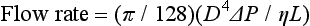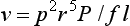Chest Tube Thoracostomy
History
Hippocrates drained an empyema using a metal tube.1 Playfair developed underwater seal drainage of chest tubes in 1875.2 Credit for the invention of the chest tube is usually given to Hewett,3 who in 1876 devised a system of continuous drainage of the empyema cavity using a rubber catheter that drained into a glass jar filled with a weakly antiseptic solution.4 Use of the chest tube was not widely adopted, however, until the 1917 influenza epidemic.5 Closed tube thoracostomy drainage of the pleural space after thoracotomy was first reported by Lilienthal in 1922.6
Anatomy and Physiology of the Pleural Space
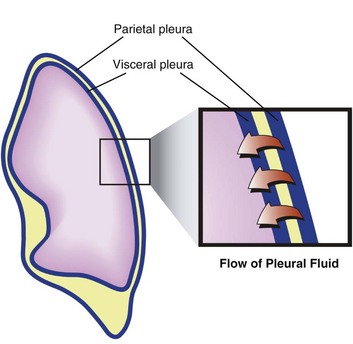
The pleural space is the interface between the chest wall and the lung and represents a critical component of pulmonary function. The visceral and parietal pleurae are composed of a single layer of mesothelium. The blood supply of the parietal pleura is of systemic origin (intercostal vessels), whereas the blood supply of the visceral pleura is of pulmonary origin (pulmonary artery and veins). The bronchial arteries may contribute significantly to the blood supply of the visceral surface.7
Pleural fluid may originate from three sources—parietal capillaries, visceral capillaries, or interstitium. Starling’s law of transcapillary exchange governs the movement of fluid across the pleural space. The pressure in the capillaries of the visceral pleura is less than that in the parietal capillaries because it drains into the pulmonary venous bed. The net hydrostatic pressure (35 cm H2O) favors movement of fluid from the parietal pleura to the pleural space (Fig. 15.1). This pressure is derived from the subtraction of −5 cm H2O (pleural pressure) from 30 cm H2O (parietal hydrostatic pressure). This net hydrostatic pressure is opposed by the net oncotic pressure (29 cm H2O), which is derived from the subtraction of 5 cm H2O (pleural oncotic pressure) from 34 cm H2O (plasma oncotic pressure). A gradient of 6 cm H2O (34 − 29 cm H2O) favors pleural fluid formation.8 The pleural lymphatics prevent the accumulation of this pleural fluid. Stomas, unique to the parietal pleura, facilitate communication between the pleural space and the capillaries. It is estimated that this mechanism allows clearance of 20 mL of pleural fluid per hour per hemithorax in a 70-kg human.9,10 The lymphatic network clears protein from the pleural space; smaller molecules can be directly absorbed by the pleural capillaries. Intercostal and diaphragmatic muscle activity influences the rate of lymphatic flow. Hypoventilation and anesthesia reduce lymphatic flow and the rate of absorption of protein.11
Drainage Systems
The original three-bottle system has been compartmentalized into a plastic unit that is easily transportable and readily pressure-adjustable, consisting of a trap bottle, a water-seal bottle, and a manometer bottle (Fig. 15.2). The trap bottle collects the pleural fluid. The water-seal bottle prevents air from returning to the pleural space during the negative pleural pressure phase on inspiration. The manometer bottle uses the distance below its fluid line to generate a negative pressure when suction is applied. For example, 20 cm of water generates a −20 cm H2O pressure.12 Modern collection and suction systems use a single compartmentalized system (Fig. 15.3).
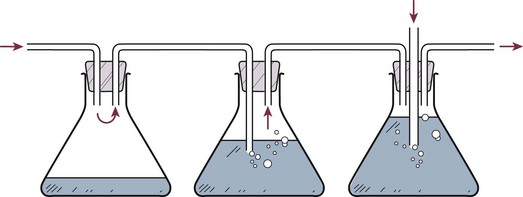
Figure 15.2 Three-bottle system consisting of (from left to right) trap bottle, water-seal bottle, and manometer bottle.
Figure 15.3 Modern drainage system.
