Chronic (Hashimoto’s) Thyroiditis
Among the inflammatory diseases in the thyroid gland, chronic thyroiditis is the most common disorder. It is also called autoimmune thyroiditis or Hashimoto’s thyroiditis. Autoimmune thyroiditis is a lifelong autoimmune disease of the thyroid gland. The enlarged thyroid gland gradually atrophies in association with the development of hypothyroidism in typical patients. The first variety of chronic thyroiditis, struma lymphomatosa, was described by Hakaru Hashimoto in 1912.1 Hashimoto reported four patients with diffuse goiter and clarified the four histologic characteristics: diffuse lymphocytic infiltration, formation of lymphoid follicles, destruction of epithelial cells, and proliferation of fibrous tissue. The term Hashimoto’s disease or Hashimoto’s thyroiditis is sometimes used to refer only to goitrous thyroiditis, but it may usually be considered, in a broad sense, a synonym of chronic thyroiditis or autoimmune thyroiditis, including atrophic and nongoitrous thyroiditis. Although the histopathology was well described, no information on its pathogenesis was available until in 1956, Rose and Witebsky2 described thyroglobulin antibodies and thyroiditis in rabbits immunized with thyroid extract. In the same year, Roitt, Doniach, and colleagues reported thyroglobulin antibodies in the serum of patients with Hashimoto’s thyroiditis.3 These observations opened a new era in immunopathology and established the value of measuring antibodies in human autoimmune disease. Hashimoto’s thyroiditis is one of a number of conditions characterized by the presence of thyroiditis.4 Salient features of some of these conditions are shown in Table 12-1. Details of suppurative thyroiditis, a bacterial or fungal infection seen in children and young adults, and Riedel’s thyroiditis, a rare condition with dense fibrosis, are not shown.
Table 12-1

F, Female; M, male; TPOAb, thyroid peroxidase antibodies.
Data from Pearce EN, Farwell AP, Braverman LE, et al: Thyroiditis, N Engl J Med 348:2646, 2003.
This chapter focuses on Hashimoto’s thyroiditis, also known as chronic lymphocytic thyroiditis, chronic autoimmune thyroiditis, and lymphadenoid goiter. In the early stage, patients are euthyroid and have no or a very small goiter. Chronic thyroiditis is subclinical, and the only evidence of autoimmune thyroiditis is the presence of antithyroid antibodies in the serum. Postmortem histologic examination has revealed that positive tests for serum antithyroid antibodies, especially antithyroid microsomal antibodies (now known as thyroid peroxidase antibodies [TPOAb]), in subjects without overt thyroid disease indicate the presence of lymphocytic infiltration into the thyroid.5 As the disease progresses, patients show a firm, diffuse goiter of small to moderate size and generally are said to have chronic autoimmune thyroiditis. Their thyroid function is variable, ranging from euthyroidism to thyrotoxicosis. A large, firm goiter develops when the disease is more advanced; this type is the classical or goitrous Hashimoto’s disease. Further progression of the immune process results eventually in atrophic thyroiditis in association with hypothyroidism. This combination represents the final stage of Hashimoto’s disease.
In the general population, the percentages of women and men with serum TPOAb and antithyroglobulin antibodies (TgAb) increase with age, from about 10% in women in reproductive age to as many as 19% or more among elderly women.6 The prevalence in men is much less—about 5%. Subclinical autoimmune thyroiditis is further evidenced by the fact that thyroid dysfunction develops after delivery in up to 50% of women with positive TPOAb measured in early pregnancy.7 Therefore, when subclinical autoimmune thyroiditis is included, chronic thyroiditis is a very common disease. One in 10 to 30 women in the general population has autoimmune thyroiditis.
Pathology
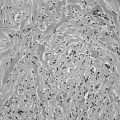
In the classic form of Hashimoto’s thyroiditis (struma lymphomatosa) with a firm, enlarged thyroid, the normal follicular structure is extensively replaced by lymphocytic and plasma cell infiltrates with the formation of lymphoid germinal centers (Fig. 12-1). Thyroid follicles remain isolated or in small clusters, are small or atrophic, and are empty or contain sparse colloid. Some persistent follicular epithelial cells are transformed into Askanazy cells, which have an eosinophilic granular cytoplasm. These cells are found in many other thyroid diseases and probably represent a damaged state of epithelial cells. Fibrosis of variable extent and lymphocytic infiltration are found in the interstitial tissue.
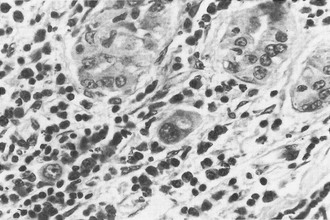
FIGURE 12-1 Hashimoto’s thyroiditis. Note atrophic follicles, absent colloid, and infiltrate of lymphocytes, plasma cells, and immunoblasts (lower left). (Hematoxylin and eosin [H&E], ×200) (From Livolsi VA. In Falk SA, ed: Thyroid disease: endocrinology, surgery, nuclear medicine and radiotherapy, New York, 1990, Raven Press.)
Autoimmune Abnormalities
Initiation of Thyroid Autoimmunity
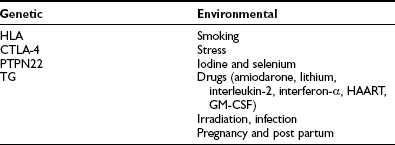
Similar to other autoimmune diseases, autoimmune thyroiditis can arise from a breakdown of self-tolerance to thyroid antigens. Immunologic self-tolerance is thought to be induced during the perinatal period, when immature lymphocytes are exposed to self-antigens.8 At this critical point, clonal deletion or induced anergy of autoreactive T cells in the thymus provides self-tolerance to autoantigens. If an abnormality occurs during this period, self-tolerance might not be induced,8 and autoimmune thyroiditis might develop. An early theory was that a genetically induced organ-specific suppressor T lymphocyte defect could deregulate a thyroid-specific helper T cell population.9 This view has required redefinition in relation to the role of regulatory T cells in thyroid autoimmunity.10 Further breakdown in self-tolerance may be induced by altered self-antigen, exposure to environmental antigens that mimic a self-antigen, polyclonal immune activation, or idiotype cross-reaction of self-antigens. These factors may augment low levels of autoimmune thyroiditis. For example, infection, drugs, or other factors might activate autoreactive helper lymphocytes. Locally produced interferon-γ (IFN-γ) may induce major histocompatibility complex (MHC) class II antigen expression on thyroid cell surfaces, which may promote autoimmunity.11 Environmental factors also play an important role in the pathogenesis of autoimmune thyroiditis. A summary of the genetic and environmental factors that predispose to the condition is shown in Table 12-2. Details are described in subsequent sections.
Environment and Thyroid Autoimmunity
Three studies all imply that smoking may in fact protect against development of TPO antibodies12 and inferentially against chronic lymphocytic thyroiditis. The mechanism for these findings is not clear, although it is known that smoking is a definite risk factor for the development of Graves’ ophthalmopathy and, to a lesser extent, Graves’ disease. Although stress is thought to contribute to the onset of Graves’ hyperthyroidism, no good data relate stressful life events to involvement in the origin of Hashimoto’s disease. Iodine administration is known to induce thyroiditis in susceptible animals by affecting the antigenicity of thyroglobulin.13,14 In contrast to iodine, it is a deficiency of selenium that has been noted to cause increased thyroid volume and reduced echogenicity, together with reduction in immune competence.15 Amiodarone contains 37% iodine, and many of its effects on the thyroid are iodine mediated; in addition, amiodarone administration results in the appearance of thyroid antibodies in patients who have preexisting thyroid autoimmunity.16 A similar situation occurs in patients who are receiving lithium therapy.17 Both of these drugs may have specific immunomodulatory effects, which exacerbate thyroid autoimmunity in Hashimoto’s thyroiditis. Interleukin (IL)-2 and Interferon-α (IFN-α) both cause changes in the immune system characterized by alterations in lymphocyte subsets, which may result in autoimmune thyroid disease.18 Highly active antiretroviral therapy (HAART) and granulocyte-macrophage colony-stimulating factor (GM-CSF) have been noted to be associated with small increases in thyroid autoimmunity in some studies.19,20 External irradiation, as an accident (e.g., Chernobyl), can result in the expression of autoimmune thyroid disease and the emergence of thyroid antibodies.21 Although infection (viral or bacterial) is an attractive factor to be considered as a cause of autoimmune thyroid disease, the data related to Hashimoto’s thyroiditis are tentative at best.22,23 The molecular mimicry hypothesis regarding the increased incidence of Yersinia enterocolitica noted in patients with Graves’ disease has not been confirmed as a causative factor, and this serology is not seen in Hashimoto’s disease. The switch in peripheral lymphocyte pattern from Th2 during pregnancy to a Th1 state post partum is associated with the so-called “immune rebound,” which is characterized by a rapid rise in titers of thyroid antibodies in women who are known to have these antibodies in early gestation. In about 25% to 30% of these women, permanent autoimmune hypothyroidism occurs. Transient postpartum thyroiditis is seen in the remaining antibody-positive women. Thus pregnancy must be regarded as a specific cause of autoimmune thyroid disease in predisposed women. From the foregoing, it will be appreciated that although environmental factors are important, it is necessary to impose these on the appropriate genetic background to initiate the immune process. This, together with an overview of the immune abnormalities seen in Hashimoto’s disease, will be discussed in the following sections.
Genetic Factors
It is widely known that autoimmune thyroid diseases (both Hashimoto’s thyroiditis and Graves’ disease) occur in families.24 Fig. 12-2 shows the age at diagnosis in 400 patients with Hashimoto’s disease (panel A) and shows that those with a positive family history present at a younger age than those who have no family history (panel B). This tendency could be due to genetic predisposition, as well as to environmental influences. Studies of genetic predisposition have revealed that autoimmune thyroid diseases are often associated with particular genetic markers. These markers include histocompatibility lymphocytic antigens, allotypes of immunoglobulin heavy chains, and variations in the T cell receptor (TCR) and the thyroid peroxidase (TPO). More recently, the association of Hashimoto’s disease with variants of CTLA-4, PTNP22, and thyroglobulin has been documented.25 These associations have been examined by linkage analysis, association analysis, and whole genome screening. Findings described in reports are not always consistent with each other, probably because of the subjects chosen and the small sizes of some studies. The main susceptibility genes are shown in Table 12-3.
