3 Clinical Features of Brain Tumors
Pathophysiology of Symptoms and Signs
Symptoms and signs are caused by a variety of mechanisms, many of which may be active in a given patient. It is the combination of these mechanisms that produce the clinical syndromes observed in patients.1,2
HERNIATION SYNDROMES
There are five common herniation syndromes.
Uncal herniation
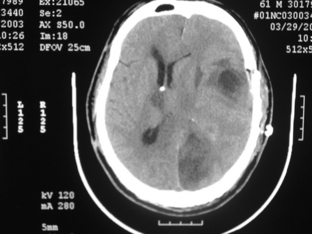
occurs when a mass lesion causes the uncus of the temporal lobe to herniate through the tentorium cerebelli. The key clinical sign of uncal herniation is ipsilateral oculomotor nerve palsy with a fixed and dilated pupil due to compression by the medial temporal lobe. Typically, a contralateral hemiparesis is also seen, but a false localizing sign of uncal herniation is an ipsilateral hemiparesis due to displacement of the brainstem to the opposite side causing compression of the contralateral cerebral peduncle against the tentorium (Kernohan notch). Additionally, unilateral or bilateral posterior cerebral artery occlusion can occur at the tentorial notch, leading to a homonymous hemianopsia or cortical blindness (Fig. 3-1).
Upward brainstem herniation
is seen in patients with posterior fossa tumors, where the superior surface of the vermis and midbrain are pushed upward, compressing the dorsal mesencephalon and cerebral aqueduct. Dorsal midbrain compression leads to impairment of vertical eye movements and a reduced level of consciousness.2,3
Factors Determining Symptoms and Signs
There are several biological factors intrinsic to the tumor itself that affect the clinical manifestations of the disease (Table 3-1). The general location of the tumor, supratentorial vs. infratentorial, or cortical vs. subcortical, will affect the presentation. If the tumor is extraaxial, it will compress the underlying brain tissue instead of destroying it as a parenchymal tumor might. The rapidity of growth will determine how soon a tumor will manifest; slow-growing tumors are insidious. Discrete tumors usually present with focal localizing symptoms as compared to diffusely infiltrative ones. The size of the tumor can affect symptoms in that even large tumors in relatively silent areas of the brain may be asymptomatic, whereas small tumors in other areas, for instance the brainstem, will present early. Some tumors can cause non-neurological symptoms, such as pituitary tumors via secretions.
TABLE 3-1 Factors Determining the Signs and Symptoms of Brain Tumors
Neurologic Symptoms and Signs
Regardless of tumor location, patients can have generalized and/or lateralizing symptoms. Generalized symptoms are non-localizing and can include headache, seizures, nausea, vomiting, dizziness, mental status changes, and visual obscurations. Focal symptoms reflect the intracranial location of the tumor and include seizures, hemiparesis, diplopia, aphasia, vertigo, incoordination, sensory abnormalities, and dysphagia (Table 3-2). Finally, there are false localizing signs, which are caused by raised intracranial pressure and which may include diplopia, tinnitus, and hearing or visual loss. These signs may suggest focal neurologic dysfunction, but actually reflect a generalized increase in intracranial pressure.
TABLE 3-2 Focal Signs and Symptoms of Brain Tumors (adapted from DeAngelis50)
GENERALIZED SYMPTOMS AND SIGNS
Headache
Headache is a very common symptom leading to neurologic referral, and when associated with a normal neurologic examination, is rarely due to a brain tumor. Intracranial neoplasms account for a small percentage (less than 10%) of patients with headache, but headache occurs in about 50% of patients with brain tumors,4,5 and in some studies the prevalence has been shown to be as high as 60% to 70%.6,7 However, most of these patients have accompanying neurologic signs. Headache is the sole presenting complaint in only 8% of patients with brain tumors.6,8 A new-onset headache of sufficient severity that the patient seeks medical assistance, in an adult without a prior history of headache, requires a full investigation including neuroimaging.