Indications and Contraindications
Simple Pneumothorax
Pneumothorax is defined as air that has entered the pleural space, either spontaneously or as a result of traumatic tears in the pleura after chest injury or after invasive procedures. Treatment of pneumothorax entails removing air from the pleural space, reexpanding the underlying lung, and preventing recurrence.13 If the patient is clinically stable, the treatment depends on the size of the pneumothorax and whether or not the patient is mechanically ventilated. If the pneumothorax is small, and the patient is not mechanically ventilated, the pneumothorax can be observed. If the pneumothorax is large, or the patient is mechanically ventilated, a chest tube should be placed.14 A large pneumothorax is defined as being greater than 15% to 20%.15 Needle aspiration has also been described as a consideration for treatment of spontaneous pneumothorax. However, a repeat chest radiograph must be obtained to assure no recurrence in which case a chest tube would be required.16
If an occult pneumothorax is identified, which is a pneumothorax found incidentally on computed tomography (CT) scan without evidence clinically or on chest radiograph, observation may be an option. The optimal management remains controversial with regard to tube placement; however, two retrospective reviews demonstrate safe observation without tube placement in patients requiring positive-pressure ventilation.17,18
Tension Pneumothorax
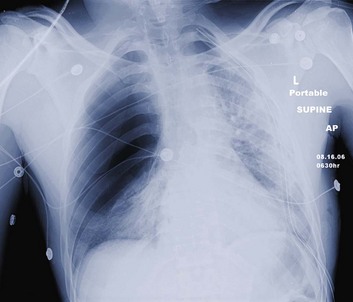
Tension pneumothorax is a life-threatening clinical situation that requires emergent and immediate treatment (Fig. 15.4). Air collects and builds up pressure in the chest cavity through a tear in the lung or bronchial tree. Air enters the chest with each mechanical or spontaneous breath, with no route for escape. Initially, the affected lung simply collapses, but as tension increases, the diaphragm flattens, and the mediastinum is shifted toward the contralateral side.19 The contralateral lung is compressed, further decreasing effective ventilation. The great vessels also are compressed, and venous return is reduced drastically. This reduction in venous return results in rapid and disastrous cardiopulmonary collapse.20 The diagnosis is ideally made on a clinical basis, and treatment is initiated without waiting for radiographic confirmation. Any tension pneumothorax should have immediate large-bore needle decompression. A readily available large-bore angiocatheter is preferentially inserted in the second intercostal space at the midclavicular line. A rush of pleural air under pressure confirms the diagnosis and location. After decompression (conversion to a simple pneumothorax), the catheter is left in until a tube thoracostomy has been placed.21
Pleural Effusions
Pleural effusions, both transudative and exudative, are frequently seen in the ICU. The incidence of pleural effusions in the ICU varies with screening methods, from approximately 8% for physical examination to more than 60% for routine ultrasound.22,23 Several factors contribute to the occurrence of pleural effusions in ICU patients. Large amounts of intravenous fluid are often administered during the first few days to patients admitted for shock. Pneumonia also is common as a reason for ICU admission and as a complication of mechanical ventilation. Heart failure, atelectasis, hypoalbuminemia, and liver disease are present in many ICU patients. In surgical ICUs, cardiac or abdominal surgery is often followed by specific, large, protracted pleural effusion; in multiple-injury patients, hemothorax is possible.24 The criteria of Light and colleagues,25 which are based on the ratio of protein or lactate dehydrogenase levels in the pleural fluid and blood, differentiate exudates from transudates with a negative predictive value of 96% and a sensitivity of 98%.24
Provided that basic rules are followed, thoracentesis is safe in ICU patients.22 A chest tube may be placed for large or symptomatic pleural effusions. The optimal drainage duration for uninfected pleural effusions has not been established. A reasonable approach may be to remove the chest tubes when drainage decreases to less than 200 mL/day.26
In the evaluation of a parapneumonic effusion or empyema, if the thickness of the pleural fluid is more than 10 mm on a decubitus radiograph, or if the pleural fluid is of similar depth and loculated, the pleural fluid should be examined to determine the stage of the effusion. Drainage of an infected pleural space is required to achieve source control as a key component of treatment (Fig. 15.5). If the fluid is removed completely and does not reaccumulate, no additional therapy need be directed toward the effusion. At the time of the initial therapeutic thoracentesis, the pleural fluid should be sent for Gram stain and culture and analysis of leukocyte, lactate dehydrogenase, glucose, and pH levels. Indicators of a poor prognosis from the pleural fluid include positive Gram stain or culture, glucose less than 60 mg/dL, lactate dehydrogenase more than three times the upper limits of normal for serum, or pH less than 7.20.
If the therapeutic thoracentesis removes all of the pleural fluid and the fluid recurs, the next step is guided by the initial pleural fluid findings. If none of the poor prognostic indicators is present, no invasive procedures are indicated if the patient is doing well clinically. If any of the poor prognostic indicators were present at the initial thoracentesis, a second therapeutic thoracentesis should be performed, and the pleural fluid should be reanalyzed. If the pleural fluid accumulates a third time, a small chest tube should be inserted into the pleural space, unless none of the poor prognostic factors were present at the time of the second thoracentesis.27 If the patient shows signs of systemic infection, and fluids have been inadequately drained, an open thoracotomy and drainage may be required.28,29
When a hemothorax is suspected, the essential management, along with appropriate resuscitation, is intercostal drainage. This achieves two objectives: first, to drain the pleural space, allowing expansion of the lung, and second, to allow assessment of rates of continuing blood loss. After satisfactory resolution of hemothorax managed with intercostal drainage alone, the drain should not be removed too promptly. Other circumstances permitting, the patient should be mobilized fully with adequate thoracic physiotherapy. These measures should allow optimal drainage of the pleural cavity.30 Complete drainage of blood also prevents empyema and fibrothorax.31
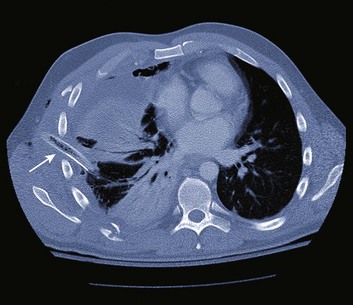
Pneumomediastinum is usually a self-limited entity (Fig. 15.6). It follows alveolar rupture into the pulmonary interstitium and is produced by an acute episode of high intrathoracic pressure. The differential diagnosis includes cardiac, pulmonary, musculoskeletal, and esophageal causes. Spontaneous pneumomediastinum is usually a self-limited clinical entity.32 Coupled with positive-pressure ventilation or subcutaneous emphysema, however, cautious observation is recommended owing to the possibility of a pneumothorax leading to tension pneumothorax.
