Chapter 152 Choroidal Osteoma
General considerations
The presence of bone within the eye is unusual and can develop in several clinical settings.1 Calcification can occur in tissues that are accessible to body fluids.2 The process of ossification occurs when an abundant blood supply is present to deliver osteoblasts to organize the architecture of Haversian systems.2,3 The choroid and the retinal pigment epithelium are the most common sites for bone formation within the eye. Intraocular ossification most often occurs as a dystrophic process in association with phthisis bulbi and in cyclitic membranes in the setting of chronic intraocular inflammation.2,4,5
The choroidal osteoma is an unusual form of benign intraocular ossification.1,6,7 In contrast to other types of intraocular ossification, it is found in otherwise healthy eyes. Interest in this tumor was stimulated by the observations of Dr Henry Van Dyk and associates who had the opportunity to study the clinical and histopathologic features of a case of an idiopathic osseous tumor of the choroid in 1975.8 The eye had been enucleated because of the clinical suspicion of an atypical malignant melanoma in a 26-year-old woman. In 1978, Gass and coworkers9 correlated the typical clinical manifestations and angiographic features of the choroidal osteoma in a series of four affected patients. Gass subsequently reported new observations in 15 patients with choroidal osteomas.10 Later, Shields and associates provided a comprehensive review on the subject of choroidal osteoma, including their own personal experiences with this tumor and Aylward and associates provided long-term follow-up of patients.1,11 Over nearly four decades, numerous clinical case descriptions of the choroidal osteoma have appeared in the literature.11–51
Definition and incidence
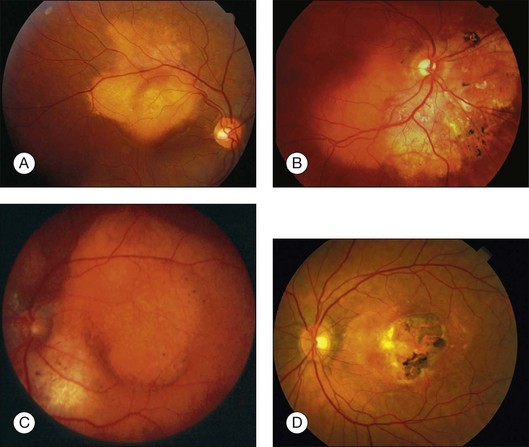
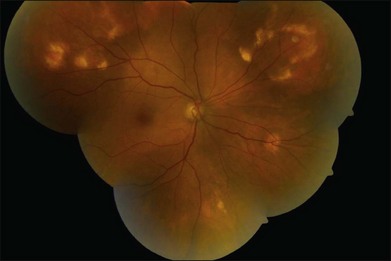
Choroidal osteoma is a benign tumor of the choroid composed of mature bone. It is typically found in healthy young females in the second or third decades of life (Fig. 152.1). However, some males,10,16,35,37,46,52 young children,21,23,24,34,38,40 and adults over age 30 years12,15,21,32,46,50 have been diagnosed with choroidal osteoma. There is no predilection for race. Most reported patients with this lesion are Caucasian, but several patients of African American24,31 and Oriental descent26,32,34,38,46,50,51 have been reported. The tumor generally occurs as a sporadic trait; however, a few cases of familial choroidal osteoma have been reported.21,23,40 The familial cases have been described in a mother and daughter,21 in a brother and sister,40 and in three siblings.23
Clinical features
Patients with a choroidal osteoma can be asymptomatic. Symptoms can include mild to severe visual blurring, metamorphopsia, and visual field defects corresponding to the location of the tumor.1,10 In one report, total blindness of both eyes was found in a young girl with bilateral choroidal osteomas.43
Choroidal osteoma is unilateral in approximately 75% of reported cases. In bilateral cases, the tumors can appear relatively symmetric or can be asymmetric, in different stages of development, growth, or decalcification (Fig. 152.2).10 Rarely, patients with initially unilateral choroidal osteoma, can later develop bilateral disease.30
The choroidal osteoma tends to be located in the juxtapapillary or peripapillary area, often with extension into the macula (Fig. 152.1). Rarely, this tumor is confined to the macular region only, without involvement of the juxtapapillary area.10,16,32,35,52 Choroidal osteoma is rarely seen in other locations of the eye and, to our knowledge, it has not been known to occur as a solitary lesion anterior to the retinal vascular arcades. Quite often choroidal osteoma is confused with idiopathic sclerochoroidal calcification, a benign, often multifocal, bilateral process that typically occurs anterior to the retinal vascular arcades, near the equator of the eye.53,54
The choroidal osteoma appears yellow-orange in color, particularly when the tumor is fully calcified. Over time, as decalcification occurs, the tumor becomes more pale yellow with overlying retinal pigment epithelial thinning and clumping. Choroidal osteoma ranges in size from approximately 2–22 mm in basal dimension and approximately 0.5–2.5 mm in elevation.1,10,11,20 In some cases, the bony tumor surface is irregularly excavated (Fig. 152.1) or elevated. The tumor shape is generally oval or round with characteristic well-defined scalloped or geographic margins. The margins can have blunt pseudopod-like projections. In some instances the tumor can be bilobed with two large plaques joined together by an isthmus.10,30
The vasculature within the choroidal osteoma is sometimes visible. Tufts of short branching vessels that originate deep within the tumor and emerge onto the tumor surface can be seen. They are especially prominent over the pale yellow portion of the tumor where the retinal pigment epithelium is thinned and depigmented.9 These large-caliber vessels are not neovascular tissue and are not associated with subretinal exudation or hemorrhage, or retinal pigment epithelial alterations.1,10 The overlying retinal vasculature and optic disc is characteristically unaffected by the osseous tumor and there are no associated vitreous or anterior segment abnormalities.