Headache related to brain tumor is a result of raised intracranial pressure causing stimulation of pain-sensitive structures in the cranial vault (Table 3-3).9,10 The pain-sensitive intracranial structures include the pachymeninges and all vascular structures in the brain; the brain parenchyma itself is insensitive to pain. Headaches occur due to involvement of the meninges and vessels by direct tumor invasion, inflammation or edema resulting from the tumor, or traction or pressure on the pain-sensitive structures by the tumor. Headache in brain tumor patients can be nonspecific, or may resemble tension-type headache, migraine, or a “classic tumor headache,” which occurs in the morning and improves spontaneously over the course of the day. Tumor headache may get progressively severe over time; this is often related to an increase in the surrounding edema rather than the actual size of the tumor.4,5
TABLE 3-3 Factors Contributing to Headache in Patients with Brain Tumor
The most common headache in brain tumor patients is similar to tension-type headache, and is characterized as a dull ache or pressure-like pain.4,6 These headaches are usually bifrontal and are often worse on the side ipsilateral to the tumor. Nausea or vomiting may be associated, and usually indicate generalized elevation of intracranial pressure. However, headaches that mimic classical migraine can also occur in patients with brain tumors. They are seen predominantly in patients with a history of migraine who then develop a brain tumor. These headaches may be associated with nausea and vomiting which, in these cases, do not necessarily indicate raised intracranial pressure.4 In some patients, a brain tumor can present as the worst headache of their life; this may be associated with intratumoral hemorrhage which is accompanied by neck stiffness, vomiting, mental status changes, and focal signs such as hemiparesis. More often, however, these severe headaches are not due to a bleed. In other cases, compression of the ophthalmic division of the trigeminal nerve or involvement of the nerve by tumor results in the typical pain of trigeminal neuralgia. Also, Gasserian ganglion involvement may cause neuralgia-type pain in the V2-3 distribution. Rarely, occipital neuralgia has been reported.11,12 Some authors have demonstrated that there is a tendency for pulsatile pain with meningioma.6 This may be related to the increased vasculature of meningiomas and innervation of the blood vessels by the trigeminal nerve.
The most common headache site in brain tumor patients is frontal, and is usually bilateral.4 This is seen commonly with supratentorial tumors or with diffuse elevation of intracranial pressure. Occipital headaches, with or without neck pain, are seen in infratentorial tumors or when there is base of skull involvement by tumor, but tumors in these locations may also give rise to frontal pain. Thus, headache has poor localizing value. However, lateralization of the tumor can be reliable when the patient complains of headache on one side of the head or the other.
Headache caused by raised intracranial pressure is distinctive in its severity, its association with nausea and vomiting, and its resistance to common analgesics.4 There may be associated papilledema, which is usually seen in children or young adults with raised intracranial pressure. Paroxysmal headaches can be seen in association with tumors of the third ventricle or pedunculated tumors intermittently obstructing CSF flow; occasionally, they are precipitated by changes in position. Rarely, Brunn syndrome can develop, which is characterized by attacks of sudden and severe headache, vomiting, and vertigo, triggered by head movement.13 Episodic headaches may also be indicative of plateau waves. These occur in patients with elevation of baseline intracranial pressure (which may be asymptomatic) and episodic symptoms, including headaches, that are triggered by a change in body position. The act of assuming the upright posture triggers a marked increase in intracranial pressure due to loss of autoregulation. The brisk rise in intracranial pressure usually resolves on its own; however, it causes transient symptoms while the pressure is elevated, typically to a level exceeding systolic blood pressure. Generally, these symptoms last five to ten minutes and resolve spontaneously. In addition to headache, common symptoms that occur with plateau waves include visual loss or obscurations, lightheadedness, loss of tone in the legs and even loss of consciousness. These symptoms are often confused with seizures, but a careful history makes the diagnosis clear. Exacerbation of headache may also occur with changes in intracranial pressure, such as with coughing, sneezing, performing Valsalva maneuver, exertion, or sexual activity; however, these exacerbations are uncommon in brain tumor patients.14
Headaches occur more commonly with infratentorial than supratentorial tumors.5,7,8 Dural-based tumors may present with headache. Subdural hematoma from dural metastases can also give rise to headache. Often, patients with supratentorial tumors without raised intracranial pressure do not have headaches despite large tumor size, and if they do have headaches, the pain is likely to be intermittent and less severe than with infratentorial tumors.4 Young patients are also more likely to suffer from headaches than older patients, possibly because age-related atrophy allows better compensation for the growing mass.
