, Seppi K, Mair KJ, et al. Seminar on choreas. Lancet Neurol. 2006;5:589-602.
80 Wild EJ, Tabrizi SJ. The differential diagnosis of chorea. Pract Neurol. 2007;7:360-373.
81 Blakeley J, Jankovic J. Secondary paroxysmal dyskinesias. Mov Disord. 2002;17:726-734.
82 Breedveld GJ, Percy AK, MacDonald ME, et al. Clinical and genetic heterogeneity in benign hereditary chorea. Neurology. 2002;59:579-584.
83 Demirkiran M, Jankovic J. Paroxysmal dyskinesias: clinical features and classification. Ann Neurol. 1995;38:571-579.
84 Hewer E, Danek A, Schoser BG, et al. McLeod myopathy revisited: more neurogenic and less benign. Brain. 2007;130:3285-3296.
85 Kanazawa I. Dentatorubral-pallidoluysian atrophy or Naito-Oyanagi disease. Neurogenetics. 1998;2:1-17.
86 Kleiner-Fisman G, Lang AE. Benign hereditary chorea revisited: a journey to understanding. Mov Disord. 2007;22:2297-2305.
87 Petrukhin K, Fischer SG, Pirastu M, et al. Mapping, cloning and genetic characterization of the region containing the Wilson disease gene. Nat Genet. 1993;5:338-343.
88 Schneider SA, Walker RH, Bhatia KP. The Huntington’s disease–like syndromes: what to consider in patients with a negative Huntington’s disease gene test. Nat Clin Pract. 2007;3:517-525.
89 Walker RH, Rasmussen A, Rudnicki D, et al. Huntington’s disease–like 2 can present as chorea-acanthocytosis. Neurology. 2003;61:1002-1004.
90 Foroud T, Gray J, Ivashina J, et al. Differences in duration of Huntington’s disease based on age at onset. J Neurol Neurosurg Psychiatry. 1999;66:52-56.
91 Penney JBJr, Young AB, Shoulson I, et al. Huntington’s disease in Venezuela: 7 years of follow-up on symptomatic and asymptomatic individuals. Mov Disord. 1990;5:93-99.
92 Avanzini G, Girotti F, Caraceni T, et al. Oculomotor disorders in Huntington’s chorea. J Neurol Neurosurg Psychiatry. 1979;42:581-589.
93 Jankovic J, Ashizawa T. Tourettism associated with Huntington’s disease. Mov Disord. 1995;10:103-105.
94 Riestra AR, Heilman KM. Visual facial grasp. Neurocase. 2004;10:363-365.
95 Naarding P, Kremer HP, Zitman FG. Huntington’s disease: a review of the literature on prevalence and treatment of neuropsychiatric phenomena. Eur Psychiatry. 2001;16:439-445.
96 Louis ED, Anderson KE, Moskowitz C, et al. Dystonia-predominant adult-onset Huntington disease: association between motor phenotype and age of onset in adults. Arch Neurol. 2000;57:1326-1330.
97 Bamford KA, Caine ED, Kido DK, et al. A prospective evaluation of cognitive decline in early Huntington’s disease: functional and radiographic correlates. Neurology. 1995;45:1867-1873.
98 Britton JW, Uitti RJ, Ahlskog JE, et al. Hereditary late-onset chorea without significant dementia: genetic evidence for substantial phenotypic variation in Huntington’s disease. Neurology. 1995;45:443-447.
99 A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. The Huntington’s Disease Collaborative Research Group. Cell. 1993;72:971-983.
100 Rubinsztein DC, Leggo J, Coles R, et al. Phenotypic characterization of individuals with 30-40 CAG repeats in the Huntington disease (HD) gene reveals HD cases with 36 repeats and apparently normal elderly individuals with 36-39 repeats. Am J Hum Genet. 1996;59:16-22.
101 Kremer B, Almqvist E, Theilmann J, et al. Sex-dependent mechanisms for expansions and contractions of the CAG repeat on affected Huntington disease chromosomes. Am J Hum Genet. 1995;57:343-350.
102 Ashizawa T, Wong LJ, Richards CS, et al. CAG repeat size and clinical presentation in Huntington’s disease. Neurology. 1994;44:1137-1143.
103 Zuhlke C, Riess O, Bockel B, et al. Mitotic stability and meiotic variability of the (CAG)n repeat in the Huntington disease gene. Hum Mol Genet. 1993;2:2063-2067.
104 Brandt J, Bylsma FW, Gross R, et al. Trinucleotide repeat length and clinical progression in Huntington’s disease. Neurology. 1996;46:527-531.
105 Stevanin G, Camuzat A, Holmes SE, et al. CAG/CTG repeat expansions at the Huntington’s disease–like 2 locus are rare in Huntington’s disease patients. Neurology. 2002;58:965-967.
106 Cardoso F, Eduardo C, Silva AP, et al. Chorea in fifty consecutive patients with rheumatic fever. Mov Disord. 1997;12:701-703.
107 Stollerman GH. Changing streptococci and prospects for the global eradication of rheumatic fever. Perspect Biol Med. 1997;40:165-189.
108 Tani LY, Veasy LG, Minich LL, et al. Rheumatic fever in children younger than 5 years: is the presentation different? Pediatrics. 2003;112:1065-1068.
109 Swedo SE, Leonard HL, Schapiro MB, et al. Sydenham’s chorea: physical and psychological symptoms of St Vitus dance. Pediatrics. 1993;91:706-713.
110 Cardoso F, Vargas AP, Oliveira LD, et al. Persistent Sydenham’s chorea. Mov Disord. 1999;14:805-807.
111 Korn-Lubetzki I, Brand A, Steiner I. Recurrence of Sydenham chorea: implications for pathogenesis. Arch Neurol. 2004;61:1261-1264.
