Chapter 158 Circumferential Cervical Spinal Fusion
The Rationale for Circumferential Surgery
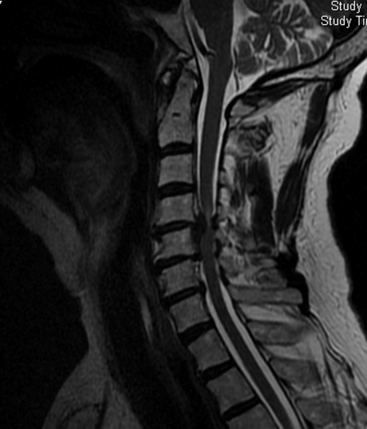
Certainly one of the most obvious reasons for choosing bidirectional surgery of the cervical spine would be that the patient presents with obvious compression of both the ventral and dorsal surfaces of the spinal cord (Fig. 158-1). If the patient is not overly frail, then one can make a rationale for this approach. There is some degree of inherent common sense to directly remove the compressive structure of the spinal cord. One variation might be surgery to treat ossification of the posterior longitudinal ligament (OPLL).1–4 Patients with dramatic anterior compression are commonly treated by posterior decompression in order to avoid the significant pitfalls of anterior surgery.
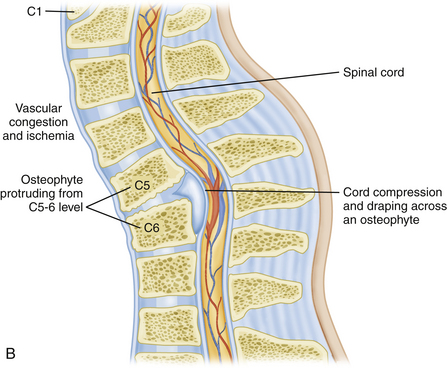
Restoration of spinal alignment has received an increasing degree of attention recently as a potential crucial factor in the outcome of patients with cervical spondylitic myelopathy (CSM).5–8 Most would agree that circumferential surgery is indicated for patients that present in frank kyphosis (Fig. 158-2). This may result from numerous factors including posterior migration of the cord, vascular perfusion, and longitudinal stresses on the cord. If a patient is left in a kyphotic position, then draping and potential anterior compression of the spinal cord are certainly possible. The classic example of this would be postlaminectomy kyphosis. Nottmeier et al. recently published results of cervical kyphotic deformity correction using a anterior-posterior reconstruction. Their patient population had a mean preoperative kyphosis of 18 degrees. They were able to achieve a mean correction of sagittal angle of 22 degrees. This resulted in a mean postoperative sagittal angle of 4 degrees. There was no reported loss of deformity correction during the follow-up period and they reported a fusion rate of 97.5%.8
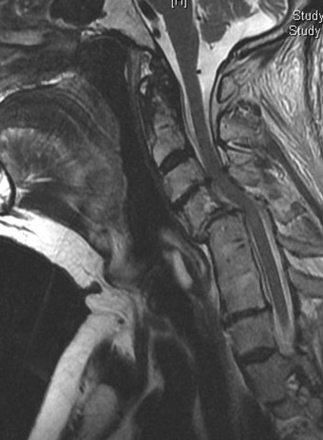
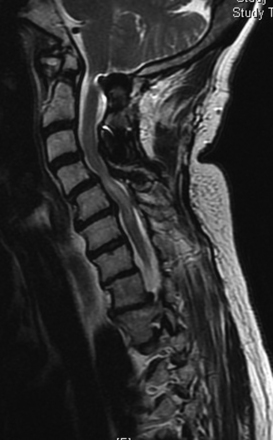
FIGURE 158-2 T2-weighted MRI in a patient with a previous fusion of C5–C7 with a pronounced anterolisthesis and kyphosis of C4–C5.