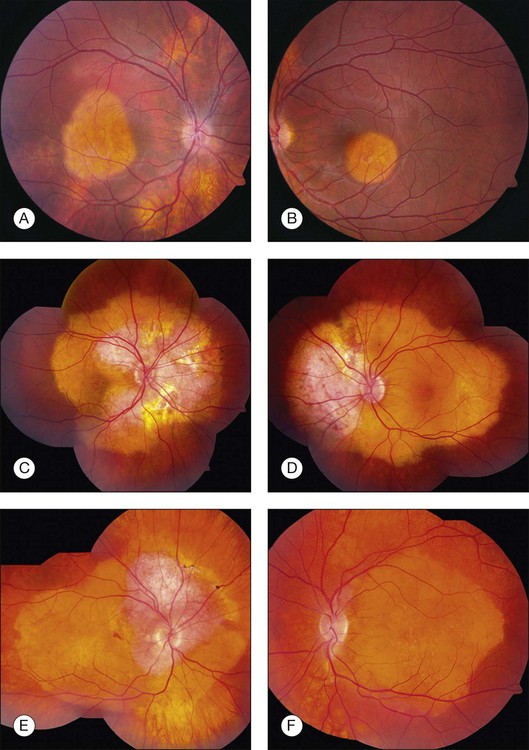
Many choroidal osteomas show evidence of slight enlargement in basal dimensions over several months to years (Fig. 152.3).11,38,41,44,48,55 Aylward and associates found tumor growth in 41% of 22 cases over a mean follow-up of 10 years.11 Shields and associates found tumor growth of 74 choroidal osteomas at 22% by 5 years and 51% by 10 years.56 In some cases, the tumor has doubled in size.10,41 Others have observed the development of a new choroidal osteoma in a previously unaffected eye.1,10
Trimble et al. reported a case of a choroidal osteoma in a 23-year-old woman, whose tumor enlarged slowly over 5 years.47 She developed a choroidal neovascular membrane that was treated with photocoagulation. Later, the tumor gradually decalcified over 18 months, leaving a bed of atrophy. Partial or complete decalcification of choroidal osteoma has been found to occur in 28% of cases by 5 years and 46% by 10 years.18,28,44,49,56 Subtle, gradual alterations in tumor color, surface pigmentation, and surface contour have also been observed.
Serous subretinal fluid can occur overlying choroidal osteoma.10 Subretinal fluid usually occurs over the macular portion of the osteoma. Careful ophthalmoscopy and fluorescein angiography should be performed for detection of choroidal neovascular membrane (CNVM) if symptoms or signs warrant.27
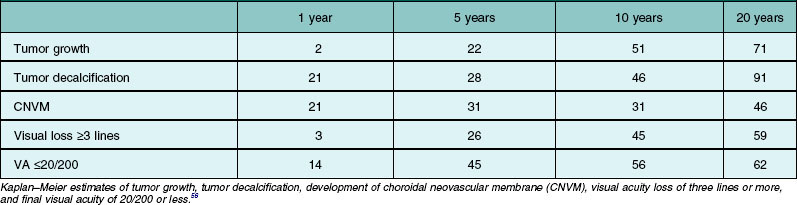
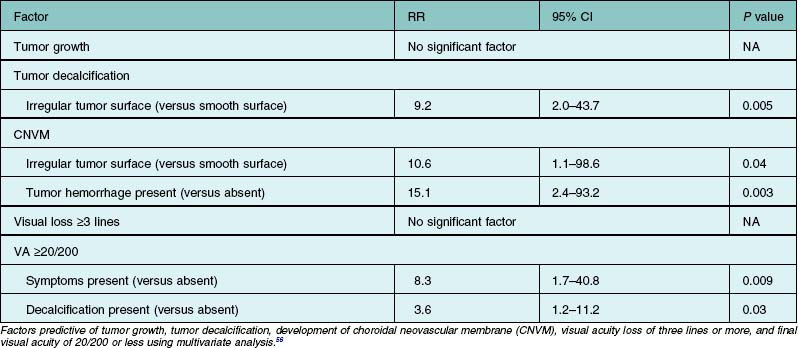
Choroidal neovascular membrane overlying osteoma has been found in 31% of cases by 5 years, 31–47% by 10 years, and 46–56% by 20 years (Fig. 152.4, Table 152.1).11,56 The CNVM is usually associated with subretinal fluid or hemorrhage, but it can be detected prior to vascular leakage as a slightly elevated gray-green subretinal tissue.15,37 Factors that predict the development of CNVM include irregular tumor surface and subretinal hemorrhage (Table 152.2).56 If the CNVM is extrafoveal then it can be treated with laser photocoagulation or photodynamic therapy.11,27 If the CNVM is in the fovea, the potential for subfoveal hemorrhage and disciform scarring often leads to a poor visual outcome.33,56 For subfoveal (and most extrafoveal) CNVM, we prefer antivascular endothelial growth factor (anti-VEGF) therapy monthly to resolve the membrane and then consolidation for extrafoveal CNVM with laser or photodynamic therapy.