Patients with a preexisting history of headache are predisposed to develop secondary headaches in the presence of brain tumors.4,6 They are likely to suffer from headaches even when controlled for raised intracranial pressure. A change in quality, severity, and location of a pre-existing headache should be considered ominous and always warrants an evaluation.
Nausea and Vomiting
Nausea and vomiting occur when the chemotactic trigger zone in the area postrema, located on the floor of the fourth ventricle, is stimulated. Vomiting usually indicates raised intracranial pressure, but it can also occur because of direct compression of the vomiting center by posterior fossa tumors such as medulloblastoma or ependymomas of the fourth ventricle. It can also occur in the absence of elevated intracranial pressure in brainstem tumors involving the nucleus solitarius. Acute headache followed by an episode of vomiting suggests increased intracranial pressure. Projectile vomiting, usually seen in posterior fossa tumors in children, is uncommon in adults. Ictus emeticus or vomiting as an ictal phenomenon can, rarely, be a manifestation of epilepsy in tumors involving the insula and mesialtemporal lobe. It has been reported with temporal lobe astrocytoma15 and mesial temporal glioma.16
Mental Status Changes
Mental and cognitive abnormalities may be specific or nonspecific.
Nonspecific mental changes are among the most common symptoms and are often subtle, particularly early in the disease course. Irritability, change in personality, emotional lability, forgetfulness, lack of enthusiasm or spontaneity, and slowed response, progressing gradually to apathy and lethargy are seen in about 16% to 34% of patients.17 These symptoms do not reflect tumor in a specific area of the brain, but, when present, are suggestive of tumors in deep structures affecting thalamocortical fibers, corpus callosum, reticular formation, and occasionally, the frontal lobes. Variations in behavior can be seen according to which area of the frontal lobe is involved; for example, apathy and abulia are seen in tumors involving the dorsolateral frontal lobe, whereas orbitofrontal tumors lead to disinhibition and impulsive behavior. Sometimes withdrawal and apathy are confused with depression, even when the patient denies feeling sad or depressed. Aggressive and impulsive behavior can be seen with lesions involving the amygdala. Occasionally, temporal lobe seizures present with behavioral disturbances. Malamud et al. have described outbursts of rage in patients with temporal lobe gliomas. Peduncular hallucinosis can occur with midbrain tumors, and has also been described in posterior fossa tumors compressing the brainstem.18,19 Behavior and personality changes predominate as the presenting complaints, particularly in primary CNS lymphoma. This is due to its predilection for the frontal lobes and a 40% incidence of multiple lesions at diagnosis. Raised intracranial pressure can present with confusion, disorientation, lethargy, and coma.
Memory Problems
Tumors of the hippocampal formation of the medial temporal lobe and diencephalon can cause amnestic syndromes. An anterograde amnesia syndrome has been reported with tumors of the corpus callosum, particularly when the splenium is involved.20,21 Korsakoff syndrome has been reported in primary CNS lymphoma involving the third ventricle.22 Astrocytomas involving both temporal lobes can cause memory deficits.23 Rarely, gliomas have been found in patients who present with transient global amnesia.24
Seizures
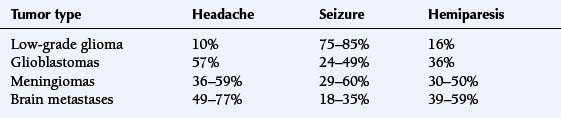
Many patients with brain tumors develop seizures, either as the presenting symptom or later in the course of their disease, usually due to tumor progression. The incidence varies depending on the patient’s age and the type of tumor. Epilepsy occurs more commonly in primary tumors (30% to 90%) than metastases (35%). Among the primary tumors, slow-growing, low-grade tumors cause seizures more often (85% of patients) than high-grade neoplasms, such as glioblastoma (49%).25–27 About 38% of patients with meningiomas develop seizures,28 typically with convexity lesions; skull base meningiomas rarely cause seizures.