Several studies have looked at the potential importance of postoperative posterior spinal cord migration. There is a concern that residual kyphosis may allow the spinal cord to be draped across the vertebral column, which may contribute to compromise of the spinal cord via vascular compromise or longitudinal stress. Lee et al. attempted to quantitatively assess preoperative factors that would predict this postoperative movement of the cord. Besides the commonly stated importance of preoperative sagittal balance, they also found significance in the relative degree of stenosis directly above the “laminectomized” level to be particularly important.9 However, the clinical importance of cord shift remains uncertain. Hatta et al. found no clinical correlation in myelopathy outcomes to cord migration.10
There has also been some attention given to the potential effects of “longitudinal stress” to the spinal cord by Uchida et al. It is interesting to consider the potential similarities of this to the physiologic derangement that has been documented to occur with tethering of the spinal cord.11,12
History
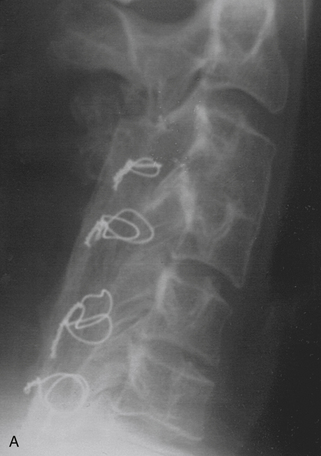
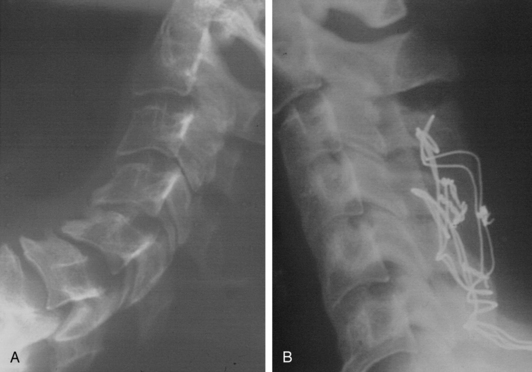
Bidirectional surgery for the cervical spine was originally reserved for cases of clear violation of stability of both the anterior and posterior elements of the cervical spine. Most cases dealt with either trauma or neoplastic disease. Instrumentation of the spine was limited to wiring techniques (Figs. 158-3, 158-4, and 158-5). Cases frequently required staging, and patients commonly required prolonged immobilization.
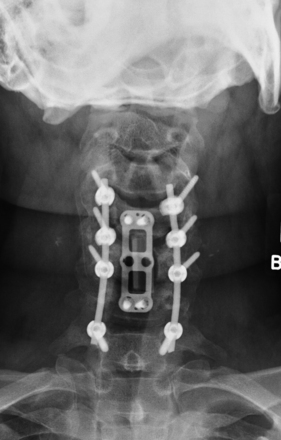
The evolution of surgical hardware has taken us from stainless steel wiring techniques to lateral mass plates and to polyaxially screw and rod fixation (Figs. 158-6 and 158-7). This has made single-stage anterior-posterior reconstructive procedures more feasible and reasonable in a larger number of patients.13
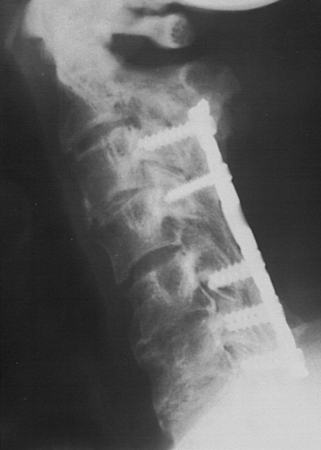
FIGURE 158-6 A case demonstrating the use of a lateral mass plate system, which became the first widely used screw system.
The surgical training for treatment of complex spinal disorders has also rapidly expanded. As a result, anterior decompression involving segmental reconstructions and corpectomies has become increasingly routine for spinal surgeons.
