Chapter 153 Circumscribed Choroidal Hemangioma
Introduction
Choroidal hemangiomas are benign vascular hamartomas which occur in two forms: circumscribed and diffuse. The circumscribed form is typically an isolated finding without systemic associations, while the diffuse form generally occurs in association with Sturge–Weber syndrome (discussed separately in Chapter 132, Phakomatoses). There have been rare reports of circumscribed choroidal hemangioma in patients with Sturge–Weber syndrome.1,2 The first histologically confirmed case of choroidal hemangioma was published by Leber in 1868.3 The incidence of the disease is difficult to estimate since most circumscribed choroidal hemangiomas only come to medical attention if patients become symptomatic or if they are discovered incidentally during routine examination; however, the disease is felt to be relatively rare, with five cases discovered upon histological examination of 4500 enucleated eyes.4 More than 90% of reported cases have been in Caucasian patients (though there have been published cases in black, Hispanic, and Asian patients), with a relatively even distribution between males and females.5,6 The mean age at diagnosis in the two largest case series ranged from 38.7 years to 47 years, considerably older than the mean age at diagnosis for diffuse choroidal hemangioma, which is typically in the first decade of life.5,6
Clinical features
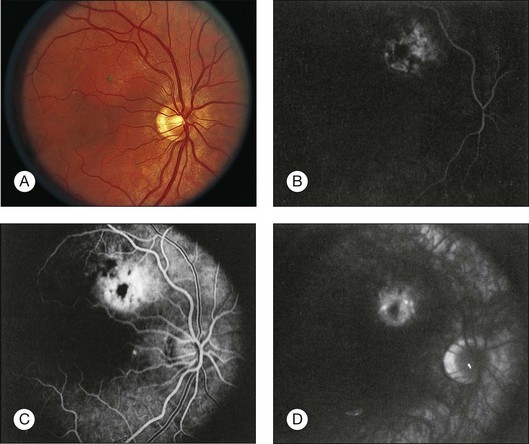
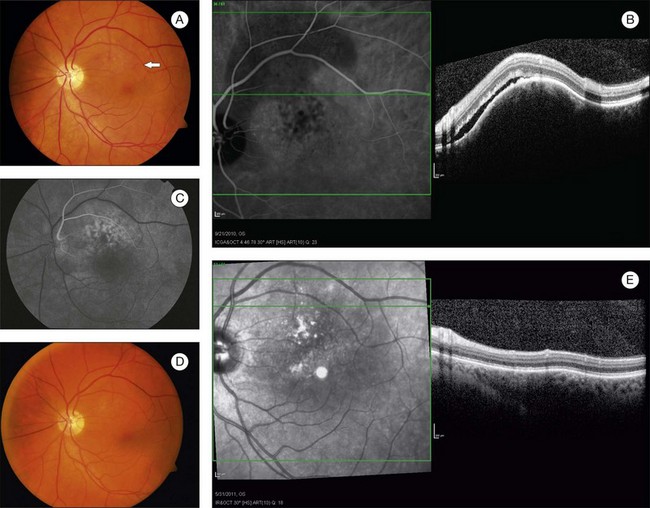
Circumscribed choroidal hemangiomas are generally orange-red elevated masses, which are found posterior to the equator (Figs 153.1–153.4). The color of the tumor has also been variably described as “salmon-colored”,7 “yellow-white”,8 and “grayish-pink”.6 Choroidal elevation may be difficult to discern on color photographs, and may be more apparent on clinical examination. Pigmentary changes have also been reported on the surface of the tumor or as a ring of pigmentation around the edge of the tumor.6 There may be accumulation of lipofuscin pigment (orange pigment) over the lesion, though this is often difficult to distinguish except with fluorescein angiography.9 In the largest published series of 200 patients by Shields et al., 67% of tumors were in the macula, 34% were between the macula and the equator, and no tumors were anterior to the equator.5 In the second-largest series of 45 patients with circumscribed choroidal hemangioma reported by Witschel and Font, all of the circumscribed tumors were located posterior to the equator.6 In the Shields series, the mean tumor diameter was 6.7 mm, and the mean tumor thickness was 3.1 mm.5 Lesions are usually solitary and unilateral, though bilateral choroidal hemangiomas have been reported.7 Overlying subretinal fluid or serous retinal detachment is a common finding, present in 47% of patients in the Witschel series,6 and 81% of the patients in the Shields series (Fig. 153.3).5 Cystoid macular edema is also a common finding, present in up to 17% of patients, and exudates, epiretinal membrane, and retinal hemorrhages have also been reported.5 Exudates are uncommon in melanocytic lesions. Choroidal neovascularization is rare but has been reported in four patients.5,10 Retinal neovascularization has also been reported in three eyes with circumscribed choroidal hemangioma.11 Patients with total retinal detachments can also develop neovascularization of the iris and angle and neovascular glaucoma.5
These lesions usually remain stationary in size, though there have been reports of gradual progressive enlargement.12,13 In five cases, there was only slight enlargement with a mean of 1.6 × 1.5 mm in basal diameter and 0.9 mm in thickness over an average of 52 months of follow-up.12 In another case with significant enlargement, the eye was enucleated due to concern for choroidal melanoma and pathologic examination revealed that the increase in size was most likely due to vascular congestion of the tumor vessels, and possibly an increase in the caliber and number of tumor vessels.13 There has also been one report of “blackening” of a circumscribed choroidal hemangioma from red-orange to dark gray after tantalum clip placement in preparation for external proton beam irradiation.14 The hemangioma subsequently returned to its original red-orange color 2 weeks after surgery (prior to proton beam irradiation), leading the authors to hypothesize that the transient color change may have been due to extravascular hemorrhage from surgical manipulation.14 Bosch and Helbig also reported blackening of a choroidal hemangioma after photodynamic therapy.15
The most commonly reported symptom is blurred vision in up to 81% of patients, though patients also note visual field deficits, metamorphopsia, and floaters.5 Presenting visual acuity ranged from 20/20–20/40 in 24% of eyes to 20/400 or worse in 34% of eyes in the Shields series, and 60% of eyes were “blind” in the Witschel series.5,6