The clinical manifestations of partial seizures depend on the location of the tumor; simple or complex partial seizures are more common in parietal, temporal, or frontal lobe lesions.29 Aphasia in the absence of any other focal signs (such as hemiparesis or facial palsy) should alert the clinician to consider seizures; an EEG may be confirmatory. Mood or behavioral changes and hallucinations may be suggestive of seizures, particularly from temporal foci; in the undiagnosed patient, these may be confused with psychiatric disorders. Nonconvulsive status epilepticus should be considered in a patient presenting with lethargy or fluctuating mental status changes. Epilepsia partialis continua of Kozhevnikov, or focal motor status epilepticus, can be problematic, and is often refractory to a single and sometimes even to multiple antiepileptic agents. Laughter as an ictal event, known as gelastic seizures, has been seen with hypothalamic hamartomas30 and has also been reported in a case of a low-grade hypothalamic astrocytoma.31
LATERALIZING SYMPTOMS AND SIGNS
Visual Problems
Lesions of the chiasm lead to bitemporal hemianopsia; the most common causes are pituitary adenoma, craniopharyngioma, meningioma, hypothalamic glioma, ectopic pinealoma or dysgerminoma, and metastases. Homonymous hemianopsia is the result of a tumor in the contralateral occipital cortex or optic tract. Tumors in the parietal lobe and adjacent temporal lobe can give rise to visual neglect on the contra-lateral side. Compression of a posterior cerebral artery directly by tumor or from herniation can result in cortical blindness. When a patient is unaware of his blindness, the condition is known as Anton syndrome. Other causes of altitudinal field defect include compression of the optic nerve by frontal tumor32 and increased pressure from a posterior fossa tumor compressing the chiasm by upward herniation.33
Prosopagnosia, or the inability to recognize faces, occurs in lesions of the right or bilateral occipitotemporal cortex. It has been documented in a case of lepto-meningeal involvement of the brain.34 Posterior parietal lesions give rise to simultanagnosia which is the inability to perceive different parts of a visual scene as a whole. Balint syndrome, a triad of optic ataxia, ocular apraxia, and simultanagnosia can occasionally be seen.
Aphasia
Language deficit or aphasia results from tumor invasion of the language areas located in the dominant hemisphere, including subcortical structures such as the basal ganglia and thalamus. Aphasia due to large brain tumors is usually associated with other focal signs such as a hemiparesis; however, a seizure can present as an isolated transient aphasia that may be mistaken for a transient ischemic attack in the undiagnosed patient. The language disturbance of many patients with brain tumors may not fit into the classical categories of aphasia as outlined in Table 3-4. This is due to the highly infiltrative nature of tumors such as gliomas and lymphomas, where there can be extensive involvement throughout the language pathways. Furthermore, tumors can occur anywhere and their growth is not defined by vascular territories. This is important to remember, as most aphasia syndromes were classified in patients with stroke. Therefore, while patients may have features suggestive of a typical Wernicke or Broca aphasia, many have a mixed picture that defies simple classification.
| Type of aphasia | Characteristics | Localization |
|---|---|---|
| Broca | Nonfluent, normal comprehension, impaired repetition | Inferior frontal gyrus |
| Wernicke | Fluent, impaired comprehension & repetition | Superior temporal gyrus |
| Global | Nonfluent, impaired comprehension & repetition | Perisylvian (frontal and temporal) |
| Conduction | Fluent, normal comprehension, impaired repetition | Insula |
| Transcortical motor | Nonfluent, normal comprehension and repetition | Anterior to Broca’s area |
| Transcortical sensory | Fluent, impaired comprehension, preserved repetition | Around Wernicke’s area |
Ataxia
Gait abnormalities can present as a hemiparetic gait, cerebellar ataxia, or frontal ataxia. A tumor in a hemisphere will cause circumduction of the contralateral leg which may be evident only on examination of gait. Tumors in the cerebellar hemisphere give rise to ipsilateral limb ataxia; the anterior portion of the anterior lobe affects the legs, whereas the posterior portion of the anterior lobe affects the arms. The patient may veer to one side; this helps localize the lesion to the ipsilateral hemisphere. Truncal ataxia results from tumors in the cerebellar vermis. Frontal lobe tumors and hydrocephalus can cause gait disturbances or apraxia.35 Lesions in the vestibular system can lead to vertigo and gait imbalance.