CNVM should be distinguished from the branching vascular tufts seen on the inner surface of the tumor. The latter are not associated with subretinal fluid, hemorrhage, or disciform scar and do not leak fluorescein on angiography as do the former. The reason for the occurrence of CNVM in eyes with choroidal osteoma is speculative. It is postulated that the thinned, degenerated retinal pigment epithelium – Bruch’s membrane complex overlying the osteoma develops breaks and neovascular fronds from the choroid grow beneath the sensory retina.27
Differential diagnosis
The ophthalmoscopic features of choroidal osteoma are classic. The most important lesions in the differential diagnosis include amelanotic choroidal melanoma, amelanotic choroidal nevus, metastatic carcinoma to the choroid, circumscribed choroidal hemangioma, disciform macular degeneration, posterior scleritis, idiopathic sclerochoroidal calcification, and choroidal cartilage.1,43,44,56–59
Amelanotic choroidal melanoma differs from choroidal osteoma in that it has a yellow-brown color, more elevation and a less well-defined margin. An amelanotic choroidal nevus can be relatively flat like a choroidal osteoma but a nevus differs in that it has less distinct margins and can have drusen on its surface. Choroidal metastasis tends to have indistinct margins and is often associated with a serous retinal detachment out of proportion to the size of the tumor.60 Furthermore, metastatic carcinoma usually occurs in middle age or older patients who have a prior history of malignancy (especially breast carcinoma). An irradiated choroidal metastasis may more closely resemble a choroidal osteoma because the irradiated metastasis appears flatter and atrophic and lacks appreciable subretinal fluid. Choroidal hemangioma can occasionally have overlying fibrous and osseous metaplasia that resembles osteoma.61,62 However, choroidal hemangioma is characteristically dome-shaped with smooth regular margins and overlying serous fluid with cystoid degeneration of the retina.
Macular degeneration is typically seen in older patients when it occurs in the spectrum of age-related macular degeneration or it is seen with other fundus findings of histoplasmosis when it occurs in the presumed ocular histoplasmosis syndrome. Macular degeneration is usually centered in the macular area and is less commonly in a juxtapapillary location. Posterior scleritis has a typical orange-brown color with indistinct margins.17 The symptom of pain and the findings of uveitis, choroidal folds, cloudy subretinal fluid, and ultrasonographic evidence of thickened sclera and choroid with retrobulbar edema aid in differentiating this entity from the choroidal osteoma.
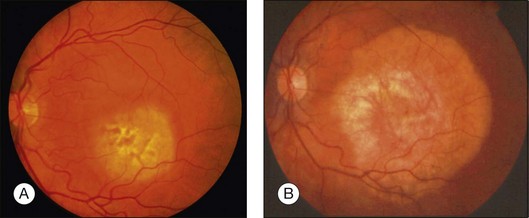
Idiopathic sclerochoroidal calcification clinically appears as an irregular calcific plaque within the choroid (Fig. 152.5).54,59 The overlying retina and vitreous are normal. These findings resemble the clinical appearance of the choroidal osteoma. However, idiopathic sclerochoroidal calcification is not typically juxtapapillary and more often occurs outside the retinal vascular arcades, is usually multifocal, and can rarely occur in patients with other systemic diseases.53 The linear nevus sebaceous syndrome can manifest calcification within the choroid, with an appearance similar to choroidal osteoma, but histopathology has shown calcified scleral cartilage, not bone.58,63
Pathology and pathogenesis
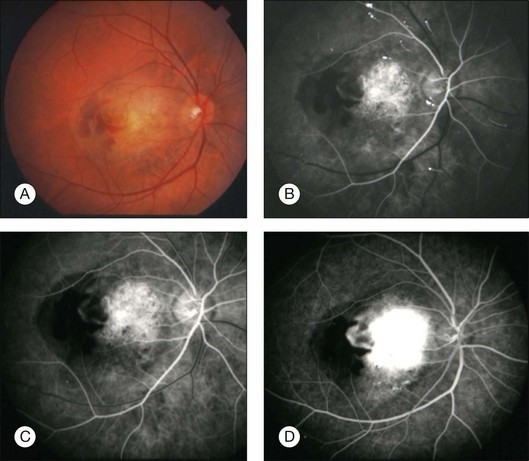
The histopathologic features of the choroidal osteoma were first defined in the case of Van Dyk and reported by both Gass and coworkers9 and Williams and coworkers.8 The mass is composed of dense bony trabeculae with endothelial-lined large cavernous spaces and small capillary blood vessels (Fig. 152.6). Osteoblasts, osteocytes, and osteoclasts are present. The intertrabecular marrow spaces contain loose fibrovascular elements, mast cells, and foamy vacuolated mesenchymal cells. The involved choriocapillaries are narrowed or obliterated in most areas. The choroidal melanocytes are displaced inwards towards the choriocapillaries and outwards towards the sclera. The overlying retinal pigment epithelium is focally depigmented and flat in some areas while clumps of pigment granules within melanophages are seen along Bruch’s membrane in other areas.
