Chapter 33 Clinical Manifestations and Treatment of Marginal Zone Lymphomas (Extranodal/Malt, Splenic, And Nodal)
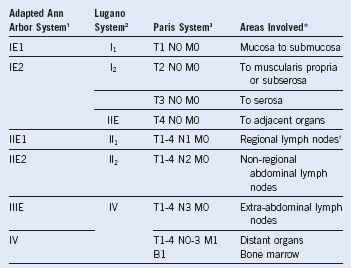
Table 33-2 ISCL/EORTC Staging System for Cutaneous Lymphomas Other Than Mycosis Fungoides and Sézary Syndrome4
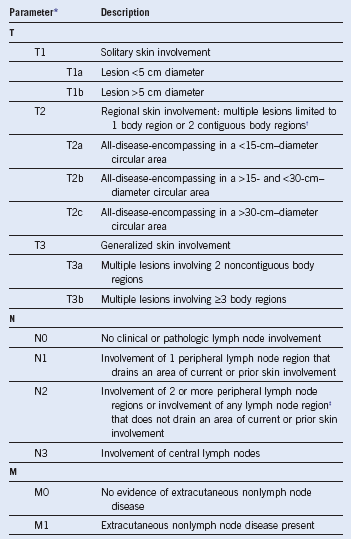
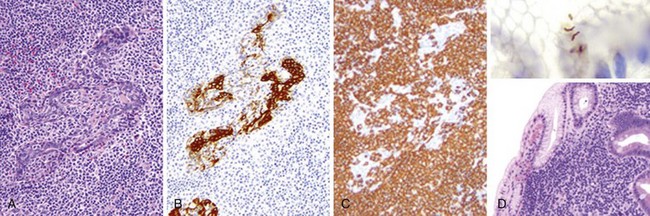
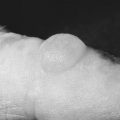
Figure 33-1 EXTRANODAL MARGINAL ZONE LYMPHOMAS OF MUCOSA-ASSOCIATED LYMPHOID TISSUE (MALT LYMPHOMAS).
Suggested Treatment Approach to Extranodal Marginal Zone Lymphoma
Suggested Treatment Approach to Splenic Marginal Zone Lymphoma
1 Radaszkiewicz T, Dragosics B, Bauer P. Gastrointestinal malignant lymphomas of the mucosa-associated lymphoid tissue: Factors relevant to prognosis. Gastroenterology. 1992;102:1628.
2 Rohatiner A, d’Amore F, Coiffier B, et al. Report on a workshop convened to discuss the pathological and staging classifications of gastrointestinal tract lymphoma. Ann Oncol. 1994;5:397.
3 Ruskone-Fourmestraux A, Dragosics B, Morgner A, et al. Paris staging system for primary gastrointestinal lymphomas. Gut. 2003;52:912.
4 Kim YH, Willemze R, Pimpinelli N, et al. TNM classification system for primary cutaneous lymphomas other than mycosis fungoides and Sezary syndrome: A proposal of the International Society for Cutaneous Lymphomas (ISCL) and the Cutaneous Lymphoma Task Force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 2007;110:479.
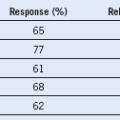
5 Ruskone-Fourmestraux A, Lavergne A, Aegerter PH, et al. Predictive factors for regression of gastric MALT lymphoma after anti-Helicobacter pylori treatment. Gut. 2001;48:297.
6 Steinbach G, Ford R, Glober G, et al. Antibiotic treatment of gastric lymphoma of mucosa-associated lymphoid tissue. An uncontrolled trial. Ann Intern Med. 1999;131:88.
7 Akamatsu T, Mochizuki T, Okiyama Y, et al. Comparison of localized gastric mucosa-associated lymphoid tissue (MALT) lymphoma with and without Helicobacter pylori infection. Helicobacter. 2006;11:86.
8 Chen LT, Lin JT, Tai JJ, et al. Long-term results of anti-Helicobacter pylori therapy in early-stage gastric high-grade transformed MALT lymphoma. J Natl Cancer Inst. 2005;97:1345.
9 Fischbach W, Goebeler-Kolve ME, Dragosics B, et al. Long term outcome of patients with gastric marginal zone B cell lymphoma of mucosa associated lymphoid tissue (MALT) following exclusive Helicobacter pylori eradication therapy: Experience from a large prospective series. Gut. 2004;53:34.
10 Lecuit M, Abachin E, Martin A, et al. Immunoproliferative small intestinal disease associated with Campylobacter jejuni. N Engl J Med. 2004;350:239.
