Chapter 390 Chronic Recurrent Aspiration
Etiology
The recurrent aspiration of small quantities of gastric, nasal, or oral contents can lead to several clinical presentations, including recurrent bronchitis or bronchiolitis; recurrent pneumonia; atelectasis; wheezing; cough; apnea; and/or laryngospasm. Pathologic outcomes include granulomatis inflammation, interstitial inflammation, fibrosis, lipoid pneumonia, and bronchiolitis obliterans. Most cases, although associated with significant morbidity, do not come to pathologic inspection, but clinically manifest as airway inflammation. Underlying disorders that are frequently associated with recurrent aspiration are listed in Table 390-1. Oropharyngeal incoordination is reportedly the most common underlying problem associated with recurrent pneumonias in hospitalized children. In one series of 238 children hospitalized with recurrent pneumonia, 48% were found to have dysphagia as the underlying problem. Lipoid pneumonia may occur after the use of home/folk remedies involving oral or nasal administration of animal or vegetable oils to treat various childhood illnesses. Lipoid pneumonia has been reported as a complication of these practices in the Middle East, Asia, India, Brazil, and Mexico. The initial underlying disease, language barriers, and a belief that these are not “medications” may delay the diagnosis.
Table 390-1 CONDITIONS PREDISPOSING TO ASPIRATION LUNG INJURY IN CHILDREN
ANATOMICAL AND MECHANICAL
NEUROMUSCULAR
MISCELLANEOUS
Gastroesophageal reflux disease (GERD) is also a common underlying finding that may predispose to recurrent respiratory disease, but it is less frequently associated with recurrent pneumonia than dysphagia. GERD is discussed in Chapter 315.1. Aspiration has also been observed in infants with respiratory symptoms but no other apparent abnormalities. Recurrent microaspiration has been reported in otherwise apparently normal newborns, especially premature infants. Aspiration is also a risk in patients suffering from acute respiratory illness from other causes, especially respiratory syncytial virus infection. These patients, when studied with modified barium swallow and videofluoroscopy, have been seen to have silent aspiration. This finding emphasizes the need for a high degree of clinical suspicion for ongoing aspiration in a child with an acute respiratory illness, being fed enterally, who deteriorates unexpectedly.
Diagnosis
Some underlying predisposing factors (see Table 390-1) are frequently clinically apparent but may require specific further evaluation. Initial assessment begins with a detailed history and physical examination. The caregiver should be asked about spitting, vomiting, arching, or epigastric discomfort in an older child, the timing of symptoms in relation to feedings, positional changes, and nocturnal symptoms such as coughing and wheezing. It is important to remember that coughing or gagging may be minimal or absent in a child with a depressed cough or gag reflex. Observation of a feeding is an essential part of the exam when a diagnosis of recurrent aspiration is being considered. Particular attention should be given to nasopharyngeal reflux, difficulty with sucking or swallowing, and associated coughing and choking. The oral cavity should be inspected for gross abnormalities and stimulated to assess the gag reflex. Drooling or excessive accumulation of secretions in the mouth suggests dysphagia. Lung auscultation may reveal transient crackles or wheezes after feeding, particularly in the dependent lung segments.
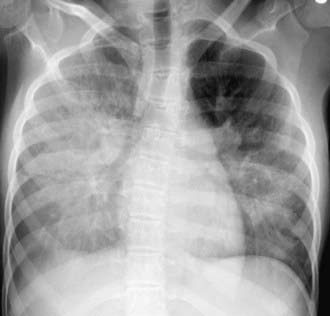
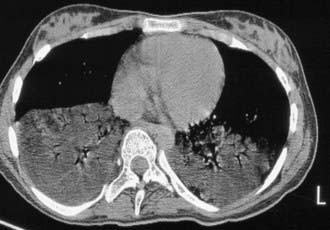
The diagnosis of recurrent microaspiration is challenging because of the lack of highly specific and sensitive tests (Table 390-2). A plain chest radiograph is the usual initial study for a child in whom recurrent aspiration is suspected.The classic findings of segmental or lobar infiltrates localized to dependent areas may be found (Fig. 390-1), but there are a wide variety of radiographic findings. These findings include diffuse infiltrates, lobar infiltrates, bronchial wall thickening, hyperinflation, and even no detectable abnormalities. CT scans, though generally not indicated to establish a diagnosis of aspiration, may show infiltrates with decreased attenuation suggestive of lipoid pneumonia (Fig. 390-2). A carefully performed barium esophagram is useful in looking for anatomic abnormalities such as vascular ring, stricture, hiatal hernia, and tracheoesophageal fistula. It also yields qualitative information about esophageal motility and, when extended, of gastric emptying. However, primarily because of the very short viewing time, the esophagram is quite insensitive and nonspecific for aspiration or GERD. A modified barium swallow study with video fluoroscopy (video fluoroscopic swallowing study) is generally considered the gold standard for evaluating the swallowing mechanism. This study is preferably done with the assistance of a pediatric feeding specialist and a caregiver in the attempt to simulate the usual feeding technique of the child. The child is seated in normal eating position, and various consistencies of barium or barium impregnated foods are offered. This study is more sensitive for demonstrating aspiration than bedside assessment or a traditional barium swallow study. The sensitivity of the modified barium swallow study is such that it occasionally detects aspiration in patients without apparent respiratory abnormalities.