Table 12-3
Susceptibility Genes for Hashimoto’s Thyroiditis
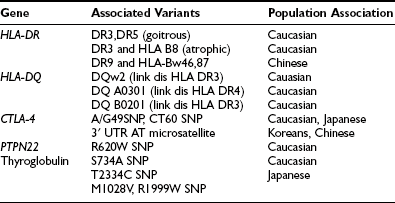
Link dis, Linkage disequilibrium; SNP, single-nucleotide polymorphism.
Data from Jacobson EM, Tomer Y: The genetic basis of thyroid autoimmunity, Thyroid 17:949–961, 2007.
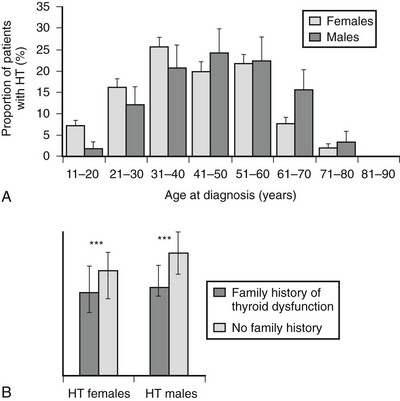
FIGURE 12-2 Age at diagnosis of Hashimoto’s thyroiditis (A) and median age at diagnosis of those with and without a family history of thyroid dysfunction (B). (From Manji N, Carr-Smith JD, Boelaert K, et al: Influences of age, gender, smoking, and family history on autoimmune thyroid disease phenotype, J Clin Endocr Metab 91:4873–4880, 2006.)
Recent studies have highlighted the possibility of association of vitamin D receptor gene polymorphisms with increased risk for Hashimoto’s disease among the Chinese26 and possibly also in the Croatian population.27 Other possible candidate susceptibility genes for Hashimoto’s include an IL-6 gene promoter polymorphism28 and a polymorphism in the IFN-γ gene, the latter being associated with severity of the disease.29 Linkage of specific TCR genes to inheritance of Hashimoto’s thyroiditis has also been reported. A specific TCR restriction fragment length polymorphism (RFLP) was increased in Hashimoto’s thyroiditis, as well as in Graves’ disease,30 and a TaqI RFLP for the Va gene of TCR was also increased.31 TCR Vbeta gene utilization was diminished, but selective expression was not observed in Hashimoto’s in contrast to Graves’ disease.32 Inheritance of specific allotypes of the immunoglobulin G (IgG) heavy chain is also seen in autoimmune thyroiditis.33 The CT60 polymorphism of CTLA-4 maps an important genetic determinant for the risk of Hashimoto’s disease across diverse populations34 and may identify patients with celiac disease who are at risk for Hashimoto’s disease and type 1 diabetes.35 Recently, X chromosome inactivation has been reported to be an important contributor to the increased risk that females may develop Hashimoto’s thyroiditis.36
Antibodies to Thyroid Antigens
Experimental autoimmune thyroiditis with histologic findings similar to those of Hashimoto’s thyroiditis can be induced in animals by immunization with human thyroglobulin in an adjuvant. It can also be produced by depleting rats of T cells followed by thyroglobulin (Tg) administration. In both these animal models, strain specificity is vital, indicating the role of MHC class II-encoded susceptibility.37
Human Tg has at least 40 antigenic epitopes, but only one or two of these bind human TgAbs.38 Although the TgAb response in autoimmune thyroid disease (AITD) is typically polyclonal on isoelectric focusing,39 TgAb from patients with AITD are specific to human Tg and are directed toward a restricted number of epitopes on Tg, unlike rabbit TgAb, which recognizes Tg from other animal species.40 However, thyroglobulin is not isolated from the immune system in the thyroid follicles but is normally present in the circulation of humans.14 Thyroglobulin-binding lymphocytes can also be detected in the fetus.15
Evidence suggests that TgAb from patients with AITD recognize Tg from normal and AITD thyroids differently, suggesting the presence of antigenic variations between the Tg from healthy and AITD thyroids.41 TgAb in AITD is mainly IgG (which consists of four subclasses) rather than IgM, which is more likely to be present in healthy individuals.42 TgAb in AITD states is predominantly of the IgG4 subtype, and the weak complementing fixing property of TgAb is probably due to the predominance of this isotype, which is a poor activator of the complement cascade.43 This implies that antithyroglobulin antibodies are not directly related to tissue damage in Hashimoto’s thyroiditis. The pattern of Tg recognition, as assessed by the use of inhibition of Tg binding by four recombinant TgAb-Fab, showed that the pattern was similar when patients with Hashimoto’s disease were compared with patients with Graves’ disease.44
TgAb are found in patients with AITD but not consistently so, being present in only about 60% of patients.42 Unlike the thyroid peroxidase antibody (TPOAb), TgAb does not fix complement as stated above, probably because the epitopes on the large molecule are widely spaced and are unable to achieve the cross-linking necessary for complement activation. Further, TgAb is also found in healthy individuals with no evidence of thyroid disease.45 A role for TgAb in the pathogenesis of AITD therefore remains unproven.
Thyroid Peroxidase
Thyroid peroxidase (TPO) evokes high-affinity, IgG-class autoantibodies (TPOAb) and TPO-specific T cells that are markers of thyroid infiltration or are implicated in thyroid destruction, respectively.46 Thyroid peroxidase (TPO), the major antigen in human Hashimoto’s disease, was shown to be identical to the previously termed microsomal antigen in the 1980s. TPOAbs in sera from patients with Hashimoto’s thyroiditis are predominantly polyclonal. Anti-TPO antibodies can induce complement-dependent cytotoxicity.47 In fact, antibodies against complement (anti-C1q) are found in patients with Hashimoto’s disease, and they correlate with thyroid-stimulating hormone (TSH) levels. Anti-C1q may be pathogenically involved in destruction in this disease independent of thyroid antibodies.48 Anti-idiotypic antibodies against TPO antibodies are occasionally found in the sera of patients with autoimmune thyroid disease and might be involved in the regulation of autoimmunity.49 A study of epitopic recognition patterns of TPOAb in healthy individuals and patients with Hashimoto’s thyroiditis has shown that specific immunodominant regions are associated with Hashimoto patients but not with normal controls. Whether the propensity to produce antibodies to certain TPO epitopes is of pathogenetic relevance is not clear.50 However, TPOAbs can damage thyroid cells as they activate the complement cascade. TPO itself appears to interact with TPO-specific T cells that are implicated in thyroid destruction. TPO antibodies that are transferred passively from mothers with Hashimoto’s thyroiditis do not seem to damage the thyroid or to affect thyroid function in the fetus or neonate.51
Thyroid-Stimulating Hormone Receptor Antibodies
Thyroid-stimulating hormone (TSH) stimulation-blocking antibody (TSBAb) can inhibit TSH action on thyroid and cause atrophic hypothyroidism in autoimmune thyroiditis.52 Although the specific epitopes for thyroid-stimulating antibody (TSAb) or TSBAb are still uncertain, the major epitope of TSBAb seems to be found in the C-terminal part of the extracellular domain (around 300 to 400 amino acids),53 probably in close proximity to that for TSAb.53 However, in vitro conversion from TSBAb to TSAb after the addition of antihuman IgG antibody suggests that TSAb and TSBAb are not determined solely by their epitopes, and the same TSHR antibody might act as a stimulator or a blocker, depending on the influence of other factors.54 TSBAb are uncommon as a cause of immune-mediated hypothyroidism, although they have been noted in a few patients with goitrous autoimmune thyroiditis, as well as in patients with atrophic chronic thyroiditis.52 Regional variations in prevalence have been noted, with the antibody reported more frequently in Japan.
Other Antibodies
The Na+/I− symporter (NIS), a membrane glycoprotein, mediates iodine uptake into the thyroid follicular cell. Although reports have described antibodies to this moiety in Hashimoto’s thyroiditis, evidence that NIS is a major autoantigen now is not generally accepted. For example, anti-NIS antibodies were found to be positive in only 15% of patients with Hashimoto’s thyroiditis.55
Antibodies to thyroxine (T4) and triiodothyronine (T3) sometimes are found in patients with autoimmune or other thyroid diseases. They are seen in 14% and 35%, respectively, of patients with primary hypothyroidism, in most of whom TGAbs are found in high titer.56 The pathogenetic significance of these antibodies is not known. Probably, they are of little importance as long as the thyroid can produce enough thyroid hormone to sustain adequate serum levels of free hormone. These antibodies interfere with measurements of serum T4 and T3, especially in assays of free T4 and free T3.57 Antibovine TSH autoantibodies, occasionally found in Graves’ disease, are also reported in Hashimoto’s thyroiditis.58 Their pathogenetic significance is unclear. They are speculated to be anti-idiotypic antibodies to anti-TSH receptor antibodies (TRAbs) in Graves’ disease. They may interfere with the measurement of TRAbs and yield unusually high or negative titers. Autoantibodies against several other thyroid components have been reported. Antibodies to colloid antigen-2, distinct from thyroglobulin, have been detected. Antibodies to cell-surface antigen (distinct for TPO) are detected by the patchy immunofluorescent staining of the cell surface or by mixed hemabsorption.