112 Berrios X, Quesney F, Morales A, et al. Are all recurrences of “pure” Sydenham chorea true recurrences of acute rheumatic fever? J Pediatr. 1985;107:867-872.
113 Ikuta N, Hirata M, Sasabe F, et al. High-signal basal ganglia on T1-weighted images in a patient with Sydenham’s chorea. Neuroradiology. 1998;40:659-661.
114 Giedd JN, Rapoport JL, Kruesi MJ, et al. Sydenham’s chorea: magnetic resonance imaging of the basal ganglia. Neurology. 1995;45:2199-2202.
115 Ford B, Greene P, Fahn S. Oral and genital tardive pain syndromes. Neurology. 1994;44:2115-2119.
116 Swartz MS, Stroup TS, McEvoy JP, et al. What CATIE found: results from the schizophrenia trial. Psychiatric Serv. 2008;59:500-506.
117 Correll CU, Leucht S, Kane JM. Lower risk for tardive dyskinesia associated with second-generation antipsychotics: a systematic review of 1-year studies. Am J Psychiatry. 2004;161:414-425.
118 Schrag A, Quinn NP, Bhatia KP, et al. Benign hereditary chorea—entity or syndrome? Mov Disord. 2000;15:280-288.
119 Krude H, Schutz B, Biebermann H, et al. Choreoathetosis, hypothyroidism, and pulmonary alterations due to human NKX2-1 haploinsufficiency. Journal Clin Invest. 2002;109:475-480.
120 Walker RH, Jung HH, Dobson-Stone C, et al. Neurologic phenotypes associated with acanthocytosis. Neurology. 2007;68:92-98.
121 Gradstein L, Danek A, Grafman J, et al. Eye movements in chorea-acanthocytosis. Invest Ophthalmol Visual Sci. 2005;46:1979-1987.
122 Schols L, Bauer P, Schmidt T, et al. Autosomal dominant cerebellar ataxias: clinical features, genetics, and pathogenesis. Lancet Neurol. 2004;3:291-304.
123 Burke JR, Wingfield MS, Lewis KE, et al. The Haw River syndrome: dentatorubropallidoluysian atrophy (DRPLA) in an African-American family. Nat Genet. 1994;7:521-524.
124 Klawans HLJr, Goetz CH, Weiner WJ. Dopamine receptor site sensitivity in hyperthyroid guinea pigs: a possible model of hyperthyroid chorea. J Neural Transm. 1973;34:187-193.
124a Curtis AR, Fey C, Morris CM, et al. Mutation in the gene encoding ferritin light polypeptide causes dominant adult-onset basal ganglia disease. Nat Genet. 2001;28:350-354.
124b Crompton DE, Chinnery PF, Bates D, et al. Spectrum of movement disorders in neuroferritinopathy. Mov Disord. 2005;20:95-99.
125 Nazabal ER, Lopez JM, Perez PA, et al. Chorea disclosing deterioration of polycythaemia vera. Postgrad Med J. 2000;76:658-659.
126 Cao M, Olsen RJ, Zu Y. Polycythemia vera: new clinicopathologic perspectives. Arch Pathol Lab Med. 2006;130:1126-1132.
127 Cardoso F. Chorea gravidarum. Arch Neurol. 2002;59:868-870.
128 Wolf RE, McBeath JG. Chorea gravidarum in systemic lupus erythematosus. J Rheumatol. 1985;12:992-993.
129 Branch DW. Antiphospholipid antibodies and pregnancy: maternal implications. Semin Perinatol. 1990;14:139-146.
130 Bordelon YM, Smith M. Movement disorders in pregnancy. Semin Neurol. 2007;27:467-475.
131 Dressler D, Thompson PD, Gledhill RF, et al. The syndrome of painful legs and moving toes. Mov Disord. 1994;9:13-21.
132 Steiner JC, DeJesus PV, Mancall EL. Painful jumping amputation stumps: pathophysiology of a “sore circuit.”. Trans Am Neurol Assoc. 1974;99:253-255.
133 Hanna MG, Davis MB, Sweeney MG, et al. Generalized chorea in two patients harboring the Friedreich’s ataxia gene trinucleotide repeat expansion. Mov Disord. 1998;13:339-340.
134 Jinnah HA, Visser JE, Harris JC, et al. Delineation of the motor disorder of Lesch-Nyhan disease. Brain. 2006;129:1201-1217.
135 Hallett M. Electrophysiologic evaluation of movement disorders. In: Aminoff M, editor. Electrodiagnosis in Clinical Neurology. New York: Churchill Livingstone; 1999:365-380.
136 Shibasaki H. Electrophysiological studies of myoclonus. Muscle Nerve. 2000;23:321-335.
137 Caviness JN, Brown P. Myoclonus: current concepts and recent advances. Lancet Neurol. 2004;3:598-607.
138 Jankovic J, Pardo R. Segmental myoclonus. Clinical and pharmacologic study. Arch Neurol. 1986;43:1025-1031.
139 Obeso JA, Rothwell JC, Marsden CD. The spectrum of cortical myoclonus. From focal reflex jerks to spontaneous motor epilepsy. Brain. 1985;108:103-124.
140 Cockerell OC, Rothwell J, Thompson PD, et al. Clinical and physiological features of epilepsia partialis continua. Cases ascertained in the UK. Brain. 1996;119:393-407.
141 Bien CG, Elger CE. Epilepsia partialis continua: semiology and differential diagnoses. Epileptic Disord. 2008;10:3-7.
142 Wilkins DE, Hallett M, Erba G. Primary generalised epileptic myoclonus: a frequent manifestation of minipolymyoclonus of central origin. J Neurol Neurosurg Psychiatry. 1985;48:506-516.
143 Kalviainen R, Khyuppenen J, Koskenkorva P, et al. Clinical picture of EPM1-Unverricht-Lundborg disease. Epilepsia. 2008;49:549-556.