Differential diagnosis
The differential diagnosis of circumscribed choroidal hemangioma includes choroidal nevus, amelanotic choroidal melanoma, choroidal metastasis, choroidal osteoma, and central serous chorioretinopathy. On clinical examination, choroidal hemangiomas have a characteristic orange-red color, unlike choroidal metastases which tend to be creamy-yellow and amelanotic melanomas which tend to be more yellow-tan.5 Ancillary testing is very helpful to distinguish these conditions. Ultrasonography shows high internal reflectivity due to the vascular component of the lesion, in contrast to choroidal melanomas which display low to medium internal reflectivity. Circumscribed choroidal hemangiomas also have a very distinct appearance on indocyanine green angiography with rapid filling and “washout” phenomenon, unlike choroidal melanoma and metastasis which have slower and less intense filling.5 On MRI, circumscribed choroidal hemangiomas show bright signal on both T1- and T2-weighted images, whereas choroidal melanomas and metastases show bright signal on T1-weighted images but low signal on T2-weighted images.5
Ancillary studies
Intravenous fluorescein angiography
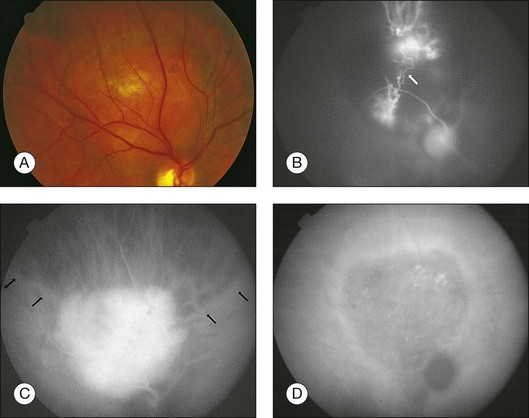
Fluorescein angiography (FA) of circumscribed choroidal hemangiomas generally reveals mild early lacy hyperfluorescence of the tumor in the pre-arterial and arterial phase, followed by moderate hyperfluorescence during the arteriovenous phase, and increasing hyperfluorescence through the late phase with variable amounts of late leakage (Figs 153.1, 153.3, 153.4).5,16,17 The FA pattern of choroidal hemangioma can be somewhat variable and similar to other amelanotic choroidal tumors, hence FA may not be diagnostic in the absence of other ancillary testing.18 FA can also be helpful for visualization of associated subretinal fluid and cystoid macular edema, which are not typically seen on indocyanine green angiography.18
Indocyanine green angiography
Indocyanine green angiography (ICGA) is particularly helpful in the diagnosis of circumscribed choroidal hemangioma since it provides better visualization of the choroidal vasculature. Circumscribed choroidal hemangiomas have a characteristic pattern of rapid onset of fluorescence around 30 s which occurs much earlier than in other choroidal tumors.18–20 The fluorescence occurs in a lacy diffuse hyperintense pattern which fills first peripherally then centrally.19 In the late frames, the tumor demonstrates loss of dye resulting in a hypofluorescent appearance compared with the surrounding choroid, which is known as the “wash out” phenomenon (Fig. 153.2).18–20 In addition, a late hyperfluorescent rim around the tumor is almost always present.18–20 Intrinsic tumor vessels are more likely to be seen with ICG than with FA, and the normal choroidal vasculature beneath the tumor is obscured, a feature which is also considered pathognomonic.18,20 Chronic lesions with significant retinal pigment epithelial changes may have blocked fluorescence on ICGA.
Ultrasonography
Circumscribed choroidal hemangiomas have a consistent characteristic appearance on ultrasonography. On B-scan ultrasonography, the hemangioma appears as an acoustically solid mass, which is almost always identical in character to the surrounding normal choroid (Fig. 153.4).5,17 The tumor is typically dome-shaped but can occasionally appear mushroom-shaped or plateau-shaped.5 On A-scan, the choroidal hemangioma demonstrates high internal reflectivity.5,17 Both of these features are useful to distinguish choroidal hemangioma from choroidal melanoma which is usually acoustically hollow with medium to low internal reflectivity.5
Neuroimaging
Magnetic resonance imaging (MRI) of choroidal hemangiomas typically shows hyperintensity in contrast to vitreous on T1-weighted images, and hyperintensity or isointensity to vitreous on T2-weighted images.5 Choroidal hemangiomas show enhancement with gadolinium contrast.5 The MRI findings are helpful for differentiation from choroidal melanoma and metastasis which show bright signal on T1-weighted images and low signal on T2-weighted images.5
Optical coherence tomography and enhanced depth imaging
