Chapter 7 Chemotherapy for Brain Tumors
Factors Influencing Delivery of Chemotherapy to the Brain
Blood–Brain Barrier
Treatment of brain tumors with systemic chemotherapy poses challenges unique to brain tumors. Concentrations of chemotherapeutic agents within central nervous system (CNS) depends on multiple factors, including ability of the agents to cross the blood–brain barrier (BBB), the volume of distribution of the drug in the brain parenchyma and the extent to which the drug is actively transported out of the brain.1 Therefore many promising compounds fail in CNS drug development due to limited access to the target sites in the brain. Foremost is the BBB, which impedes delivery of adequate concentrations of most chemotherapeutic agents to the tumor; others include the brain tumor barrier (BTB), blood–cerebrospinal fluid (CSF) barrier, and brain–CSF barrier.2 Paul Ehrlich first described the BBB in 1985 when he noted that all body tissues except the brain were stained when certain vital dyes were injected intravenously into animals.3
The BBB critically controls the passage of drugs or other compounds from the blood to the CNS and protects the brain from the foreign and undesirable molecules. The major component of the BBB is a monolayer of brain capillary endothelial cells. The restriction of brain penetration arises from the presence of tight junctions between adjacent endothelial cells and interaction between astrocytes and endothelial cells.4 In contrast to other blood vessels in the body, the endothelial cells of brain capillaries lack intercellular fenestrations, and have high electrical resistance and low ionic permeability rendering them relatively impermeable to many water-soluble compounds.2,4 The principal route to cross the BBB is via the lipid-mediated transport of small nonpolar molecules through the BBB by passive diffusion or less frequently by catalyzed transport.5 For a drug to successfully reach the brain parenchyma requires uptake across the luminal (blood-facing) membrane into the endothelial cells, transport across the transcellular membrane, and finally efflux across the abluminal side (brain parenchyma–facing membrane) into the interstitial fluid. The key to successful chemotherapy of brain tumors is adequate drug delivery to the tumor-infiltrated brain around the tumor and the individual tumor cells. To cross the BBB, chemotherapeutic drugs administered systemically must be less than approximately 200 daltons in size, lipid soluble, not bound to plasma proteins, and minimally ionized.2,4 As a result, there is a positive correlation between lipophilicity of the drug and its ability to cross the BBB.
Role of Steroids
Steroids are important in the management of patients with brain tumors particularly in patients with bulky disease and those who have hydrocephalus. Dexamethasone has the best CNS penetration of all the steroids and is most commonly used in practice.6 Steroids decrease CSF production and cerebral blood flow and help to reduce vasogenic edema associated with the tumor. However, the steroid can impair delivery of the chemotherapeutic agents to the tumor.7 The assessment of response to treatment can also be affected by steroid use. Steroids can potentially decrease the degree of gadolinium enhancement that is a surrogate for the leakiness of the blood vessels and decrease the measurable volume of the tumor.8 Guidelines for determining response criteria to chemotherapy now require that the patient be on the same or a lower steroid dose as compared to baseline before determining an objective response.9,10
Mechanisms of Drug Resistance and Strategies to Overcome Resistance
Efflux Transporters
However, uptake can be lower than predicted for drugs as they are subject to extrusion from the brain by active BBB efflux transporters. Drug transporters belong to two major superfamilies, ABC (adenosine triphosphate binding cassette) and SLC (solute carrier) transporters.11,12 ABC transporters are integral membrane proteins, many of which are located in the plasma membrane are primary active transporters, and they couple ATP hydrolysis to active efflux of their substrates against concentration gradients.13 The most extensively studied BBB transporter of the ABC family is P-glycoprotein (P-gp) initially discovered in 1976,14 but members of the multidrug resistance-associated proteins (MRP),15 family and breast cancer resistance protein (BCRP)16 have also been identified in brain endothelial cells and choroid plexus epithelial cells. Anticancer agents were among the first drugs identified to be substrates of BBB efflux transporters, that is, of Pgp as well as MRPs and BCRP.
DNA Repair Enzymes
Methyl guanine methyl transferase (MGMT) is an enzyme that removes chloroethylation or methylation damage from the O(6) position of guanine following alkylating chemotherapy, and hence is involved in DNA-repair.17 Clinical response to alkylating agents such as temozolomide (TMZ) in GBM patients has been correlated to the activity of the MGMT repair enzyme.18 The MGMT gene may be silenced by methylation of its promoter that prevents repair of DNA damage and increases the lethal effect of chemotherapy. O(6)-benzylguanine (O(6)-BG) is an AGT substrate that inhibits AGT by suicide inactivation, and based on these findings, clinical trials with agents such as O6-benzylguanine (O6BG) added to alkylating agents have been pursued that deplete MGMT.19,20 Unfortunately, this approach has been limited by systemic toxicities to date, as the combined toxicity of O6BG and TMZ has required significant dose reductions in TMZ, the presumed active agent for cancer cell death.20
Poly (ADP-ribose) polymerase-1 (PARP-1) is an enzyme that catalyzes the transfer of β-nicotinamide adenine dinucleotide (NAD+) to poly(ADP-ribose).21 PARP-1 enzyme catalyzes the synthesis of polymers for DNA repair after injury, and PARP-1 influences both direct repair and base excision repair of DNA after injury from alkylating agents or ionizing radiation and is a key enzyme in the DNA repair pathways complementary to and downstream of MGMT.21 Hence, the PARP-1 enzyme inhibition is an attractive target for glioma therapy. PARP-1 inhibitors have also been shown to overcome resistance to TMZ in both mismatch repair-proficient and -deficient glioma cells in culture, and numerous PARP-1 inhibitors are in clinical trials in patients with high-grade gliomas.22,23
Strategies to Improve Drug Delivery to Treat Brain Tumors
Intra-Arterial Chemotherapy
The goal of this approach is to deliver chemotherapy intra-arterially so that the tissue perfused by that artery is exposed to higher plasma concentrations of the drug during the first passage through the circulation. The principal advantage to this approach is to maximize the amount of drug crossing through the BBB and minimize systemic side effects. Theoretical modeling suggests that intra-arterial infusion can produce a 10-fold increase in peak drug concentrations as compared to intravenous infusion.24 However, two phase-III trials failed to show a survival benefit for intra-arterial chemotherapy (IAC).25,26 A large trial of over 300 patients with newly diagnosed malignant glioma was conducted by the Brain Tumor Study Group (BTSG) trial to assess the efficacy of IAC chemotherapy in which nutrients were randomly assigned to IAC or intravenous (IV) BCNU with or without IV 5-fluorouracil (5-FU) and radiation therapy (RT). This study was closed early when an interim analysis showed shorter survival times in patients receiving IAC.26 The side effects of the IAC in these two studies included catheter-related complications such as bleeding, infection, thrombosis, treatment-related neurotoxicity, leukoencephalopathy, cortical necrosis, and ipsilateral blindness.25,26
Intra–Cerebrospinal Fluid Chemotherapy
Intra-CSF chemotherapy involves administration of drugs either into the lateral ventricle, usually through a surgically implanted subcutaneous device, such as an Ommaya reservoir27 or instilling the drug into the lumbar subarachnoid space (i.e., intrathecal therapy). The benefit of this approach is that small doses of chemotherapeutic agents given intrathecally can produce high concentrations within the CSF with minimal systemic toxicity. However, abnormal CSF flow and obstruction due to tumor or scarring from prior surgical interventions impair its utility in the treatment for primary brain tumors. Intrathecal administration of drugs has limited penetration into the brain parenchyma, and is generally employed in treatment or prophylaxis of leptomeningeal disease.28 Side effects include increased risks of neurotoxicity (especially with radiation) and chemical meningitis.