Chest Tube Size
If the diameter of the tube is doubled, flow increases by a factor of 16, implying that a small increase in the size of the drainage tubes would result in substantial improvements in the flow rates.33
where v is the flow, r is the radius, l is the length, P is the pressure, and f is the friction factor.12,34–36
In an in vitro study, Park and coworkers37 measured flow rates of different viscosity fluids (serous, blood, pus) through catheters of different diameters, ranging from 6F to 18F, and found that flow rates increased for larger catheters as predicted by Poiseuille’s law. At catheter sizes larger than 7F, however, the differences were small.
Reports on drainage techniques suggest that before drainage of a collection, diagnostic needle aspiration should be performed, initially with a 22-gauge needle and, if this is unsuccessful, subsequently with a 20-gauge or 18-gauge cannula.38 It has been postulated that if pus can be aspirated by such a needle, it should be drainable through a catheter twice the size (i.e., >6F).39 As such, if drain patency can be maintained, the maximum flow rate of the catheter is unlikely to be the limiting factor for most pleural collections.33
Given the variety of liquids and accompanying pleural debris that may be drained by a chest tube, no single formula exists for flow of these many materials. The principal determinants of airflow through a tube, bore and length, are logically key determinants of flow for various pleural liquids, including blood and pus. Chest tube selection must take into account not only what material is being drained but also its rate of formation. Ongoing production of more viscous fluids requires a larger bore tube than for a similar volume of air produced.40
In our institution, we have had good success with small tubes placed under radiologic guidance into empyema cavities. These small catheters also have complemented larger tubes that have drained most of the chest but have left small residual pockets, which would be difficult to access blindly with large, less flexible tubes.41
Technique of Insertion
Mild sedation or anxiolysis is typically needed before the procedure, and intravenous access is essential for the ability to give intravenous pain medication. If the procedure is done carefully and with good local anesthesia, there is usually minimal to no need for intravenous sedation. Coagulation parameters (i.e., prothrombin time, partial thromboplastin, and platelets) should be confirmed to be normal or, if abnormal, adequate for coagulation (typically platelet count 50,000/µL and international normalized ratio ≤1.5 IU). Most hospitals have closed tube thoracostomy trays premade; however, the components that should be available are as follows42:
4. Needles—21-gauge, 23-gauge, 25-gauge
5. Local anesthesia—1% or 2% 10 mL lidocaine
7. Sutures—2-0 silk (to anchor tube) and 3-0 nylon (to close site when tube is removed if desired)
9. Drain sponge dressings and 4 × 4 dressings
12. Underwater seal collection system
13. Sterile chest tube—sizes 24F and 32F (depending on what is found to be drained; blood or pus should be 32F, air or fluid can be 24F)
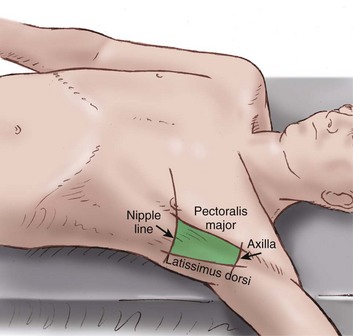
Insertion should be in the “safe triangle” illustrated in Figure 15.7. This is the triangle formed by the anterior border of the latissimus dorsi, the lateral border of the pectoralis major muscle, a line superior to the horizontal level of the nipple, and an apex below the axilla. The most common position for chest tube insertion is in the midaxillary line, through the safe triangle. This position minimizes risk to underlying structures such as the internal mammary artery and avoids damage to muscle and breast tissue resulting in scarring. A more posterior position may be chosen if suggested by the presence of a loculus, although this site is more uncomfortable for the patient, and there is a risk of the drain kinking.43
The patient should be prepared and draped in sterile fashion with a wide field (Fig. 15.8), and the operator should be wearing sterile gown, gloves, hat, and mask. Local anesthesia should be instilled with a 23-gauge or 25-gauge needle making a skin wheal and allowing 2 to 5 minutes for anesthetic to take effect. Deeper infiltration follows with a 21-gauge needle to the intercostal muscles, the area over the rib, periosteum, and parietal pleura (when air or fluid is aspirated, withdraw the needle slightly and reinfiltrate to ensure anesthetizing the pleura).42
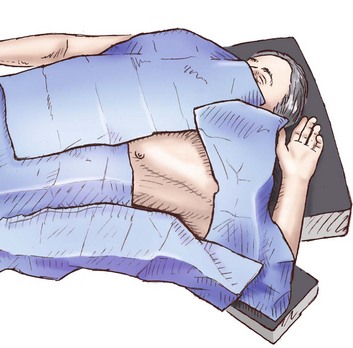
Figure 15.8 Prepared field for chest tube placement.