Technique
Imaging
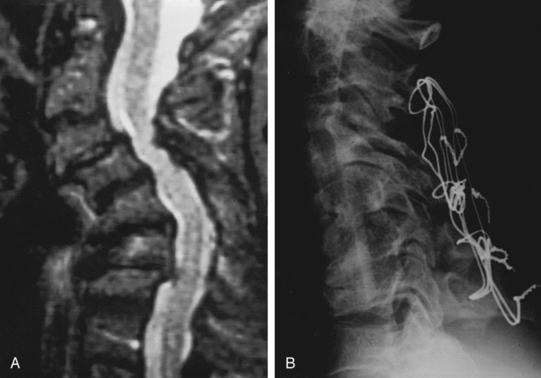
Patients should be evaluated with preoperative plain x-rays in addition to the expected magnetic resonance imaging (MRI) and or computed tomography (CT) myelogram. Abnormal signal on MRI images may also offer some prognostic information regarding potential patient recovery. Mastronardi et al. studied patients with spondylotic myelopathy and found that T2 signal changes are potentially reversible with decompression. T1-weighted signal changes were associated with the worst prognosis and were thought to represent irreversible damage to the cord (Figs. 158-3 and 158-8). Patients with myelopathy that had no signal abnormalities on their preoperative MRI were found to have the best prognosis.14 This is an interesting concept to include in the debate of whether patients with cervical cord compression should be considered for early treatment or for a conservative approach during follow-up.
Anesthesia
Preoperatively the patient should be assessed regarding potential instability, range of motion, and exacerbation of neurologic symptoms associated with neck movement. The findings will determine whether the patient can be put to sleep with standard techniques or may require an awake fiber-optic intubation. This concern cannot be overemphasized in the ankylosing spondylitis patient. This pathology can produce alarming instability with devastating neurologic morbidity. All patients with ankylosing spondylitis should be considered for intubation with awake fiber-optic techniques. The authors believe that it is crucial to discuss the issues with the anesthesia team, so that no miscommunication occurs.
Neuromonitoring
Somatosensory potentials can be used with muscular paralytics and are not quite as sensitive to inhalational anesthetics. The posterior columns are monitored. One potential drawback to SSEPs is that a significant amount of time can elapse before the surgeon is alerted to dysfunction of the tissue.15
Anterior Approach
Standard operative exposure is used, with surgeon preference dictating a right- or left-sided incision. Much has been passionately debated on the topic of side of surgical approach. Although anatomic studies may be able to demonstrate some theoretical benefits to a left-sided approach, the clinical literature does not seem to reflect a significant difference in outcome.16 If the patient has had prior surgery, then the same side should be used to avoid a catastrophic bilateral recurrent laryngeal nerve injury. Any need to approach from the opposite side of a previous surgery should be cleared prior to surgery by direct laryngoscopic examination. A vertical incision is usually preferred, as this allows for adequate tissue dissection and exposure of multiple levels. A horizontal incision can certainly be used for less extensive anterior cases. An extensive subplatysmal dissection, along with a wide opening of the superficial cervical fascia will facilitate the exposure.
Once the standard dissection has been completed, self-retaining retractors may be placed. It is a good practice to release the retractors several times to avoid excessive pressure and ischemia to the underlying tissues. The anesthesiologist may also consider deflating and reinflating the endotracheal tube balloon. These maneuvers are believed to reduce patient dysphagia and recurrent laryngeal nerve (RLN) morbidity. Apfelbaum and colleagues reported on a series of 900 consecutive patients over a 12-year period. They began to institute a protocol of deflating the cuff of the endotracheal tube so that it would be recentered in the larynx. After this protocol was adopted, they observed a drop in the incidence of RLN injury from 6.4% to 1.69%.17 Multiple levels of surgery and female gender have been shown to increase the risk of dysphagia at 6 months follow-up.18,19
Once proper exposure has been obtained, then the appropriate decompression may be carried out. Whenever possible the authors prefer to perform segmental reconstruction via multiple diskectomies rather than corpectomies. This helps to ensure adequate creation of lordosis, as well as improving load sharing of the anterior column. When this technique does not allow for adequate decompression, then corpectomies are used. If the extent of decompression requires the use of corpectomy, then attempts should be made to create lordosis. The authors commonly do this by cutting a fibular shaft allograft on an angle. Structural cages are also manufactured with angled end pieces to contribute to lordosis.