Manipulating BBB Permeability or Methods to Cause BBB Disruption
Various agents have been used to modify BBB and/or BTB in an attempt to increase the drug concentration in the tumor.29 Drug delivery to brain tumors can potentially be improved by increasing the permeability of the BBB with hyperosmolar solutions such as mannitol and vasoactive compounds such as bradykinin analogues that induce an osmotic opening of the BBB and BTB.29,30 Hyperosmolar solutions increase capillary permeability by temporarily opening the intercellular tight junctions of the brain endothelium that results in increased movement of water soluble substances. Complications with this approach include increased risk of stroke and seizures,31 and no clinical benefit has been demonstrated with this approach so far.32
High-Dose Chemotherapy
High-dose chemotherapy (HDCT) is theoretically promising as increases the peak concentration of unbound drug in the circulation and can result in greater transfer of drug across the BBB. The associated myelosuppression seen with this approach requires use of autologous hematopoietic cell rescue and treatment-related morbidity is substantial. The survival using this approach is similar to that achieved by conventional chemotherapy or targeted therapy and its use remains investigational.33,34
Wafers/Implantable Polymers
Surgical implantation of solid-phase reagents permits constant drug delivery into the tumor without significant systemic or local side effects. The most commonly used “wafer” is a copolymer matrix with carmustine that is implanted directly into the tumor resection cavity at the time of surgical intervention and has been approved for use in patients with malignant gliomas. This therapy has been approved for patients with newly diagnosed and recurrent high-grade gliomas.35,36
Convection-Enhanced Delivery
Convection-enhanced delivery (CED) involves direct intratumoral infusion with various chemotherapeutic drugs and has been designed to use pharmacological agents that would not normally cross the BBB, and this approach is particularly useful for the delivery of large molecules.37 Drugs are delivered through one to several catheters placed stereotactically directly within the tumor mass or around the tumor or the resection cavity and it allows distribution of substances throughout the interstitium via positive-pressure infusion.38 Several classes of drugs are amenable to this technology including chemotherapeutics or targeted drugs.39 Two multicenter randomized controlled trials in patients with recurrent GBM (PRECISE and TransMID) demonstrated that CED of agents was safe and well tolerated.40 However, no survival benefit was seen in PRECISE, a phase III trial to assess the efficacy of this approach in patients with GBM upon first relapse compared to treatment with carmustine wafers.40 Results of the TransMID trial have not been presented or published yet.
Various CNS Tumors
Low-Grade Gliomas
Low-grade gliomas (LGGs) comprise approximately 20% of CNS glial tumors with approximately 1800 new cases diagnosed each year in the United States.41 Oligodendrogliomas represent 3.7% of all primary brain and CNS tumors.41 Patients with LGGs typically present between the second and fourth decades of life. The optimal role of surgical resection in the long-term outcome of patients with LGG remains controversial, and the debate about the effect on outcome of its timing and extent persists. Nevertheless, surgery continues to be indispensable to provide tissue for histopathologic diagnosis and importantly molecular characterization that is prognostic and helps determine therapy approach. Retrospective studies have shown that more extensive resection rather than simple debulking is more beneficial and that greater than 99% resection yields improved overall survival (OS) and progression-free survival (PFS).42
Radiation Therapy
The value of RT in managing LGGs is controversial. This is due to prolonged natural history of LGGs and these patients are more likely to live long enough to suffer from the late effects of RT. In addition, dose of RT to treat LGGs is not clear. The most commonly used RT for the treatment of LGGs is 54 Gy with a range of 45 to 60 Gy, based on results of the European Organization for Research and Treatment of Cancer (EORTC) trial 2284443 and the North Central Cancer Treatment Group/Radiation Therapy Oncology Group(RTOG)/Eastern Cooperative Oncology Group study.44
In the EORTC trial, there was no significant difference in OS and PFS in patients of LGG treated with 59.4 Gy in 33 treatments or 45 Gy in 25 treatments.43 In the multigroup trial there was no survival benefit of using 64.8 Gy compared to 50.4 Gy.44 A higher dose of RT (64.8 Gy) resulted in higher rates of radiation necrosis, and consequently doses above 60 Gy are avoided in this patient group.44
Moreover, the benefit of RT is limited to improvement in PFS without translating into any improvement in OS as was demonstrated by the EORTC trial 22845.45 This study evaluated the role of up-front RT versus observation in LGG. A total of 311 patients were treated with immediate RT (54 Gy in 6 weeks) or no therapy until progression.45 Up-front RT significantly prolonged the median PFS (5.4 years vs. 3.7 years), but did not result in improvement in OS (7.4 years vs. 7.2 years).45 This suggests that radiation may have a comparable effect whether it is administered early or at subsequent tumor progression.
Chemotherapy
There is no level 1 evidence that postoperative chemotherapy significantly prolongs survival in patients with LGGs. The RTOG 98-02 was a three-arm trial in which 111 patients with a favorable prognosis (age <40 years and gross tumor resection) were followed with observation following surgery. A total of 251 patients with an unfavorable prognosis (age ≥40 years or those who had subtotal resection or biopsy only) were randomized to receive RT (54 Gy in 30 fractions) followed by six cycles of procarbazine, lomustine, and vincristine (PCV), or the same dose of RT only. At the last update of the trial presented at the American Society of Clinical Oncology meeting in 2008, PFS was increased with adjuvant PCV chemotherapy; however, the OS was similar in the two groups.46 However, after 2 years, the addition of chemotherapy to RT did result in a significant OS and PFS advantage suggesting a delayed advantage to chemotherapy.46
Recently, TMZ has been increasingly been used to treat this patient population. In a retrospective review of 149 patients with LGGs treated with TMZ, a partial response (PR) rate of 15% and a minor response (MR) rate of 38% were reported.47 In addition stable disease (SD) was seen in 37% and PD in 10% with a median PFS of 28 months. Tissue from 86 patients showed that codeletion of 1p/19q was associated with a significantly higher response rate (RR), a longer response to TMZ, especially with 1p/19q codeletion improved PFS and OS.47 These results provide strong evidence that LGGs respond to TMZ. Besides LOH 1p/19q, methylation status of the MGMT promoter (MGMTP) predicts response to TMZ in LGG.48 The LGG patients with methylation of MGMTP had an improved PFS compared to those with unmethylated MGMTP when treated with TMZ (p < 0.0001).48
Malignant Glioma
Malignant gliomas (MG) or high-grade gliomas (HGG) include WHO grade IV gliomas, also known as GBM, and WHO grade III gliomas referred to as anaplastic gliomas (AG) (anaplastic astrocytoma [AA], anaplastic oligodendroglioma [AO], and anaplastic oligoastrocytoma [AOA]).49 The goals of surgery are to establish a histological diagnosis and relieve mass effect. Biopsy alone is used in situations where the lesion is not amenable to resection, or the patient’s overall clinical condition will not permit surgery. However, maximal surgical resection while preserving neurological function is preferred.