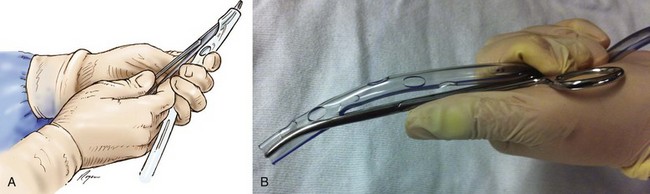
A 1- to 1.5-cm incision is made parallel to the rib and down to the subcutaneous fat at the lower border of the rib space to allow for a small tunnel or tract to prevent air from being drawn around the tube and to close the incision when the tube is removed. A vertical or horizontal mattress suture is placed through the incision, tying a knot at the free ends of the suture. This can be used later when the chest tube is removed to close the skin incision. A tract is created using a small curved hemostat by inserting it closed into the incision and gently spreading. It should be removed and reinserted with each spreading maneuver.44 This needs to be done slowly and carefully to prevent pain; this is done multiple times continuously using gentle forward pressure toward the upper border of the rib at the intercostal space to be entered. The right hand opens and closes the instrument, and the left hand is placed close to the tip to prevent plunging into the chest (Fig. 15.9). When the intercostal muscle has been dissected, the pleura is entered. There is a rush of air or fluid. When possible, a gloved finger should be inserted into the chest cavity to ensure there are no adhesions between the lung and the chest wall. This is most important if the patient has had a history of multiple chest tubes or thoracic surgery.
Before beginning the procedure, the tube should be prepared and placed on the sterile field within easy reach. A closed clamp may be passed through the distal hole and out the end of the tube to facilitate placement (Fig. 15.10). This technique may be easier than trying to open the clamp in the intercostal space to advance the chest tube. The tube can then be passed over the clamp in a Seldinger-type technique. Another method is to place the tube in the clamp prior to insertion into the chest. The tube should be positioned against the outside curve of the clamp (see Fig. 15.10). When placed, the tube should be clamped at the distal end to prevent leakage. The tube should be connected to the tubing in a sterile fashion. The clamp can then be removed. Reexpansion of a longstanding pneumothorax may be painful, and reexpansion should be done slowly with use of intermittent clamping. This also is true for chronic pneumothorax and pleural effusions to avoid reexpansion pulmonary edema. Onset of coughing may be a sign of onset of reexpansion pulmonary edema, at which point the clamp should be reapplied. Reexpansion pulmonary edema is discussed later in the section on complications. The tube should be anchored in place with heavy silk suture, and sterile dressings should be applied. A chest radiograph should be obtained and reviewed.
Management of Thoracostomy Tubes
When a thoracic drain has been placed, a chest radiograph should be obtained immediately and reviewed. This is done to confirm placement and assess success of intervention. The amount and character of the drainage or air leak or both should be assessed. A chest radiograph should be done every day for as long as the tube is in place.45,46 There is an ongoing debate as to whether to place a tube to water seal or suction when an air leak is present. In two studies of postoperative patients, it was shown that water seal was superior to suction in stopping air leak.47,48 In the presence of a large air leak, however, if the pneumothorax increases or a subcutaneous emphysema develops, the tube should be placed back to suction (usually 20 cm H2O), and a chest radiograph should be immediately obtained. If there is no air leak for 24 hours, and the drainage is less than 2 mL/kg/day, it is safe to remove the tube.49 One protocol option begins with thoracostomy tubes placed to 20 cm H2O suction immediately after insertion.45 Tubes are usually assessed for air leak and drainage. Suction is continued if an air leak is present, or if the drainage is greater than or equal to 200 mL in 24 hours. If these criteria are not met, the tubes are placed to water seal. Chest radiographs are not obtained routinely on water seal or before removal. If no air leak is present after 6 hours on water seal, the tube is removed.50 If a tube is nonfunctioning, it should be removed. If one chooses to place the tube to water seal before removal, a chest radiograph obtained 3 hours after water seal excludes development of a clinically significant pneumothorax.51
Clamping a chest drain before removal may be necessary. If an air leak is small and intermittent, clamping followed by chest film may help determine if the patient is likely to develop a pneumothorax after removal.52 The house staff and nursing staff must be fully informed, however, so the tube can be immediately unclamped if there is any respiratory difficulty. Clamping of the chest tube in the face of massive hemothorax (1500 mL on placement) also has been advocated; however, in a study creating hemothorax spontaneously in piglets, chest tube clamping did not decrease hemorrhage or mortality rate but worsened gas exchange without improving hypotension.53
There are no data to support prophylactic antibiotic use for chest tube placement in the ICU in nontrauma patients. In traumatic hemothorax, multiple factors, including the condition under which the tube is inserted (emergent or urgent), the mechanism of injury, retained hemothorax, and ventilator care, contribute to development of pleural space infection. The incidence of empyema ranges between 0% and 18% and is decreased with the use of prophylactic antibiotics. Administration of antibiotics for longer than 24 hours does not reduce this risk further.54