The pathogenesis of the choroidal osteoma is unknown. The speculated pathogeneses include possible choristomatous, inflammatory, traumatic, hormonal, metabolic, environmental, or hereditary etiology. Noble suggested that there might be a congenital, possibly inherited, abnormality of the juxtapapillary choroid that predisposes the patient to the development of this tumor.40
Intraocular inflammation has been known to cause dystrophic intraocular calcification and ossification.64,65 A focal peripapillary choroiditis could possibly lead to dystrophic calcium deposition and eventually to a lesion compatible with a choroidal osteoma.48 Choroidal osteomas have been reported in children after eosinophilic granulomatosis,35 sclerouveitis with optic nerve edema,48 and orbital inflammatory pseudotumor.31 Although these three cases are well documented, the majority of choroidal osteomas develop in otherwise clinically normal eyes.
Several reports have noted ocular trauma preceding choroidal osteoma.10,30 Osseous metaplasia of the retinal pigment epithelium may have occurred in these cases; however the relationship to trauma is speculative.
The occurrence of this tumor predominantly in young adult women raises the question of a hormonal influence.1,11 Most of the women are diagnosed after puberty but several cases of prepubertal girls have been documented.10,24,31 Also several male patients have been reported.14,16,35,37,46,50,52 No endocrinologic work-up has been revealing in these patients.
There have been no consistently abnormal serum calcium, phosphorus, or alkaline phosphatase levels detected in patients with choroidal osteomas. Katz and Gass31 reported a patient with transient mild secondary hypoparathyroidism (decreased serum calcium and increased serum phosphorus and increased alkaline phosphatase) and multiple choroidal osteomas, but classically parathyroid abnormalities cause a picture of sclerochoroidal calcification, not choroidal osteomas.
Choroidal osteomas were not reported or at least recognized in the older literature. The possibility that recent environmental toxins, exposures, or drugs may cause this tumor has been suggested. No consistent drug use or geographic location has been found. Choroidal osteomas have been misdiagnosed in the earlier literature as one prominent textbook, Tumors of the Eye, showed an ossified choroidal hemangioma in 1947 that actually proved to be a choroidal osteoma upon later review.8,66 It is possible that several cases in the older literature may have been misdiagnosed prior to the description of the choroidal osteoma in 1978.
Cunha in 1984 reported a familial case of choroidal osteomas seen in a 37-year-old mother and her 5-year-old daughter.21 The mother had poor vision from 11 years old. Eting and Savir in 1992 reported an atypical fulminant course of bilateral choroidal osteomas in a 5-year-old boy and his 7-year-old sister and both siblings developed severe bilateral visual loss.23 Noble in 1990 reported bilateral choroidal osteomas in 9-year-old twin brothers and their 11-year-old sister.40 The mother had a yellow mottled fundus but no clear evidence of osteoma. These three occurrences seem to be more than coincidental for this rare tumor and may indicate a possible hereditary or environmental component for some choroidal osteomas.
Diagnostic approaches
Fluorescein angiography
Fluorescein angiography of a choroidal osteoma shows mild patchy early hyperfluorescence of the tumor that evolves to a diffuse intense late staining (Fig. 152.5). The tumor can show early hyperfluorescence of the vascular tufts without leakage.1,7,10 This fluorescence fades slightly in the later stages of angiography.
Indocyanine green angiography
Indocyanine green angiography (ICGA) shows early hypofluorescence of the choroidal mass.36,51,57 The large-caliber choroidal vessels can traverse the peripheral portion of the osteoma and even transect a pseudopod. Fine diffuse fluorescence of the mass is seen by 2 minutes into the study and seems to emanate from ill-defined multifocal points within the area of the mass, perhaps related to the fine perforating vessels of the tumor. The fluorescence gradually becomes confluent and almost isofluorescent in the late frames.