Glioblastoma
Radiation
Even patients who undergo a gross total resection of their glioblastoma (GBM) have a high recurrence rate, and for over three decades adjuvant radiation therapy has been the standard approach for GBMs. The efficacy of radiation was initially established in the 1970s in a trial of over 300 patients with an AG addition of adjuvant whole-brain radiation therapy (WBRT) to surgical resection resulted in increased median survival from 14 to 36 weeks.50 A seminal analysis of patients treated in the previous Brain Tumor Cooperative Group Trials had established the standard radiation dose to be 60 Gy in the late 1970s,51 and dose escalation above 60 Gy with WBRT has not been shown to provide further additional benefit.52,53 Predominant mode of recurrence in patients with high-grade gliomas treated with radiation has been local failure within 2 cm of the enhancing tumor.54–56 Serious side effects of WBRT such as progressive and irreversible radiation necrosis, with accompanying small blood vessel injury, and demyelination led to the utilization of involved field radiation therapy (IFRT) as the standard approach for adjuvant RT to minimize toxicity. The pattern of treatment failure seen with IFRT are similar to those seen with WBRT and are mostly local failures within 2 cm of the initial tumor.55 In the United States, the RTOG treatment volumes generally used that deliver a 46-Gy dose to the peritumoral edema with a 2-cm margin and a 14-Gy boost to the enhancing tumor with a 2.5-cm margin. In Europe, a full 60 Gy are delivered to a 2- to 3-cm margin around the enhancing tumor without any field reduction. At present, T2 or fluid-attenuated, inversion-recovery magnetic resonance imaging (MRI) sequences are used to identify peritumoral edema, and the T1 sequence with contrast images is used to identify the enhancing portion of the tumor. If the tumor margin is defined upon contrast enhancement, typically a margin of 2.0 to 3.0 cm is used, and a margin of 1.0 to 2.0 cm is used if the RT field is defined by the T2-weighted MRI abnormality. Use of metabolic imaging such as positron emission tomography (PET), MR diffusion and MR perfusion, and MR spectroscopy are promising as they represent areas of activity that may need different treatment planning as compared to that defined by the traditional MRI sequences.57,58 However, they are still largely investigational at present, and are employed to define boost volumes rather than primary target volumes. Further advances in imaging will likely change the method of tumor delineation.
Intensity-modulated RT (IMRT) is a technique that utilizes software and modification of standard linear accelerator output to deliver varied intensity of radiation across each treatment field. IMRT is beneficial especially when the tumor is juxtaposed to radiation-sensitive structures. IMRT is increasingly used these days as it may reduce radiation-related adverse effects59 and can escalate the radiation dose delivered to the tumor. However, as of this writing, no proven benefit has been demonstrated by delivering doses in excess of 60 Gy.59,60
Chemotherapy
The benefit of adjuvant temozolomide (Stupp regimen) was established in a seminal phase III trial, when 573 patients with newly diagnosed GBM were randomly assigned to postoperative involved-field RT (60 Gy in daily 30 fractions) versus the RT plus concurrent temozolomide (TMZ) (75 mg/m2 daily up to 49 days) followed by up to six cycles of adjuvant TMZ (150 to 200 mg/m2 daily for 5 days, every 28 days).61 This study demonstrated a benefit with adjuvant TMZ with a 2.5-month median improvement of OS (12.1 months for RT alone vs. 14.6 months for RT plus TMZ). At 2 years, 26.5% of patients treated with TMZ plus RT were alive, compared with 10.4% of patients treated with RT alone. This benefit was even more impressive at 5 years when 9.8% of patients treated with TMZ plus RT were alive compared to 1.9% patients treated with RT alone.62 However, this study did not include patients older than 70 years of age, which constitutes 20% of all patients with newly diagnosed GBMs (discussed below). It also excluded patients with low performance status who were not independent in activities of daily living, a group constituting at least 10% of all newly diagnosed patients with GBMs in which the treatment plan needs to be tailor made according to the patient’s ability to tolerate RT and or chemotherapy.
As previously mentioned, MGMT is an enzyme responsible for DNA repair following alkylating chemotherapy. During the course of tumor development, the MGMT gene may be silenced by methylation of its promoter, which prevents repair of DNA damage and increases the effectiveness of alkylating agents such as TMZ. MGMT was determined retrospectively from the tumor tissue of 206 patients and appeared to be a prognostic factor for improved survival and was predictive of benefit from chemotherapy.18 For those with MGMT methylation, the 2-year survival rates were 49% and 24% with combination therapy and with RT alone, respectively, while for those without MGMT methylation, the 2-year survival rates were 15% and 2%, respectively. Biodegradable wafers impregnated with carmustine (Gliadel® wafer) that function as a slow-release carrier system for local drug delivery implanted at the time of resection are approved therapy for patients with newly diagnosed MG.35,36 In a phase III trial, 240 newly diagnosed malignant glioma patients were randomized to placement of up to eight carmustine wafers or a placebo, followed by standard RT.35,36 Patients receiving carmustine polymer had a statistically significant increase in median survival (13.9 vs. 11.6 months). This difference in survival, however, was not statistically significant when the analysis was restricted to GBM.35,36 Additional toxicities with Gliadel included increase in the incidence of CSF leak and intracranial hypertension compared to placebo.35,36
Bevacizumab is a monoclonal antibody that binds vascular endothelial growth factor (VEGF), which plays a critical role in the development of the abnormal vasculature observed in tumors including malignant gliomas.63 Two phase II trials that have been reported evaluating the addition of Bevacizumab to standard RT and TMZ (Stupp regimen).64,65 In a study conducted at Duke, a total of 125 patients with newly diagnosed GBM received standard radiation therapy, TMZ, and bevacizumab. At a 21-month follow-up, the median PFS of 13.8 months and median OS of 21.3 months was reported.65 These results are similar to the median PFS and OS of 13.6 and 19.6 months, respectively, in a phase II trial of bevacizumab plus TMZ and RT in patients with newly diagnosed GBM reported by Albert Lai and colleagues.64 These compare with PFS and OS of 7.6 and 21.1 months in the University of California, Los Angeles/KPLA control cohort.64 These two studies demonstrate that patients treated with BEV and TMZ during and after RT may show improved PFS without improved OS compared to the historical control group. The two phase III ongoing studies, RTOG 0825 (NCT00884741) and Hoffmann-La Roche Study (NCT00943826) will help answer the question of whether adding bevacizumab to TMZ and radiotherapy will improve survival of patients with GBM.