Obtaining a chest film after removal of a chest tube has been standard practice at most institutions. Timing usually ranges from 6 to 24 hours after the tube is removed. Two retrospective studies and one prostpective study concluded that it is unnecessary to obtain routine chest radiographs after chest tube removal.55–57 Other authors advocate obtaining a single upright chest radiograph 24 hours after chest tube removal to evaluate for recurrence of hemothorax or pneumothorax.56 In a mechanically ventilated patient, a chest film should be obtained after chest drain removal. A study by Pizano and colleagues58 of 214 patients undergoing positive-pressure ventilation concluded that the number of clinically significant pneumothoraces after chest tube removal seems to be small. The concern persists, however, regarding expansion of a small pneumothorax into a tension pneumothorax. Failure to diagnose a large and expanding pneumothorax could lead to a life-threatening situation. Pizano and colleagues58 supported obtaining a chest film within 3 hours after chest tube removal. It also seemed safe to remove a chest tube from patients undergoing positive-pressure ventilation if standard removal criteria were met.58,59
Routine milking and stripping of chest tubes is performed primarily in postoperative cardiac surgical patients. The data do not support routine milking and stripping unless there is clot in the tubing. A significant negative pressure can be generated in the chest during the procedure and could be detrimental. Few such complications are cited in the literature, however.60
Postplacement chest radiograph may raise the question of placement in the fissure of the lung, potentially compromising function. This may lead to manipulation to change position. Chest tubes appearing to be in the pleural fissure on plain radiograph function as effectively, however, as tubes located elsewhere in the pleural space.61,62
The positioning of the tubing connecting the chest tube to the drainage system is important. In one study, three tubing positions were studied: straight, coiled, and dependent loop (allowing fluid to collect in a dependent loop with the loop left alone in some, and periodically lifted and drained at 15-minute intervals in others). It was found that the dependent loop left alone did not drain adequately. The straight and coiled positions were optimal for drainage of fluid. If the dependent loop cannot be avoided, lifting and draining it every 15 minutes would maintain adequate drainage.63
Removal of a chest tube is associated with pain. In one study, 4 mg of intravenous morphine was given 20 minutes before removal versus 30 mg of intravenous ketorolac given 60 minutes before removal. Either of these regimens was found to reduce pain substantially during chest tube removal without causing adverse sedative effects.64 Another study compared topical lidocaine-prilocaine cream (EMLA) versus intravenous morphine. The investigators found that topical EMLA cream was more effective, but it had to be applied 3 hours before chest tube removal.65
Removal of the chest tube must be timed with the breathing pattern of the patient. Some authors advocate removal at end inspiration, whereas others recommend removal at end expiration. The reason some advocate end inspiration is that when the tube is removed the patient may gasp from the pain and may be more likely to suck in air through the site. In one study, a similar rate of postremoval pneumothorax was found. Both methods were found to be safe.66 There are two ways to close the sites after the tube has been removed. If sutures were placed, they are tied down. If no sutures were placed, an occlusive dressing must be made with 4 × 4 dressings and tape and petroleum jelly gauze.
Complications
Complications of chest tube placement include improper positioning, bleeding, nerve damage, injury to diaphragm or abdominal organs, mechanical problems, pain, and bronchopleural fistula. There are multiple case reports identifying issues including atrial fibrillation, pleurocutaneous fistula, extrathoracic herniation of a lung bulla, chylothorax, and neuropathies.67–76 Any structure or organ in the chest or upper abdomen can be damaged or perforated with chest tube insertion. The heart, lung, aorta, vena cava, pulmonary artery, nerves, liver, spleen, and stomach all are vulnerable. Damage is more likely to occur with the use of a trocar, but can occur if the clamp dissecting through the intercostal space is not controlled by the operator. A sudden thrust into the chest, especially in a small chest, can easily reach the mediastinum.
Reexpansion pulmonary edema is a rare and potentially lethal complication of tube placement for pneumothorax, pleural effusion, and severe atelectasis (Fig. 15.11).77 The estimated mortality rate is 20%.78 Onset of symptoms is often immediate, but can be delayed 24 hours.78 Severe coughing heralds the development of pulmonary edema. The patient becomes tachypneic and tachycardic as hypoxia increases. The patient does not respond to oxygen therapy because blood is shunted past fluid-filled alveoli. Rarely, bilateral or contralateral edema develops.79 The pathophysiology is complex. Multiple factors contribute to a capillary bed with increased permeability. An inflammatory response occurs when the lung reexpands. This response is believed to be secondary to expansion-related mechanical injury to the alveolar-capillary membrane and reperfusion injury as blood flow returns to the now fully expanded lung.80 Patients 20 to 39 years old are more susceptible.81 The duration of pneumothorax also has been implicated in the incidence of reexpansion pulmonary edema.82 Early case series found that spontaneous pneumothorax was present an average of 14 days with a minimum of 3 days before edema would develop.83 The severity of the pneumothorax may be more predictive than its duration in developing pulmonary edema. In the series by Matsuura and coworkers,81 no patient with a pneumothorax less than 30% of the lung field developed pulmonary edema. Seventeen percent of patients with total collapse and 44% of patients with tension pneumothorax had this complication. Some authors support slow reexpansion by lower negative pressure as beneficial, whereas others support the idea that it is not so much the degree of negative pressure as the rate of reexpansion that is important.82 In clinically stable patients with a large (30% of the lung field) primary pneumothorax, the American College of Chest Physicians recommends either small-bore (≤14F) catheter or 16F to 22F chest tube placement.14 Connection to a Heimlich valve or a water-seal device is recommended. If the lung fails to reexpand, suction is deemed appropriate.