144 Eldridge R, Iivanainen M, Stern R, et al. “Baltic” myoclonus epilepsy: hereditary disorder of childhood made worse by phenytoin. Lancet. 1983;2:838-842.
145 Footitt DR, Quinn N, Kocen RS, et al. Familial Lafora body disease of late onset: report of four cases in one family and a review of the literature. J Neurol. 1997;244:40-44.
146 Mole SE, Williams RE, Goebel HH. Correlations between genotype, ultrastructural morphology and clinical phenotype in the neuronal ceroid lipofuscinoses. Neurogenetics. 2005;6:107-126.
147 Rapin I. Myoclonus in neuronal storage and Lafora diseases. Adv Neurol. 1986;43:65-85.
148 Pranzatelli MR. The neurobiology of the opsoclonus-myoclonus syndrome. Clin Neuropharmacol. 1992;15:186-228.
149 Dalmau J, Rosenfeld MR. Paraneoplastic syndromes of the CNS. Lancet Neurol. 2008;7:327-340.
150 Lance JW, Adams RD. The syndrome of intention or action myoclonus as a sequel to hypoxic encephalopathy. Brain. 1963;86:111-136.
151 Hallett M. Physiology of human posthypoxic myoclonus. Mov Disord. 2000;15(suppl 1)):8-13.
152 Satoyoshi E, Kinoshita M, Takazawa Y, et al. [Case of intention myoclonus (Lance-Adams syndrome) and a dramatic effect of L-5-hydroxytryptophan.]. Rinsho Shinkeigaku. 1976;16:654-660.
153 De Lean J, Richardson JC, Rewcastle NB. Pathological findings in a case of hypoxic myoclonus treated with 5-hydroxytryptophan and a decarboxylase inhibitor. Adv Neurol. 1986;43:215-223.
154 Andermann F, Keene DL, Andermann E, et al. Startle disease or hyperekplexia: further delineation of the syndrome. Brain. 1980;103:985-997.
155 Meinck HM. Startle and its disorders. Neurophysiol Clin. 2006;36:357-364.
156 Brown P, Rothwell JC, Thompson PD, et al. The hyperekplexias and their relationship to the normal startle reflex. Brain. 1991;114:1903-1928.
157 Saint-Hilaire MH, Saint-Hilaire JM, Granger L. Jumping Frenchmen of Maine. Neurology. 1986;36:1269-1271.
158 Tanner CM, Chamberland J. Latah in Jakarta, Indonesia. Mov Disord. 2001;16:526-529.
159 McFarling DA. The “Ragin’ Cajuns” of Louisiana. Mov Disord. 2001;16:531-532.
160 Nishida H, Shinbo Y, Motomura H. [A study of pathogenesis and symptoms of Tourette’s syndrome—mainly on the importance of “startle reflex” through Latah reaction. Seishin Shinkeigaku Zasshi. 1994;96:26-47.
161 Aguglia U, Tinuper P, Gastaut H. Startle-induced epileptic seizures. Epilepsia. 1984;25:712-720.
162 Karama S, Lal S. Tardive dyskinesia following brief exposure to risperidone—a case study. Eur Psychiatry. 2004;19:391-392.
163 Chouinard G, Jones BD. Early onset of tardive dyskinesia: case report. Am J Psychiatry. 1979;136:1323-1324.
164 Mink JW. Paroxysmal dyskinesias. Curr Opin Pediatr. 2007;19:652-656.
165 Bruno MK, Lee HY, Auburger GW, et al. Genotype-phenotype correlation of paroxysmal nonkinesigenic dyskinesia. Neurology. 2007;68:1782-1789.
166 Lance JW. Familial paroxysmal dystonic choreoathetosis and its differentiation from related syndromes. Ann Neurol. 1977;2:285-293.
167 Derry CP, Duncan JS, Berkovic SF. Paroxysmal motor disorders of sleep: the clinical spectrum and differentiation from epilepsy. Epilepsia. 2006;47:1775-1791.
168 Szepetowski P, Rochette J, Berquin P, et al. Familial infantile convulsions and paroxysmal choreoathetosis: a new neurological syndrome linked to the pericentromeric region of human chromosome 16. Am J Hum Genet. 1997;61:889-898.
169 Kato H, Kobayashi K, Kohari S, et al. Paroxysmal kinesigenic choreoathetosis and paroxysmal dystonic choreoathetosis in a patient with familial idiopathic hypoparathyroidism. Tohoku J Exp Med. 1987;151:233-239.
170 Inghilleri M, Conte A, Frasca V, et al. Belly dance syndrome due to spinal myoclonus. Mov Disord. 2006;21:394-396.
171 Kono I, Ueda Y, Araki K, et al. Spinal myoclonus resembling belly dance. Mov Disord. 1994;9:325-329.
172 Iliceto G, Thompson PD, Day BL, et al. Diaphragmatic flutter, the moving umbilicus syndrome, and “belly dancer’s” dyskinesia. Mov Disord. 1990;5:15-22.
173 Cohen AJ, Leckman JF. Sensory phenomena associated with Gilles de la Tourette’s syndrome. J Clin Psychiatry. 1992;53:319-323.
174 Chee KY, Sachdev P. A controlled study of sensory tics in Gilles de 1a Tourette syndrome and obsessive-compulsive disorder using a structured interview. J Neurol Neurosurg Psychiatry. 1997;62:188-192.
175 Lang A. Patient perception of tics and other movement disorders. Neurology. 1991;41:223-228.
176 Chouinard S, Ford B. Adult onset tic disorders. J Neurol Neurosurg Psychiatry. 2000;68:738-743.
177 Definitions and classification of tic disorders. The Tourette Syndrome Classification Study Group. Arch Neurol. 1993;50:1013-1016.
178 Leckman JF, Zhang H, Vitale A, et al. Course of tic severity in Tourette syndrome: the first two decades. Pediatrics. 1998;102:14-19.