Recurrent Glioblastoma
Progression versus Pseudoprogression
Despite recent advances in therapeutics, most patients with GBM develop tumor recurrence after the above therapy. Recurrence is suspected when a previously stable patient develops new neurologic signs and symptoms or when surveillance MRI with gadolinium imaging shows increased tumor size or new enhancement usually accompanied with increased edema. However, clinical and imaging changes may be due to complications such as infection, a decrease in steroid use or radiation necrosis (also referred to as “pseudoprogression”). Radiation necrosis is a well-known late effect of RT of the brain that can mimic tumor recurrence. Pseudoprogression is a similar effect of transient increase in tumor enhancement that has been described after combined chemoradiotherapy and that occurs more rapidly and dramatically than after radiation alone. Pseudoprogression has been noted to occur between 20% and 30% of the cases in recent series.66–68 It has been suggested that “pseudoprogression may occur more frequently in patients with methylation of the MGMT promoter as it increases the effect of chemoradiotherapy on residual tumor and that this translates to the transient worsening of imaging characteristics.69 In the same series, survival in patients with pseudoprogression was significantly better than survival in those whose scans were initially stable (38 months vs. 20.2 months).69 Various novel imaging modalities such as magnetic resonance perfusion with or without spectroscopy, and PET are used to help distinguish between pseudoprogression and true early progression of disease, but are not always reliable, and none has yet been widely accepted as standard practice. In most cases, repeat imaging is helpful to distinguish between the two, while in select cases surgery may be necessary to relieve mass effect and obtain a tissue diagnosis.
Therapeutic Options
When a tumor reaches a certain size, the requirements for oxygen and nutrients lead to the growth of new blood vessels and tumors can promote the formation of new vessels through the process of angiogenesis.70 GBMs are among the most vascular tumors known and hence therapy directed against tumor-associated vasculature is a promising strategy.70 Bevacizumab is a monoclonal antibody directed toward VEGF, and is the prototype of antiangiogenic agents in clinical use for treatment of GBM. Bevacizumab was approved by the Food and Drug Administration (FDA) for recurrent GB in the United States based on two trials of bevacizumab as a single agent or combined with irinotecan in recurrent GBM patients after initial treatment with chemoradiation and adjuvant TMZ. In a randomized, phase II clinical trial, 167 patients with recurrent GBM were treated with bevacizumab alone or bevacizumab in combination with irinotecan; there was no statistically significant difference in the median OS in the group treated with bevacizumab alone (9.2 months) compared to the those treated with combination of bevacizumab and irinotecan (8.7 months).71 The objective response rate was 25.9% in patients who received bevacizumab monotherapy, and there were no complete responses per the outside review. Median duration of response was 4.2 months among the responders and the 6-month PFS (PFS-6) was 36%. The second study by the National Cancer Institute involved 48 recurrent high-grade glioma heavily pretreated patients treated with bevacizumab alone.10 The objective response as determined by independent review was 19.6% and median duration of response was 3.9 months in responders.72 The FDA approved bevacizumab as a single agent based on improvement in objective response rate in these studies, although no increased survival was seen.
Potent anti-VEGF activity of bevacizumab results in normalization of permeable tumor vessels producing rapid and marked reduction in edema and contrast enhancement on neuroimaging.73 This effect of rapid and dramatic improvements in MRI can occur within days of initiation of treatment with antiangiogenic agents such bevacizumab, cediranib, sunitinib, sorafenib, and aflibercept, and is partly a result of reduced vascular permeability to contrast agents rather than a true antitumor effect. These imaging changes can make evaluation of tumor response and progression difficult if one relies on commonly used MacDonald criteria of two-dimensional measurement of enhancing disease. In addition, a subset of patients treated with bevacizumab develop tumor recurrence observed as an increase in the nonenhancing component on T2-weighted/fluid-attenuated inversion recovery (FLAIR) sequences. This likely reflects a phenotypically invasive tumor recurrence pattern due to co-option of normal cerebral vessels and diffuse perivascular spread of tumor cells. Hence, the Response Assessment in Neuro-Oncology Working Group proposed a new standardized response criterion that takes into consideration the challenges of nonenhancing signal abnormality changes, pseudoprogression, and pseudoresponse.10
Carmustine polymer wafers (Gliadel) may prolong survival and has been approved for use after surgery in locally recurrent high-grade glioma.74 A prospective, randomized phase-III trial demonstrated a modest increase in OS from 23 weeks in those patients who received placebo wafers compared to 31 weeks receiving Gliadel.74 However, the study included recurrent low- and higher-grade gliomas, and the benefit in the GBM subgroup was smaller than in the whole cohort. This study predated the use of chemoradiation and adjuvant TMZ, and the benefit of this approach in recurrent GBM patients treated with prior TMZ is unclear.