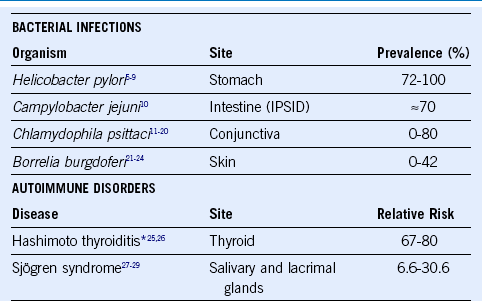
11 Chanudet E, Zhou Y, Bacon CM, et al. Chlamydia psittaci is variably associated with ocular adnexal MALT lymphoma in different geographical regions. J Pathol. 2006;209:344.
12 Daibata M, Nemoto Y, Togitani K, et al. Absence of Chlamydia psittaciin ocular adnexal lymphoma from Japanese patients. Br J Haematol. 2006;132:651.
13 deCremoux P, Subtil A, Ferreri AJ, et al. Re: Evidence for an association between Chlamydia psittaci and ocular adnexal lymphomas. J Natl Cancer Inst. 2006;98:365.
14 Ferreri AJ, Guidoboni M, Ponzoni M, et al. Evidence for an association between Chlamydia psittaci and ocular adnexal lymphomas. J Natl Cancer Inst. 2004;96:586.
15 Ferreri AJ, Ponzoni M, Guidoboni M, et al. Bacteria-eradicating therapy with doxycycline in ocular adnexal MALT lymphoma: A multicenter prospective trial. J Natl Cancer Inst. 2006;98:1375.
16 Gracia E, Froesch P, Mazzucchelli L, et al. Low prevalence of Chlamydia psittaci in ocular adnexal lymphomas from Cuban patients. Leuk Lymphoma. 2007;48:104.
17 Liu YC, Ohyashiki JH, Ito Y, et al. Chlamydia psittaci in ocular adnexal lymphoma: Japanese experience. Leuk Res. 2006;30:1587.
18 Mulder MM, Heddema ER, Pannekoek Y, et al. No evidence for an association of ocular adnexal lymphoma with Chlamydia psittaci in a cohort of patients from the Netherlands. Leuk Res. 2006;30:1305.
19 Rosado MF, Byrne GE, Jr., Ding F, et al. Ocular adnexal lymphoma: A clinicopathologic study of a large cohort of patients with no evidence for an association with Chlamydia psittaci. Blood. 2006;107:467.
20 Vargas RL, Fallone E, Felgar RE, et al. Is there an association between ocular adnexal lymphoma and infection with Chlamydia psittaci? The University of Rochester experience. Leuk Res. 2006;30:547.
21 Li C, Inagaki H, Kuo TT, et al. Primary cutaneous marginal zone B-cell lymphoma: A molecular and clinicopathologic study of 24 asian cases. Am J Surg Pathol. 2003;27:1061.
22 Wood GS, Kamath NV, Guitart J, et al. Absence of Borrelia burgdorferi DNA in cutaneous B-cell lymphomas from the United States. J Cutan Pathol. 2001;28:502.
23 Goodlad JR, Davidson MM, Hollowood K, et al. Primary cutaneous B-cell lymphoma and Borrelia burgdorferi infection in patients from the Highlands of Scotland. Am J Surg Pathol. 2000;24:1279.
24 Cerroni L, Zochling N, Putz B, et al. Infection by Borrelia burgdorferi and cutaneous B-cell lymphoma. J Cutan Pathol. 1997;24:457.
25 Holm LE, Blomgren H, Lowhagen T. Cancer risks in patients with chronic lymphocytic thyroiditis. N Engl J Med. 1985;312:601.
26 Kato I, Tajima K, Suchi T, et al. Chronic thyroiditis as a risk factor of B-cell lymphoma in the thyroid gland. Jpn J Cancer Res. 1985;76:1085.
27 Anderson LA, Gadalla S, Morton LM, et al. Population-based study of autoimmune conditions and the risk of specific lymphoid malignancies. Int J Cancer. 2009;125:398.
28 Smedby KE, Hjalgrim H, Askling J, et al. Autoimmune and chronic inflammatory disorders and risk of non-Hodgkin lymphoma by subtype. J Natl Cancer Inst. 2006;98:51.
29 Smedby KE, Vajdic CM, Falster M, et al. Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: A pooled analysis within the InterLymph Consortium. Blood. 2008;111:4029.