In choroidal tumors, traditional time-domain and spectral-domain optical coherence tomography (SD-OCT) are most useful for visualizing secondary changes to the retina and retinal pigment epithelium (RPE).21 In choroidal hemangiomas, OCT can be used to demonstrate macular edema, epiretinal membranes, and subretinal fluid (Fig. 153.3). Until recently, wavelength-dependent light scattering and decreasing sensitivity and resolution with increased displacement from zero delay prevented detailed imaging of the choroid and sclera with OCT.22 Spaide and colleagues described a method of placing the SD-OCT closer to the patient to purposefully image deeper layers, producing a detailed inverted image of the choroid.22 Torres et al. have used this enhanced depth imaging (EDI) technique to look at a variety of choroidal tumors, including three circumscribed choroidal hemangiomas.23 Circumscribed choroidal hemangiomas in this series showed low to medium homogeneous reflectivity with intrinsic spaces.23
Autofluorescence
Fundus autofluorescence (AF) is an imaging technique which visualizes lipofuscin distribution in the RPE cell monolayer.24 AF is increased in dysfunctional RPE and decreased in areas of photoreceptor loss, and has been useful in age-related macular degeneration (AMD), Best disease, and various chorioretinal inflammatory disorders.24 A study of AF in 34 eyes with choroidal hemangiomas (27 of which were circumscribed) found that the intrinsic tumor AF of untreated circumscribed choroidal hemangiomas was either iso-autofluorescent (58%) or hypo-autofluorescent (42%), and treated circumscribed choroidal hemangiomas were all hypo-autofluorescent (100%).25 The extrinsic AF of the overlying retinal pigment epithelium and retinal alterations showed hyper-autofluorescence of orange pigment in two cases of untreated circumscribed choroidal hemangioma, and hypo-autofluorescence of RPE hyperplasia, atrophy, and fibrous metaplasia.25
Pathology
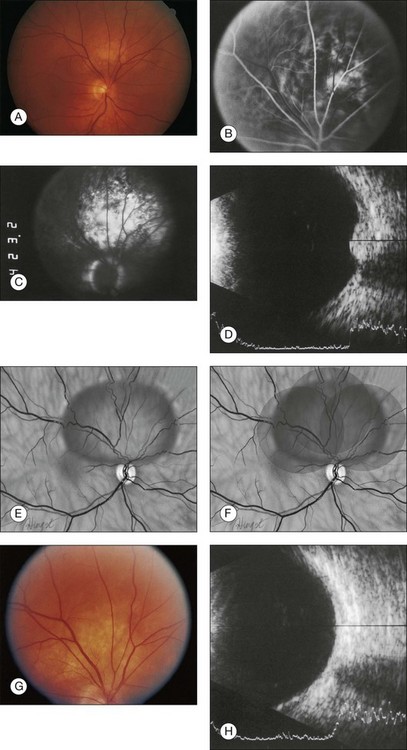
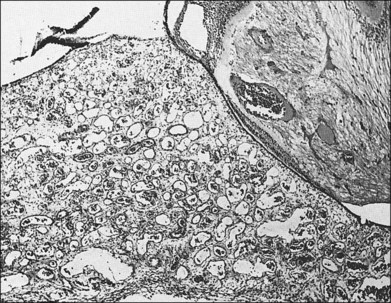
Choroidal hemangiomas are hamartomas, benign vascular tumors which are composed of tissue elements normally found in a given location. Hemangiomas of the choroid are classified histopathologically according to the prevailing type or types of vessels within the tumor: capillary, cavernous, or mixed.6 The capillary type is composed of small vessels separated by loose connective tissue (Fig. 153.5), whereas the cavernous type is composed of larger vessels separated by relatively little connective tissue (Fig. 153.6). The mixed type shows both capillary and cavernous features. Choroidal hemangiomas are notable for the lack of cellular proliferation of the elements of the vessel walls, supporting the idea that these tumors are nonproliferative lesions.6
Witschel and Font, using the files of the Armed Forces Institute of Pathology (AFIP), studied 45 eyes containing a circumscribed choroidal hemangioma.6 They found 20 tumors of the cavernous type, 22 tumors of the mixed type, and only three of the capillary type.6 This finding is in contrast to the diffuse type of choroidal hemangioma associated with Sturge–Weber syndrome, in which mixed type tumors were found in all 17 cases studied.6
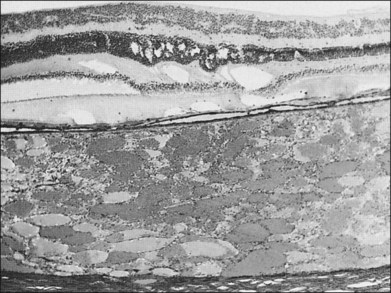
The circumscribed choroidal hemangioma pushes choroidal melanocytes toward the sclera and choriocapillaris, as well as toward the periphery of the tumor. Irregular pigmentation alterations in the overlying retinal pigment epithelium (RPE) are common and may include changes ranging from disorganization and proliferation of the RPE to the formation of fibrous plaques on the inner surface of the choroid (Fig. 153.7), which produces a grayish-white appearance in some of these tumors.6,26 Ossification overlying the tumor can occur and is a degenerative change due to transformation and proliferation of the retinal pigment epithelial cells overlying the choroidal hemangioma (rather than within the tumor) and is probably correlated with the length of time the tumor has been present.6
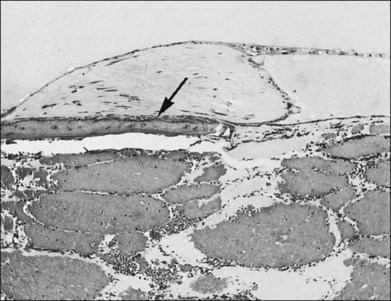
The retina overlying the tumor is affected to some extent by any tumor, even those that are very small (Fig. 153.8). The retinal changes that result include mild edema or cystic degeneration, loss of photoreceptors, gliosis, and, occasionally, invasion of the retina by RPE.6,26 Histological studies demonstrate that the orange or orange-yellow spots over the surface of these tumors are due to lipofuscin. This lipofuscin is contained in macrophages located in both the RPE and the outer plexiform layer.9
Except in those cases of severe secondary glaucoma, the ganglion cells and the nerve fiber layer are always preserved despite severe retinal changes.6 Serous detachments of the retina are not an uncommon finding in eyes studied histopathologically.
Treatment
Asymptomatic circumscribed choroidal hemangiomas do not require treatment and can be managed with observation alone.5 Observation may also be indicated in cases of subfoveal tumors which have resulted in hyperopic amblyopia.5 For symptomatic circumscribed choroidal hemangiomas with exudative retinal detachment or cystoid macular edema, a variety of treatment modalities are available.