179 Robertson MM. The Gilles de la Tourette syndrome: the current status. Br J Psychiatry. 1989;154:147-169.
180 Kurlan R, Como PG, Miller B, et al. The behavioral spectrum of tic disorders: a community-based study. Neurology. 2002;59:414-420.
181 Comings DE, Comings BG. A controlled study of Tourette syndrome. I. Attention-deficit disorder, learning disorders, and school problems. Am J Hum Genet. 1987;41:701-741.
182 Singer HS. Tourette’s syndrome: from behaviour to biology. Lancet Neurol. 2005;4:149-159.
183 Eapen V, Robertson MM, Alsobrook JP2nd, et al. Obsessive compulsive symptoms in Gilles de la Tourette syndrome and obsessive compulsive disorder: differences by diagnosis and family history. Am J Med Genet. 1997;74:432-438.
184 Jankovic J, Sekula S. Dermatological manifestations of Tourette syndrome and obsessive-compulsive disorder. Arch Dermatol. 1998;134:113-114.
185 Robertson M, Doran M, Trimble M, et al. The treatment of Gilles de la Tourette syndrome by limbic leucotomy. J Neurol Neurosurg Psychiatry. 1990;53:691-694.
186 Pauls DL. An update on the genetics of Gilles de la Tourette syndrome. J Psychosom Res. 2003;55:7-12.
187 Pauls DL, Cohen DJ, Kidd KK, et al. Tourette syndrome and neuropsychiatric disorders: is there a genetic relationship? Am J Hum Genet. 1988;43:206-217.
188 Price RA, Kidd KK, Cohen DJ, et al. A twin study of Tourette syndrome. Arch Gen Psychiatry. 1985;42:815-820.
189 Curtis D, Robertson MM, Gurling HM. Autosomal dominant gene transmission in a large kindred with Gilles de la Tourette syndrome. Br J Psychiatry. 1992;160:845-849.
190 Pauls DL, Raymond CL, Stevenson JM, et al. A family study of Gilles de la Tourette syndrome. Am J Hum Genet. 1991;48:154-163.
191 Schuerholz LJ, Baumgardner TL, Singer HS, et al. Neuropsychological status of children with Tourette’s syndrome with and without attention deficit hyperactivity disorder. Neurology. 1996;46:958-965.
192 Peterson BS, Skudlarski P, Anderson AW, et al. A functional magnetic resonance imaging study of tic suppression in Tourette syndrome. Arch Gen Psychiatry. 1998;55:326-333.
193 Evidente VG. Is it a tic or Tourette’s? Clues for differentiating simple from more complex tic disorders. Postgrad Med. 2000;108:175-176. 179-182
194 Sotero de Menezes MA, Rho JM, Murphy P, et al. Lamotrigine-induced tic disorder: report of five pediatric cases. Epilepsia. 2000;41:862-867.
195 Marsalek M. Tardive drug-induced extrapyramidal syndromes. Pharmacopsychiatry. 2000;33(suppl 1)):14-33.
196 Canitano R, Vivanti G. Tics and Tourette syndrome in autism spectrum disorders. Autism. 2007;11:19-28.
197 Mejia NI, Jankovic J. Secondary tics and tourettism. Rev Bras Psiquiatr. 2005;27:11-17.
198 Krauss JK, Jankovic J. Tics secondary to craniocerebral trauma. Mov Disord. 1997;12:776-782.
199 Gibb WR, Lees AJ. The clinical phenomenon of akathisia. J Neurol Neurosurg Psychiatry. 1986;49:861-866.
200 Kompoliti K, Goetz CG. Hyperkinetic movement disorders misdiagnosed as tics in Gilles de la Tourette syndrome. Mov Disord. 1998;13:477-480.
201 Poewe W, Hogl B. Akathisia, restless legs and periodic limb movements in sleep in Parkinson’s disease. Neurology. 2004;63:S12-S16.
202 Bassetti CL, Mauerhofer D, Gugger M, et al. Restless legs syndrome: a clinical study of 55 patients. Eur Neurol. 2001;45:67-74.
203 Hening WA, Walters AS, Wagner M, et al. Circadian rhythm of motor restlessness and sensory symptoms in the idiopathic restless legs syndrome. Sleep. 1999;22:901-912.
204 Phillips B, Young T, Finn L, et al. Epidemiology of restless legs symptoms in adults. Arch Intern Med. 2000;160:2137-2141.
205 Walters AS, Hickey K, Maltzman J, et al. A questionnaire study of 138 patients with restless legs syndrome: the “Night-Walkers” survey. Neurology. 1996;46:92-95.
206 Lipinski JFJr, Mallya G, Zimmerman P, et al. Fluoxetine-induced akathisia: clinical and theoretical implications. J Clin Psychiatry. 1989;50:339-342.
207 Jimenez-Jimenez FJ, Garcia-Ruiz PJ, Molina JA. Drug-induced movement disorders. Drug Saf. 1997;16:180-204.
208 Onishi H, Yamamoto W, Wada M, et al. Detection and treatment of akathisia in advanced cancer patients during adjuvant analgesic therapy with tricyclic antidepressants: case reports and review of the literature. Palliat Supporte Care. 2007;5:411-414.
209 Korff S, Harvey BH. Animal models of obsessive-compulsive disorder: rationale to understanding psychobiology and pharmacology. Psychiatr Clin North Am. 2006;29:371-390.
210 FitzGerald PM, Jankovic J, Percy AK. Rett syndrome and associated movement disorders. Mov Disord. 1990;5:195-202.
211 Ridley RM. The psychology of perseverative and stereotyped behaviour. Prog Neurobiol. 1994;44:221-231.
212 Lees AJ. Facial mannerisms and tics. Adv Neurol. 1988;49:255-261.
213 Jankovic J. Stereotypies. In: Marsden CD, Fahn S, editors. Movement Disorders 3. Boston: Buterworth-Heinemann; 1994:503-517.
