Chapter 40 Clinical Approach to Infections in the Compromised Host
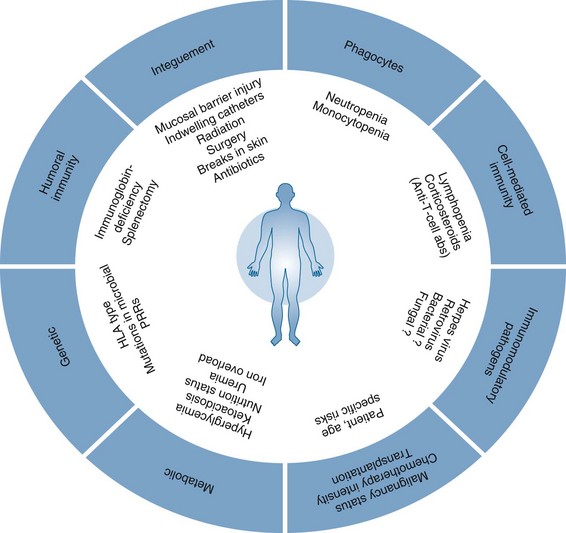
Figure 40-1 CONSTELLATION OF FACTORS CONTRIBUTING TO INCREASED RISK FOR INFECTION IN IMMUNOCOMPROMISED HOSTS.
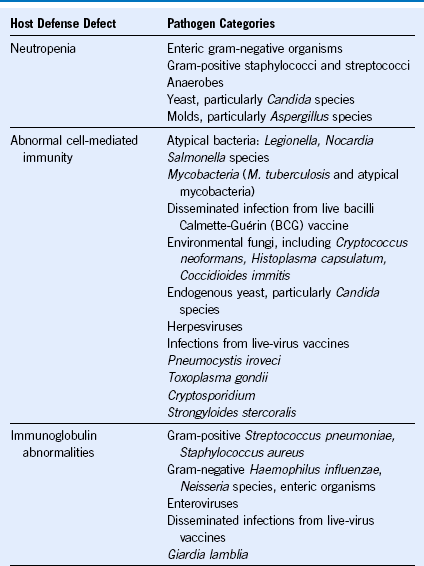
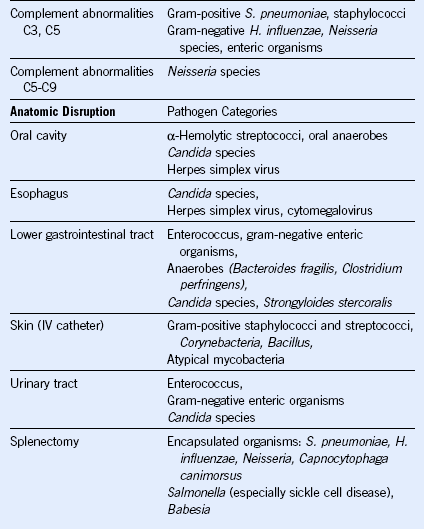
Table 40-1 Malignant and Select Nonmalignant Hematologic Diseases and Their Associated Infection-Predisposing Host Defects
| Hematologic Condition | Infection-Predisposing Host Defects |
|---|---|
| Acute myeloid leukemia | Neutropenia; therapies such as dose-intensive chemotherapy and hematopoietic stem cell transplant may result in additional anatomic disruptions, cell-mediated defects, and humoral defects |
| Acute lymphocytic leukemia | Neutropenia; therapy effects similar to acute myeloid leukemia |
| Hairy cell leukemia | Neutropenia (also monocytopenia); abnormal humoral immunity; T-cell suppressing therapy |
| Chronic lymphocytic leukemia | Hypogammaglobulinemia; abnormal cell-mediated immunity |
| Chronic myeloid leukemia | No prominent host defects unless aggressive therapy, advanced stage, or postsplenectomy |
| Multiple myeloma | Hypogammaglobulinemia; other host defects may occur with aggressive therapy or advanced stage |
| Hodgkin/non-Hodgkin lymphomas | Abnormal cell-mediated immunity, therapy-related neutropenia, splenic dysfunction (if splenectomy or radiation) |
| Myelodysplastic syndromes | Functional or absolute neutropenia |
| Aplastic anemia | Neutropenia; abnormal cell-mediated immunity from immunosuppressive therapies (e.g., steroids, antithymocyte globulin, cyclosporine, hematopoietic stem cell transplantation) |
| Paroxysmal nocturnal hemoglobinuria | Deficient Fc receptor may contribute to abnormal cell-mediated immunity |
| Hemolytic states (thalassemia) | Gallstones may serve as a nidus for infection; splenic dysfunction or splenectomy |
| Sickle cell disease | Can be neutropenic with aplastic crisis; bone infarcts may serve as a nidus for infection; splenic dysfunction with poor complement activation and opsonization from autosplenectomy |
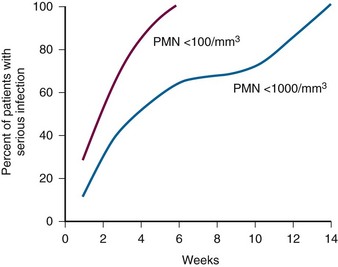
Figure 40-2 RELATIONSHIP OF SEVERITY AND DURATION OF NEUTROPENIA TO THE RISK FOR DEVELOPING A SERIOUS INFECTION.