Photodynamic therapy
Photodynamic therapy (PDT) is currently considered to be the preferred treatment for symptomatic circumscribed choroidal hemangioma with exudative retinal detachment (Figs 153.3, 153.4).27 Circumscribed choroidal hemangioma is a prototypic disease for photodynamic therapy treatment since verteporfin is sequestered in abnormal large-caliber vessels distinct from the normal choriocapillaris. In contrast to laser photocoagulation, radiation therapies, and transpupillary thermotherapy, PDT allows selective targeting of choroidal tumor vessels leading to tumor regression with minimal damage to neural retina.28 For this reason, PDT is also suitable for treatment of subfoveal choroidal hemangiomas.29 Laser photocoagulation, in contrast, does not penetrate beyond the surface of the tumor.30 Radiation therapies can induce tumor regression but carry additional risks (cataract, optic neuropathy, radiation retinopathy) and often require invasive surgeries such as tantalum clip placement for proton beam and plaque placement and removal for brachytherapy. PDT is typically performed with administration of intravenous verteporfin at a dose of 6 mg/m2 before treatment with laser wavelengths of 689, 690, or 692 nm at an intensity of 600 mW/cm2 with duration ranging from 83–166 seconds (50–100 J/cm2).31–36 The systemic and ocular safety of PDT has been well-documented in the Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) trial37 and related studies.
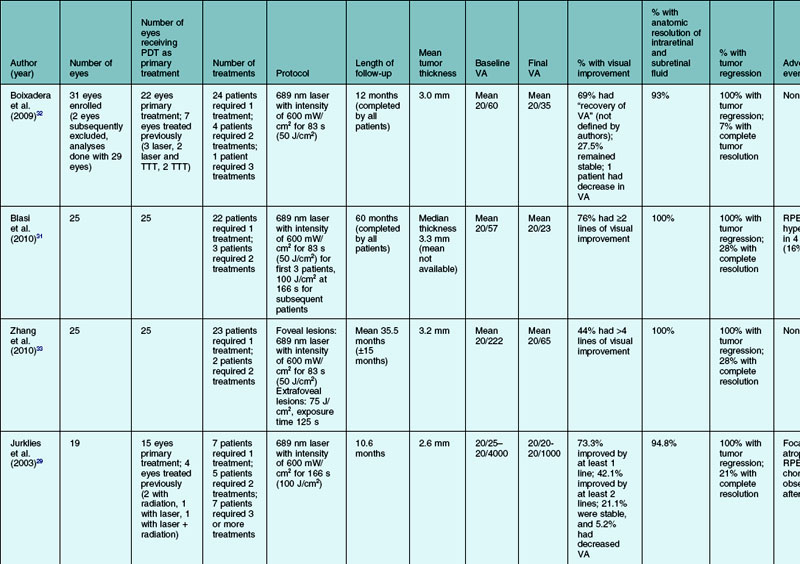
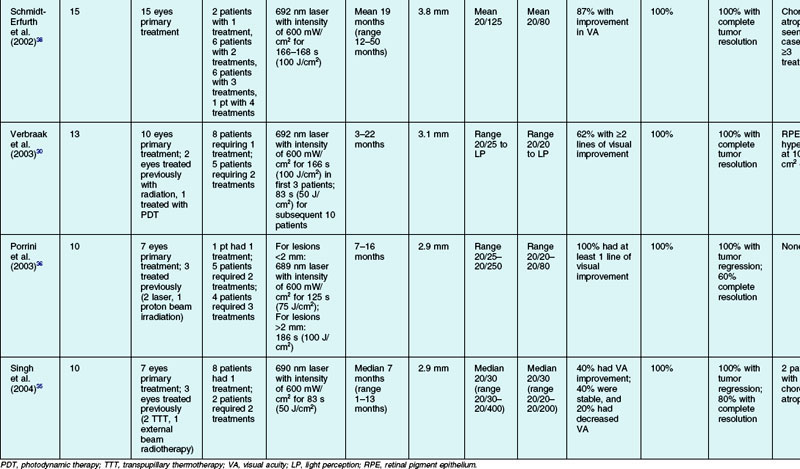
There have been numerous case series of patients with circumscribed choroidal hemangioma treated with PDT. The results of the studies with ten or more subjects are summarized in Table 153.1, which reviews the results of PDT treatments in 146 patients, not including the numbers that have been treated in smaller case series. In the more recent studies, PDT is increasingly chosen as the primary therapy.31,33 In all but two of the case series, the majority of patients received one PDT treatment.29–33,35 The two series where the vast majority of patients required more than one PDT treatment were the studies by Schmidt-Erfurth et al. and Porrini et al. where the treatment goal was defined as complete resolution of the tumor, not just resolution of subretinal and intraretinal fluid.36,38 Follow-up ranged from 1 to 60 months in the eight studies.29–33,35,36,38 The baseline tumor thickness ranged between 2.6 mm and 3.8 mm.29–33,35,36,38 Some degree of visual acuity improvement was seen in 40–100% of patients.29–33,35,36,38 Some 93–100% of patients had resolution of subretinal and/or intraretinal exudation.29–33,35,36,38 A total of 100% of the patients had some amount of tumor regression, and 7–100% had complete tumor resolution.29–33,35,36,38
While Schmidt-Erfurth et al. and Porrini et al. have suggested that the goal of therapy is elimination of subretinal fluid and tumor regression, most other authors have concluded that tumor elimination is not the ultimate goal and retreatment is only necessary for persistent or recurrent exudation or visual loss.29,32,36 Higher numbers of PDT treatments may increase the risk of choroidal atrophy and neurosensory retinal degeneration.38 The number of PDT treatments has also been shown to be inversely associated with visual acuity improvement; whether this is because larger tumors are more likely to require more PDT treatments or if this reflects a side-effect of PDT treatment is unknown.29
Radiation
Radiation treatment is felt to be most useful for patients with extensive retinal detachment that is difficult to treat with PDT. It was previously also used in patients with subfoveal choroidal hemangiomas not amenable to laser photocoagulation, though this role has been supplanted by PDT. Radiation therapy often results in retinal reattachment and tumor regression, though this can be accompanied by radiation-induced side-effects such as cataract, radiation retinopathy, and optic neuropathy.39 External beam radiotherapy treats the entire choroid with a homogeneous dose of radiation.39 Plaque brachytherapy allows more targeted treatment of the hemangioma but the dose is not homogeneous with higher doses at the tumor base than the tumor apex.39 Moreover, plaque brachytherapy requires two surgeries, one for plaque placement and one for plaque removal. Proton beam radiotherapy requires only one surgery for tantalum clip placement, and delivers a homogeneous dose of radiation to the tumor with sparing of surrounding healthy tissue.39 Negative considerations typically include the need to travel to a center and most likely, greater cost compared with other treatments. Stereotactic radiotherapy does not require any surgical intervention, though the case series are quite small and clinical experience with this procedure is limited.