214 Ringman JM, Jankovic J. Occurrence of tics in Asperger’s syndrome and autistic disorder. J Child Neurol. 2000;15:394-400.
215 Stacy M, Cardoso F, Jankovic J. Tardive stereotypy and other movement disorders in tardive dyskinesias. Neurology. 1993;43:937-941.
216 Mandelbaum DE, Stevens M, Rosenberg E, et al. Sensorimotor performance in school-age children with autism, developmental language disorder, or low IQ. Dev Med Child Neurol. 2006;48:33-39.
217 MacDonald R, Green G, Mansfield R, et al. Stereotypy in young children with autism and typically developing children. Res Dev Disabil. 2007;28:266-277.
218 Stoppelbein L, Greening L, Kakooza A. The importance of catatonia and stereotypies in autistic spectrum disorders. Int Rev Neurobiol. 2006;72:103-118.
219 Teitelbaum P, Teitelbaum O, Nye J, et al. Movement analysis in infancy may be useful for early diagnosis of autism. Proc Natl Acad Sci U S A. 1998;95:13982-13987.
220 Kozinetz CA, Skender ML, MacNaughton N, et al. Epidemiology of Rett syndrome: a population-based registry. Pediatrics. 1993;91:445-450.
221 Ager S, Fyfe S, Christodoulou J, et al. Predictors of scoliosis in Rett syndrome. J Child Neurol. 2006;21:809-813.
222 Percy AK, Lane JB, Childers J, et al. Rett syndrome: North American database. J Child Neurol. 2007;22:1338-1341.
223 Hagberg B, Hanefeld F, Percy A, et al. An update on clinically applicable diagnostic criteria in Rett syndrome. Comments to Rett Syndrome Clinical Criteria Consensus Panel Satellite to European Paediatric Neurology Society Meeting, Baden Baden, Germany, 11 September 2001. Eur J Paediatr Neurol. 2002;6:293-297.
224 Murakami JW, Courchesne E, Haas RH, et al. Cerebellar and cerebral abnormalities in Rett syndrome: a quantitative MR analysis. AJR Am J Roentgenol. 1992;159:177-183.
225 Moersch FP, Woltman HW. Progressive fluctuating muscular rigidity and spasm (“stiff-man” syndrome); report of a case and some observations in 13 other cases. Mayo Clin Proc. 1956;31:421-427.
226 Brown P, Marsden CD. The stiff man and stiff man plus syndromes. J Neurol. 1999;246:648-652.
227 Walikonis JE, Lennon VA. Radioimmunoassay for glutamic acid decarboxylase (GAD65) autoantibodies as a diagnostic aid for stiff-man syndrome and a correlate of susceptibility to type 1 diabetes mellitus. Mayo Clin Proc. 1998;73:1161-1166.
228 Barker RA, Revesz T, Thom M, et al. Review of 23 patients affected by the stiff man syndrome: clinical subdivision into stiff trunk (man) syndrome, stiff limb syndrome, and progressive encephalomyelitis with rigidity. J Neurol Neurosurg Psychiatry. 1998;65:633-640.
229 Leigh PN, Rothwell JC, Traub M, et al. A patient with reflex myoclonus and muscle rigidity: “jerking stiff-man syndrome.”. J Neurol Neurosurg Psychiatry. 1980;43:1125-1131.
230 Solimena M, Folli F, Aparisi R, et al. Autoantibodies to GABA-ergic neurons and pancreatic beta cells in stiff-man syndrome. N Engl J Med. 1990;322:1555-1560.
231 Maddison P. Neuromyotonia. Clin Neurophysiol. 2006;117:2118-2127.
232 Isaacs H, Frere G. Syndrome of continuous muscle fibre activity. Histochemical, nerve terminal and end-plate study of two cases. South Afr Med J. 1974;48:1601-1607.
233 Hart IK. Acquired neuromyotonia: a new autoantibody-mediated neuronal potassium channelopathy. Am J Med Sci. 2000;319:209-216.
234 Odabasi Z, Joy JL, Claussen GC, et al. Isaacs’ syndrome associated with chronic inflammatory demyelinating polyneuropathy. Muscle Nerve. 1996;19:210-215.
235 Walsh JC. Neuromyotonia: an unusual presentation of intrathoracic malignancy. J Neurol Neurosurg Psychiatry. 1976;39:1086-1091.
236 Liu YC, Wu ZA, Wang SJ, et al. Transient syndrome of continuous muscle fiber activity associated with staphylococcal infection. Mov Disord. 1998;13:609-611.
237 Maddison P, Lawn N, Mills KR, et al. Acquired neuromyotonia in a patient with spinal epidural abscess. Muscle Nerve. 1998;21:672-674.
238 Brick JF, Gutmann L, Brick J, et al. Timber rattlesnake venom-induced myokymia: evidence for peripheral nerve origin. Neurology. 1987;37:1545-1546.
239 Voiculescu V, Ionescu-Drinca M, Vasilescu C. Neuromyotonia in alcoholic patients with gastrectomy. Rom J Neurol Psychiatry. 1995;33:3-6.
240 Bonduelle M. [Charcot. Dates. Legend and reality.]. Hist Sci Med. 1994;28:289-295.
241 Gowers WR. A Manual of Diseases of the Nervous System, 2nd ed. Darien, CT: Hafner; 1970.
242 Hughes AJ, Bishop S, Kleedorfer B, et al. Subcutaneous apomorphine in Parkinson’s disease: response to chronic administration for up to five years. Mov Disord. 1993;8:165-170.
243 Hoehn MM, Yahr MD. Parkinsonism: onset, progression, and mortality. 1967. Neurology. 1998;50:318. and 16 pages following
244 Calne DB, Stoessl AJ. Early parkinsonism. Clin Neuropharmacol. 1986;9(suppl 2)):S3-S8.
245 Golbe LI. Young-onset Parkinson’s disease: a clinical review. Neurology. 1991;41:168-173.
246 Schrag A, Schott JM. Epidemiological, clinical, and genetic characteristics of early-onset parkinsonism. Lancet Neurol. 2006;5:355-363.