Anterior plating is commonly used by most surgeons to encourage fusion and prevent graft subsidence. Anterior instrumentation is not used by the authors in cases without obvious, overt instability, so as to reduce possible dysphagia and avoid load shielding of the anterior column.20
Posterior Approach
Decompression is performed via a standard laminectomy. Most cases of cervical spondylitic myelopathy only require removal of the roof of the C7 lamina. The authors typically preserve the rest of the lamina, along with the C7 spinous process. This seems to allow terminating the construct at the C7 pedicle. Care is also taken to preserve the muscular attachments to the C7 spinous process. Muscular preservation has generally received more attention in relation to laminoplasty, but the authors believe that it is also important to avoid junctional breakdown at the cervicothoracic region.20 If there is any doubt of the structural integrity of the C7-T1 level, then one should consider incorporating the thoracic spine into the construct.
Instrumentation of the posterior elements is typically performed with standard lateral mass instrumentation from C3–C6. Screw implantation is generally preferred with a Magerl or similar technique.21 For the termination of the construct, where the forces will be the highest, the authors prefer to use the pedicle of C7, as it generally allows for a very strong fixation point.22 We have also observed that the standard 3.5-mm screws are susceptible to fracture at the neck of the screw. This complication has not generally been observed since we started using 4.0-mm diameter screws. Although the C7 pedicle can be found using external landmarks, the use of internal landmarks can reduce the rate of perforation with minimal increase in intraoperative time.23 A laminotomy is performed on the superior aspect of the C7 lamina, along with a C6–C7 foraminotomy. A micro ball–tipped probe is then used to palpate the C7 pedicle. This region can be associated with a significant epidural venous plexus. Gelfoam and thrombin or Flo-Seal are generally kept on the surgical field to minimize blood loss. There is a significant downward angle of the C7 pedicle relative to the C6 lateral mass. Because of this we commonly skip instrumentation of the C6 lateral mass, in order to properly use the pedicle of C7.
Complications
The actual risk of performing circumferential surgery has been somewhat nebulous as the technique has expanded. Several papers have attempted to detail the morbidity to the patient. Gok et al. retrospectively reviewed lone anterior cases versus combined anterior posterior cases and found a similar rate of complications.13 Boakye et al. completed an extensive review of 58,115 admissions of patients with cervical spondylitic myelopathy from 1993 to 2002. While this study did not separate out specifics of circumferential surgery, it does contain some interesting data. The overall mortality rate was 0.6%. The addition of just one postoperative complication increased the mortality rate by 20-fold. This also translated into an average increased stay of 4 days and $10,000 in additional charges. Patients older than 84 years of age had a 40-fold increase in adverse outcomes.24
Explanations for dysphagia following the anterior cervical approach include direct pressure to the esophagus, denervation injury, and local tissue ischemia. Histologic studies also show that the development of fibrosis within the esophageal wall may play an important role. Injury to the recurrent laryngeal nerve has also been associated with dysphagia, but appears to occur at an incidence of 0.07% to 11%. Because the right nerve also innervates the esophagus, it has been suggested that the left approach is preferred.25–27
Dysphonia can is also associated with the anterior cervical approach. This risk increases with multilevel procedures. The higher the level of the cervical spine also has a transition of anatomy to the more delicate tissues of the retropharyngeal space. Because circumferential cases involve the patient commonly being placed in the prone position for a significant length of time after the completion of the anterior approach, edema and hematomas can be a concern. The authors generally recommend the use of an anterior drain and intraoperative corticosteroids. Airway compromise can also occur because of these issues. The appearance of significant neck and facial edema should prompt a low threshold for the patient to remain intubated. Sagi et al. have shown that there is an increased risk of airway complication with anterior cervical surgery in patients who have undergone multilevel surgery, operative time greater than 5 hours, and blood loss of greater than 300 ml. This obviously describes the typical patient for an anterior posterior reconstruction. This paper also showed that the mean time for these problems to develop was 36 hours postoperatively.28 It is the authors’ observation that the patients undergoing anterior posterior reconstructions tend to develop these complications early in the postoperative period. This is probably because of the time that the airway was in the dependent position during the posterior portion of the procedure. Obviously, one should have a low threshold for concern and action over issues of upper airway difficulty in these patients. Direct observation along with lateral x-rays generally provide the best assessment of the patient.