External beam
Lens-sparing external beam radiotherapy has been used for the treatment of circumscribed choroidal hemangioma. The largest series was reported by Schilling et al. in 1997, where they treated 36 eyes with a dose of 20 Gy.40 A total of 63.8% of the patients had resolution of subretinal fluid, and visual acuity was stable or improved in 78% of patients.40 There were four cases of subretinal fibrosis leading to decreased final visual acuity, but no reports of radiation-induced side-effects such as radiation retinopathy or cataract over a mean follow-up period of 4.5 years.40 Ritland et al. treated nine eyes with circumscribed choroidal hemangioma with 20–24 Gy resulting in resolution of subretinal fluid, stable or improved visual acuity, and tumor regression in all cases.41 Over the mean follow-up time of 3.6 years, no radiation-induced side-effects were observed.41 Shields et al. reported two patients treated with external beam radiotherapy, both of whom had stable or improved visual acuity.5 Madreperla et al. also reported two patients treated with lens-sparing external beam radiotherapy, one of whom had resolution of subretinal fluid.42
Plaque brachytherapy
Plaque brachytherapy with palladium-103, cobalt-60, ruthenium-106, and iodine-125 have all been reported for the treatment of circumscribed choroidal hemangioma.5,42–46 Compared with external beam radiotherapy, plaque brachytherapy offers more localized delivery of radiation to the tumor itself, minimizing radiation-induced side effects.43 The disadvantages of plaque brachytherapy are the necessity of two surgeries for plaque placement and removal, as well as heterogeneous radiation dose to the tumor base and apex. Zografos et al. reported the largest series of 39 patients with circumscribed choroidal hemangioma treated with cobalt-60 plaque brachytherapy who had 100% retinal reattachment.46 Complications included pigment migration into the treated area, subretinal fibrosis, and an areolar atrophic scar.46 Shields et al. reported the second-largest series of 15 patients treated with plaque radiotherapy, 100% of whom had resolution of subretinal fluid, and 53% of whom had stable or improved visual acuity.5 Lopez-Caballero et al. also treated eight patients with iodine-125 plaque brachytherapy, with 100% resolution of subretinal fluid, 100% with some decrease in tumor thickness, and 75% with stable visual acuity.45 Three patients developed radiation retinopathy, and one patient developed subretinal fibrosis.46 Madreperla et al. treated two patients with iodine-125 and six patients with ruthenium-106 plaque brachytherapy, all of whom had resolution of subretinal fluid and 75% of whom had final vision better than 6/12.42 Aizman et al. treated five patients with palladium-103 plaque brachytherapy and all five patients had resolution of subretinal fluid with some degree of tumor regression and three patients had improvement in visual acuity.43 Chao et al. also reported one case where iodine-125 plaque brachytherapy was used as an alternative to enucleation in a patient with no light perception vision, total retinal detachment, and iris neovascularization; the post-treatment visual acuity remained no light perception but the retinal detachment and iris neovascularization resolved.44
Proton beam
The advantage of proton beam therapy is the localized delivery of a homogeneous dose of radiation; however, surgical intervention is usually necessary prior to radiation treatment to place tantalum clips for tumor localization. The largest series of 71 patients treated with proton beam therapy was reported by Levy-Gabriel et al. in 2009.47 After treatment with 20 cobalt gray equivalents, retinal reattachment was achieved in 100% of patients, tumor regression in 91.5% of patients, and visual acuity improved by two or more lines in 52%.47 Complications included cataract in 28% of patients and radiation-induced maculopathy in 8%.47 In 2004, Frau et al. reported a series of 17 patients with circumscribed choroidal hemangiomas with serous retinal detachments treated primarily with low-dose proton therapy at 20 cobalt gray equivalents.39 At 2-year follow-up, 94% of patients had visual acuity improvement of two or more lines, and 65% of patients had complete tumor resolution.39 Earlier series reported results with higher doses of radiation; Hannouche et al. reported a series of 13 patients with circumscribed choroidal hemangiomas with serous retinal detachments who underwent proton therapy at a total dose of 30 cobalt gray equivalents.48 All patients had resolution of subretinal fluid, and 62% had improvement of visual acuity by two or more lines.48 Zografos et al. treated 48 eyes with circumscribed choroidal hemangioma and all of the patients had resolution of exudative retinal detachment at last follow-up (6 months to 9 years).49 The radiation dose ranged from 16.4 to 27.3 Gy, and the three patients treated with 27.3 Gy all developed optic neuropathy.49 Finally, there is one recent report from Chan et al. where they used a nonsurgical light-field technique without surgical tumor localization at doses of 15–30 cobalt gray equivalents with good results.50
Stereotactic radiosurgery
Two different Korean groups have reported on the use of gamma knife radiosurgery for the treatment of choroidal hemangioma.51–53 Kim et al. have reported on the use of the Leksell gamma knife for symptomatic choroidal hemangiomas with associated exudative retinal detachments in a total of seven patients with choroidal hemangiomas, three of whom had the circumscribed form.52 Compared with other forms of radiation treatment, stereotactic radiosurgery provides accurately focused radiation with a single session treatment period and less effect on surrounding structures such as the lens and optic nerve.52 Gamma knife radiosurgery can also be used for large choroidal hemangiomas where transpupillary thermotherapy, PDT, and photocoagulation may not be feasible.52 In the three patients with circumscribed choroidal hemangioma treated with a marginal dose of 10 Gy, the exudative retinal detachment resolved completely within 3 months and visual acuity improved in all three cases.52 Song et al. also published their results with gamma knife radiosurgery for choroidal hemangioma with exudative retinal detachment, two of which were circumscribed, and they found that the retinal detachment resolved in both cases, but vision worsened in one patient and was stable in the other.51 The marginal dose used in this series was 26.7 Gy.51