The mainstay of therapy remains oxygenation, a low threshold for mechanical ventilation with positive end-expiratory pressure, diuresis if it can be tolerated, and hemodynamic support. Reexpansion pulmonary edema usually resolves in 24 to 72 hours.80
Imaging
Areas in the chest that require chest tube drainage may be loculated owing to adhesions from pneumonias or multiple chest tube placements, making it difficult and dangerous to place a drain blindly into the chest. The use of ultrasound or CT guidance can be invaluable.84 Ultrasound and CT also can be used to verify location of tubes previously placed. In one study, 51 pigtail catheters placed under radiologic guidance (CT or ultrasound) were reviewed, with an overall success rate of 88%. The specific success rates were 92%, 85%, and 91% for loculated pleural effusion, pneumothorax, and empyema, respectively. Complications were few and minor.85
A chest radiograph should be performed immediately after chest drain placement, but the position of the tube may still be in question. CT has proved to be useful when this occurs (Fig. 15.12). Placement locations such as intraparenchymal, intrafissural, mediastinal, chest wall, and abdominal may be identified.86 In a study in which CT revealed 28 malpositioned chest tubes among 76 tubes placed in 54 patients, frontal chest radiograph revealed only 6 of the 28.87
References
1. Hippocrates. Genuine Works, Vol. 2 (trans. by Francis Adams). New York: William Wood & Company; 1886.
2. Playfair, GE. Case of empyema treated by aspiration and subsequently by drainage: Recovery. BMJ. 1875; 1:45.
3. Hewett, C. Drainage for empyema. BMJ. 1876; 1:317.
4. Hewett, C. Thoracentesis: The plan of continuous aspiration. BMJ. 1876; 1(793):317.
5. Graham, EA, Bell, RD. Open pneumothorax: Its relation to the treatment of empyema: War medicine. Am J Med Sci. 1918; 156:839.
6. Lilienthal, H. Pulmonary resection for bronchiectasis. Ann Surg. 1922; 75:257.
7. Albertine, KH, Wiener-Kronish, JP, Roos, PJ, et al. Structure, blood supply, and lymphatic vessels of the sheep’s visceral pleura. Am J Anat. 1982; 165:277.
8. Miserocchi, G, Agostini, E. Pleural liquid and surface pressures at various lung volumes. Respir Physiol. 1980; 39:315.
9. Broaddus, VC, Staub, NC. Pleural liquid protein turnover in health and disease. Semin Respir Med. 1987; 9:7.
10. Broaddus, VC, Weiner-Kronish, JP, Berthiauma, Y, et al. Removal of pleural liquid and protein by lymphatics in awake sheep. J Appl Physiol. 1988; 64:384.
11. Kinasewitz, GT, Fishman, AP. Influence of alterations in Starling forces of visceral pleural fluid movement. J Appl Physiol. 1981; 51:671.
12. Miller, KS, Sahn, FA. Chest tubes: Indication, technique, management and complications. Chest. 1987; 91:258–264.
13. Jenkinson, SG. Pneumothorax. Clin Chest Med. 1985; 6:153–161.
14. Baumann, MH, Strange, C, Heffner, JE, et al. Management of spontaneous pneumothorax. American College of Chest Physicians Delphi Consensus Statement. Chest. 2001; 119:590–602.
15. Putukian, M. Pneumothorax and pneumomediastinum. Clin Sports Med. 2004; 23:443–454.
16. Zehtabchi, S, Rios, CL. Management of emergency department patients with primary spontaneous pneumothorax: Needle aspiration or tube thoracostomy? Ann Emerg Med. 2008; 51(1):91–100.
17. Wilson, H, Ellsmere, J, Tallon, J, Kirkpatrick, A. Occult pneumothorax in the blunt trauma patient: Tube thoracostomy or observation? Injury. 2009; 40(9):928–931.
18. Barrios, C, Tran, T, Malinoski, D, et al. Successful management of occult pneumothorax without tube thoracostomy despite positive pressure ventilation. Am Surg. 2008; 74(10):958–961.
19. Sabiston, DC, Spencer, FC. Gibbon’s Surgery of the Chest, 3rd ed. Philadelphia: WB Saunders; 1975.
20. Vulkich, DJ. Pneumothorax, hemothorax, and other abnormalities of the pleural space. Emerg Med Clin North Am. 1983; 1:431–448.
21. Gilbert, TB, McGrath, BJ, Soberman, M. Chest tubes: Indications, placement, management, and complications. J Intensive Care Med. 1993; 8:73–86.
22. Fartoukh, M, Azoula, E, Galliot, R, et al. Clinically documented pleural effusions in medical ICU patients: How useful is routine thoracentesis. Chest. 2002; 121:178–184.
23. Mattison, LE, Coppage, L, Alderman, DF, et al. Pleural effusions in the medical ICU: Prevalence, causes, and clinical implications. Chest. 1997; 111:1018–1023.
24. Azoulay, E. Pleural effusions in the intensive care unit. Curr Opin Pulm Med. 2003; 9:291–297.
25. Light, RW, Macgregor, MI, Luchsinger, PC, et al. Pleural effusions: The diagnostic separation of transudates and exudates. Ann Intern Med. 1972; 77:507–513.
26. Younes, RN, Gross, JL, Aguiar, S, et al. When to remove a chest tube? A randomized study with subsequent prospective consecutive validation. J Am Coll Surg. 2002; 195:658–662.
27. Light, RW. The management of parapneumonic effusions and empyema. Curr Opin Pulm Med. 1998; 4:227–229.
28. Roper, WH, Waring, JJ. Primary serofibrinous pleural effusion in military personnel. Am Rev Tuberc. 1955; 71:616–635.
29. Pablo, A, Villena, V, Echave-Sustaeta, J, et al. Are pleural fluid parameters related to the development of residual pleural thickening in tuberculosis? Chest. 1997; 112:1293–1297.