Considerable interest has been expressed in past years in the concept of direct growth-stimulating antibodies or growth-blocking antibodies found in goitrous Hashimoto’s thyroiditis and in primary myxedema, respectively.37 However, contoversy regarding the assay has impeded progress.59 Following the development of a sensitive bioassay for growth-stimulating antibody, which did report positive findings in goitrous Hashimoto’s disease, no studies have explored the use of this assay in greater detail. Autoantibodies against other cellular components, so-called natural antibodies (not always thyroid cell-specific), have also been reported, for example, antibodies to tubulin and calmodulin60 and the ganglioside asialo-GM1, present in the plasma membrane of human thyroid.61 Measurement of natural antibodies (to DNA, actin, myoglobin, myosin, trinitrophenyl hapten, and tubulin) has shown that around 50% of a series of Hashimoto’s patients are positive for one or more of these antibodies, and this positivity appears to correlate with thyroid antibody activity.62 Antibodies to other organs related to autoimmune disease (e.g., islet cells, adrenal cortex, gastric mucosa, parathyroid) are found in autoimmune thyroiditis in higher incidence than in the general population.60 (See section on other autoimmune diseases.)
Cellular Abnormalities
It has become clear that regulatory T cells (T-reg) (with CD4+/CD25 surface expression) play an important role in the maintenance of immune tolerance and the prevention of its breakdown. T-reg express FoxP3, a specific gene marker for the inhibition of expression of inflammatory cytokines. Hence, in vivo depletion of this subset exacerbates autimmune disease in the Hashimoto’s mouse animal model. The development of transgenic animal models of autoimmune thyroiditis has suggested that T-regs have a role in the progression of Graves’ disease to Hashimoto’s thyroiditis and subsequent hypothyroidism.63 In addition to the predominance of the Th1 lymphocyte subset, the B7-1 (CD80) molecule preferentially acts as a co-stimulator for the generation of Th1 cells and has been recognized on thyrocytes in Hashimoto’s disease.64 This phenomenon, together with expression of MHC class II and intercellular adhesion molecule (ICAM)-1,65 leads to T cell differentiation from Th0 to Th1 cells and maintenance of thyroid autoimmunity. Thyroid cells in autoimmune thyroid disease also produce several cytokines (e.g., IL-1, IL-6, tumor necrosis factor-β [TNF-β]) that influence lymphocytic responses (vide infra).
In studies of intrathyroid-infiltrating lymphocytic populations, T lymphocytes predominate over B cells and CD8+ T lymphocytes are increased in Hashimoto’s thyroiditis. Analysis of the gene for the variable region of the a chain (Va gene) of the TCR suggested that the infiltrating T cells are a highly restricted population,66 raising the possibility of a clonal restriction in this autoimmune disease. However, this has not been confirmed.67 Several in vitro cytotoxic mechanisms have been demonstrated. Cytotoxic T cells that are specific for thyroid epithelial cells are observed and antibody-mediated cell cytotoxicity activity can be found in the sera of patients with Hashimoto’s disease. In addition, complement-dependent cytotoxic antibodies are found in sera from these patients, and natural killer lymphocyte counts correlate inversely with thyroid hormone levels. These factors undoubtedly contribute to thyroid follicular cellular damage. Their importance in the whole destructive cascade has been overshadowed to some extent by the data related to apoptosis of thyroid cells (vide infra).
Cytokines
Cytokines are known to have a wide variety of inflammatory and immunomodulatory effects; therefore, it might be thought that many steps in thyroid autoimmunity are mediated and/or modulated by cytokines.68
Characterization of T cells into Th1 and Th2 based on different patterns of cytokine production has aided the understanding of immunologic events in Hashimoto’s disease, although the picture in the human is not as clear as in animals. Cytokines associated with Th1 and Th2 are shown in Table 12-4. In general, the pattern of T cell response in autoimmune hypothyroidism is a Th1 pattern. Following activation by autoantigen recognition, thyroid-specific lymphocytes produce IL-2 with induction of T cell proliferation and differentiation. IFN-γ is also produced and natural killer cells activated. Thyroid autoimmunity occurs in humans who received IL-2 or IFN-γ for the treatment of cancer or viral hepatitis.69,70 A broader range of cytokines then is produced from these activated lymphocytes, resulting in amplification and perpetuation of the immune response. In addition, some cytokines stimulate human lymphocyte antigen (HLA) class II expression on thyroid epithelial cells, which may be important for the amplification and progression of the disease. Data from in vitro effects of cytokines on cultured thyroid cells also indicate that some cytokines (e.g., TNF-α, which is mainly produced by monocytes) may be directly cytotoxic to thyroid cells in vivo. The mechanisms of cytokine interaction are not simple. For example, autoantibodies and complement may promote mixed Th1/Th2 cell cytokine response by enhancing the uptake of autoantigens by antigen presenting cells.71 Another molecule, CTLA-4, whose gene is now a known risk factor for autoimmune thyroid disease, is expressed by activated T and B lymphocytes. Levels of serum CTLA-4 are elevated in autoimmune thyroid disease, and this may play a pathogenetic role in this condition.72 It should be noted that some cytokines (e.g., IL-1, IL-6) are produced by thyrocytes, as well as by lymphocytes. Thus, interleukins produced by thyrocytes would stimulate intrathyroidal lymphocytes. IL-1 stimulates T cells to release lymphokines and has many other inflammatory effects. IL-1 can induce thyrocyte apoptosis through Fas-Fas ligand interaction (vide infra).
Table 12-4
Cytokine Pattern from Th1 and Th2 CD4+ Cells
| Th1 | Th2 | |
| IL-1 | ++ | ++ |
| IL-2 | +++ | − |
| IL-4 | − | +++ |
| Il-5 | − | +++ |
| Il-10 | + | +++ |
| IL-13 | − | +++ |
| IFN-γ | +++ |
Chemokines
Chemokines are a group of low molecular weight proteins that recruit leukocyte subtypes and other cell types to sites of inflammation. They do this by interacting with G protein–coupled receptors, and they play important roles in initiating and maintaining immunologic responses in Hashimoto’s thyroiditis, as well as in other autoimmune endocrine diseases. The main chemokines found in the thyroid in autoimmune thyroid disease are shown in Table 12-5. In human Hashimoto’s disease, there is increased expression of CXCL9 and CXCL10, and the serum level of the latter is higher. The role of chemokines is being further investigated with the use of animal models, particularly a transgenic mouse model expressing CCL21. This mouse does not develop hypothyroidism, despite significant lymphoid infiltration.73 Further work in this area is awaited with interest.
Table 12-5
Chemokines Found in the Thyroid in Autoimmune Thyroid Disease
| Type | Name | Receptor |
| CXC | CXCL1 | CXCR1, CXCR2 |
| CXCL8 | CXCR1, CXCR2 | |
| CXCL9 | CXCR3 | |
| CXCL10 | CXCR3 | |
| CXCL11 | CXCR3, CXCR3B, CXCR7 | |
| CXCL12 | CXCR4 | |
| CXCL13 | CXCR5 | |
| CXCL14 | Unknown | |
| CC | CCL2 | CCR2 |
| CCL3 | CCR1, CCR5 | |
| CCL4 | CCR5 | |
| CCL5 | CCR1, CCR3, CCR5 | |
| CCL18 | Unknown | |
| CCL21 | CCR7 | |
| CCL22 | CCR4 | |
| CCL19 | CCR7 |
C, Cysteine; L, ligand; R, receptor; X, amino acid residue.
CXC have X between two cysteine molecules.
Data from Weetman AR: Cellular immune responses in autoimmune thyroid disease, Clin Endocrinol 61:405–413, 2004; Kimura H, Caturegli P: Chemokine orchestration of autoimmune thyroiditis, Thyroid 17:1005–1011, 2007.
Mechanism for Development of Hypothyroidism
Mounting evidence indicates that destruction of thyroid follicles occurs by the mechanism of apoptosis.74 Apoptosis or programmed cell death is characterized by progressive digestion of the cell and its genetic material by caspases, which are proteolytic enzymes. Thyroid cells express apoptotic ligands and receptors, for example, TNF, Fas, and TRAIL (tumor necrosis–related apoptosis-inducing ligand). In the normal situation, no cell death occurs, but when the thyroid follicular cells are in contact with infiltrating cytotoxic lymphocytes expressing FasL and Bcl-2, apoptosis develops. Fas is an apoptosis-signaling receptor molecule that is found on the surface of a number of cell types.75 Interaction of Fas with its ligand regulates a number of physiologic and pathologic processes involved in cell death. Although Fas ligand is expressed in thyrocytes from both normal controls and patients with Hashimoto’s thyroiditis, Fas is expressed only in thyrocytes from patients with Hashimoto’s thyroiditis.76 Interleukin-1β, which is abundantly produced by the glands of patients with Hashimoto’s thyroiditis, induced Fas expression in normal thyrocytes, and cross-linking of Fas resulted in massive thyrocyte apoptosis. The interaction of the Fas/FasL, which is inhibited in normal thyrocytes, can be activated by proinflammatory cytokines (e.g., IL-1β, IFN-γ, TNF-α), which will arise from Th1 cytokines. Soluble Fas molecules lack the transmembrane domain because of alternative splicing and block Fas-mediated apoptosis. Decreased serum levels of soluble Fas in Hashimoto’s disease may also induce destruction of thyroid cells by promoting their apoptosis.77 Increased serum TSH may inhibit Fas-mediated apoptosis of thyrocytes,78 and TSBAb may block the inhibitory action of TSH toward Fas-mediated apoptosis, thus inducing thyroid atrophy. Indeed, the expression of FasL on thyrocytes correlated postively with TRAb in Graves’ thyrocytes, but no correlation was found with antibody titer in Hashimoto’s thyrocytes.79 However, serum levels of Fas correlate with TPOAb and TSH in Hashimoto’s patients,80 and soluble CD40 correlates with TPOAb.81 Transgenic expression of FasL on thyroid follicular cells actually prevents autoimmune thyroiditis, possibly through inhibition of lymphocyte infiltation.82 Mutations of Fas, which induce loss of function, were found in thyroid lymphocytes in 38.1% of patients with Hashimoto’s thyroiditis83 and in 65.4% of patients with malignant lymphoma,84 which usually develops from Hashimoto’s thyroiditis.