247 Lander ES, Schork NJ. Genetic dissection of complex traits. Science. 1994;265:2037-2048.
248 Hubble JP, Cao T, Hassanein RE, et al. Risk factors for Parkinson’s disease. Neurology. 1993;43:1693-1697.
249 Priyadarshi A, Khuder SA, Schaub EA, et al. Environmental risk factors and Parkinson’s disease: a metaanalysis. Environ Res. 2001;86:122-127.
250 Ritz B, Ascherio A, Checkoway H, et al. Pooled analysis of tobacco use and risk of Parkinson disease. Arch Neurol. 2007;64:990-997.
251 Langston JW, Ballard P, Tetrud JW, et al. Chronic parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219:979-980.
252 Burns RS, Chiueh CC, Markey SP, et al. A primate model of parkinsonism: selective destruction of dopaminergic neurons in the pars compacta of the substantia nigra by N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Proc Natl Acad Sci U S A. 1983;80:4546-4550.
253 Fearnley JM, Lees AJ. Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain. 1991;114:2283-2301.
254 Maraganore DM, Harding AE, Marsden CD. A clinical and genetic study of familial Parkinson’s disease. Mov Disord. 1991;6:205-211.
255 Elbaz A, Grigoletto F, Baldereschi M, et al. Familial aggregation of Parkinson’s disease: a population-based case-control study in Europe. EUROPARKINSON Study Group. Neurology. 1999;52:1876-1882.
256 Payami H, Larsen K, Bernard S, et al. Increased risk of Parkinson’s disease in parents and siblings of patients. Ann Neurol. 1994;36:659-661.
257 Calne S, Schoenberg B, Martin W, et al. Familial Parkinson’s disease: possible role of environmental factors. Can J Neurol Sci. 1987;14:303-305.
258 Jenner P. Genetic susceptibility and the occurrence of Parkinson’s disease. Parkinsonism Relat Disord. 1999;5:173-177.
259 Scott WK, Nance MA, Watts RL, et al. Complete genomic screen in Parkinson disease: evidence for multiple genes. JAMA. 2001;286:2239-2244.
260 Kitada T, Asakawa S, Hattori N, et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605-608.
261 Zimprich A, Biskup S, Leitner P, et al. Mutations in LRRK2 cause autosomal dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44:601-607.
262 Valente EM, Abou-Sleiman PM, Caputo V, et al. Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science. 2004;304:1158-1160.
263 Polymeropoulos MH, Lavedan C, Leroy E, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045-2047.
264 Klein C, Lohmann-Hedrich K, Rogaeva E, et al. Deciphering the role of heterozygous mutations in genes associated with parkinsonism. Lancet Neurol. 2007;6:652-662.
265 DeLong MR, Wichmann T. Circuits and circuit disorders of the basal ganglia. Arch Neurol. 2007;64:20-24.
266 Gale JT, Amirnovin R, Williams ZM, et al. From symphony to cacophony: pathophysiology of the human basal ganglia in Parkinson disease. Neurosci Biobehav Rev. 2008;32:378-387.
267 Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol. 1996;50:381-425.
268 Uhlhaas PJ, Singer W. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron. 2006;52:155-168.
269 Broussolle E, Krack P, Thobois S, et al. Contribution of Jules Froment to the study of parkinsonian rigidity. Mov Disord. 2007;22:909-914.
270 Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson’s disease. Arch Neurol. 1999;56:33-39.
271 Jellinger KA. Neuropathological spectrum of synucleinopathies. Mov Disord. 2003;18(suppl 6)):S2-S12.
272 Ince P, McKeith I. Dementia with Lewy bodies. In: Dickson DW, editor. Neurodegeneration: The Molecular Pathology of Dementia. Basel: International Society of Neuropathology Press; 2003:188-199.
273 Kosaka K. Diffuse Lewy body disease. Neuropathology. 2000;20(suppl):S73-S78.
274 Manford M, Andermann F. Complex visual hallucinations. Clinical and neurobiological insights. Brain. 1998;121:1819-1840.
275 Lewis AJ, Gawel MJ. Diffuse Lewy body disease with dementia and oculomotor dysfunction. Mov Disord. 1990;5:143-147.
276 Burkhardt CR, Filley CM, Kleinschmidt-DeMasters BK, et al. Diffuse Lewy body disease and progressive dementia. Neurology. 1988;38:1520-1528.
277 Steele JC, Richardson JC, Olszewski J. Progressive supranuclear palsy. A heterogeneous degeneration involving the brain stem, basal ganglia and cerebellum with vertical gaze and pseudobulbar palsy, nuchal dystonia and dementia. Arch Neurol. 1964;10:333-359.
278 Rajput A, Robinson CA, Rajput AH. Essential tremor course and disability: a clinicopathologic study of 20 cases. Neurology. 2004;62:932-936.
279 Popescu A, Lippa C. Parkinsonian syndromes: Parkinson’s disease dementia, dementia with Lewy bodies and progressive supranuclear palsy. Clin Neurosci Res. 2004;3:461-468.
280 Garbutt S, Riley DE, Kumar AN, et al. Abnormalities of optokinetic nystagmus in progressive supranuclear palsy. J Neurol Neurosurg Psychiatry. 2004;75:1386-1394.
281 Ishino H, Higashi H, Kuroda S, et al. Motor nuclear involvement in progressive supranuclear palsy. J Neurol Sci. 1974;22:235-244.
282 Muller J, Wenning GK, Verny M, et al. Progression of dysarthria and dysphagia in postmortem-confirmed parkinsonian disorders. Arch Neurol. 2001;58:259-264.