30. Perry, GW. Management of haemothorax. Ann R Coll Surg Engl. 1996; 78:325–326.
31. Muslim, M, Bilal, A, Salim, M, et al. Tube thorocostomy: Management and outcome in patients with penetrating chest trauma. J Ayub Med Coll Abbottabad. 2008; 20(4):108–111.
32. Koullias, GJ, Korkolis, DP, Wang, XJ, et al. Current assessment and management of spontaneous pneumomediastinum: Experience in 24 adult patients. Eur J Cardiothorac Surg. 2004; 25:852–855.
33. Tattersall, DJ, Traill, ZC, Gleeson, FV. Chest drains: Does size matter? Clin Radiol. 2000; 55:415–421.
34. Baumann, MH, Strange, C. Treatment of spontaneous pneumothorax: A more aggressive approach? Chest. 1997; 112:789–804.
35. Batchelder, TL, Morris, KA. Critical factors in determining adequate pleural drainage in both the operated and nonoperated chest. Am Surg. 1962; 28:296–302.
36. Swenson, EW, Birath, G, Ahbeck, A. Resistance to air flow in bronchospirometric catheters. J Thorac Surg. 1957; 33:275–281.
37. Park, JK, Kraus, FC, Haaga, JR. Fluid flow during percutaneous drainage procedures: An in vitro study of the effects of fluid viscosity, catheter size, and adjunctive urokinase. Am J Roentgenol. 1993; 160:165–169.
38. van Sonnenberg, E, Ferruci, JT, Mueller, PR, et al. Percutaneous drainage of abscesses and fluid collections: Technique, results and applications. Radiology. 1982; 142:1–10.
39. Meuller, PR, van Sonnenberg, E, Ferruci, JT. Percutaneous drainage of 250 abdominal abscesses and fluid collections: Part II. Current procedural concepts. Radiology. 1984; 151:343–347.
40. Baumann, MH. What size chest tube? What drainage system is ideal? And other chest tube management questions. Curr Opin Pulm Med. 2003; 9:276–281.
41. Rivera, L, O’Reilly, EB, Sise, MJ, et al. Small catheter tube thoracostomy: Effective in managing chest trauma in stable patients. J Trauma. 2009; 66(2):393–399.
42. Tomlinson, MA, Treasure, T. Insertion of a chest drain: How to do it. Br J Hosp Med. 1997; 58:248–252.
43. Laws, D, Neville, E, Duffy, J. BTS guidelines for the insertion of a chest tube. on behalf of the British Thoracic Society Pleural Disease Group, a subgroup of the British Thoracic Society Standards of Care Committee. Thorax. 2003; 58(Suppl II):53–59.
44. Parmar, JM. How to insert a chest drain. Br J Hosp Med. 1989; 42:231–233.
45. Martino, K, Merrit, S, Boyakye, K, et al. Prospective randomized trial of thoracostomy removal algorithms. J Trauma. 1999; 46:369.
46. Mattox, KL. Per hospital care of the patient with an injured chest. Surg Clin North Am. 1989; 69:21.
47. Cerfolio, RJ, Tummala, RP, Holman, WL, et al. A prospective algorithm for the management of air leaks after pulmonary resection. Ann Thorac Surg. 1998; 66:1726–1731.
48. Cerfolio, RJ, Bass, C, Katholi, CR. Prospective randomized trial compares suction versus water seal for air leaks. Ann Thorac Surg. 2001; 71:1613–1617.
49. Davis, JW, Mackersie, RC, Hoyt, DB, et al. Randomized study of algorithms for discontinuing tube thoracostomy drainage. J Am Coll Surg. 1994; 179:553.
50. Adrales, G, Huynh, T, Broering, B, et al. A thoracostomy tube guideline improves management efficiency in trauma patients. J Trauma Inj Infec Crit Care. 2002; 52:210–216.
51. Schulman, CI, Cohn, SM, Blackbourne, L, et al. How long should you wait for a chest radiograph after placing a chest tube on water seal? A prospective study. J Trauma Inj Infect Crit Care. 2005; 59:92–95.
52. Funk, GA, Petrey, LB, Foreman, ML. Clamping thoracostomy tubes: A heretical notion? Proc Baylor Univ Med Cent. 2009; 22(3):215–217.
53. Ali, J, Qi, W. Effectiveness of chest tube clamping in massive hemothorax. J Trauma Inj Infect Crit Care. 1995; 38:59–63.
54. Luchette, FA, Barrie, PS, Oswanski, MF, et al. Practice management guidelines for prophylactic antibiotic use in tube thoracostomy for traumatic hemopneumothorax: The EAST Practice Management Guidelines Work Group. Eastern Association for Trauma. J Trauma Inj Infect Crit Care. 2000; 48:753–757.
55. Palesty, JA, McKelvey, AA, Dudrick, SJ. The efficacy of x-rays after chest tube removal. Am J Surg. 2000; 179:13–16.
56. Pacanowski, JP, Waack, ML, Daley, BJ, et al. Is routine roentgenography needed after closed tube thoracostomy removal? J Trauma Inj Infect Crit Care. 2000; 48:684–688.
57. Whitehouse, MR, Patel, A, Morgan, JA. The necessity of routine post-thoracostomy tube chest radiographs in post-operative thoracic surgery patients. Surgeon. 2009; 7(2):79–81.
58. Pizano, LR, Houghton, DE, Cohn, SM, et al. When should a chest radiograph be obtained after chest tube removal in mechanically ventilated patients? A prospective study. J Trauma Inj Infect Crit Care. 2002; 53:1073–1077.