The TRAIL molecule is a member of the TNF group that exists in membrane-bound and soluble form. It induces apoptosis through interaction with its receptors via a caspase-dependent pathway. Molecular regulation of apoptosis in the thyroid is unknown, but it is reasonable to suggest that defects in T regulatory cells, referred to in the section on cellular events, are related to the apoptosis. Indeed, the expression of Fas in CD4+ T-reg cells is greater in severe thyroiditis.85 In summary, Fas/FasL-mediated apoptosis plays an important role in the active stage of the autoimmune process in Hashimoto’s thyroiditis and may contribute to the irreversible damage of thyrocytes. Furthermore, soluble CD40 may serve as a marker for these events.
Other mechanisms of cell death in autoimmune thyroiditis include exocytic granules that contain perforin and granzyme B and complement activation. Complement activation may not kill cells but may help to maintain the inflammatory disease by exacerbating the immune process.86 A schematic overview of the immunopathologic process of the development of thyroid autoimmunity is shown in Fig. 12-3. Inevitably, this is simplistic, and many molecular details still are not clear.
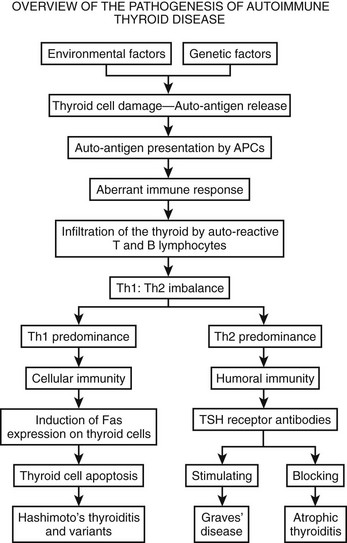
FIGURE 12-3 Development of Hashimoto’s thyroiditis and Graves’ disease is shown, in addition to the effect of thyroid-stimulating hormone (TSH) receptor blocking antibody. (Adapted from Fontoulakis and Tsatsoulis: on the pathogenesis of autoimmune thyroid disease: a unifying hypothesis, Clin Endocr 60, 397–409, 2004.)
Clinical Features
Most patients with Hashimoto’s thyroiditis are euthyroid or have subclinical hypothyroidism with goiter and circulating thyroid antibodies. As time passes, overt hypothyroidism will develop at a rate of about 5% per year.6 The goiter becomes smaller and occasionally atrophies (atrophic thyroiditis). Histologically, fibrosis spreads, and few thyroid follicles remain in this stage of thyroiditis. The patient is usually an asymptomatic female. A goiter may be found incidentally at medical examination, but some patients present with discomfort related to the presence of a goiter. Thyroid enlargement in thyroiditis is usually lobular and irregular. It may be smooth or bosselated. The consistency is often firm and in some cases hard, suggesting a diagnosis of thyroid cancer. In rare cases, thyroid enlargement can encircle the trachea, causing compressive symptoms such as dysphagia, hoaresness, and dysphonia. Very rarely, the goiter is painful, suggesting a diagnosis of subacute thyroiditis. Some patients present with an apparent single thyroid nodule, even in the presence of circulating thyroid antibodies. In this situation, full investigation of the nodule must be performed to exclude malignancy, as the two conditions may coincide. In cases of diagnostic uncertainty, a fine needle biopsy, together with thyroid ultrasound, will clarify the problem. Although most patients will be euthyroid at presentation, some may even be hyperthyroid. Elderly patients are more likely to be found hypothyroid, although the presence of thyroid antibodies does not indicate progression to hypothyroidism at the same rate as in younger patients.87 As was already mentioned, the presence of circulating thyroid antibodies, particularly TPOAb, is the hallmark of Hashimoto’s thyroiditis; however, a minority of patients also have thyroid receptor blocking as well as stimulating immunoglobulins. Hypothyroidism then will be related to the blocking antibodies and the autoimmune process. In some instances, however, a patient may become hyperthyroid because of the stimulating antibodies after many years of replacement of levothyroxine for autoimmune hypothyroidism. Patients with an acute aggravation of thyroid autoimmunity are subject to destruction-induced thyrotoxicosis. These episodes usually are followed by transient hypothyroidism. High iodine ingestion can aggravate autoimmune thyroiditis, thus inducing hypothyroidism.
In children, Hashimoto’s disease is less common and titers of antithyroid antibodies are usually lower than in adult patients. Autoimmune thyroid disease is uncommon in children and adolescents, and the prevalence of self-limiting autoimmune thyroiditis is significant.88 They usually have a small symptomless goiter, and hypothyroidism is uncommon; thyroid dysfunction reverts to normal in up to 50% during follow-up.89–91
Relation to Silent and Postpartum Thyroiditis
Another form of thyroiditis, also believed to be of autoimmune cause, is variably referred to as painless, silent, occult, subacute, subacute nonsuppurative, and atypical (silent) subacute thyroiditis; it is also known as hyperthyroiditis, transient thyrotoxicosis with low thyroidal radioactive iodine uptake (RAIU), and lymphocytic thyroiditis with spontaneously resolving hyperthyroidism. No agreement has been reached on an inclusive name. Features of this disease entity overlap those of De Quervain’s thyroiditis and Hashimoto’s thyroiditis. The clinical course resembles that of De Quervain’s (subacute) thyroiditis, except that no neck or thyroid pain is present and the erythrocyte sedimentation rate is not elevated, at least not to a very high level. Histologically, the condition cannot be differentiated from a milder form of Hashimoto’s disease. The clinical features of the condition are firstly characterized by an initial phase of hyperthyroidism due to leakage of intrathyroidal hormones into the circulation after damage to thyroid epithelial cells from inflammation.92 Thus, thyroid radioactive iodine uptake is low. This transient hyperthyroid phase is followed by hypothyroidism, which may be transient or, in a minority, permanent. A significant percentage of patients with silent thyroiditis have personal or family histories of autoimmune thyroid disease. Most patients have a complete remission, but persistent hypothyroidism develops in some.93 Recurrence of disease is common in silent thyroiditis but very rare in subacute thyroiditis. It appears that silent thyroiditis is caused by the exacerbation of autoimmune thyroiditis induced by aggravating factors. Thyroiditis frequently recurs, and seasonal allergic rhinitis is reported to be an initiation factor,94 as well as vigorous massage of the neck.95
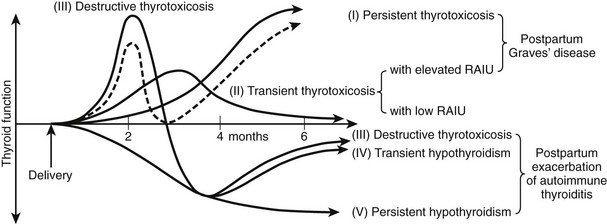
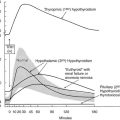
The occurrence of lassitude and other symptoms of hypothyroidism related to the postpartum period was first noted in 1948.96 These complaints were treated successfully with thyroid extract. The syndrome was later confirmed as postpartum thyroiditis (PPT), and the immune nature of the condition has been discussed.97 Postpartum thyroiditis is essentially silent thyroiditis in the postpartum period. The term postpartum thyroiditis relates to destructive thyroiditis that occurs during the first 12 months postpartum and not to Graves’ disease, although the two conditions may be seen concurrently. The origin is related to the change in immune status that follows delivery associated with the development or exacerbation of thyroid dysfunction (Fig. 12-4). PPT with thyroid dysfunction (i.e., postpartum thyroid dysfunction [PPTD]) is characterized by an episode of transient hyperthyroidism followed by transient hypothyroidism. The former presents at about 14 weeks’ postpartum and is followed by transient hypothyroidism at a median of 19 weeks. Very occasionally, the hypothyroid state is seen before hyperthyroidism is noted. The thyroid dysfunction that occurs in up to 50% of TPO-antibody positive women ascertained at around 14 weeks’ gestation involves 19% with hyperthyroidism alone, 49% with hypothyroidism alone, and the remaining 32% with hyperthyroidism followed by hypothyroidism (i.e., biphasic). Not all women manifest both thyroid states, and the hyperthyroid episode may escape detection as it may be of short duration. PPTD is almost always associated with the presence of antithyroid antibodies, usually anti-TPO antibodies that rise in titer at about 6 weeks’ postpartum. Anti-Tg antibody occurs in about 15% and is the sole antibody in less than 5%. However, postpartum thyroid dysfunction has been described in small numbers of women who have not been shown to have circulating thyroid antibodies.98 Although the clinical manifestations of the hyperthyroid state are not usually severe, lack of energy and irritability are particularly prominent, even in thyroid antibody–positive women who do not develop thyroid dysfunction. In contrast, the symptoms of the hypothyroid phase may be profound. Many classic hypothyroid symptoms occur before the onset of thyroid hormone reduction and persist even when recovery of hormone levels is seen. Postpartum thyroiditis can also occur in women receiving T4 therapy before pregnancy and after miscarriage. Abundant evidence indicates that postpartum thyroiditis is an immunologically related disease.98 Biopsy of the thyroid in this syndrome shows lymphocytic infiltration similar to that seen in Hashimoto’s thyroiditis.99
Relation to Graves’ Disease
After treatment, patients with Graves’ disease often progress to hypothyroidism,100 possibly because of the autoimmune tissue destruction described above. Another mechanism for the appearance of hypothyroidism is an increase in TSBAb, which has been found in 11.1% of thyrotoxic patients with Graves’ disease who had blocking activity to TSH-induced adenylate cyclase stimulation but no TSAb activity,101 that is, some patients with Graves’ disease have both stimulating and blocking antibodies. When they have predominantly stimulating antibodies, hyperthyroidism develops, and when blocking antibodies become predominant, patients progress to hypothyroidism. The clinical features of patients depend on the balance among stimulating, blocking, and destructive aspects of humoral and cellular immunity. When stimulating factors are predominant, Graves’ thyrotoxicosis develops. Predominance of destructive factors, such as antibody dependent cytotoxicity (ADCC), T lymphocyte cytotoxicity, lymphotoxin (TNF), and cytotoxic antibody, may produce Hashimoto’s disease or myxedema. Blocking factors such as TSBAb also cause a reduction in thyroid function. Once thyroid cells are destroyed completely, stimulating factors are ineffective. Clinically, it is important to recognize that patients who are receiving levothyroxine for hypothyroidism due to Hashimoto’s thyroiditis may develop thyrotoxicosis due to Graves’ disease because of the presence of TSAb.102 This implies of course that viable thyroid tissue is present that has not been completely destroyed by the destructive factors mentioned above.