283 Riley DE, Fogt N, Leigh RJ. The syndrome of “pure akinesia” and its relationship to progressive supranuclear palsy. Neurology. 1994;44:1025-1029.
284 Williams DR, Holton JL, Strand K, et al. Pure akinesia with gait freezing: a third clinical phenotype of progressive supranuclear palsy. Mov Disord. 2007;22:2235-2241.
285 Wenning GK, Litvan I, Jankovic J, et al. Natural history and survival of 14 patients with corticobasal degeneration confirmed at postmortem examination. J Neurol Neurosurg Psychiatry. 1998;64:184-189.
286 Fitzgerald DB, Drago V, Jeong Y, et al. Asymmetrical alien hands in corticobasal degeneration. Mov Disord. 2007;22:581-584.
287 Fisher CM. Alien hand phenomena: a review with the addition of six personal cases. Can J Neurol Sci. 2000;27:192-203.
288 Banks G, Short P, Martinez J, et al. The alien hand syndrome. Clinical and postmortem findings. Arch Neurol. 1989;46:456-459.
289 Leiguarda R, Starkstein S, Nogues M, et al. Paroxysmal alien hand syndrome. J Neurol Neurosurg Psychiatry. 1993;56:788-792.
290 Brazdil M, Kuba R, Rektor I. Rostral cingulate motor area and paroxysmal alien hand syndrome. J Neurol Neurosurg Psychiatry. 2006;77:992-993.
291 Green RC, Goldstein FC, Mirra SS, et al. Slowly progressive apraxia in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1995;59:312-315.
292 Graham NL, Bak TH, Hodges JR. Corticobasal degeneration as a cognitive disorder. Mov Disord. 2003;18:1224-1232.
293 Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. The Consensus Committee of the American Autonomic Society and the American Academy of Neurology. Neurology. 1996;46:1470.
294 Gilman S, Low PA, Quinn N, et al. Consensus statement on the diagnosis of multiple system atrophy. J Neurol Sci. 1999;163:94-98.
295 Albanese A, Colosimo C, Bentivoglio AR, et al. Multiple system atrophy presenting as parkinsonism: clinical features and diagnostic criteria. J Neurol Neurosurg Psychiatry. 1995;59:144-151.
296 Colosimo C, Albanese A, Hughes AJ, et al. Some specific clinical features differentiate multiple system atrophy (striatonigral variety) from Parkinson’s disease. Arch Neurol. 1995;52:294-298.
297 Anderson T, Luxon L, Quinn N, et al. Oculomotor function in multiple system atrophy: clinical and laboratory features in 30 patients. Mov Disord. 2008;23:977-984.
298 Geser F, Colosimo C, Wenning GK. Multiple system atrophy. In: Beal MF, Lang A, Ludolph AC, editors. Neurodegenerative Diseases: Neurobiology, Pathogenesis and Therapeutics. Cambridge: Cambridge University Press; 2005:623-662.
299 Paviour DC, Williams D, Fowler CJ, et al. Is sphincter electromyography a helpful investigation in the diagnosis of multiple system atrophy? A retrospective study with pathological diagnosis. Mov Disord. 2005;20:1425-1430.
300 Litvan I, Booth V, Wenning GK, et al. Retrospective application of a set of clinical diagnostic criteria for the diagnosis of multiple system atrophy. J Neural Transm. 1998;105:217-227.
301 Watanabe H, Saito Y, Terao S, et al. Progression and prognosis in multiple system atrophy: an analysis of 230 Japanese patients. Brain. 2002;125:1070-1083.
302 Schrag A, Good CD, Miszkiel K, et al. Differentiation of atypical parkinsonian syndromes with routine MRI. Neurology. 2000;54:697-702.
303 Almasy L, Bressman SB, Raymond D, et al. Idiopathic torsion dystonia linked to chromosome 8 in two Mennonite families. Ann Neurol. 1997;42:670-673.
304 Auburger G, Ratzlaff T, Lunkes A, et al. A gene for autosomal dominant paroxysmal choreoathetosis/spasticity (CSE) maps to the vicinity of a potassium channel gene cluster on chromosome 1p, probably within 2 cM between D1S443 and D1S197. Genomics. 1996;31:90-94.
305 Fink JK, Rainer S, Wilkowski J, et al. Paroxysmal dystonic choreoathetosis: tight linkage to chromosome 2q. Am J Hum Genet. 1996;59:140-145.
306 Fouad GT, Servidei S, Durcan S, et al. A gene for familial paroxysmal dyskinesia (FPD1) maps to chromosome 2q. Am J Hum Genet. 1996;59:135-139.
307 Grimes DA, Han F, Lang AE, et al. A novel locus for inherited myoclonus-dystonia on 18p11. Neurology. 2002;59:1183-1186.
308 Ichinose H, Ohye T, Takahashi E, et al. Hereditary progressive dystonia with marked diurnal fluctuation caused by mutations in the GTP cyclohydrolase I gene. Nat Genet. 1994;8:236-242.
309 Khan NL, Wood NW, Bhatia KP. Autosomal recessive, DYT2-like primary torsion dystonia: a new family. Neurology. 2003;61:1801-1803.
310 Klein C, Brin MF, Kramer P, et al. Association of a missense change in the D2 dopamine receptor with myoclonus dystonia. Proc Natl Acad Sci U S A. 1999;96:5173-5176.
311 Leube B, Rudnicki D, Ratzlaff T, et al. Idiopathic torsion dystonia: assignment of a gene to chromosome 18p in a German family with adult onset, autosomal dominant inheritance and purely focal distribution. Hum Mol Genet. 1996;5:1673-1677.
312 Nolte D, Niemann S, Muller U. Specific sequence changes in multiple transcript system DYT3 are associated with X-linked dystonia parkinsonism. Proc Natl Acad Sci U S A. 2003;100:10347-10352.