Conclusions
Circumferential cervical reconstructions are major procedures associated with multiple complications that must be taken into account for each individual patient. Patients who require extensive decompression of the neural element, with or without kyphosis, can be treated with a reasonable morbidity when compared to unidirectional cervical procedures.13,23
Albert T., Klein G.R., Joffe D., et al. Use of cervicothoracic junction pedicle screws for reconstruction of complex cervical spine pathology. Spine. 1998;23(14):1596-1599.
Albert T.J., Vacarro A. Postlaminectomy kyphosis. Spine. 1998;23(24):2738-2745.
Apfelbaum R., Kriskovich M., Haller J., et al. On the incidence, cause and prevention of recurrent laryngeal nerve palsies during anterior cervical spine surgery. Spine. 2000;25(22):2906-2912.
Bazaz R., Lee M.J., Yoo J.U., et al. Incidence of dysphagia after anterior cervical spine surgery: a prospective study. Spine. 2002;27(22):2453-2458.
Beutler W., Sweeney C., Connolly P., et al. Recurrent laryngeal nerve injury with anterior cervical spine surgery: risk with laterality of surgical approach. Spine. 2001;26(12):1337-1342.
Boakye M., Patil C.G., Santarelli J., et al. Cervical spondylotic myelopathy: complications and outcomes after spinal fusion. Neurosurgery. 2008;62(2):455-461.
Edwards C.C.2nd, Heller J.G., Murakami H., et al. Corpectomy versus laminoplasty for multilevel cervical myelopathy: an independent matched-cohort anaylysis. Spine. 2002;1;27(11):1168-1175.
Epstein N. Posterior approaches in the management of cervical spondylosis and ossification of the posterior longitudinal ligament. Surg Neurol. 2002;58(3-4):194-207.
Gok B., Sciubba D.M., McLoughlin G.S., et al. Surgical treatment of cervical spondylotic myelopathy with anterior compression: a review of 67 cases. J Neurosurg Spine. 2008;9(2):152-157.
Hatta Y., Shiraishi T., Hase H., et al. Is posterior spinal cord shift by extensive posterior decompression clinically significant for multisegmental cervical spondylotic myelopathy? Spine. 2005;1;30(21):2414-2419.
Heller J.G., Jeffords P.. Internal fixation of the cervical spine. C. Posterior instrumentation of the lower cervical spine, The Adult and Pediatric Spine, 3rd ed. Philadelphia, Lippincott, Williams & Wilkins, 2004 , 803-813
Lee J.Y., Sharan A., Baron E.M., et al. Quantitative prediction of spinal cord drift after cervical laminectomy and arthrodesis. Spine. 2006;15;31(16):1795-1798.
Lee S.K.S., Lee G.Y.F., Wong G.T.H., et al. Prolonged and severe dysphagia following anterior cervical surgery. J Clin Neuroscience. 2003;11(4):424-427.
Martin-Benlloch J.A., Maruenda-Paulino J.L., Barra-Pla A., et al. Expansive laminoplasty as a method for managing cervical multilevel spondylotic myelopathy. Spine. 2003;28(70):680-684.
Mastronardi L., Elsawaf A., Roperto R., et al. Prognostic relevance of the postoperative evolution of intramedullary spinal cord changes in signal intensity on magnetic resonance imaging after anterior decompression for cervical spondylotic myelopathy. J Neurosurg Spine. 2007;7(6):615-622.
Meylearts S.A., Jacobs M.J., van Iterson V., et al. Comparison of transcranial motor evoked potentials and somatosensory evoked potentials during thoracoabdominal aortic aneurysm repair. Ann Surg. 1999;230(6):742-749.
Nottmeier E.W., Deen H.G., Patel N., et al. Cervical kyphotic deformity correction using 360-degree reconstruction. J Spinal Disord Tech. 2009;22:385-391.
Ogawa Y., Toyama Y., Chiba K., et al. Long-term results of expansive open-door laminoplasty for ossification of the posterior longitudinal ligament of the cervical spine. J Neurosurg. 2004;1(2):168-174.