Relation to Other Autoimmune Diseases
Patients with other autoimmune diseases are often found to be positive for thyroglobulin antibodies; these disorders are thus associated with autoimmune thyroid disease.4 The incidence is higher than in the general population. Autoimmune thyroid disease is found in association with both organ-specific autoimmune disease (e.g., vitiligo, myasthenia gravis, thrombocytopenic purpura, alopecia, Sjögren’s syndrome)103 and systemic autoimmune disease (e.g., rheumatoid arthritis, systemic lupus erythematosus, progressive systemic sclerosis). An association with other endocrine autoimmune diseases (such as insulin-dependent diabetes mellitus, autoimmune adrenalitis, autoimmune hypoparathyroidism, and autoimmune hypophysitis) is also found in autoimmune thyroiditis (Table 12-6). Such autoimmunity may occur simultaneously in multiple organs (polyendocrine autoimmune disease). During the past decade, it has been realized that celiac disease is more common in patients with Hashimoto’s disease than was previously thought. In one recent study, 15% of patients with Hashimoto’s disease had positive serology for celiac disease, and, conversely, 21% of patients with celiac disease had positive thyroid antibodies. Screening of patients with Hashimoto’s disease for celiac disease and vice versa should be considered.104 It is important to note that the increased frequency of chronic anemia in patients with autoimmune thyroid disease is due to concomitant autoimmune gastrointestinal disease.105 On the other hand, the frequency with which patients with autoimmune thyroid disease suffer from another autoimmune disease is low, except for autoimmune gastritis and low B12 levels.106 Antiparietal cell antibodies and/or anti-intrinsic factor antibodies are found in about one third of patients with autoimmune thyroid disease.
Table 12-6
Risk for Other Autoimmune Diseases in Hashimoto’s Thyroiditis
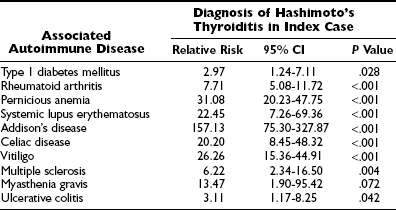
Relative risk of diagnosis of other autoimmune diseases in index cases.
Data from Boelaert, Newby, Simmonds, et al: The quantification of other autoimmune diseases in subjects with autoimmune thyroid disease: a large cross-sectional study, Amer J Med, 2009.
Hashimoto’s Encephalopathy
Hashimoto’s encephalopathy or encephalitis is a rare complication of Hashimoto’s thyroiditis. It is a steroid-responsive, relapsing encephalopathy associated with thyroid antibodies. It is more common in females and has been reported in children, adults, and the elderly.107 Neurologic complications sometimes are associated with thyroid dysfunction, but patients with this encephalopathy are usually euthyroid. It is a treatable, steroid-responsive, progressive or relapsing encephalopathy associated with elevation of thyroid-specific autoantibodies.108 The condition was first described in 1966109 and may present as a subacute or acute encephalopathy with seizures and stroke-like episodes, often in association with myoclonus and tremor.110 Seizures, cognitive decline, and behavioral problems are seen in chidren.111 It is associated with abnormal electroencephalogram (EEG) and high cerebrospinal fluid proteins without pleocytosis. Some patients have significant residual disability.112 Antibody to α-enolase has been identified in some patients,113,114 but this antibody is frequently found in other autoimmune diseases as well. Although this condition may represent an association of an uncommon autoimmune encephalopathy with autoimmune thyroid disease,115 the finding of TPOAb binding to specific astrocytes is of interest.116 The autoimmune response to enzymes identified as the IgG autoimmune response in cerebrospinal fluid (CSF) from patients may indicate mechanisms of vascular and or neuronal damage in this autoimmune inflammatory condition.117
Relation to Thyroid Cancer
The association between autoimmune thyroiditis and B cell lymphoma is well recognized. Sequence similarity between clonal bands in Hashimoto’s thyroiditis and thyroid lymphoma (as determined by polymerase chain reaction [PCR] and sequence determination of immunoglobulin heavy chain gene rearrangements) suggests strongly that primary thyroid lymphoma may evolve from Hashimoto’s thyroiditis.118 It has been generally assumed that differentiated thyroid cancer is not more frequent in patients with Hashimoto’s disease, but several reports, including two recent large studies,119,120 have shown that patients with Hashimoto’s disease may be up to three times more likely to have papillary thyroid cancer. Molecular studies have shown that the RET/PTC-RAS-BRAF cascade is expressed more commonly in non-neoplastic follicular cells in thyroiditis, indicating overlapping molecular mechanisms that regulate early tumor development and inflammation in Hashimoto’s thyroiditis.121,122
Diagnosis
The diagnosis of autoimmune thyroiditis is usually simple to make by clinical observation and serologic tests, especially in overt hypothyroidism. A goiter that may be diffuse or nodular and positive antithyroid antibodies (anti-TPO antibodies and/or antithyroglobulin antibodies) with no evidence of other thyroid disease lead to the diagnosis of goitrous Hashimoto’s thyroiditis. Tests of thyroid function (free T4, free T3, and TSH) might not be helpful because thyroiditis is subclinical in about 90% of patients. About 90% of patients with Hashimoto’s disease have positive TPOAbs, but no difference is seen in titers and in the prevalence of antibodies between euthyroid and hypothyroid patients. In patients who are found to have thyroid antibodies but no palpable goiter, a thyroid ultrasound will reveal a hypoechoic pattern consistent with Hashimoto’s thyroiditis (Fig. 12-5). In patients who have primary hypothyroidism with an atrophic thyroid, the existence of blocking-type anti–thyrotropin receptor (TSHR) antibodies (TSBAbs) can be assessed, although their prevalence is low even in Japan.123 Histologic examination is confirmative but not necessary for the diagnosis and management of thyroiditis because lymphocytic infiltration into the thyroid is observed in all seropositive patients.5 Conversely, TPOAb or TgAb is detectable in more than 95% of histologically confirmed cases of Hashimoto’s disease. Indeed, detection of the antibodies alone is sufficient for a diagnosis of autoimmune thyroid disease. Antibodies are also found in about 10% of individuals in the general population with no clinical manifestations, and these patients should be considered to have subclinical autoimmune thyroiditis. For the small number of seronegative patients who have overt or latent hypothyroidism, the diagnosis of Hashimoto’s thyroiditis is likely and can be confirmed by ultrasound examination. Fine needle aspiration biopsy will show cytologic evidence of thyroiditis (Fig. 12-6), but histologic examination is the only way to absolutely prove its existence. Thyroid biopsy will be necessary if the goiter is rapidly increasing and is very hard or fixed, that is, when thyroid tumors are suspected.
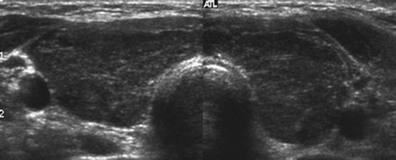
FIGURE 12-5 Ultrasound of patient with Hashimoto’s thyroiditis. Note bilateral hypoechogenicity. (Courtesy Dr. Peter Smyth.)
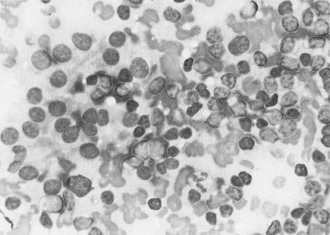
FIGURE 12-6 Hashimoto’s thyroiditis: fine needle aspiration biopsy. Note loose clusters of follicular epithelial cells and a range of lymphoid cells. (From Orell SR, Philips J: Monographs in clinical cytology. In Orell SR, ed: The thyroid fine-needle biopsy and cytological diagnosis of thyroid lesions, vol 14, Basel, Switzerland, 1997, Karger, p 68.)
Natural History
Patients may experience periods of transient hypothyroidism under certain conditions. In some cases of Hashimoto’s thyroiditis, the hypothyroidism seems to be reversible.124 This transient hypothyroidism may occur in the course of Hashimoto’s thyroiditis when destruction of the thyroid is slow. Hypothyroidism is often transient when goiter is present and the serum thyroglobulin concentration is high.125 The thyroid response to TSH after the administration of thyrotropin-releasing hormone can be used to evaluate potential recovery from hypothyroidism in patients undergoing T4 therapy.126 In a region where iodine-containing food (such as seaweed) is common, as in Japan, excessive dietary iodine intake (1000 mg/day or more) can cause transient hypothyroidism in patients with subclinical autoimmune thyroiditis. This condition is easily reversible with reduction of iodine intake.127
A rare but important complication of Hashimoto’s thyroiditis is malignant lymphoma. The tumor arises in patients with Hashimoto’s thyroiditis in around 40% to 80% of cases128; hence, it has a female preponderance. The patient is usually in the sixth decade or older and presents with a rapidly enlarging neck mass on a background of previously diagnosed autoimmune thyroiditis, often while on levothyroxine treatment. Rapid diagnosis is made by fine needle biopsy and external radiotherapy, and chemotherapy can result in dramatic shrinkage of the tumor with relief of pressure symptoms and remission in most cases.