| EVALUATION | BENEFITS | LIMITATIONS |
|---|---|---|
| Chest radiograph | Inexpensive and widely availableAssesses accumulation of injury over time | Insensitive to early subtle changes of lung injury |
| HRCT | Sensitive in detecting lung injury, such as bronchiectasis, tree-in-bud opacities, and bronchial thickeningLess radiation than conventional CTAssesses accumulation of injury over time | More radiation exposure than plain radiographExpensive |
| VSS | Evaluates all phases of swallowingEvaluates multiple consistenciesFeeding recommendations made at time of study | Information limited if child consumes only small quantitiesDifficult to perform in child who has not been feeding by mouthRadiation exposure proportional to study durationCannot be performed at bedsideLimited evaluation of anatomyEvaluates one moment in timeExpensive |
| FEES/with sensory testing | Ability to thoroughly evaluate functional anatomyEvaluates multiple consistenciesCan assess risk of aspiration in nonorally feeding child; airway protective reflexes can be assessedFeeding recommendations made at time of studyVisual feedback for caregiversCan be performed at bedsideNo radiation exposure | Blind to oesophageal phase and actual swallowInvasive and may not represent physiological swallowing conditionsEvaluates one moment in timeNot widely availableExpensive |
| BAL | Evaluates anatomy of entire upper and lower airwaysSamples the endorgan of damageSample available for multiple cytological and microbiological testsBecoming more widely available | Uncertainty regarding interpretation of lipid-laden macrophage indexIndex cumbersome to calculateRequires sedation or anaesthesiaInvasiveExpensive |
| Esophageal pH monitoring | Current gold standard for diagnosis of GOREstablished normative data in children | Blind to majority of reflux eventsDifficult to establish causal relationship between GOR and aspirationSomewhat invasiveEvaluates one moment in time |
| Esophageal impedance monitoring | Likely future gold standard for diagnosis of GERD with supra-esophageal manifestationsAble to detect acid and nonacid reflux eventsDetects proximal reflux eventsAble to evaluate for GERD without stopping medications | Lack of normative data for childrenSomewhat invasiveExpensive and cumbersome to interpretNot widely availableEvaluates one moment in time |
| Gastrooesophageal scintigraphy | Performed under physiological conditionsLow radiation exposure | Poor sensitivityMay not differentiate between aspiration from dysphagia or GERD |
| Radionuclide salivagram | Child does not have to be challenged with food bolusLow radiation exposure | Unknown sensitivityUnknown relationship to disease outcomesEvaluates one moment in time |
| Dye studies | Can be constructed as screening test or confirmatory testCan evaluate aspiration of secretions or feedsRepeating over time allows for broader evaluation | Uncertainty in interpretation owing to variability of techniqueCan only be performed in children with tracheostomies |
HRCT: high-resolution computed tomography; VSS: videofluoroscopic swallow study; FEES: fibreoptic-endoscopic evaluation of swallowing; BAL: bronchoalveolar lavage; CT: computed tomography; GERD, gastroesophageal reflux disease.
From Boesch RP, Daines C, Willging JP, et al: Advances in the diagnosis and management of chronic pulmonary aspiration in children, Eur Respir J 28:847–861, 2006.
Boesch RP, Caines C, Willging JP, et al. Advances in the diagnosis and management of chronic pulmonary aspiration in children. Eur Respir J. 2006;28:847-861.
Celedón JC, Litonjua A, Ryan L, et al. Bottle feeding in the bed or crib before sleep time and wheezing in early childhood. Pediatrics. 2002;110:1-5.
Furuya M, Moreno-Cordova V, Ramirez-Figueroa J, et al. Cutoff valve of lipid-laden alveolar macrophages for diagnosing aspiration in infants in children. Pediatr Pulmonol. 2007;42:452-457.
Heuschkel RB, Fletcher K, Hill A, et al. Isolated neonatal swallowing dysfunction: a case series and review of the literature. Dig Dis Sci. 2003;48:30-35.
Hoffman LR, Yen EH, Kanne JP, et al. Lipoid pneumonia due to Mexican folk remedies. Arch Pediatr Adolesc Med. 2005;159:1043-1048.
Khoshoo V, Ross G, Kelly B, et al. Benefits of thickened feeds in previously healthy infants with respiratory syncytial viral bronchiolitis. Pediatr Pulmonol. 2001;31:301-302.
Newman LA, Keckley C, Petersen MC, et al. Swallow function and medical diagnoses in infants suspected of dysphagia. Pediatrics. 2001;108:E106.
Mercado-Deane MG, Burton EM, Harlow SA, et al. Swallowing dysfunction in infants less than 1 year of age. Pediatr Radiol. 2001;31:423-428.
Owayaed AF, Campbell DM, Wange GG. Underlying causes of recurrent pneumonia in children. Arch Pediatr Adolesc Med. 2000;154:190-194.
Sheikh S, Allen E, Shell R, et al. Chronic aspiration without gastroesophageal reflux as a cause of chronic respiratory symptoms in neurologically normal infants. Chest. 2001;120:1190-1195.
Takamizawa S, Tsugawa C, Nishijima E, et al. Laryngotracheal separation for intractable aspiration pneumonia in neurologically impaired children: experience with 11 cases. J Pediatr Surg. 2003;38:975-977.