Other options for patients who have contraindication to bevacizumab or prior to therapy with bevacizumab include rechallenge with alternative dosing schedules of TMZ.75 One of the mechanisms of resistance to TMZ occurs through direct repair of DNA damage by MGMT enzyme, and an effective strategy to overcome such form of resistance is to deplete tumor cell MGMT. TMZ rechallenge with alternative doses and dosing schedules that deliver higher cumulative doses over prolonged periods of time can result in depletion of MGMT,76 and has been shown to be directly toxic to endothelial cells.77 Commonly used TMZ dosing schedules are 75 to 100 mg/m2 (21 days on/7 days off), 150 mg/m2 (7 days on/7 days off), and 50 mg/m2 daily dosing. Similar responses seen in patients with high and low levels of tumor MGMT support the rationale that these regimens may overcome MGMT-mediated resistance.78
Other chemotherapy options for recurrent GBM include nitrosoureas (e.g., carmustine, fotemustine) either as single agent or in combination (most commonly used regimen, PCV) that have shown activity in previously treated patients.79–81 Other chemotherapeutic agents used in this patient population includes carboplatin, etoposide, and irinotecan, which have demonstrated modest efficacy as single agents or in combination regimens.82–85 Recently in a randomized phase III trial of 325 recurrent GBM patients, lomustine was found to be superior to the pan-VEFG receptor inhibitor, cediranib.86
Molecularly Targeted Therapy
In the past decade there has been substantial growth in the number of novel therapies due to increased understanding of the molecular pathways involved in glioma formation and progression. Malignant transformation in gliomas is often the result of the sequential accumulation of genetic aberrations and proliferation of growth factor signaling pathways that include the vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), and platelet-derived growth factor (PDGF).87
A number of agents that target VEGF have been developed including bevacizumab. Tyrosine kinase inhibitors that target VEGF pathway include cediranib,88,89 and adnectin.90–92 Small-molecule EGFR inhibitors such as gefitinib and erlotinib are well tolerated in patients with recurrent HGG, but responses have been disappointing.93,94 There are a number of agents that target different signal transduction pathways including PI3K/AKT/mTOR,95,96 RAF-MEK-ERK,97,98 PDGF,99,100, SRC,101 and PKC102 pathways are undergoing trials in patients with high-grade gliomas (Table 7-1). Results from clinical trials with most of these molecular-targeted therapies with the exception of bevacizumab have been disappointing so far. This is likely due to the complexity of the molecular abnormalities in recurrent high-grade gliomas, the redundancy of the signaling pathways, and the inability of many of these agents to cross the BBB.
TABLE 7-1 Selected Molecularly Targeted Agents in Clinical Trials in High-Grade Gliomas
| Drug Name | Type of Drug | Targets |
|---|---|---|
| ABT-888 | Tyrosine kinase inhibitor | PARP-1, PARP-2 |
| Aflibercept | Soluble decoy receptor | VEGF-A,B, PlGF |
| AMG 102 | Thrombospondin-1 mimetic peptide | FGFR, VEGFR2 |
| Bevacizumab | Monoclonal antibody | VEGF-A |
| Brivanib | Monoclonal antibody | FGF pathway |
| Cediranib | Tyrosine kinase inhibitor | VEGFR1–3, PDGFRβ, c-Kit |
| Cetuximab (Erbitux) | Monoclonal antibody | EGFR |
| CT-322 | Fibronectin (adnectin)-based inhibitor | VEGFR1–3 |
| Dasatinib | Immunomodulatory and anti-inflammatory | PDGFRβ, BCR-ABL, c-Kit |
| Erlotinib (OSI-774) | Tyrosine kinase inhibitor | EGFR |
| Everolimus (RAD-001) | Tyrosine kinase inhibitor | mTOR |
| Gefitinib (ZD1839) | Tyrosine kinase inhibitor | EGFR |
| Imatinib | Tyrosine kinase inhibitor | PDGFRβ, Flt3, c-Kit |
| IMC-1121B | Monoclonal antibody | VEGR |
| Lapatinib (GW-572016) | Tyrosine kinase inhibitor | EGFR |
| Lenalidomide | Tyrosine kinase inhibitor | PDGFRβ, Src, BCR-ABL, c-Kit, EphA2 |
| Lonafarnib (SCH-66336) | Farnesyl tranferase inhibitor | Ras |
| Pazopanib (GW786034) | Tyrosine kinase inhibitor | VEGFR1–3, PDGFRβ, c-Kit |
| Sorafenib | Tyrosine kinase inhibitor | VEGFR2,3, BRAF, PDGFRβ, c-Kit, Ras, p38α |
| Sunitinib | Tyrosine kinase inhibitor | VEGFR2, PDGFRβ, Flt3, c-Kit |
| Tandutinib (MLN518) | Tyrosine kinase inhibitor | COX-2 |
| Temsirolimus (CC!-779) | Tyrosine kinase inhibitor | mTOR |
| Tipifarnib (R115777) | Farnesyl tranferase inhibitor | Ras |
| Vandetanib (ZD6474) | Tyrosine kinase inhibitor | VEGFR2, EGFR, RET |
| Vatalanib (PTK787) | Tyrosine kinase inhibitor | VEGFR1–3, PDGFRβ, c-Kit |
| XL-184 | Tyrosine kinase inhibitor | VEGFR2, Met, RET, c-Kit, Flt3, Tie-2 |
| XL-765 | Tyrosine kinase inhibitor | mTOR, P13K |
Source: Modified from Ahluwalia MS, Gladson CL. Progress on antiangiogenic therapy for patients with malignant glioma. J Oncol. 2010;2010:689018.
Elderly Patients
The incidence of elderly patients with high-grade gliomas is increasing. The optimal treatment for this group of patients is unknown as they tend to respond less well to standard chemotherapy and have poorer prognosis than younger patients. A recent population-based analysis of GBM patients aged 65 years and older showed a median survival of only 4 months.103
The optimal dose and schedule of RT in the elderly has not been determined. RT benefit in the elderly was demonstrated in a prospective randomized clinical trial in which 85 patients aged 70 and older with grade-III or -IV gliomas had good performance status. Patients treated with IFRT (50 Gy in 1.8-Gy fractions) had a modest survival benefit over those who received supportive care alone (median survival of 29.1 weeks vs. 16.9 weeks).104 No further deterioration in performance status, quality of life, or cognitive function was seen in the patients treated with radiation.104
An abbreviated course of RT may be appropriate treatment in select groups of elderly patients, especially for those with poor performance status. This was demonstrated in a prospective randomized study of 100 patients with GBM aged 60 years and older who were either randomized to treatment with 40 Gy in 15 fractions over 3 weeks or 60 Gy in 30 fractions over 6 weeks. There was no difference in median survival between the two groups, 5.6 months in the short-course RT group versus 5.1 months in the 6-week RT group, fewer patients discontinued the abbreviated RT schedule (10% vs. 26%), and the short-course RT was better tolerated—only 23% of patients required corticosteroid increases, compared with 49% in the 6-week RT.105
As previously mentioned, the EORTC/NCIC study that established the current standard of care for GBM, RT with concomitant TMZ followed by adjuvant TMZ, excluded patients aged more than 70 years. Due to concerns regarding increased toxicity with combined chemoradiation therapy in older patients, chemotherapy with TMZ has been studied as an alternative to RT.106 At the American Society for Clinical Oncology (ASCO) meeting in 2010, the Nordic Elderly Trial reported that 342 GBM patients aged 60 years and older were randomly assigned to either receive 60 Gy in 30 fractions or 34 Gy in 10 fractions RT or chemotherapy with TMZ (200 mg/m2 for 5 days with cycles repeated every 28 days).107 There was no significant difference in OS between the three treatment arms, with median survival being 8 months for TMZ, 7.5 months for hypofractionated RT, and 6 months for 6-week RT (p = 0.14). The study suggested that hypofractionated RT is preferable to standard 6-week RT in elderly patients, which is consistent with a previous Canadian study.105
The Neurooncology Working Group (NOA) of the German Cancer Society randomized 373 anaplastic astrocytoma or GBM patients aged 65 years and older and Karnofsky performance status (KPS) of 60 or higher to treatment with (1) IFRT of 54 to 60 Gy or (2) TMZ 100 mg/m2 in a 1 week on/1 week off108 regime. The primary aim of the trial was to demonstrate that TMZ treatment is comparable to RT. Longer OS was seen in patients initially managed with RT (median survival 293 vs. 245 days in those treated with dose-dense TMZ).108 The patients in the TMZ arm had an increased risk of death compared with patients in the RT arm, as well as an increased incidence of adverse events, suggesting that RT is preferable in this population.