Treatment
No practical way has been found to manipulate the autoimmune abnormality itself. In a study in which euthyroid patients with Hashimoto’s thyroiditis were randomly allocated to levothyroxine treatment or simple follow-up, a significant increase in free T4 and a significant decrease in TSH and antithyroglobulin antibody anti–thyroid peroxidase antibody levels were noted after 15 months in the T4-treated group. Although no change in cytologic findings was seen, ultrasonography showed a decrease in thyroid volume in l-thyroxine–receiving patients, whereas an increase was detected in patients who were followed without treatment. It seems therefore that thyroid hormone therapy may be beneficial in patients with Hashimoto’s thyroiditis, even if they are euthyroid.129 Recently it was observed that the administration of selenium to patients with Hashimoto’s thyroiditis is accompanied by a significant fall in titers of thyroid antibodies compared with a control group.130 However, no evidence of reversal of the pathology was found. Hypothyroidism in patients with large goiters and high iodine uptake is often transient,124 especially in patients younger than 30 years. Restriction of high iodine ingestion may be effective in these patients, but surgical resection may be required occasionally. Such patients can be treated successfully with radioiodine, with a size reduction of around 50%.131 At present, the major approach is to treat the associated hypothyroidism or to attempt to shrink the goiter to release pressure symptoms or simply for cosmetic reasons. T4 replacement therapy is not always necessary in euthyroid patients with small or moderate goiters. These patients should be examined once a year to detect the later development of hypothyroidism. When hypothyroidism develops, patients should be treated with T4. The daily replacement dose is 100 to 200 mcg/day (about 2 mcg/kg/day). In patients with long-standing hypothyroidism, replacement therapy should be initiated with a small dose of T4 and should be built up gradually until a satisfactory maintenance dose is achieved. Hypothyroidism in patients with postpartum thyroiditis is usually transient. In these patients, lifelong T4 therapy is not necessary. T3 rather than T4 therapy may be useful for a short period to quickly relieve hypothyroid symptoms in some cases.
References
1. Hashimoto, H. Zur Kenntniss der lymphomatösen Veränderung der Schilddrüse (Struma lymphomatosa). Arch Klin Chir. 1912;97:219.
2. Rose, NR, Witebsky, E. Studies in organ specificity. V. Changes in the thyroid glands of rabbits following activ immunisation with rabbit thyroid extracts. J Immunol. 1956;76:417–427.
3. Roit, IM, Doniach, D, Campbell, PN, et al. Autoantibodies in Hashimoto’s disease (lymphadenoid goitre). Lancet. 1956;ii:820–821.
4. Pearce, EN, Farwell, AP, Braverman, LE, et al. Thyroiditis. N Engl J Med. 2003;348:2646–2655.
5. Yoshida, H, Amino, N, Yagawa, K, et al. Association of serum antithyroid antibodies with lymphocytic infiltration of the thyroid gland: Studies of seventy autopsied cases. J Clin Endocrinol Metab. 1978;46:859.
6. Vanderpump, MPJ. The epidemiology of thyroid disease. In: Braverman LE, Utiger RD, eds. Werner & Ingbar’s The Thyroid A Fundamental and Clinical Text. ed 9. Philadelphia: Lippincott Williams & Wilkins; 2005:398–406.
7. Lazarus, JH. Sporadic and Postpartum thyroiditis. In: Braverman LE, Utiger RD, eds. Werner & Ingbar’s The Thyroid A Fundamental and Clinical Text. ed 9. Philadelphia: Lippincott Williams & Wilkins; 2005:524–535.
8. Nossal, GJ, Pike, BL. Evidence for the clonal abortion theory of B-lymphocyte tolerance. 1975. J Immunol. 2007;179:5619–5632.
9. Volpe, R, Iitaka, M. Evidence for an antigen-specific defect in suppressor T-lymphocytes in autoimmune thyroid disease. Exp Clin Endocrinol. 1991;97:133–138.
10. Quaratino, S, Badami, E. Regulatory T cells and thyroid autoimmunity. In: Wiersinga WM, Drexhage HA, Weetman AP, et al, eds. The Thyroid and Autoimmunity. Stuttgart: Verlag; 2007:74–81.
11. Bottazzo, GF, Pujol-Borrell, R, Hanafusa, T, et al. Role of aberrant HLA-DR expression and antigen presentation in induction of endocrine autoimmunity. Lancet. 1983;2:1115–1119.
12. Krassas, GE, Wiersinga, WM. Smoking and autoimmune thyroid disease: the plot thickens. Eur J Endocrinol. 2006 Jun;154:777–780.
13. Rose, NR, Bonita, R, Burek, CL. Iodine: an environmental trigger of thyroiditis. Autoimmun Rev. 2002;1:97–103.
14. Camargo, R, Tomimori, E, Neves, S, et al. Thyroid and the environment: exposure to excessive nutritional iodine increases the prevalence of thyroid disorders in Sao Paulo, Brazil. Eur J Endocrinol. 2008 Jun 27. [in press].
15. Bartalena, L, Tanda, ML, Piantanida, A, et al. Environment and thyroid autoimmunity. In: Wiersinga WM, Drexhage HA, Weetman AP, et al, eds. The Thyroid and Autoimmunity. Stuttgart: Verlag; 2007:60–73.
16. Martino, E, Bartalena, L, Bogazzi, F, et al. The effects of amiodarone on the thyroid. Endocr Rev. 2001;22:240–254.
17. Lazarus, JH. The effects of lithium therapy on thyroid and thyrotropin-releasing hormone. Thyroid. 1998;8:909–913.
18. Sharma, RB, Burek, CL, Cihakova, D, et al. Environmental factors in autoimmune endocrinopathies. In: Weetman AP, ed. Autoimmune Diseases in Endocrinology. Totowa: Humana Press; 2008:35–75.
19. Chen, F, Day, SL, Metcalfe, RA, et al. Characteristics of autoimmune thyroid disease occurring as a late complication of immune reconstitution in patients with advanced immunodeficiency virus (HIV) disease. Medicine. 2005;84:98–106.
20. Hoekman, K, von Blomberg-von dar, F, Wagstaff, J, et al. Reversible thyroid dysfunction during treatment with GM-CSF. Lancet. 1991;338:541–542.
21. Eheman, CR, Garbe, P, Tuttle, RM. Autoimmune thyroid disease associated with thyroid irradiation. Thyroid. 2003;13:453–464.
22. Mori, K, Munakata, Y, Saito, T, et al. Intrathyroidal persistence of human parvovirus B19 DNA in a patient with Hashimoto’s thyroiditis. J Infect. 2007;55:29–31.
23. Thomas, D, Liakos, V, Michou, V, et al. Detection of herpes virus DNA in post-operative thyroid tissue specimens of patients with autoimmune thyroid disease. Exp Clin Endocrinol Diabetes. 2008;116:35–39.
24. Manji, N, Carr-Smith, JD, Boelaert, K, et al. Influences of age, gender, smoking, and family history on autoimmune thyroid disease phenotype. J Clin Endocrinol Metab. 2006;91:4873–4880.
25. Jacobson, EM, Tomer, Y. The genetic basis of thyroid autoimmunity. Thyroid. 2007;17:949–961.
26. Lin, WY, Wan, L, Tsai, CH, et al. Vitamin D receptor gene polymorphisms are associated with risk of Hashimoto’s thyroiditis Chinese patients in Taiwan. J Clin Lab Anal. 2006;20:109–112.
27. Stefanic, M, Papi , S, Suver, M, et al. Association of vitamin D receptor gene 3′-variants with Hashimoto’s thyroiditis in the Croatian population. Int J Immunogenet. 2008;35:125–131.
, S, Suver, M, et al. Association of vitamin D receptor gene 3′-variants with Hashimoto’s thyroiditis in the Croatian population. Int J Immunogenet. 2008;35:125–131.
28. Chen, RH, Chang, CT, Chen, WC, et al. Proinflammatory cytokine gene polymorphisms among Hashimoto’s thyroiditis patients. J Clin Lab Anal. 2006;20:260–265.
29. Ito, C, Watanabe, M, Okuda, N, et al. Association between the severity of Hashimoto’s disease and the functional +874A/T polymorphism in the interferon-gamma gene. Endocr J. 2006;53:473–478.
30. Ito, M, Tanimoto, M, Kamura, H, et al. Association of HLA antigen and restriction fragment length polymorphism of T cell receptor beta-chain gene with Graves’ disease and Hashimoto’s thyroiditis. J Clin Endocrinol Metab. 1989;69:100–104.
31. Weetman, AP, So, AK, Roe, C, et al. T-cell receptor alpha chain V region polymorphism linked to primary autoimmune hypothyroidism but not Graves’ disease. Hum Immunol. 1987;20:167–173.
32. Zhang, J, Zhang, M, Wang, Y. Infiltrating T-lymphocyte receptor V beta gene family utilization in autoimmune thyroid disease. J Int Med Res. 2006;34:585–595.
33. Tamai, H, Uno, H, Hirota, Y, et al. Immunogenetics of Hashimoto’s and Graves’ diseases. J Clin Endocrinol Metab. 1985;60:62–66.
34. Kavvoura, FK, Akamizu, T, Awata, TJ, et al. Cytotoxic T-lymphocyte associated antigen 4 gene polymorphisms and autoimmune thyroid disease: a meta-analysis. J Clin Endocrinol Metab. 2007;92:3162–3170.
35. Dallos, T, Avbelj, M, Barák, L, et al. CTLA-4 gene polymorphisms predispose to autoimmune endocrinopathies but not to celiac disease. Neuro Endocrinol Lett. 2008;29:334–340.
36. Yin, X, Latif, R, Tomer, Y, Davies, TF. Thyroid epigenetics: X chromosome inactivation in patients with autoimmune thyroid disease. Ann N Y Acad Sci. 2007;1110:193–200.
37. Ludgate, M. Animal models of autoimmune disease. In: Weetman AP, ed. Autoimmune Diseases in Endocrinology. Totowa: Humana Press; 2008:79–93.
38. Roitt, IM, Campbell, PN, Doniach, D. The nature of the thyroid autoantibodies present in patients with Hashimoto’s thyroiditis (lymphadenoid goitre). Biochem J. 1958;69:248–256.