Primary Intra-axial Tumors
Astrocytomas
are graded by the World Health Organization (WHO) classification scheme. In order of increasing malignancy, they are classified as pilocytic (WHO grade I), fibrillary (WHO grade II), anaplastic astrocytomas (WHO grade III) and glioblastoma multiforme (WHO grade IV). Low grade tumors usually present with seizures. High-grade tumors are more likely to present with focal symptoms and deficits (Table 3-5). Patients with grade III astrocytomas and glioblastomas present with headaches (53% to 57%), seizures (57% of patients with grade III, 24% of patients with GBM), hemiparesis (25% to 36%), aphasia (23% to 36%), and visual problems (21%).17 Memory loss and cognitive changes are more frequent in GBM (39%), as compared to grade III tumors (23%).17 Low-grade tumors almost always present with a seizure (75% to 85%). Typically, this occurs in a young adult, and the neurologic examination is otherwise normal, even if the lesion is found to be quite large on neuroimaging. Preservation of neurologic function can be attributed to the infiltrative nature of these lesions and the lack of tissue destruction. Other presenting symptoms for pilocytic astrocytomas are headaches (27%) and visual complaints (12%).36
Oligodendrogliomas
are slower growing than astrocytomas. They are found only as either grade II or grade III lesions. The low-grade tumors typically present with seizures alone, and these patients can go for over a decade before other neurologic symptoms develop. In 72% of patients, a seizure is the initial presenting complaint37,38; seizures occur in 90% of patients at some point during the disease course.38 Headaches are the initial symptom in 10% of patients, whereas hemiparesis and aphasia occur only in 3% of patients at diagnosis.38 In patients who have oligodendrogliomas with a combined loss of 1p and 19q, 93% present with low-grade tumors and seizures of long standing duration.39 The high-grade oligodendrogliomas present similarly to the high-grade astrocytomas.
Brainstem gliomas
present with cranial nerve palsies (91%),40 followed by the subsequent development of long-tract signs. Headaches with nausea are seen in 57% of patients.40 Brainstem gliomas may be associated with neurofibromatosis type 1.
Medulloblastomas
are seen usually in children and young adults. Obstructive hydrocephalus is common at presentation. Clinically, patients of all ages present with headache, nausea, and vomiting. In adults, papilledema is seen in about 75% of patients, cerebellar symptoms in 45%, and nystagmus in 50%.41
Primary CNS lymphomas
often present with behavioral and cognitive changes (43%). Headaches, nausea, and vomiting suggestive of raised intracranial pressure (33%) and focal deficits (70%) are seen, although seizures are less common (14%), as these tumors typically involve the deep structures of brain.42 Ocular involvement leads to visual complaints.
Primary Extra-axial Tumors
Meningiomas
are usually benign and very slow-growing; they are often very large by the time they are symptomatic. They typically involve the cortical convexity or skull base. The symptoms depend on the location of the tumor, but headaches (36% to 59%), seizures (29% to 60%), and personality changes (22%) may develop due to compression of the underlying brain tissue (Table 3-5). Hemiparesis is more frequently seen with malignant meningiomas (50%) than benign (30%).5,27,43
Vestibular schwannomas
present with hearing loss (75%), tinnitus (18%), unsteadiness of gait (20%), vertigo (16%), and/or ipsilateral facial numbness or weakness.44 Bilateral vestibular schwannomas are pathognomonic for neurofibromatosis type 2.
Pituitary tumors
may be adenomas, carcinomas, or metastases. Adenomas are the most common and are usually benign. When the tumors are of microadenoma size (<1 cm), patients often present with hormone hypersecretion (e.g., ACTH, growth hormone, prolactin). Alternatively, macroadenomas (>1 cm) usually present with hormone hyposecretion and compression of neighboring structures, particularly the optic chiasm, causing visual field defects (68%) including bitemporal hemianopia (16%).45 Table 3-6 outlines endocrine disturbances associated with pituitary tumors.