Ultrasonography
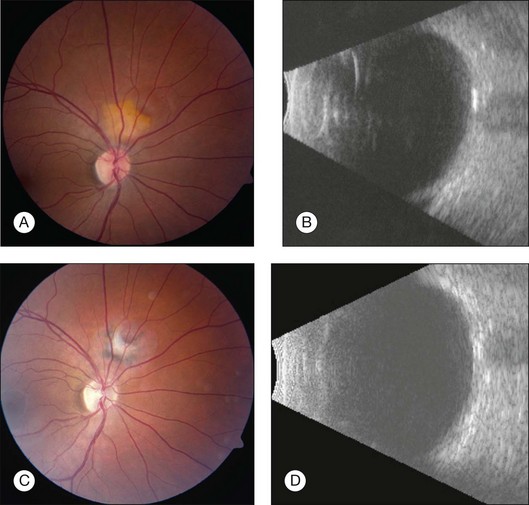
Ultrasonography is useful for differentiating choroidal osteomas from other clinically similar lesions. A-scan ultrasonography demonstrates a high-intensity echo spike from the inner surface of the tumor with decreased amplitude of the orbital soft tissue echoes immediately posterior to the tumor. B-scan ultrasonography demonstrates a slightly elevated, highly reflective choroidal mass which persists at lower scanning sensitivity after the other soft tissue echoes have disappeared (Fig. 152.7). Acoustic shadowing of the orbit occurs just posterior to the choroidal mass, giving the appearance of a pseudo-optic nerve.1,7
Optical coherence tomography and autofluorescence
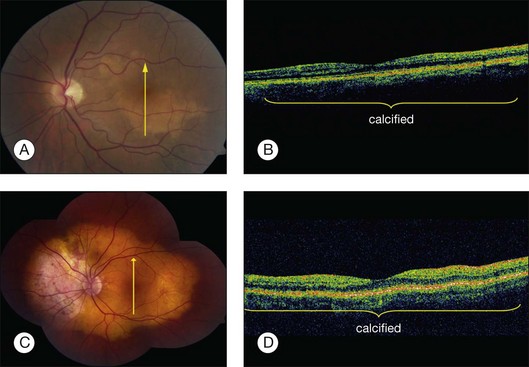
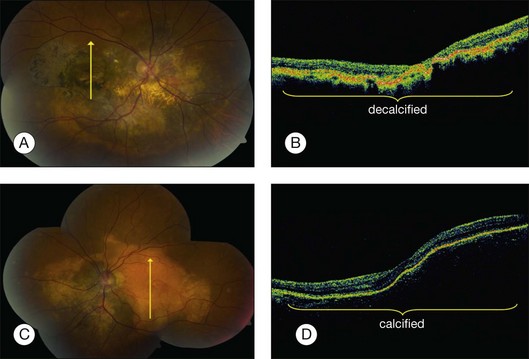
Optical coherence tomography (OCT) is a high-resolution imaging method for the retina with cross-sectional resolution under 10 µm. OCT reveals only minor choroidal details. Choroidal osteoma shows dense, abrupt reflection of light on OCT and the overlying choroidal neovascularization, subretinal fluid, retinal edema, and retinal thinning can be clearly visualized on OCT (Fig. 152.6).26,67 The retina shows profound outer-layer thinning and photoreceptor loss over decalcified osteoma whereas these features are not as advanced over the calcified portion (Fig. 152.8).67 Recently, the SD-OCT features have been illustrated,68,69 as well as the fundus autofluorescence findings.70
Roentgenography
Roentgenograms of the orbit demonstrate the presence of a radiodensity corresponding to the ossified tumor.10,30 The density may be difficult to visualize on plain films. Roentgenograms are rarely performed for diagnostic purposes in cases of choroidal osteoma, because this imaging mode has been replaced by computed tomography.
Computed tomography
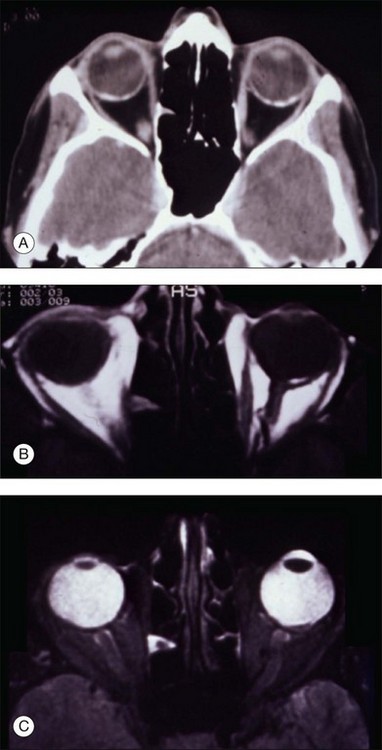
Computed tomography of choroidal osteomas shows characteristic features of a radiopaque plaque of bone density at the level of the affected choroid (Fig. 152.8).1,7
Magnetic resonance imaging
Magnetic resonance imaging with surface coil of choroidal osteoma reveals a bright signal on T1-weighted images and an area of relative low intensity on T2-weighted images (Fig. 152.8). On contrast T1-weighted scans, the tumor shows enhancement.22
Radioactive phosphorus uptake
The radioactive phosphorus uptake (32P) test is not usually performed in cases of choroidal osteoma. In a few cases in which it had been performed, the results were strongly positive.8 The choroidal osteoma can give positive results on 32P testing because the bone in the tumor accumulates phosphorus and incorporates it into its structure.