39. Nye, L, De Carvalho, LP, Roitt, IM. An investigation of the clonality of human autoimmune thyroglobulin antibodies and their light chains. Clin Exp Immunol. 1981;46:161–170.
40. Chan, CT, Byfield, PG, Himsworth, RL, et al. Human autoantibodies to thyroglobulin are directed towards a restricted number of human specific epitopes. Clin Exp Immunol. 1987;69:516–523.
41. Kim, PS, Dunn, AD, Dunn, JT. Altered immunoreactivity of thyroglobulin in thyroid disease. J Clin Endocrinol Metab. 1988;67:161–168.
42. McIntosh, RS, Asghar, MS, Weetman, AP. The antibody response in human autoimmune thyroid disease. Clin Sci. 1997;92:529–541.
43. Parkes, AB, McLachlan, SM, Bird, P, et al. The distribution of microsomal and thyroglobulin antibody activity among the IgG subclasses. Clin Exp Immunol. 1984;57:239–243.
44. Latrofa, F, Ricci, D, Grasso, L, et al. Characterization of thyroglobulin epitopes in patients with autoimmune and non-autoimmune thyroid diseases using recombinant human monoclonal thyroglobulin autoantibodies. J Clin Endocrinol Metab. 2007;93:591–596.
45. Guilbert, B, Dighiero, G, Avrameas, S. Naturally occurring antibodies against nine common antigens in human sera. I. Detection, isolation and characterization. J Immunol. 1982;128:2779–2787.
46. McLachlan, SM, Rapoport, B. Thyroid peroxidase as an autoantigen. Thyroid. 2007;17:939–948.
47. Khoury, EL, Hammond, L, Bottazzo, GF, et al. Presence of the organ-specific “microsomal” autoantigen on the surface of human thyroid cells in culture: Its involvement in complement-mediated cytotoxicity. Clin Exp Immunol. 1981;45:316–328.
48. Potlukova, E, Jiskra, J, Limanova, Z, et al. Autoantibodies against complement C1q correlate with the thyroid function in patients with autoimmune thyroid disease. Clin Exp Immunol. 2008 Jul;153(1):96–101.
49. Tandon, N, Jayne, DR, McGregor, AM, et al. Analysis of anti-idiotypic antibodies against anti-microsomal antibodies in patients with thyroid autoimmunity. J Autoimmun. 1992;5:557–570.
50. Nielsen, CH, Brix, TH, Gardas, A, et al. Epitope recognition patterns of thyroid peroxidase autoantibodies in healthy individuals and patients with Hashimoto’s thyroiditis. Clin Endocrinol (Oxf). 2008. [in press].
51. Tamaki, H, Amino, N, Aozasa, M, et al. Effective method for prediction of transient hypothyroidism in neonates born to mothers with chronic thyroiditis. Am J Perinatol. 1989;6:296–303.
52. Konishi, J, Iida, Y, Kasagi, K, et al. Primary myxedema with thyrotrophin-binding inhibitor immunoglobulins. Clinical and laboratory findings in 15 patients. Ann Intern Med. 1985;103:26–31.
53. Rees Smith, B, Sanders, J, Furmaniak, J. TSH receptor antibodies. Thyroid. 2008;17:923–938.
54. Amino, N, Watanabe, Y, Tamaki, H, et al. In-vitro conversion of blocking type anti-TSH receptor antibody to the stimulating type by anti-human IgG antibodies. Clin Endocrinol (Oxf). 1987;27:615–624.
55. Endo, T, Kogai, T, Nakazato, M, et al. Autoantibody against Na+/I-symporter in the sera of patients with autoimmune thyroid disease. Biochem Biophys Res Commun. 1996;224:92–95.
56. Staeheli, V, Vallotton, MB, Burger, A. Detection of human anti-thyroxine and anti-triiodothyronine antibodies in different thyroid conditions. J Clin Endocrinol Metab. 1975;41:669–675.
57. John, R, Othman, S, Parkes, AB, et al. Interference in thyroid function tests in postpartum thyroiditis. Clin Chem. 1991;37:1397–1400.
58. Sakata, S, Takuno, H, Nagai, K, et al. Anti-bovine thyrotropin autoantibodies in patients with Hashimoto’s thyroiditis, subacute thyroiditis, and systemic lupus erythematosus. J Endocrinol Invest. 1991;14:123–130.
59. Zakaria, M, MacKenzie, J. Do thyroid growth-promoting immunoglobulins exist? J Clin Endocrinol Metab. 1990;70:308–310.
60. DeGroot, LJ, Quintans, J. The causes of autoimmune thyroid disease. Endocr Rev. 1989;10:537–562.
61. Sawada, K, Sakurami, T, Imura, H, et al. Anti-asialo-GM1 antibody in sera from patients with Graves’ disease and Hashimoto’s thyroiditis. Lancet. 1980;2:198.
62. Jasani, B, Parkes, AB, Lazarus, JH. Natural antibody status in patients with Hashimoto’s thyroiditis. J Clin Lab Immunol. 1999;51:9–20.
63. McLachlan, SM, Nagayama, Y, Pichurin, PN, et al. The link between Graves’ disease and Hashimoto’s thyroiditis: a role for regulatory T cells. Endocrinology. 2007;148:5724–5733.
64. Battifora, M, Pesce, G, Paolieri, F, et al. B7.1 costimulatory molecule is expressed on thyroid follicular cells in Hashimoto’s thyroiditis, but not in Graves’ disease. J Clin Endocrinol Metab. 1998;83:4130–4139.
65. Weetman, AP, Freeman, M, Borysiewicz, LK, et al. Functional analysis of intercellular adhesion molecule-1-expressing human thyroid cells. Eur J Immunol. 1990;20:271–275.
66. Davies, TF, Martin, A, Concepcion, ES, et al. Evidence of limited variability of antigen receptors on intrathyroidal T cells in autoimmune thyroid disease [see comments]. N Engl J Med. 1991;325:238–244.
67. McIntosh, RS, Tandon, N, Pickerill, AP, et al. IL-2 receptor-positive intrathyroidal lymphocytes in Graves’ disease. Ananlysis of V alpha transcript microheterogeneity. J Immunol. 1993;151:3884–3893.
68. Nagataki, S, Eguchi, K. Cytokines and immune regulation in thyroid autoimmunity. Autoimmunity. 1992;13:27–34.
69. Atkins, MB, Mier, JW, Parkinson, DR. Hypothyroidism after treatment with interleukin-2 and lymphokine-activated killer cells. N Engl J Med. 1988;16(318):1557–1563.
70. Nagayama, Y, Ohta, K, Tsuruta, M, et al. Exacerbation of thyroid autoimmunity by interferon alpha treatment in patients with chronic viral hepatitis: Our studies and review of the literature. Endocr J. 1994;41:565–572.
71. Nielsen, CH, Hegedüs, L, Rieneck, K, et al. Production of interleukin (IL)-5 and IL-10 accompanies T helper cell type 1 (Th1) cytokine responses to a major thyroid self-antigen, thyroglobulin, in health and autoimmune thyroid disease. Clin Exp Immunol. 2007;147:287–295.
72. Saverino, D, Brizzolara, R, Simone, R, et al. Soluble CTLA-4 in autoimmune thyroid diseases: relationship with clinical status and possible role in the immune response dysregulation. Clin Immunol. 2007;123:190–198.
73. Marinkovic, T, Garin, A, Yokota, Y, et al. Interaction of mature CD3+CD4+ T cells with dendritic cells triggers the development of tertiary lymphoid structures in the thyroid. J Clin Invest. 2006;116:2622–2632.
74. Wang, SH, Baker, JR. The role of apoptosis in thyroid autoimmunity. Thyroid. 2007;17:975–979.
75. Itoh, N, Yonehara, S, Ishii, A, et al. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991;66:233–243.
76. Giordano, C, Stassi, G, De Maria, R, et al. Potential involvement of Fas and its ligand in the pathogenesis of Hashimoto’s thyroiditis. Science. 1997;275:960–963.
77. Shimaoka, Y, Hidaka, Y, Okumura, M, et al. Serum concentration of soluble Fas in patients with autoimmune thyroid diseases. Thyroid. 1998;8:43–47.
78. Kawakami, A, Eguchi, K, Matsuoka, N, et al. Thyroid-stimulating hormone inhibits Fas antigen-mediated apoptosis of human thyrocytes in vitro. Endocrinology. 1996;137:3163–3169.
79. Bossowski, A, Czarnocka, B, Bardadin, K, et al. Identification of apoptotic proteins in thyroid gland from patients with Graves’ disease and Hashimoto’s thyroiditis. Autoimmunity. 2008;41:163–173.
80. My liwiec, J, Okota, M, Nikołajuk, A, et al. Soluble Fas, Fas ligand and Bcl-2 in autoimmune thyroid diseases: relation to humoral immune response markers. Adv Med Sci. 2006;51:119–122.
liwiec, J, Okota, M, Nikołajuk, A, et al. Soluble Fas, Fas ligand and Bcl-2 in autoimmune thyroid diseases: relation to humoral immune response markers. Adv Med Sci. 2006;51:119–122.
81. Mysliwiec, J, Oklota, M, Nikolajuk, A, et al. Serum CD40/CD40L system in Graves’ disease and Hashimoto’s thyroiditis related to soluble Fas, FasL and humoral markers of autoimmune response. J Immunol Invest. 2007;36:247–257.
82. Batteux, F, Lores, P, Bucchini, D, et al. Transgenic expression of Fas ligand on thyroid follicular cells prevents autoimmune thyroiditis. J Immunol. 2000;164:1681–1688.
83. Dong, Z, Takakuwa, T, Takayama, H, et al. Fas and Fas ligand gene mutations in Hashimoto’s thyroiditis. Lab Invest. 2002;82:1611–1616.
84. Takakuwa, T, Dong, Z, Takayama, H, et al. Frequent mutations of Fas gene in thyroid lymphoma. Cancer Res. 2001;61:1382–1385.