TABLE 3-6 Endocrine Disturbances Associated with Pituitary Tumors
| Hormone | Endocrine abnormality |
|---|---|
| Hypersecretion | |
| Prolactin | Amenorrhea, galactorrhea (in women) |
| Impotence (in men) | |
| Growth hormone | Acromegaly |
| TSH | Hyperthyroidism |
| ACTH | Cushing syndrome, Nelson syndrome |
| FSH, LH | Hypogonadism, asymptomatic |
| Hyposecretion | |
| FSH, LH | Impotence, loss of libido, osteoporosis |
| Growth hormone | Central obesity, reduced muscle mass |
| Premature atherosclerosis | |
| Psychiatric manifestations | |
| TSH | Hypothyroidism |
| ACTH | Addison disease |
| Vasopressin | Polydipsia, polyuria, nocturia |
TSH: Thyroid stimulating hormone
ACTH: Adrenocorticotropic hormone
FSH: Follicle-stimulating hormone
LH: Luteinizing hormone
Brain Metastases
Cancer can spread to any part of the central nervous system. Often, patients with no known cancer present with symptoms from brain metastases.2 Certain cancers, such as lung, breast, and melanoma, have a predilection to metastasize to the nervous system. Autopsy studies have shown that 24% to 29% of patients dying from systemic cancer have intracranial metastases; 15% are intraparenchymal, 9% are dural, and 8% are leptomeningeal metastases.46,47
The site and nature of the CNS metastasis depends on the primary cancer; for example, lung cancer commonly leads to brain metastases, whereas prostate cancer is most likely to produce skull lesions and dural metastases. Lymphoma and leukemia usually cause leptomeningeal metastases.47
Brain metastases
The most common cancers associated with metastases to the brain are lung, breast, and melanoma.46,47 Common symptoms and signs are headache (49% to 77%), hemiparesis (39% to 59%), cognitive changes (22% to 58%), seizures (18% to 35%), aphasia (10% to 18%), and gait ataxia (19%) (Table 3-5).2,5,27,48
Dural Metastases
Intracranial dural metastases result from extension of skull lesions or hematogenous spread. Prostate and breast cancer are the most common offenders.46,47 Symptoms are usually based on the tumor site; however, other presentations of dural involvement include venous sinus invasion or compression leading to venous infarcts, hemorrhage and hydrocephalus, malignant subdural effusions, and ophthalmoplegia from cranial nerve involvement in the cavernous sinus. Common symptoms and signs are headaches (39%), cranial neuropathies (30%), visual problems (16%), mental status changes (16%), and seizures (11%).49
Leptomeningeal Metastases
Lymphoma, leukemia, breast and lung cancer, and melanoma frequently metastasize to the leptomeninges.46,47 Cranial nerve involvement leading to diplopia (20%), peripheral facial weakness (26%), sensorineural hearing loss (20%), and bulbar weakness (4%) are common manifestations. Metastases to the leptomeninges over the cerebral cortex can give rise to seizures (15%).2 Hydrocephalus and symptoms of raised intracranial pressure can result from leptomeningeal infiltration at the base of the brain or by infiltration of the arachnoid villi obstructing CSF flow and reabsorption.
1. L.M. DeAngelis, P.H. Gutin, S.A. Leibel, J.B. Posner. Intracranial Tumors: Diagnosis and Treatment. London: Martin Dunitz, 2002;65-96.
2. L.M. DeAngelis, J.B. Posner, editors. Neurologic Complications of Cancer. New York: Oxford University Press. 2008:141-281.
3. J.B. Posner, C.B. Saper, N.D. Schiff, F. Plum, editors. Plum and Posner’s Diagnosis of Stupor and Coma, 4th ed. New York: Oxford University Press. 2007:88-118.
4. P.A. Forsyth, J.B. Posner. Headaches in patients with brain tumors: a study of 111 patients. Neurology. 1993;43:1678-1683.
5. Z. Pfund, L. Szapary, O. Jaszberenyi, F. Nagy, J. Czopf. Headache in intracranial tumors. Cephalalgia. 1999;19:787-790.
6. C.J. Schankin, U. Ferrari, V.M. Reinisch, T. Birnbaum, R. Goldbrunner, A. Straube. Characteristics of brain tumour-associated headache. Cephalalgia. 2007;27:904-911.
7. N. Suwanwela, K. Phanthumchinda, S. Kaoropthum. Headache in brain tumor: a cross-sectional study. Headache. 1994;34:435-438.
8. A. Vazquez-Barquero, F.J. Ibanez, S. Herrera, J.M. Izquierdo, J. Berciano, J. Pascual. Isolated headache as the presenting clinical manifestation of intracranial tumors: a prospective study. Cephalalgia. 1994;14:270-272.