Kivela et al. have also published a series of five patients with perifoveolar and peripapillary circumscribed choroidal hemangiomas treated with 20 Gy delivered stereotactically with a linear accelerator.54 Exudative retinal detachments resolved within 6 months in four eyes and within 20 months in the fifth eye, and visual acuity improved or remained stable in four of five eyes.54
Transpupillary thermotherapy
Transpupillary thermotherapy (TTT) utilizes diode laser to raise the temperature within treated tumor tissue, causing heat-induced sclerosis of vascular channels and eventually tumor regression and resolution of subretinal fluid.55 TTT differs from laser photocoagulation in that the goal is optimal heat penetration rather than coagulation.56 TTT is felt to be most appropriate for circumscribed choroidal hemangiomas with shallow subretinal fluid which are posterior to the equator, with a tumor base <10 mm and tumor thickness <4 mm.55 If the tumor is larger than these dimensions or if there is extensive subretinal fluid, tumor visualization may be difficult and prevent accurate focusing of the laser beam.55 TTT may not be ideal in cases where the tumor margin touches the optic disc given the risk of thermal papillitis, which was reported in two of 80 eyes having TTT for juxtapapillary choroidal melanoma.57 The use of TTT for subfoveal tumors is also questionable since TTT is not selective for abnormal tissue and may have effects on normal retina.55
Gunduz published a review in 2004 of 38 cases (ten cases managed by the author and 28 cases from the published literature) of circumscribed choroidal hemangioma primarily treated with TTT. He found that all tumors ceased leaking after TTT with resolution of subretinal fluid.55 Of these, 42% of tumors showed complete regression, 53% demonstrated partial regression by at least 10%, and 5% showed no change in tumor thickness.55 After excluding 12 patients whose pretreatment visual acuities were less than 20/400, visual acuity improved by two or more Snellen lines in 77% of eyes and remained unchanged in the remaining 23% of eyes.55 Complications included branch retinal vein occlusion in one eye, cystoid macular edema in three eyes, preretinal fibrosis in two eyes, and focal iris atrophy in three eyes.55 While all of the cases reviewed by Gunduz were <4 mm thick and <10 mm in tumor diameter, there has been one report by Rishi et al. of a large subfoveal circumscribed choroidal hemangioma 6 mm in height and 14 mm in diameter with near total exudative retinal detachment treated with TTT which showed resolution of subretinal fluid.55,58 Visual acuity improved from light perception to 10/200 at 6 months.58 There is also one report by Kamal et al. where indocyanine green dye was injected prior to TTT to enhance heat uptake.59
Laser photocoagulation
Laser photocoagulation with xenon arc or argon laser was one of the earlier primary treatments of circumscribed choroidal hemangioma prior to the development of PDT.5 In the Shields’ series of 86 patients who were treated with argon laser photocoagulation, 62% of patients had complete resolution of subretinal fluid, and 71% of patients had visual stability or improvement.5 An earlier case series in 1989 by Anand et al., where 42 patients underwent treatment with argon or xenon laser showed a 79.2% rate of initial visual acuity and subretinal fluid improvement, but a 40% rate of recurrent subretinal fluid after initial treatment.60 All of the patients had resolution of subretinal fluid with retreatment at the end of the follow-up period.60 Another series by Madreperla et al. describes 13 patients treated with laser photocoagulation where 38% had vision better than 6/12 and 46% had no subretinal fluid at 1 year.42 However, laser photocoagulation cannot be used to treat subfoveal lesions and laser photocoagulation does not reduce tumor size, resulting in a higher rate of recurrent exudation.27 Patients who have failed laser photocoagulation treatment often require subsequent radiation treatment or PDT.
Anti-VEGF injection
We have identified one published report of circumscribed choroidal hemangioma treated with intravitreal injection of bevacizumab.61 Sagong et al. reported three patients with circumscribed choroidal hemangioma with subretinal fluid treated with intravitreal bevacizumab.61 One of these patients had prior laser photocoagulation 13 years ago with recent recurrence of subretinal fluid; this patient was treated with bevacizumab alone and had resolution of subretinal fluid within 1 month.61 Two other patients with no prior treatment underwent a combination of intravitreal bevacizumab first and PDT 1 week later.61 In both cases, the author noted a reduction of subretinal fluid after bevacizumab injection and prior to PDT, and in both cases there was resolution of subretinal fluid within 2 weeks of PDT treatment.61 The authors hypothesize that the use of bevacizumab prior to PDT reduces tumor thickness through resorption of subretinal fluid, and this may maximize the effect of PDT.61
1 Scott IU, Alexandrakis G, Cordahi GJ, et al. Diffuse and circumscribed choroidal hemangiomas in a patient with Sturge-Weber syndrome. Arch Ophthalmol. 1999;117:406–407.