85. Marazuela, M, García-López, MA, Figueroa-Vega, N, et al. Regulatory T cells in human autoimmune thyroid disease. J Clin Endocrinol Metab. 2006;91:3639–3646.
86. Tandon, N, Motgan, BP, Weetman, AP. Expression and function of multiple regulators of complement activation in autoimmune thyroid disease. Immunology. 1994;81:643–647.
87. Lazarus, JH, Burr, ML, McGregor, AM, et al. The prevalence and progression of autimmune thyroid disease in the elderly. Acta Endocrinol. 1984;106:199–202.
88. Weetman, AP. The thyroid and autoimmunity in children and adolescents. In: Krassas GE, Rivkees SA, Kiess W, eds. Diseases of the Thyroid in Childhood and Adolescence. Basel: Karger; 2007:104–117.
89. Jaruratanasirikul, S, Leethanaporn, K, Khuntigij, P, et al. The clinical course of Hashimoto’s thryoiditis in children and adolescents: 6 years longitudinal follow-up. J Pediatr Endocrinol Metab. 2001;14:177–184.
90. Radetti, G, Gottardi, E, Bona, G, et al. The natural history of euthyroid Hashimoto’s thyroiditis in children. Study Group for Thyroid Diseases of the Italian Society for Pediatric Endocrinology and Diabetes. J Pediatr. 2006;149:827–832.
91. Wang, SY, Tung, YC, Tsai, WY, et al. Long-term outcome of hormonal status in Taiwanese children with Hashimoto’s thyroiditis. Eur J Pediatr. 2006;165:481–483.
92. Woolf, PD. Transient painless thyroiditis with hyperthyroidism: a variant of lymphocytic thyroiditis. Endocr Rev. 1980;4:411–420.
93. Nikolai, TF, Coombs, GJ, McKenzie, AK. Lymphocytic thyroiditis with spontaneously resolving hyperthyroidism and subacute thyroiditis: Long-term follow-up. Arch Intern Med. 1981;141:1455–1458.
94. Yamamoto, M, Shibuya, N, Chen, LC, et al. Seasonal recurrence of transient hypothyroidism in a patient with autoimmune thyroiditis. Endocrinol Jpn. 1988;35:135–142.
95. Tachi, J, Amino, N, Miyai, K. Massage therapy on neck: A contributing factor for destructive thyrotoxicosis? Thyroidology. 1990;2:25–27.
96. Roberton, HEW. Lassitude, coldness, and hair changes following pregnancy, and their response to treatment with thyroid extract. Brit Med J. 1948;2:93–94.
97. Lazarus, JH, Premawardhana, LDKE. Postpartum Thyroiditis. In: Weetman AP, ed. Contemporary Endocrinology: Autoimmune Diseases in Endocrinology. New Jersey, USA: Humana Press Inc.; 2008:177–192.
98. Muller, AF, Drexhage, HA, Berghout, A. Postpartum thyroiditis and autoimmune thyroiditis in women of childbearing age: recent insights and consequences for antenatal and postnatal care. Endocr Rev. 2001;22:605–630.
99. Mizukami, Y, Michigishi, T, Nonomura, A, et al. Postpartum thyroiditis. A clinical, histologic, and immunopathologic study of 15 cases. Am J Clin Pathol. 1993;100:200–205.
100. Wood, LC, Ingbar, SH. Hypothyroidism as a late sequela in patient with Graves’ disease treated with antithyroid agents. J Clin Invest. 1979;64:1429–1436.
101. Macchia, E, Concetti, R, Carone, G, et al. Demonstration of blocking immunoglobulins G, having a heterogeneous behaviour, in sera of patients with Graves’ disease: Possible coexistence of different autoantibodies directed to the TSH receptor. Clin Endocrinol. 1988;28:147–156.
102. Gavras, I, Thomson, J. Late thyrotoxicosis complicating autoimmune thyroiditis. Acta Endocrinol. 1972;69:41–46.
103. Karsh, J, Pavlidis, N, Weintraub, BD, et al. Thyroid disease in Sjogren’s syndrome. Arthritis Rheum. 1980;23:1326–1329.
104. Hadithi, M, de Boer, H, Meijer, JW, et al. Coeliac disease in Dutch patients with Hashimoto’s thyroiditis and vice versa. World J Gastroenterol. 2007;13:1715–1722.
105. Sibilla, R, Santaguida, MG, Virili, C, et al. Chronic unexplained anaemia in isolated autoimmune thyroid disease or associated with autoimmune related disorders. Clin Endocrinol. 2008;68:640–645.
106. Ness-Abramof, R, Nabriski, DA, Braverman, LE, et al. Prevalence and evaluation of B12 deficiency in patients with autoimmune thyroid disease. Am J Med Sci. 2006;33:119–122.
107. Mocellin, R, Walterfang, M, Velakoulis, D. Hashimoto’s encephalopathy: epidemiology, pathogenesis and management. CNS Drugs. 2007;21:799–811.
108. Peschen-Rosin, R, Schabet, M, Dichgans, J. Manifestation of Hashimoto’s encephalopathy years before onset of thyroid disease. Eur Neurol. 1999;41:79–84.
109. Brain, L, Jellinek, EH, Ball, K. Hashimoto’s disease and encephalopathy. Lancet. 1966;2:512–514.
110. Pozo-Rosich, P, Villoslada, P, Canton, A, et al. Reversible white matter alterations in encephalopathy associated with autoimmune thyroid disease. J Neurol. 2002;249:1063–1065.
111. Gayatri, NA, Whitehouse, WP. Pilot survey of Hashimoto’s encephalopathy in children. Dev Med Child Neurol. 2005;47:556–558.
112. Canton, A, de Fabregas, O, Tintore, M, et al. Encephalopathy associated to autoimmune thyroid disease: A more appropriate term for an underestimated condition? J Neurol Sci. 2000;176:65–69.
113. Ochi, H, Horiuchi, I, Araki, N, et al. Proteomic analysis of human brain identifies fienolase as a nobel autoantigen in Hashimoto’s encephalopathy. FEBS Lett. 2002;528:197–202.
114. Yoneda, M, Fujii, A, Ito, A. High prevalence of serum autoantibodies against the amino terminal of alpha-enolase in Hashimoto’s encephalopathy. J Neuroimmunol. 2007;185:195–200.
115. Sawka, AM, Fatourechi, V, Boeve, BF, et al. Rarity of encephalopathy associated with autoimmune thyroiditis: A case series from Mayo Clinic from 1950 to 1996. Thyroid. 2002;12:393–398.
116. Blanchin, S, Coffin, C, Viader, F, et al. Anti-thyroperoxidase antibodies from patients with Hashimoto’s encephalopathy bind to cerebellar astrocytes. J Neuroimmunol. 2007;192:13–20.
117. Gini, B, Laura, L, Riccardo, C, et al. Novel autoantigens recognised by CSF IgG from Hashimoto’s encephalitis revealed by a proteomic approach. J neuroimmunol. 2008;196:153–158.
118. Moshynska, OV, Saxena, A. Clonal relationship between Hashimoto thyroiditis and thyroid lymphoma. J Clin Pathol. 2008;61:438–444.
119. Cipolla, C, Sandonato, L, Graceffa, G, et al. Hashimoto thyroiditis coexistent with papillary thyroid carcinoma. Am Surg. 2005;71:874–878.
120. Larson, SD, Jackson, LN, Riall, TS, et al. Increased incidence of well-differentiated thyroid cancer associated with Hashimoto thyroiditis and the role of the PI3k/Akt pathway. J Am Coll Surg. 2007;204:764–773.
121. Rhoden, KJ, Unger, K, Salvatore, G, et al. RET/papillary thyroid cancer rearrangement in nonneoplastic thyrocytes: follicular cells of Hashimoto’s thyroiditis share low-level recombination events with a subset of papillary carcinoma. J Clin Endocrinol Metab. 2006;91:2414–2423.
122. Kang, DY, Kim, KH, Kim, JM, et al. High prevalence of RET, RAS, and ERK expression in Hashimoto’s thyroiditis and in papillary thyroid carcinoma in the Korean population. Thyroid. 2007;17:1031–1038.
123. Tamaki, H, Amino, N, Kimura, M, et al. Low prevalence of thyrotropin receptor antibody in primary hypothyroidism in Japan. J Clin Endocrinol Metab. 1990;71:1382–1386.
124. Yoshinari, M, Okamura, K, Tokuyama, T, et al. Clinical importance of reversibility in primary goitrous hypothyroidism. Br Med J. 1983;287:720–722.
125. Sato, K, Okamura, K, Ikenoue, H, et al. TSH dependent elevation of serum thyroglobulin in reversible primary hypothyroidism. Clin Endocrinol. 1988;29:231–237.
126. Takasu, N, Komiya, I, Asawa, T, et al. Test for recovery from hypothyroidism during thyroxine therapy in Hashimoto’s thyroiditis. Lancet. 1990;336:1084–1086.
127. Tajiri, J, Higashi, K, Morita, M, et al. Studies of hypothyroidism in patients with high iodine intake. J Clin Endocrinol Metab. 1986;63:412–417.
128. Wirtzfeld, DA, Winston, JS, Hicks, WL, Jr., et al. Clinical presentation and treatment of non-Hodgkin’s lymphoma of the thyroid gland. Ann Surg Oncol. 2001;8:338–341.
129. Aksoy, DY, Kerimoglu, U, Okur, H, et al. Effects of prophylactic thyroid hormone replacement in euthyroid Hashimoto’s thyroiditis. Endocr J. 2005;52:337–343.
130. Mazokopakis, EE, Papadakis, JA, Papadomanolaki, MG, et al. Effects of 12 months treatment with L-selenomethionine on serum anti-TPO Levels in Patients with Hashimoto’s thyroiditis. Thyroid. 2007;17:609–612.
131. Tajiri, J. Radioactive iodine therapy for goitrous Hashimoto’s thyroiditis. J Clin Endocrinol Metab. 2006;91:4497–4500.