313 Segawa M, Hosaka A, Miyagawa F, et al. Hereditary progressive dystonia with marked diurnal fluctuation. Adv Neurol. 1976;14:215-233.
314 Valente EM, Bentivoglio AR, Cassetta E, et al. Identification of a novel primary torsion dystonia locus (DYT13) on chromosome 1p36 in an Italian family with cranial-cervical or upper limb onset. Neurol Sci. 2001;22:95-96.
315 Zimprich A, Grabowski M, Asmus F, et al. Mutations in the gene encoding epsilon-sarcoglycan cause myoclonus-dystonia syndrome. Nat Genet. 2001;29:66-69.
316 Nutt JG, Muenter MD, Melton LJ3rd, et al. Epidemiology of dystonia in Rochester, Minnesota. Adv Neurol. 1988;50:361-365.
317 Bressman SB, de Leon D, Brin MF, et al. Idiopathic dystonia among Ashkenazi Jews: evidence for autosomal dominant inheritance. Ann Neurol. 1989;26:612-620.
318 Lee LV, Maranon E, Demaisip C, et al. The natural history of sex-linked recessive dystonia parkinsonism of Panay, Philippines (XDP). Parkinsonism Relat Disord. 2002;9:29-38.
319 Kramer PL, Mineta M, Klein C, et al. Rapid-onset dystonia-parkinsonism: linkage to chromosome 19q13. Ann Neurol. 1999;46:176-182.
320 Segawa M. Hereditary progressive dystonia with marked diurnal fluctuation. Brain Dev. 2000;22:S65-S80.
321 Vidailhet M, Tassin J, Durif F, et al. A major locus for several phenotypes of myoclonus-dystonia on chromosome 7q. Neurology. 2001;56:1213-1216.
322 Tezenas du Montcel S, Clot F, Vidailhet M, et al. Epsilon sarcoglycan mutations and phenotype in French patients with myoclonic syndromes. J Med Genet. 2006;43:394-400.
323 Brusse E, Maat-Kievit JA, van Swieten JC. Diagnosis and management of early- and late-onset cerebellar ataxia. Clin Genet. 2007;71:12-24.
324 Browne DL, Gancher ST, Nutt JG, et al. Episodic ataxia/myokymia syndrome is associated with point mutations in the human potassium channel gene, KCNA1. Nat Genet. 1994;8:136-140.
325 Ophoff RA, Terwindt GM, Vergouwe MN, et al. Familial hemiplegic migraine and episodic ataxia type-2 are caused by mutations in the Ca2+ channel gene CACNL1A4. Cell. 1996;87:543-552.
326 Bradley JL, Blake JC, Chamberlain S, et al. Clinical, biochemical and molecular genetic correlations in Friedreich’s ataxia. Hum Mol Genet. 2000;9:275-282.
327 Hagerman PJ, Hagerman RJ. Fragile X–associated tremor/ataxia syndrome (FXTAS). Ment Retard Dev Disabil Res Rev. 2004;10:25-30.
328 Hewer R. The heart in Friedreich’s ataxia. Br Heart J. 1969;31:5-14.
328a Crawford TO, Skolasky RL, Fernandez R, et al. Survival probability in ataxia telangiectasia. Arch Dis Child. 2006;91:610-611.
328b Chun HH, Gatti RA. Ataxia-telangiectasia, an evolving phenotype. DNA Repair (Amst). 2004;3:1187-1196.
329 Biton S, Barzilai A, Shiloh Y. The neurological phenotype of ataxia-telangiectasia: solving a persistent puzzle. DNA Repair (Amst). 2008;7:1028-1038.
330 Hagerman PJ, Hagerman RJ. The fragile-X premutation: a maturing perspective. Am J Hum Genet. 2004;74:805-816.
331 Walshe JM, Yealland M. Wilson’s disease: the problem of delayed diagnosis. J Neurol Neurosurg Psychiatry. 1992;55:692-696.
332 Brewer GJ. Recognition, diagnosis, and management of Wilson’s disease. Proc Soc Exp Biol Med. 2000;223:39-46.
333 Strickland GT, Leu ML. Wilson’s disease. Clinical and laboratory manifestations in 40 patients. Medicine (Baltimore). 1975;54:113-137.
334 Walshe JM. Wilson’s disease. The presenting symptoms. Arch Dis Child. 1962;37:253-256.
335 Olivarez L, Caggana M, Pass KA, et al. Estimate of the frequency of Wilson’s disease in the US Caucasian population: a mutation analysis approach. Ann Hum Genet. 2001;65:459-463.
336 Reilly M, Daly L, Hutchinson M. An epidemiological study of Wilson’s disease in the Republic of Ireland. J Neurol Neurosurg Psychiatry. 1993;56:298-300.
337 Gibbs K, Walshe JM. A study of the caeruloplasmin concentrations found in 75 patients with Wilson’s disease, their kinships and various control groups. Q J Med. 1979;48:447-463.
338 Sinha S, Taly AB, Ravishankar S, et al. Wilson’s disease: cranial MRI observations and clinical correlation. Neuroradiology. 2006;48:613-621.
339 Miyajima H, Kohno S, Takahashi Y, et al. Estimation of the gene frequency of aceruloplasminemia in Japan. Neurology. 1999;53:617-619.
340 Miyajima H, Nishimura Y, Mizoguchi K, et al. Familial apoceruloplasmin deficiency associated with blepharospasm and retinal degeneration. Neurology. 1987;37:761-767.
341 Gregory A, Hayflick SJ. Neurodegeneration with brain iron accumulation. Folia Neuropathol. 2005;43:286-296.
342 Strecker K, Hesse S, Wegner F, et al. Eye of the tiger sign in multiple system atrophy. Eur J Neurol. 2007;14:e1-e2.
343 Molinuevo JL, Munoz E, Valldeoriola F, et al. The eye of the tiger sign in cortical-basal ganglionic degeneration. Mov Disord. 1999;14:169-171.