Management
The management of choroidal osteoma includes prevention of tumor growth, induction of decalcification of tumor, and treatment of choroidal neovascularization. There is currently no known systemic metabolic or hormonal method for altering the growth of the choroidal osteoma. For choroidal osteoma in the subfoveal region, the goal is to maintain calcification of the mass so that the outer retinal structures remain intact.67 Some have advocated oral calcium supplements in these cases but there is no confirmatory study. For osteoma in the extrafoveal region, the goal is to decalcify it so that it cannot grow towards the foveola. Decalcified osteoma does not grow in the direction of decalcification. Decalcification can be achieved with ablative laser photocoagulation or photodynamic therapy.1,14,19,27,29,39,42,71 Our current preferred treatment for extrafoveal osteoma is photodynamic therapy, as has been published, with decalcification occurring slowly over 1 year.71
Management of related CNVM has previously been somewhat successful with laser photocoagulation, but it often requires several treatments and visual acuity can decline.14,19,27,29,39,67 Subretinal surgical removal of subretinal neovascular membrane has been performed, but visual acuity may not be retained.25 Photodynamic therapy using verteporfin has been found successful for management of CNVM over osteoma (Fig. 152.9).16,52 In one case, transfoveal photodynamic therapy allowed for resolution of subretinal fluid and visual acuity stabilization of 20/30 at 6 months follow-up.16,52 More recently, anti-VEGF medications have been used for CNVM over osteoma with some success and visual preservation, but long-term reinjection might be necessary.72,73
Prognosis
The visual prognosis is quite varied and unpredictable. Most patients with extrafoveal choroidal osteoma maintain good visual acuity. Some patients with a subfoveal choroidal osteoma retain good visual acuity for months to years. In an analysis of 74 affected eyes, visual loss of three or more lines occurred in 26% by 5 years, 45% by 10 years, and 59% by 20 years. Poor visual acuity of 20/200 or worse was found in 14% at 1 year, 45% at 5 years, and 56% at 10 years (Table 152.1).67 The reason for visual loss includes subretinal fluid, subretinal blood, retinal pigment epithelial atrophy or mottling, and photoreceptor atrophy, particularly over decalcified osteomas. Factors that statistically predict poor vision include symptoms and decalcification (Table 152.2).67 The prognosis for life in the patient with choroidal osteoma is no different from the general population.1
1 Shields CL, Shields JA, Augsburger JJ. Review: choroidal osteoma. Surv Ophthalmol. 1988;33:17–27.
2 Duke-Elder S, Perkins ES. System of ophthalmology, Vol 1X. Diseases of the uveal tract. St Louis: Mosby; 1966. p. 740–8
3 Hogan MJ, Zimmerman LE. Ophthalmic pathology. Philadelphia: WB Saunders; 1962. p. 63
4 Green WR. Uveal tract. Ophthalmic pathology. An atlas and textbook. 4th ed. Spencer WH, Bilyk J, Eagle RC, et al, eds. Ophthalmic pathology. An atlas and textbook. Philadelphia: WB Saunders; 1996;Vol. 3:1439–2217.
5 Monselise M, Rapaport I, Romem M, et al. Intraocular ossification. Ophthalmologica Basel. 1985;190:225–229.
6 Shields JA, Shields CL. Atlas of intraocular tumors. Philadelphia: Lippincott Williams and Wilkins; 1999. p. 53–335
7 Shields JA, Shields CL. Osseous tumors of the uvea. Intraocular tumors: A text and atlas. Philadelphia: WB Saunders; 1992. p. 261–72
8 Williams AT, Font RL, Van Dyk HJ, et al. Osseous choristoma of the choroid simulating a choroidal melanoma. Arch Ophthalmol. 1978;96:1874–1877.
9 Gass JDM, Guerry RK, Jack RL, et al. Choroidal osteoma. Arch Ophthalmol. 1978;96:428–435.
10 Gass JDM. New observations concerning choroidal osteomas. Int Ophthalmol. 1979;2:71–84.
11 Aylward GW, Chang TS, Pautler SE, et al. A long-term follow-up of choroidal osteoma. Arch Ophthalmol. 1998;116:1337–1341.
12 Amalric P. Osteomas choroidens. Bull Soc Ophthalmol Fr. 1980;80:47–50.
13 Augsburger JJ, Shields JA, Rife CJ. Bilateral choroidal osteoma after nine years. Can J Ophthalmol. 1979;14:281–284.
14 Avila MP, El-Markabi H, Azzolini C, et al. Bilateral choroidal osteoma with subretinal neovascularization. Ann Ophthalmol. 1984;16:381–385.