2 Cheung D, Grey R, Rennie I. Circumscribed choroidal haemangioma in a patient with Sturge Weber syndrome. Eye (Lond). 2000;14:238–240.
3 Leber T. Fall von cavernosem Sarcom der Aderhaut. von Graefes Arch Ophth. 1868;14:221–227.
4 Hill E, Dart RO. Cavernous hemangioma of the choroid: report of five cases in the Registry of Ophthalmic Pathology, Army Medical Museum, Washington, DC. Trans Am Ophthalmol Soc. 1936;34:122–133.
5 Shields CL, Honavar SG, Shields JA, et al. Circumscribed choroidal hemangioma: clinical manifestations and factors predictive of visual outcome in 200 consecutive cases. Ophthalmology. 2001;108:2237–2248.
6 Witschel H, Font RL. Hemangioma of the choroid. A clinicopathologic study of 71 cases and a review of the literature. Surv Ophthalmol. 1976;20:415–431.
7 Schepens CL, Schwartz A. Intraocular tumors. I. Bilateral hemangioma of the choroid. AMA Arch Ophthalmol. 1958;60:72–83.
8 Hogan MJ. Choroidal hemangioma. Arch Ophthalmol. 1964;71:69–70.
9 Shields JA, Rodrigues MM, Sarin LK, et al. Lipofuscin pigment over benign and malignant choroidal tumors. Trans Sect Ophthalmol Am Acad Ophthalmol Otolaryngol. 1976;81:871–881.
10 Ruby AJ, Jampol LM, Goldberg MF, et al. Choroidal neovascularization associated with choroidal hemangiomas. Arch Ophthalmol. 1992;110:658–661.
11 Leys AM, Silva R, Inhoffen W, et al. Neovascular growth following photodynamic therapy for choroidal hemangioma and neovascular regression after intravitreous injection of triamcinolone. Retina. 2006;26:693–697.
12 Medlock RD, Augsburger JJ, Wilkinson CP, et al. Enlargement of circumscribed choroidal hemangiomas. Retina. 1991;11:385–388.
13 Shields JA, Stephens RF, Eagle RC, et al. Progressive enlargement of a circumscribed choroidal hemangioma. A clinicopathologic correlation. Arch Ophthalmol. 1992;110:1276–1278.
14 Schalenbourg A, Zografos L. Blackening of choroidal hemangioma after tantalum clip surgery. Arch Ophthalmol. 2007;125:1136.
15 Bosch MM, Helbig H. Blackening of a choroidal hemangioma after photodynamic therapy. Klin Monbl Augenheilkd. 2005;222:258–260.
16 Norton EW, Gutman F. Fluorescein angiography and hemangiomas of the choroid. Arch Ophthalmol. 1967;78:121–125.
17 Singh AD, Kaiser PK, Sears JE. Choroidal hemangioma. Ophthalmol Clin North Am. 2005;18:151–161.
18 Arevalo JF, Shields CL, Shields JA, et al. Circumscribed choroidal hemangioma: characteristic features with indocyanine green videoangiography. Ophthalmology. 2000;107:344–350.
19 Shields CL, Shields JA, De Potter P. Patterns of indocyanine green videoangiography of choroidal tumours. Br J Ophthalmol. 1995;79:237–245.
20 Schalenbourg A, Piguet B, Zografos L. Indocyanine green angiographic findings in choroidal hemangiomas: A study of 75 cases. Ophthalmologica. 2000;214:246–252.
21 Shields CL, Materin MA, Shields JA. Review of optical coherence tomography for intraocular tumors. Curr Opin Ophthalmol. 2005;16:141–154.
22 Spaide RF, Koizumi H, Pozzoni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008;146:496–500.
23 Torres VL, Brugnoni N, Kaiser PK, et al. Optical coherence tomography enhanced depth imaging of choroidal tumors. Am J Ophthalmol. 2011;151:586–593.
24 Schmitz-Valckenberg S, Holz FG, Bird AC, et al. Fundus autofluorescence imaging: review and perspectives. Retina. 2008;28:385–409.
25 Ramasubramanian A, Shields CL, Harmon SA, et al. Autofluorescence of choroidal hemangioma in 34 consecutive eyes. Retina. 2010;30:16–22.
26 Jones IS, Cleasby GW. Hemangioma of the choroid: a clinicopathologic analysis. Am J Ophthalmol. 1959;48:612–628.
27 Tsipursky MS, Golchet PR, Jampol LM. Photodynamic therapy of choroidal hemangioma in sturge-weber syndrome, with a review of treatments for diffuse and circumscribed choroidal hemangiomas. Surv Ophthalmol. 2011;56:68–85.
28 Schmidt-Erfurth U, Hasan T, Gragoudas E, et al. Vascular targeting in photodynamic occlusion of subretinal vessels. Ophthalmology. 1994;101:1953–1961.
29 Jurklies B, Anastassiou G, Ortmans S, et al. Photodynamic therapy using verteporfin in circumscribed choroidal haemangioma. Br J Ophthalmol. 2003;87:84–89.
30 Verbraak FD, Schlingemann RO, Keunen JE, et al. Longstanding symptomatic choroidal hemangioma managed with limited PDT as initial or salvage therapy. Graefes Arch Clin Exp Ophthalmol. 2003;241:891–898.
31 Blasi MA, Tiberti AC, Scupola A, et al. Photodynamic therapy with verteporfin for symptomatic circumscribed choroidal hemangioma: five-year outcomes. Ophthalmology. 2010;117:1630–1637.