(Courtesy GE Body.)
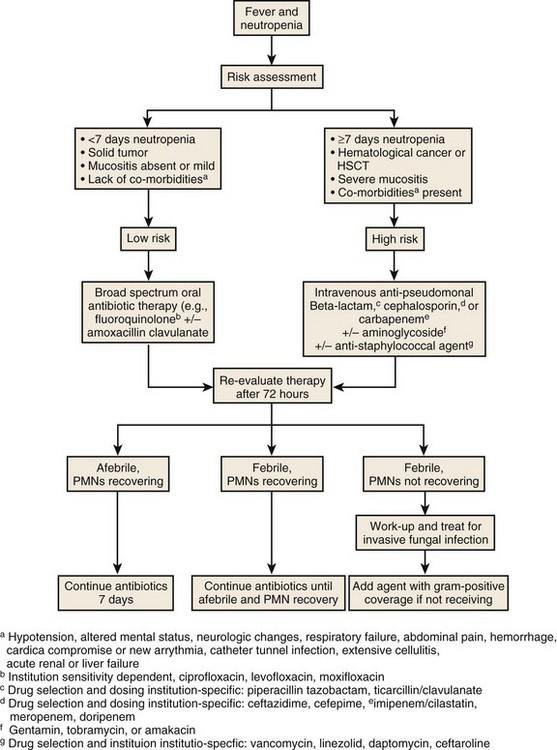
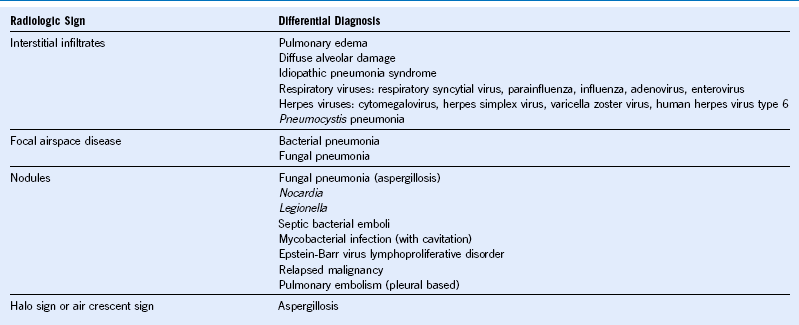
Approach to Pulmonary Infiltrates
• Cytology, with the attendant Fite and Gomori methenamine silver stains
• Bacterial (Gram) stain and aerobic culture
• Mycobacterial (acid-fast bacillus) stain and culture
• Fungal (potassium hydroxide or calcofluor) stain and culture
• Viral cultures for herpes viruses and respiratory viruses
• Rapid test for cytomegalovirus (shell vial or polymerase chain reaction [PCR])
• Rapid test for respiratory viruses during respiratory virus season (pooled respiratory virus shell vial, respiratory syncytial virus antigen, influenza A/B antigen)
Treatment of Drug-Resistant Gram-Negative Bacilli
• Pseudomonas aeruginosa is the paradigm for drug resistance among gram-negative bacilli and often rapidly develops pan-resistance to antimicrobials. Serious pseudomonal infections are generally treated with two antimicrobial agents, although convincing data supporting this approach are lacking.
• Extended-spectrum β-lactamase (ESBL) production among Enterobacteriacae such as Escherichia coli and Klebsiella pneumoniae renders these organisms resistant to non-carbapenem β-lactam antibiotics. Treatment is generally with a carbapenem (e.g., meropenem), although a quinolone can sometimes be used.
• The presence of a Klebsiella pneumoniae carbapenemase (KPC) can occur among species other than K. pneumoniae and creates resistance to all β-lactam antibiotics including carbapenems. Resistance to quinolones is also typical, so therapy is limited to the polymyxins (e.g., colistin), or to tigecycline and the aminoglycosides depending on the organism’s antibiotic susceptibility pattern.
• Like P. aeruginosa, A. baumannii can develop resistance to all known antimicrobials via a variety of mechanisms. This generally occurs among critically ill patients following prolonged stays in the intensive care unit. Treatment options may include polymyxins and sulbactam, a β-lactamase inhibitor that possesses some activity against drug-resistant Acinetobacter.
• Enterobacter, Serratia, and Citrobacter species can elaborate an AmpC β-lactamase following initiation of β-lactam therapy, which can result in a relapsing infection following an initial response. Thus clinicians treating such infections with penicillins or cephalosporins should closely monitor patients for the emergence of resistance. If resistance does emerge, AmpC-producing organisms can be treated with a carbapenem.
• Patients with multidrug-resistant gram-negative infections should be cared for in such a manner as to minimize spread of these dangerous organisms to other patients. Compliance with infection control protocols is paramount.
• Knowledge of the local epidemiology and antibiogram are important for preemptive and empiric antibiotic choices, especially against gram-negative rods
• A carefully designed and executed antibiotic stewardship program could curtail unnecessary antibiotic use, thereby decreasing antibiotic selection pressure with a resulting decrease in bacterial resistance rates.