15 Baarsma GS, Craandyk A. Osteoma of the choroid. Doc Ophthalmol. 1981;92:205–212.
16 Battaglia Parodi M, Da Pozzo S, Toto L, et al. Photodynamic therapy for choroidal neovascularization associated with choroidal osteoma. Retina. 2001;2:660–661.
17 Benson WE, Shields JA, Tasman W, et al. Posterior scleritis. A cause of diagnostic confusion. Arch Ophthalmol. 1979;97:1482–1486.
18 Buettner H. Spontaneous involution of a choroidal osteoma [letter]. Arch Ophthalmol. 1990;108:1517–1518.
19 Burke JF, Brockhurst RJ. Argon laser photocoagulation of subretinal neovascular membrane associated with osteoma of choroid. Retina. 1983;3:304–307.
20 Coston TO, Wilkinson CP. Choroidal osteoma. Am J Ophthalmol. 1978;86:368–372.
21 Cunha SL. Osseous choristoma of the choroid. A familial disease. Arch Ophthalmol. 1984;102:1052–1054.
22 DePotter P, Shields JA, Shields CL, et al. Magnetic resonance imaging of choroidal osteoma. Retina. 1991;11:221–223.
23 Eting E, Savir H. An atypical fulminant course of choroidal osteoma in two siblings. Am J Ophthalmol. 1992;113:52–55.
24 Fava GE, Brown GC, Shields JA, et al. Choroidal osteoma in a 6 year old child. J Pediatr Ophthalmol Strabismus. 1980;17:203–205.
25 Foster BS, Fernandez-Suntay JP, Dryja TP, et al. Surgical removal and histopathologic findings of a subfoveal neovascular membrane associated with choroidal osteoma. Arch Ophthalmol. 2003;121:273–276.
26 Fukasawa A, Iijima H. Optical coherence tomography of choroidal osteoma. Am J Ophthalmol. 2002;133:419–421.
27 Grand MG, Burgess DB, Singerman LJ, et al. Choroidal osteoma. Treatment of associated subretinal neovascular membranes. Retina. 1984;4:84–90.
28 Gurelik G, Lonneville Y, Safak N, et al. A case of choroidal osteoma with subsequent laser induced decalcification. Int Ophthalmol. 2001;24:41–43.
29 Hoffman ME, Sorr EM. Photocoagulation of subretinal neovascularization associated with choroidal osteoma. Arch Ophthalmol. 1987;105:998–999.
30 Joffe L, Shields JA, Fitzgerald JR. Osseous choristoma of the choroid. Arch Ophthalmol. 1978;96:1809–1812.
31 Katz RS, Gass JDM. Multiple choroidal osteomas developing in association with recurrent orbital inflammatory pseudotumor. Arch Ophthalmol. 1983;101:1724–1727.
32 Kayazawa F, Shimamoto S. Choroidal osteoma. Two cases in Japanese women. Ann Ophthalmol. 1981;13:1053–1056.
33 Kelinske M, Weinstein G. Bilateral choroidal osteomas. Am J Ophthalmol. 1981;92:676–680.
34 Kida Y, Shibuya Y, Oguni M, et al. Choroidal osteoma in an infant. Am J Ophthalmol. 1997;124:119–120.
35 Kline LB, Skalka HW, Davidson JD, et al. Bilateral choroidal osteomas associated with fatal systemic illness. Am J Ophthalmol. 1982;93:192–197.
36 Lafaut BA, Mestdagh C, Kohno T, et al. Indocyanine green angiography in choroidal osteoma. Graefes Arch Clin Exp Ophthalmol. 1997;235:330–337.
37 Laibovitz RA. An unusual case of intraocular calcification. Choroidal osteoma. Ann Ophthalmol. 1979;11:1077–1080.
38 Mizota A, Tanabe R, Adachi-Usami E. Rapid enlargement of choroidal osteoma in a 3-year-old girl. Arch Ophthalmol. 1998;116:1128–1129.
39 Morrison DL, Magargal LE, Ehrlich DR, et al. Review of choroidal osteoma: Successful krypton red laser photocoagulation of an associated subretinal neovascular membrane involving the fovea. Ophthal Surg. 1987;18:299–303.
40 Noble KG. Bilateral choroidal osteoma in three siblings. Am J Ophthalmol. 1990;109:656–660.
41 Pamer Z, Kovacs B. A case of a fast-growing bilateral choroidal osteoma. Retina. 2001;21:657–659.
42 Rose SJ, Burke JF, Brockhurst RJ. Argon laser photoablation of a choroidal osteoma. Retina. 1991;11:224–228.
43 Shields JA, Shields CL, Ellis J, et al. Bilateral choroidal osteoma associated with bilateral total blindness. Retina. 1996;16:445–447.