59. Tawil, I, Gonda, JM, King, RD, et al. Impact of positive pressure ventilation on thoracostomy tube removal. J Trauma. 2010; 68(4):818–821.
60. Teplitz, L. Update: Are milking and stripping chest tubes necessary? Focus Crit Care. 1991; 18:506–511.
61. Curtin, JJ, Goodman, LR, Quebbeman, EJ, et al. Thoracostomy tubes after acute chest injury: Relationship between location in a pleural fissure and function. Am J Roentgenol. 1994; 163:1339–1342.
62. Meyers, L, Jones, J. Towards evidence based emergency medicine: Best BETs from the Manchester Royal Infirmary. BET 3: Do patients with a thoracostomy tube placed in the lung fissure need an additional thoracostomy tube placed? Emerg Med J. 2008; 25(8):523–525.
63. Schmelz, JO, Johnson, D, Norton, JM, et al. Effects of position of chest drainage tube on volume drained and pressure. Am J Crit Care. 1999; 8:319–323.
64. Puntillo, K, Ley, SJ. Appropriately timed analgesics control pain due to chest tube removal. Am J Crit Care. 2004; 13:292–304.
65. Valenzuela, RC, Rosen, DA. Topical lidocaine-prilocaine cream (EMLA) for thoracostomy tube removal. Anesth Analg. 1999; 88:1107–1108.
66. Bell, RL, Ovadia, P, Abdullah, F, et al. Chest tube removal: End-inspiration or end-expiration? J Trauma Inj Infect Crit Care. 2001; 50:674–677.
67. Maritz, D, Wallis, L, Hardcastle, T. Complications of tube thoracostomy for chest trauma. S Afr Med J. 2009; 99(2):114–117.
68. Hsu, KF, Wang, HB, Hsieh, CB. Refractory atrial fibrillation following tube thoracostomy. Can Med Assoc J. 2010; 182(3):280.
69. Menger, R, Telford, G, Kim, P, et al. Complications following thoracic trauma managed with tube thoracostomy. Injury. 2012; 43(1):46–50.
70. Lin, MT, Shih, JY, Lee, YC, Yang, PC. Pleurocutaneous fistula after tube thoracostomy: Sonographic findings. J Clin Ultrasound. 2008; 36(8):523–525.
71. Okur, E, Tezel, C, Baysungur, V, Halezeroglu, S. Extrathoracic herniation of a lung bulla through a tube thoracostomy site. Interact Cardiovasc Thorac Surg. 2008; 7(6):1210–1211.
72. Limsukon, A, Yick, D, Kamangar, N. Chylothorax: A rare complication of tube thoracostomy. J Emerg Med. 2011; 40(3):280–282.
73. Iribhogbe, PE, Uwuigbe, O. Complications of tube thoracostomy using advanced trauma life support technique in chest trauma. West Afr J Med. 2011; 30(5):369–372.
74. Rosing, JH, Lance, S, Wong, MS. Ulnar neuropathy after tube thoracostomy for pneumothorax. J Emerg Med. 2010; 43(4):e223–225.
75. Shaikhrezai, K, Zamvar, V. Hazards of tube thoracostomy in patients on a ventilator. J Cardiothorac Surg. 2011; 6:39.
76. Kesieme, EB, Dongo, A, Ezemba, N, et al. Tube thoracostomy: Complications and its management. Pulm Med. 2012; 2012:256878.
77. Sherman, SC. Reexpansion pulmonary edema: A case report and review of the current literature. J Emerg Med. 2003; 24:23–27.
78. Mahfood, S, Hix, WR, Aaron, BL, et al. Reexpansion pulmonary edema. Ann Thorac Surg. 1988; 45:340–345.
79. Heller, B, Grathwohl, M. Contralateral reexpansion pulmonary edema. South Med J. 2000; 93:828–831.
80. Trachiotis, GD, Vricella, LA, Aaron, BL, et al. Reexpansion pulmonary edema: Updated in 1997. Ann Thorac Surg. 1997; 63:1206–1207.
81. Matsuura, Y, Nomimura, T, Murakami, H, et al. Clinical analysis of reexpansion pulmonary edema. Chest. 1991; 100:1562–1566.
82. Murphy, K, Tomlanovich, MC. Unilateral pulmonary edema after drainage of a spontaneous pneumothorax: Case report and review of the world literature. J Emerg Med. 1983; 1:29–36.
83. Kassis, E, Philipsen, E, Clausen, K. Unilateral pulmonary edema following spontaneous pneumothorax. Eur J Respir Dis. 1981; 62:102–106.
84. Iberti, TJ, Stern, PM. Chest tube thoracostomy. Crit Care Clin. 1992; 8:879–895.
85. Cantin, L. Chest tube drainage under radiological guidance for pleural effusion and pneumothorax in a tertiary care university teaching hospital: Review of 51 cases. Can Respir J. 2005; 12:29–33.
86. Gayer, G, Rozenman, J, Hoffmann, C, et al. CT diagnosis of malpositioned chest tubes. Br J Radiol. 2000; 73:786–790.
87. Lim, KE, Tai, SC, Chan, CY, et al. Diagnosis of malpositioned chest tubes after emergency tube thoracostomy: Is computed tomography more accurate than chest radiograph? Clin Imaging. 2005; 29:401–405.