9. F.M. Cutrer. Pain-sensitive cranial structures:chemical anatomy. In: S.D. Silberstein, R.B. Lipton, D.J. Dalessio, editors. Wolff’s Headache and other head pain. New York: Oxford University Press; 2001:50-56.
10. B. Fricke, K.H. Andres, M. Von During. Nerve fibers innervating the cranial and spinal meninges: morphology of nerve fiber terminals and their structural integration. Microsc Res Tech. 2001;53:96-105.
11. I. Garza. Craniocervical junction schwannoma mimicking occipital neuralgia. Headache. 2007;47:1204-1205.
12. E.J. Piovesan, L.C. Werneck, H.A. Teive, F. Navarro, P.A. Kowacs. Neurophysiology of pain in tentorial irritation: description of a case secondary to medulloblastoma. Arq Neuropsiquiatr. 1998;56:677-682.
13. M. Krasnianski, T. Muller, K. Stock, S. Zierz. Bruns syndrome caused by intraventricular tumor. Eur J Med Res. 2007;12:582-584.
14. J. Pascual, F. Iglesias, A. Oterino, A. Vazquez-Barquero, J. Berciano. Cough, exertional, and sexual headaches: an analysis of 72 benign and symptomatic cases. Neurology. 1996;46:1520-1524.
15. C. Chen, D.J. Yen, C.H. Yiu, Y.H. Shih, H.Y. Yu, M.S. Su. Ictal vomiting in partial seizures of temporal lobe origin. Eur Neurol. 1999;42:235-239.
16. B. Schauble, J.W. Britton, B.P. Mullan, J. Watson, F.W. Sharbrough, W.R. Marsh. Ictal vomiting in association with left temporal lobe seizures in a left hemisphere language-dominant patient. Epilepsia. 2002;43:1432-1435.
17. S.M. Chang, I.F. Parney, W. Huang, et al. Patterns of care for adults with newly diagnosed malignant glioma. JAMA. 2005;293:557-564.
18. C. Leiva-Santana, P.T. Jerez-Garcia, M.A. del Real-Francia, R.M. Sanchez. Peduncular hallucinosis associated with a space occupying lesion of the brain stem. Rev Neurol. 1999;28:1174-1176.
19. S.S. Nadvi, J.R. van Dellen. Transient peduncular hallucinations secondary to brain stem compression by a medulloblastoma. Surg Neurol. 1994;41:250-252.
20. J. Bustamante, F. Lopera. Tumour of the corpus callosum: the association between interhemispheric disconnection and an anterograde amnesia syndrome. Rev Neurol. 2006;43:207-212.
21. P. Rudge, E.K. Warrington. Selective impairment of memory and visual perception in splenial tumours. Brain. 1991;114(Pt 1B):349-360.
22. C. Toth, C. Voll, R. Macaulay. Primary CNS lymphoma as a cause of Korsakoff syndrome. Surg Neurol. 2002;57:41-45.
23. J.S. Gillespie, J.J. Craig, C.S. McKinstry. Bilateral astrocytoma involving the limbic system precipitating disabling amnesia and seizures. Seizure. 2000;9:301-303.
24. J.R. Shuping, J.F. Toole, E. AlexanderJr. Transient global amnesia due to glioma in the dominant hemisphere. Neurology. 1980;30:88-90.
25. S.T. Herman. Epilepsy after brain insult: targeting epileptogenesis. Neurology. 2002;59:S21-S26.
26. K. Lote, A.E. Stenwig, K. Skullerud, H. Hirschberg. Prevalence and prognostic significance of epilepsy in patients with gliomas. Eur J Cancer. 1998;34:98-102.
27. M.S. van Breemen, E.B. Wilms, C.J. Vecht. Epilepsy in patients with brain tumours: epidemiology, mechanisms, and management. Lancet Neurol. 2007;6:421-430.
28. A. Liigant, S. Haldre, A. Oun, et al. Seizure disorders in patients with brain tumors. Eur Neurol. 2001;45:46-51.
29. L.M. Lynam, M.K. Lyons, J.F. Drazkowski, et al. Frequency of seizures in patients with newly diagnosed brain tumors: a retrospective review. Clin Neurol Neurosurg. 2007;109:634-638.