Another reasonable alternative for treating elderly patients with GBM and poor performance status is therapy with TMZ as demonstrated by the ANOCEF “TAG” trial, a phase II trial of TMZ in elderly patients with GBM and poor performance status (KPS < 70).109 In this multicenter, prospective phase-II trial, 70 patients (10 centers) with a median age of 77 years were treated with TMZ. A PFS-6 of 29% and median OS of 25 weeks observed in this study compared favorably with an expected 12 to 16 weeks from a purely supportive approach.
Anaplastic Astrocytoma
Chemotherapy
The role of adjuvant chemotherapy following surgery in patients with AA is less clear, as there is no level 1 evidence that such treatment improves survival. The EORTC/NCIC phase III trial that established the survival benefit with TMZ excluded patients with AA and no randomized trial has evaluated the benefit of adjuvant chemotherapy in addition to RT in patients with AA. TMZ has been shown to be active in patients with AA in a retrospective analysis of 109 patients from two consecutive trials; adjuvant TMZ was as effective and less toxic than PCV.110 In the analysis, outcomes of 49 patients treated with PCV and 60 with TMZ were compared, and there was no significant difference in the 2-year PFS and the median PFS and OS between the two groups. Adjuvant chemotherapy was discontinued prematurely less often with TMZ than PCV because of toxicity (0% vs. 37%). Due to its better toxicity profile, TMZ has replaced PCV as the treatment regimen for these patients.
Adjuvant Chemotherapy versus Adjuvant RT
A German phase III trial (NOA-4) compared adjuvant chemotherapy to adjuvant RT in a phase III trial of 318 patients with grade III gliomas.111 Following surgical resection, the patients were randomized to adjuvant RT with chemotherapy on progression or to adjuvant chemotherapy (either PCV or TMZ) with RT on progression of the disease. There was no difference in time to treatment failure in the different groups. Similar outcomes were seen in the patients treated with TMZ or PCV.111
Recurrent Anaplastic Astrocytoma
TMZ has demonstrated good single-agent activity with an acceptable safety profile, and improved QOL in patients with recurrent AA.112 In a multicenter phase II trial of recurrent AA treated with TMZ demonstrated a PFS-6 of 46% and objective response rate of 35%.112 Other options for these patients include treatment with bevacizumab and irinotecan.113,114 In phase II trials, this regimen has a demonstrated RR of 55% to 66% with a median PFS-6 of 56% to 61% with an acceptable safety profile.113,114
Oligodendroglial Tumors
Oligodendroglial tumors constitute 5% to 20% of all glial tumors.115 Oligodendroglial tumors exhibit more sensitivity to chemotherapy as compared to nonoligodendroglial tumors. This chemosensitivity was initially reported in 1988 when Cairncross and Macdonald reported dramatic responses to chemotherapy in eight consecutive patients with recurrent AO.116 TMZ is preferred over the PCV regimen these days due to better patient tolerance, ease of administration, and improved patient compliance. There are no randomized trials comparing PCV and TMZ for efficacy in oligodendroglial tumors. Molecular genetic studies have revealed an association between radiographic responses and allelic loss of chromosome 1p, often in association with loss of chromosome 19q.117 The 1p/19q codeletion that is associated with sensitivity to chemotherapy is mediated by an unbalanced translocation of 19p to 1q. Randomized studies have shown that 1p/19q codeletion is associated with a better outcome with RT. Multiple studies have established the activity of PCV in patients with both low-grade and anaplastic oligodendroglial tumors.118–120
Two prospective, randomized, controlled trials of AO and AOA patients have shown that PCV chemotherapy prior to RT does not result in a survival benefit, although it does lead to a longer PFS time. In the RTOG 94-02 trial, patients were randomized to either four cycles of up-front intensified PCV chemotherapy followed by RT or RT only.121 In the second trial, EORTC 26951, patients were randomized to RT followed by adjuvant PCV or RT alone.122 In both the trials, there was improvement in the PFS that did not translate to a survival benefit. In both trials, most of the patients randomized to the RT arm received PCV at progression, which helps explain the longer PFS without improvement in survival time.
Medulloblastoma
Medulloblastomas are the most common malignant brain tumors in children and account for approximately 20% of all pediatric CNS tumors and 40% of all posterior fossa tumors.123 The peak incidence of medulloblastoma is between 5 and 9 years of age,123 with 10% of cases diagnosed within the first year of life, and the incidence decreases with age. Medulloblastomas account for 1% to 3% of all brain tumors in adults.
Dissemination throughout the cerebrospinal fluid, at least at the microscopic level, is assumed for all PNETs, and craniospinal RT is an integral component of the initial management of patients with medulloblastoma, both to control residual posterior fossa disease and to treat any disease that has spread along the craniospinal axis. However, toxicity to the brain and spinal cord limits the doses used.124 Medulloblastoma are quite chemosensitive and respond to a variety of cytotoxic drugs.125 The most active agents include cisplatin, cyclophosphamide, carboplatin, and methotrexate (MTX). However, since chemotherapy is generally administered after craniospinal RT, the use of MTX has largely been abandoned outside of the infant population because of neurologic complications of leukoencepalopathy.126,127
The medical treatment of children with medulloblastoma can be divided into 3 main categories: standard risk medulloblastoma in patients older than 3 years of age, high-risk medulloblastoma in patients older than 3 years of age, and medulloblastoma in infants and young children. The average-risk medulloblastoma are children aged 3 years and older who have undergone a complete or near complete resection, have negative cerebrospinal fluid cytology, and have no evidence of distant metastases. In absence of a clinical trial which is preferred, children are treated with a radiation (23.4 Gy to the craniospinal axis with a posterior fossa boost for a total dose of 55.8 Gy). RT is typically followed by eight cycles of adjuvant chemotherapy with cisplatin, vincristine, and either cyclophosphamide or lomustine.128 This is based on the 5-year, event-free and OS rates of 81% and 86%, respectively, of 379 evaluable children with average-risk medulloblastomas who were treated with this regimen in a phase II trial.128
In adults, the clinical experience with medulloblastoma is limited, and treatment is done based on experience in children. The optimal treatment for adults with metastatic, unresectable, or recurrent medulloblastoma is unknown, and these patients should be treated in a clinical trial whenever possible. If a clinical trial is not available, a combined modality treatment including craniospinal RT and adjuvant chemotherapy is used. A phase II study involving 95 adults with medulloblastoma patients over a 20-year period was reported recently at ASCO 2010 meeting.129 In this study, low-risk patients defined as no residual disease following surgery were treated only with craniospinal RT while high-risk patients (residual disease following surgery or distant metastases) treated with two cycles of “up-front chemotherapy” (cisplatin, etoposide, and cyclophosphamide) before craniospinal radiation followed by maintenance chemotherapy. For low-risk patients, 10-year PFS and OS rates of 46% and 65%, respectively, were observed, whereas in those with high-risk disease, the 10-year PFS and OS rates were 36% and 45%, respectively.129 The value of adjuvant chemotherapy in low-risk patients and benefits of preirradiation chemotherapy compared with postirradiation chemotherapy is unknown.