44 Shields JA, Shields CL, DePotter P, et al. Progressive enlargement of a choroidal osteoma. Arch Ophthalmol. 1995;113:819–820.
45 Teich SA, Walsh JB. Choroidal osteoma. Ophthalmology. 1981;88:696–698.
46 Totsuka H, Minoda K. Choroidal osteoma. Folia Ophthalmol Jpn. 1980;31:427–435.
47 Trimble SN, Schatz H, Schneider GB. Spontaneous decalcification of a choroidal osteoma. Ophthalmology. 1988;95:631–634.
48 Trimble SN, Schatz H. Choroidal osteoma after intraocular inflammation. Am J Ophthalmol. 1983;96:759–764.
49 Trimble SN, Schatz H. Decalcification of a choroidal osteoma. Br J Ophthalmol. 1991;75:61–63.
50 Tsukahara I, Hayashi M. Osseous choristoma of the choroid. Jap J Ophthalmol. 1980;24:90–95.
51 Yuzawa M, Kawamura A, Haruyama M, et al. Indocyanine green video-angiographic findings in choroidal osteoma. Eur J Ophthalmol. 1994;4:191–198.
52 Baum MD, Pilkerton AR, Berler DK, et al. Choroidal osteoma. Ann Ophthalmol. 1979;11:1849–1851.
53 Shields JA, Shields CL. Sclerochoroidal calcification: the 2001 Harold Gifford Lecture. Retina. 2002;22:251–261.
54 Sivalingam A, Shields CL, Shields JA, et al. Idiopathic sclerochoroidal calcification. Ophthalmology. 1991;98:720–724.
55 Jackson WE, Freed S. Unilateral osseous choristoma of the choroid. Ann of Ophthalmol. 1984;16:134–136.
56 Shields CL, Sun H, Demirci H, et al. Factors predictive of tumor growth, tumor decalcification, choroidal neovascularization and visual outcome in 74 eyes with choroidal osteoma. Arch Ophthalmol. 2005;123:658–666.
57 Shields CL, Shields JA, DePotter P. Patterns of indocyanine green videoangiography of choroidal tumours. Br J Ophthalmol. 1995;79:237–245.
58 Shields JA, Shields CL, Eagle RC, Jr., et al. Ocular manifestations of the organoid nevus syndrome. Ophthalmology. 1997;104:549–557.
59 Shields JA, Shields CL. Intraocular tumors: A text and atlas. Philadelphia: WB Saunders; 1992. p. 85–489
60 Shields CL, Shields JA, Gross N, et al. Survey of 520 eyes with uveal metastases. Ophthalmology. 1997;104:1265–1276.
61 Shields CL, Honavar SG, Shields JA, et al. Circumscribed choroidal hemangioma. Clinical manifestations and factors predictive of visual outcome in 200 consecutive cases. Ophthalmology. 2001;108:2237–2248.
62 Witschel H, Font RL. Hemangioma of the choroid. A clinicopathologic study of 71 cases and a review of the literature. Surv Ophthalmol. 1976;20:415–431.
63 Lambert HM, Sipperly JO, Shore JW, et al. Linear nevus sebaceous syndrome. Ophthalmol. 1987;94:278–282.
64 Finklestein EM, Boniuk M. Intraocular ossification and hematopoiesis. Am J Ophthalmol. 1969;68:683–690.
65 Zeiter HJ. Calcification and ossification of ocular tissue. Am J Ophthalmol. 1962;53:265–274.
66 Reese AB. Tumors of the eye, 3rd ed. New York: Harper & Row; 1976. p. 266–7
67 Shields CL, Perez B, Materin MA, et al. Optical coherence tomography of choroidal osteoma in 22 cases. Evidence for photoreceptor atrophy over the decalcified portion of the tumor. Ophthalmology. 2007;114:e53–e58.
68 Freton A, Finger PT. Spectral domain-optical coherence tomography analysis of choroidal osteoma. Br J Ophthalmol. 2012;96:224–228.
69 Sayanagi K, Pelayes DE, Kaiser PK, et al. 3D Spectral domain optical coherence tomography findings in choroidal tumors. Eur J Ophthalmol. 2011;21:271–275.
70 Ascaso FJ, Villén L. Fundus autofluorescence imaging findings in choroidal osteoma. Retina. 2011;31:1004–1005.
71 Shields CL, Materin MA, Mehta S, et al. Regression of extrafoveal choroidal osteoma following photodynamic therapy. Arch Ophthalmol. 2007;126:135–137.
72 Shields CL, Salazar P, Demirci H, et al. Intravitreal bevacizumab (Avastin) and ranibizumab (Lucentis) for choroidal neovascularization overlying choroidal osteoma. Retinal Cases & Brief Reports. 2008;2:18–20.
73 Kubota-Taniai M, Oshitari T, Handa M, et al. Long-term success of intravitreal bevacizumab for choroidal neovascularization associated with choroidal osteoma. Clin Ophthalmol. 2011;5:1051–1055.