32 Boixadera A, Garcia-Arumi J, Martinez-Castillo V, et al. Prospective clinical trial evaluating the efficacy of photodynamic therapy for symptomatic circumscribed choroidal hemangioma. Ophthalmology. 2009;116:100–105.
33 Zhang Y, Liu W, Fang Y, et al. Photodynamic therapy for symptomatic circumscribed macular choroidal hemangioma in Chinese patients. Am J Ophthalmol. 2010;150:710–715.
34 Barbazetto I, Schmidt-Erfurth U. Photodynamic therapy of choroidal hemangioma: two case reports. Graefes Arch Clin Exp Ophthalmol. 2000;238:214–221.
35 Singh AD, Kaiser PK, Sears JE, et al. Photodynamic therapy of circumscribed choroidal haemangioma. Br J Ophthalmol. 2004;88:1414–1418.
36 Porrini G, Giovannini A, Amato G, et al. Photodynamic therapy of circumscribed choroidal hemangioma. Ophthalmology. 2003;110:674–680.
37 Bressler NM. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: two-year results of 2 randomized clinical trials-tap report 2. Arch Ophthalmol. 2001;119:198–207.
38 Schmidt-Erfurth UM, Michels S, Kusserow C, et al. Photodynamic therapy for symptomatic choroidal hemangioma: visual and anatomic results. Ophthalmology. 2002;109:2284–2294.
39 Frau E, Rumen F, Noel G, et al. Low-dose proton beam therapy for circumscribed choroidal hemangiomas. Arch Ophthalmol. 2004;122:1471–1475.
40 Schilling H, Sauerwein W, Lommatzsch A, et al. Long-term results after low dose ocular irradiation for choroidal haemangiomas. Br J Ophthalmol. 1997;81:267–273.
41 Ritland JS, Eide N, Tausjo J. External beam irradiation therapy for choroidal haemangiomas. Visual and anatomical results after a dose of 20 to 25 Gy. Acta Ophthalmol Scand. 2001;79:184–186.
42 Madreperla SA, Hungerford JL, Plowman PN, et al. Choroidal hemangiomas: visual and anatomic results of treatment by photocoagulation or radiation therapy. Ophthalmology. 1997;104:1773–1779.
43 Aizman A, Finger PT, Shabto U, et al. Palladium 103 (103Pd) plaque radiation therapy for circumscribed choroidal hemangioma with retinal detachment. Arch Ophthalmol. 2004;122:1652–1656.
44 Chao AN, Shields CL, Shields JA, et al. Plaque radiotherapy for choroidal hemangioma with total retinal detachment and iris neovascularization. Retina. 2001;21:682–684.
45 Lopez-Caballero C, Saornil MA, De Frutos J, et al. High-dose iodine-125 episcleral brachytherapy for circumscribed choroidal haemangioma. Br J Ophthalmol. 2010;94:470–473.
46 Zografos L, Bercher L, Chamot L, et al. Cobalt-60 treatment of choroidal hemangiomas. Am J Ophthalmol. 1996;121:190–199.
47 Levy-Gabriel C, Rouic LL, Plancher C, et al. Long-term results of low-dose proton beam therapy for circumscribed choroidal hemangiomas. Retina. 2009;29:170–175.
48 Hannouche D, Frau E, Desjardins L, et al. Efficacy of proton therapy in circumscribed choroidal hemangiomas associated with serious retinal detachment. Ophthalmology. 1997;104:1780–1784.
49 Zografos L, Egger E, Bercher L, et al. Proton beam irradiation of choroidal hemangiomas. Am J Ophthalmol. 1998;126:261–268.
50 Chan RV, Yonekawa Y, Lane AM, et al. Proton beam irradiation using a light-field technique for the treatment of choroidal hemangiomas. Ophthalmologica. 2010;224:209–216.
51 Song WK, Byeon SH, Kim SS, et al. Gamma knife radiosurgery for choroidal haemangiomas with extensive exudative retinal detachment. Br J Ophthalmol. 2009;93:836–837.
52 Kim YT, Kang SW, Lee JI. Gamma knife radiosurgery for choroidal hemangioma. Int J Radiat Oncol Biol Phys. 2011;81:1399–1404.
53 Kong DS, Lee JI, Kang SW. Gamma knife radiosurgery for choroidal hemangioma. Am J Ophthalmol. 2007;144:319–322.
54 Kivela T, Tenhunen M, Joensuu T, et al. Stereotactic radiotherapy of symptomatic circumscribed choroidal hemangiomas. Ophthalmology. 2003;110:1977–1982.
55 Gunduz K. Transpupillary thermotherapy in the management of circumscribed choroidal hemangioma. Surv Ophthalmol. 2004;49:316–327.
56 Garcia-Arumi J, Ramsay LS, Guraya BC. Transpupillary thermotherapy for circumscribed choroidal hemangiomas. Ophthalmology. 2000;107:351–357.
57 Shields CL, Shields JA, Perez N, et al. Primary transpupillary thermotherapy for small choroidal melanoma in 256 consecutive cases: outcomes and limitations. Ophthalmology. 2002;109:225–234.
58 Rishi P, Sharma T. Transpupillary thermotherapy for large-sized subfoveal circumscribed choroidal hemangioma. Retina. 2006;26:974–976.
59 Kamal A, Watts AR, Rennie IG. Indocyanine green enhanced transpupillary thermotherapy of circumscribed choroidal haemangioma. Eye (Lond). 2000;14(Pt 5):701–705.
60 Anand R, Augsburger JJ, Shields JA. Circumscribed choroidal hemangiomas. Arch Ophthalmol. 1989;107:1338–1342.
61 Sagong M, Lee J, Chang W. Application of intravitreal bevacizumab for circumscribed choroidal hemangioma. Korean J Ophthalmol. 2009;23:127–131.