Recurrent Disease
Most patients with medulloblastoma that relapse do so within 2 years of completing therapy, and a significant proportion of them relapse with disseminated disease. It is imperative to perform CSF cytology and MRI of the brain and entire spine with and without gadolinium at the time of relapse to determine the full extent of disease. Bone scan and bone marrow examination should be considered in appropriate cases. For infants and young children treated with chemotherapy only at the time of up-front therapy, radiation therapy is an option for patients with local recurrence, albeit at a high cost. High-dose chemotherapy followed by autologous stem cell rescue has also been used effectively as a salvage option.130,131
Meningiomas
Meningiomas are the most common type of primary brain tumors in adults, accounting for one third of total brain tumors.132 Current management for meningiomas consists of surgery, RT, and stereotactic radiosurgery. These approaches are effective in achieving tumor control for most of patients with WHO grade I tumors and a subset of patients with WHO grade II tumors. However, there are limited treatment options for patients with inoperable or higher grade meningiomas who develop recurrent disease following surgery and RT. Chemotherapy use in meningioma is mostly limited to patients who have exhausted all surgical and radiotherapy options.132 The most commonly used agents in treatment of meningiomas include hydroxyurea,133,134 somatostatin analogues,135 and hormonal agents, such as progesterone receptor inhibitors.
Hydroxyurea is an oral ribonucleotide reductase inhibitor that arrests meningioma cell growth in the S phase of the cell cycle.136 Despite initial promising preliminary data, response rates have generally been less than 5%.133,134,137 Chamberlain and colleagues treated 16 patients with recurrent meningiomas with monthly injections of a sustained-release somatostatin preparation (sandostatin LAR).135 Approximately 60% of the patients either had a partial response or achieved stable disease.135 This study has generated considerable interest in the therapeutic potential of somatostatin analogues and a clinical trial involving pasireotide (SOM230), a novel somatostatin analog with a wider somatostatin receptor spectrum (including subtypes 1, 2, 3, and 5) and higher affinity (particularly for subtypes 1, 3, and 5) than sandostatin, is being evaluated in patients with recurrent meningiomas. Although initial studies of the antiprogesterone mifepristone (RU486) were encouraging,138 a prospective randomized SWOG multicenter study failed to demonstrate any benefit.139 In this study of 180 patients, treatment with mifepristone did not result in any improvement in PFS or OS compared to placebo.139
The importance of dysregulated cell signaling as a cause of neoplastic transformation is increasingly apparent and recent studies have identified aberrant expression of critical signaling molecules in meningioma cells. Hence a number of targeted agents have been evaluated in meningiomas. A phase II trial of PTK787 (vatalanib) in 25 patients with recurrent or progressive meningiomas demonstrated partial response in 1 patient (4.0%) and stable disease in 15 patients (60.0%).140 Overall PFS-6 was 57.2% and median time to progression was 7.5 months (intent to treat). Median OS was 26.9 months. A phase II trial of sunitinib (SU011248) of 50 patients with recurrent meningiomas showed a median PFS of 5.1 months and PFS-6 of 36%.141 These studies with targeted therapy suggest that targeted therapy may have a better role than cytotoxic therapies. More studies are warranted as it is likely that these novel therapies will complement traditional approaches such as surgery and radiotherapy and lead to more effective treatments for patients with meningiomas.
Brain Metastasis
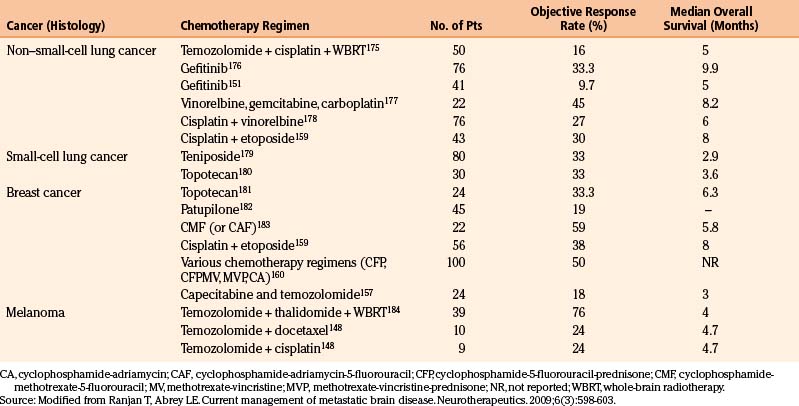
The main goals of systemic therapy in patients of brain metastasis (BM) includes control of the existing BM (local brain control), prevention of future BM (distant brain control), and control of the systemic disease (systemic control). Systemic therapies are used either alone or in combination with radiation and generally selected based on their efficacy in specific tumor histology and their ability to successfully penetrate the BBB. Most commonly utilized drugs include cytotoxics like TMZ,142 methotrexate,143 capecitabine,144 topotecan,145 and targeted agents such as lapatinib146 given their ability to cross the BBB and the sensitivity of tumors that commonly metastasize to the brain to these agents. Complications of systemic chemotherapy include myelosuppression, fatigue, immune suppression, gastrointestinal dysfunction, or drug-specific toxicities. Currently, the role of chemotherapy in the management of brain metastases is limited. Many commonly seen brain metastases (from melanoma and non–small-cell lung cancer (NSCLC), are relatively chemoresistant, and the patients often develop BM after use of the most effective chemotherapeutic agents (Table 7.2).