30. G.D. Cascino, F. Andermann, S.F. Berkovic, et al. Gelastic seizures and hypothalamic hamartomas: evaluation of patients undergoing chronic intracranial EEG monitoring and outcome of surgical treatment. Neurology. 1993;43:747-750.
31. G. Coppola, D. Spagnoli, N. Sciscio, F. Russo, R.M. Villani. Gelastic seizures and low-grade hypothalamic astrocytoma: a case report. Brain and Development. 2002;24:183-186.
32. R.S. Newsom, P. Simcock, H. Zambarakji. Cerebral metastasis presenting with altitudinal field defect. J Neuroophthalmol. 1999;19:10-11.
33. B. Fierro, M.G. Castiglione, G. Turrisi, G. Savettieri. Inferior altitudinal hemianopia associated with a tumor in the posterior fossa: report of a case. Ital J Neurol Sci. 1984;5:89-91.
34. M. Miura, N. Iijima, K. Hayashida, K. Kitazawa, K. Ishii, S. Ohara. [Case of leptomeningeal carcinomatosis effectively treated with intrathecal chemotherapy using ventriculoperitoneal shunt]. Rinsho Shinkeigaku. 2006;46:404-409.
35. J.B. Terry, R.N. Rosenberg. Frontal lobe ataxia. Surg Neurol. 1995;44:583-588.
36. P.A. Forsyth, E.G. Shaw, B.W. Scheithauer, J.R. O’Fallon, D.D. LaytonJr, J.A. Katzmann. Supratentorial pilocytic astrocytomas. A clinicopathologic, prognostic, and flow cytometric study of 51 patients. Cancer. 1993;72:1335-1342.
37. C. Lebrun, D. Fontaine, A. Ramaioli, et al. Long-term outcome of oligodendrogliomas. Neurology. 2004;62:1783-1787.
38. J.D. Olson, E. Riedel, L.M. DeAngelis. Long-term outcome of low-grade oligodendroglioma and mixed glioma. Neurology. 2000;54:1442-1448.
39. M.J. van den Bent, L.H. Looijenga, K. Langenberg, et al. Chromosomal anomalies in oligodendroglial tumors are correlated with clinical features. Cancer. 2003;97:1276-1284.
40. S. Nishio, M. Fukui, J. Tateishi. Brain stem gliomas: a clinicopathological analysis of 23 histologically proven cases. J Neurooncol. 1988;6:245-250.
41. A. Maleci, L. Cervoni, R. Delfini. Medulloblastoma in children and in adults: a comparative study. Acta Neurochir (Wien). 1992;119:62-67.
42. B. Bataille, V. Delwail, E. Menet, et al. Primary intracerebral malignant lymphoma: report of 248 cases. J Neurosurg. 2000;92:261-266.
43. M. Rohringer, G.R. Sutherland, D.F. Louw, A.A. Sima. Incidence and clinicopathological features of meningioma. J Neurosurg. 1989;71:665-672.
44. S.I. Rosenberg. Natural history of acoustic neuromas. Laryngoscope. 2000;110:497-508.
45. A.A. Halle, R.D. Drewry, J.T. Robertson. Ocular manifestations of pituitary adenomas. South Med J. 1983;76:732-735.
46. S. Hojo, A. Hirano. Pathology of Metastases Affecting the Central Nervous System. In: K. Takakura, K. Sano, S. Hojo, A. Hirano, editors. Metastatic tumors of the central nervous system. Tokyo-New York: Igaku-Shoin, 1982.
47. J.B. Posner, N.L. Chernik. Intracranial metastases from systemic cancer. Adv Neurol. 1978;19:579-592.
48. K. Takakura, K. Sano. Clinical features of intracranial metastatic tumors. In: K. Takakura, K. Sano, S. Hojo, A. Hirano, editors. Metastatic tumors of the central nervous system. Tokyo-New York: Igaku-Shoin; 1982:112-137.
49. L. Nayak, F.M. Iwamoto, L.E. Abrey. Intracranial Dural Metastases. Neurology. 2008;70(Suppl. 1):A160.
50. L.M. DeAngelis. Tumors of the central nervous system and intracranial hypertension and hypotension. In: L. Goldman, D. Ausiello, editors. Cecil Textbook of Medicine. 23rd ed. Philadelphia: Saunders; 2008:1437-1449.