Rhee J.M., Kraiwattanapong C., Hutton W., et al. a comparison of pedicle and lateral mass screw construct stiffness at the cervicothoracic junction: a biomechanical study. Spine. 2005;30(21):636-640.
Riew K.D., Hilibrand A.S., Palumbo M.A., et al. Anterior cervical corpectomy in patients with previously managed myelopathy with a laminectomy. J Bone Joint Surg Am. 1999;81(7):950-957.
Sagi C., Beutler W., Carroll E., et al. Airway complications associated with surgery on the anterior cervical spine. Spine. 2002;27(9):949-953.
Smith-Hammond C.A., New K.C., Pietrobon R., et al. Prospective analysis of incidence and risk factors of dysphagia in spine surgery patients: comparison of anterior cervical, posterior cervical and lumbar procedures. Spine. 2004;29(13):144-146.
Stewart M., Johnston R.A., Stewart I., Wilson J.A. Swallowing performance following anterior cervical spine surgery. Br J Neurosurg. 1995;9(5):605-610.
Takeuchi T., Shono Y. Importance of preserving the C7 spinous process and attached nuchal ligament in French-door laminoplasty to reduce postoperative axial symptoms. Eur Spine J. 2007;16(9):1417-1422.
Uchida K., Baba H. Importance of sagittal balance in determining the outcome of anterior versus posterior surgery for cervical spondylotic myelopathy. J Neurosurg Spine. 2009;11:518-520.
Uchida K., Nakajima H., Sato R., et al. Cevical spondylotic myelopathy associated with kyphosis or sagittal alignment: outcome after anterior or posterior decompression. J Neurosurg Spine. 2009;11:521-528.
Yamada S., Won D.J., Yamada S.M., et al. Pathophysiology of tethered cord syndrome: correlation with symptomatology. Neurosurg Focus. 2004;16(2):E6.
Yue W.M., Brodner W., Highland T., et al. Persistent swallowing and voice problems after anterior cervical discectomies and fusion with allograft and plating: a 5- to 11-year follow-up study. Eur Spine J. 2005;14(7):677-682.
1. Ogawa Y., Toyama Y., Chiba K., et al. Long-term results of expansive open-door laminoplasty for ossification of the posterior longitudinal ligament of the cervical spine. J Neurosurg. 2004;1(2):168-174.
2. Martin-Benlloch J.A., Maruenda-Paulino J.L., Barra-Pla A., et al. Expansive laminoplasty as a method for managing cervical multilevel spondylotic myelopathy. Spine. 2003;28(70):680-684.
3. Epstein N. Posterior approaches in the management of cervical spondylosis and ossification of the posterior longitudinal ligament. Surg Neurol. 2002;58(3-4):194-207.
4. Edwards C.C.2nd, Heller J.G., Murakami H., et al. Corpectomy versus laminoplasty for multilevel cervical myelopathy: an independent matched-cohort anaylysis. Spine. 2002;1;27(11):1168-1175.
5. Riew K.D., Hilibrand A.S., Palumbo M.A., et al. Anterior cervical corpectomy in patients with previously managed myelopathy with a laminectomy. J Bone Joint Surg Am. 1999;81(7):950-957.
6. Uchida K., Nakajima H., Sato R., et al. Cevical spondylotic myelopathy associated with kyphosis or sagittal alignment: outcome after anterior or posterior decompression. J Neurosurg Spine. 2009;11:521-528.
7. Albert T.J., Vacarro A. Postlaminectomy kyphosis. Spine. 1998;23(24):2738-2745.
8. Nottmeier E.W., Deen H.G., Patel N., et al. cervical kyphotic deformity correction using 360-degree reconstruction. J Spinal Disord Tech. 2009;22:385-391.
9. Lee J.Y., Sharan A., Baron E.M., et al. Quantitative prediction of spinal cord drift after cervical laminectomy and arthrodesis. Spine. 2006;15;31(16):1795-1798.
10. Hatta Y., Shiraishi T., Hase H., et al. Is posterior spinal cord shift by extensive posterior decompression clinically significant for multisegmental cervical spondylotic myelopathy? Spine. 2005;1;30(21):2414-2419.
11. Yamada S., Won D.J., Yamada S.M., et al. Pathophysiology of tethered cord syndrome: correlation with symptomatology. Neurosurg Focus. 2004;16(2):E6.
12. Uchida K., Baba H. Importance of sagittal balance in determining the outcome of anterior versus posterior surgery for cervical spondylotic myelopathy. J Neurosurg Spine. 2009;11:518-520.
13. Gok B., Sciubba D.M., McLoughlin G.S., et al. Surgical treatment of cervical spondylotic myelopathy with anterior compression: a review of 67 cases. J Neurosurg Spine. 2008;9(2):152-157.
14. Mastronardi L., Elsawaf A., Roperto R., et al. Prognostic relevance of the postoperative evolution of intramedullary spinal cord changes in signal intensity on magnetic resonance imaging after anterior decompression for cervical spondylotic myelopathy. J Neurosurg Spine. 2007;7(6):615-622.
15. Meylearts S.A., Jacobs M.J., van Iterson V., et al. Comparison of transcranial motor evoked potentials and somatosensory evoked potentials during thoracoabdominal aortic aneurysm repair. Ann Surg. 1999;230(6):742-749.
16. Beutler W., Sweeney C., Connolly P., et al. Recurrent laryngeal nerve injury with anterior cervical spine surgery: risk with laterality of surgical approach. Spine. 2001;26(12):1337-1342.
17. Apfelbaum R., Kriskovich M., Haller J., et al. On the incidence, cause and prevention of recurrent laryngeal nerve palsies during anterior cervical spine surgery. Spine. 2000;25(22):2906-2912.
18. Bazaz R., Lee M.J., Yoo J.U., et al. Incidence of dysphagia after anterior cervical spine surgery: a prospective study. Spine. 2002;27(22):2453-2458.
19. Yue W.M., Brodner W., Highland T., et al. Persistent swallowing and voice problems after anterior cervical discectomies and fusion with allograft and plating: a 5- to 11-year follow-up study. Eur Spine J. 2005;14(7):677-682.
20. Takeuchi T., Shono Y. Importance of preserving the C7 spinous process and attached nuchal ligament in French-door laminoplasty to reduce postoperative axial symptoms. Eur Spine J. 2007;16(9):1417-1422.
21. Heller J.G., Jeffords P. Internal fixation of the cervical spine. C. Posterior instrumentation of the lower cervical spine. In The Adult and Pediatric Spine, 3rd ed., Philadelphia: Lippincott, Williams & Wilkins; 2004:803-813.
22. Rhee J.M., Kraiwattanapong C., Hutton W., et al. A comparison of pedicle and lateral mass screw construct stiffness at the cervicothoracic junction: a biomechanical study. Spine. 2005;30(21):636-640.
23. Albert T., Klein G.R., Joffe D., et al. Use of cervicothoracic junction pedicle screws for reconstruction of complex cervical spine pathology. Spine. 1998;23(14):1596-1599.
24. Boakye M., Patil C.G., Santarelli J., et al. Cervical spondylotic myelopathy: complications and outcomes after spinal fusion. Neurosurgery. 2008;62(2):455-461.
25. Stewart M., Johnston R.A., Stewart I., Wilson J.A. Swallowing performance following anterior cervical spine surgery. Br J Neurosurg. 1995;9(5):605-610.
26. Smith-Hammond C.A., New K.C., Pietrobon R., et al. Prospective analysis of incidence and risk factors of dysphagia in spine surgery patients: comparison of anterior cervical, posterior cervical and lumbar procedures. Spine. 2004;29(13):144-146.
27. Lee S.K.S., Lee G.Y.F., Wong G.T.H., et al. Prolonged and severe dysphagia following anterior cervical surgery. J Clin Neuroscience. 2003;11(4):424-427.
28. Sagi C., Beutler W., Carroll E., et al. Airway complications associated with surgery on the anterior cervical spine. Spine. 2002;27(9):949-953.