Non–Small-Cell Lung Cancer
Response rates of patients with non–small-cell lung cancer (NSCLC) with BM chemotherapy are highest in patients without any prior history of systemic treatment or RT. Chemotherapeutic agents commonly used in this patient population include platinum agents (cisplatin147), premetexed,148 TMZ,149 topotecan,150 and targeted agents such as gefitinib151 and erlotinib.152 The response rates to these chemotherapies range between 5% and 38%.148 Increasingly, targeted therapies such as gefitinib and erlotinib are being used in the treatment of NSCLC patients with BM. In a prospective study of 41 consecutive NSCLC patients with measurable BM, four patients treated with gefitinib (250 mg daily) demonstrated partial response (PR; 10%) and SD in seven patients (17%).151 Median duration of PR was 13.5 months and a median PFS of 3 months was observed.151 Patients who are more likely to have an activating mutation of the EGFR have reported substantially higher response rates to these therapies.152 In a study of the 23 Korean patients, never-smokers with adenocarcinoma of the lung, 16 (69.6%) achieved a PR, and 3 experienced SD.
Small-Cell Lung Cancer
Varied response rates between 27% to 82% have been reported when previously untreated patients of small-cell lung cancer (SCLC) that present with BM are treated with chemotherapy.153–155 Response rates in previously treated patients with brain metastases are considerably lower and comparable to the response rates seen with extracranial disease in patients with SCLC with second-line chemotherapy.153 To further improve the response rates, combination approach of chemotherapy and RT was compared to chemotherapy alone.156 In a phase III EORTC trial, 120 patients with BM from SCLC were randomized to treatment with teniposide alone or teniposide and RT. Although the response rate in the combined modality group was significantly higher (57%) than in the teniposide-alone group (22%), this did not result in a prolongation of survival, likely due to progression of disease outside the brain.
Breast Cancer
Chemotherapeutic agents used in the treatment of brain metastases in patients with breast cancer include capecitabine,157 TMZ,158 etoposide,159 and platinum agents.159 One hundred consecutive breast cancer patients treated with different combinations of chemotherapy showed an objective response rate of 50% and a median duration of response of 7 months.160 However, in this study less than 10% of patients had received adjuvant chemotherapy and approximately half had not received chemotherapy for metastatic disease, and hence it is difficult to extrapolate these response rates today, as most of the patients with breast cancer have been heavily pretreated by the time they develop brain metastasis. Recently targeted agents such as lapatinib are increasingly being used in the treatment of HER2 positive breast cancer patients. Two single-arm clinical trials have shown promising outcomes in these traditionally poor-prognosis patients when treated with lapatinib, an orally active, small-molecule inhibitor of epidermal growth factor receptor (EGFR) and HER2, in patients with refractory CNS metastases.161,162 An objective RR of 20% was seen in patients who entered the lapatinib-plus-capecitabine extension.161
Melanoma
Brain metastasis from melanoma is generally resistant to cytotoxic chemotherapy, and systemic treatment has been ineffective in the management of melanoma BM until recent approval of ipilimumab in patients with metastatic melonoma. In a phase II trial of metastatic melanoma patients with brain metastasis, impressive activity was seen with ipilimumab. In this phase II trial, 51 patients were treated in Arm 1, of which five patients had a global PR and four SD, for an overall global disease control rate of 17.6% in this cohort of patients. Similar response rates were seen in patients with CNS disease compared to systemic disease. This offers an exciting option for these patients with dismal outcomes. TMZ has systemic activity in patients with advanced melanoma, and although objective responses to TMZ have been reported, the overall response rate is less than 10%.163,164 A combination approach of chemotherapy and RT was compared to chemotherapy alone in a prospective randomized phase III trial of fotemustine plus WBRT versus fotemustine alone in patients with BM from malignant melanoma.165 Although the addition of fotemustine to WBRT significantly increased the median time to progression of BM, it did not improve the RR or OS.165
Leptomeningeal Metastasis
Leptomeningeal metastasis (LM) or neoplastic meningitis (NM) refers to the dissemination of cancer to the arachnoid mater, CSF, and pia mater, which occurs in approximately 2% to 8% of all patients with cancer.166 LM is diagnosed in 1% to 5% of patients with solid tumors, 5% to 15% of patients with leukemia (often referred to as leukemic meningitis), and lymphoma (called lymphomatous meningitis), and 1% to 2% of patients with primary brain tumors.167 Breast, lung, and melanoma are the most common cancers that metastasize and involve the leptomeninges. LM usually occurs in patients with progressive systemic cancer (>70%), but can be the first manifestation of cancer in ~5% of the patients.168
Radiotherapy
External beam radiotherapy (RT) is often used in patients with LM.169 RT is used for palliation of symptoms (cauda equina syndrome), correction of CSF flow abnormalities and if bulky disease is present when IFRT is the modality used. RT is used to treat bulky disease as intrathecal chemotherapy is limited by diffusion to 3-mm penetration into tumor nodules and is not effective in bulky disease.170
Intrathecal Chemotherapy
Intrathecal (IT) chemotherapy is the mainstay of treatment of LM. The most commonly used IT chemotherapeutic agents for LM methotrexate (MTX),171 cytarabine (Ara-C),172 depocyt ®,173 and less commonly, thiotepa.174 Usual schedule includes induction phase (4–6 weeks), followed by consolidation phase and maintenance phase. Complications of IT include those related to the ventricular reservoir and due to chemotherapy itself.170 Chemical aseptic meningitis is the most frequent complication observed, and leukoencephalopathy can occur, especially when the combination of RT and IT is used.173
Brandes A.A., Franceschi E., Tosoni A., et al. Efficacy of tailored treatment for high- and low-risk medulloblastoma in adults: a large prospective phase II trial. ASCO Meeting Abstracts. 2010;28:2003.
Brem H., Piantadosi S., Burger P.C., et al. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The Polymer-Brain Tumor Treatment Group. Lancet. 1995;345:1008-1012.
Cairncross G., Berkey B., Shaw E., et al. Phase III trial of chemotherapy plus radiotherapy compared with radiotherapy alone for pure and mixed anaplastic oligodendroglioma: Intergroup Radiation Therapy Oncology Group Trial 9402. J Clin Oncol. 2006;24:2707-2714.
Chamberlain M.C., Glantz M.J., Fadul C.E. Recurrent meningioma: salvage therapy with long-acting somatostatin analogue. Neurology. 2007;69:969-973.
Friedman H.S., Prados M.D., Wen P.Y., et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733-4740.
Glantz M.J., Jaeckle K.A., Chamberlain M.C., et al. A randomized controlled trial comparing intrathecal sustained-release cytarabine (DepoCyt) to intrathecal methotrexate in patients with neoplastic meningitis from solid tumors. Clin Cancer Res. 1999;5:3394-3402.
Hegi M.E., Diserens A.C., Gorlia T., et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997-1003.
Karim A.B., Maat B., Hatlevoll R., et al. A randomized trial on dose-response in radiation therapy of low-grade cerebral glioma: European Organization for Research and Treatment of Cancer (EORTC) Study 22844. Int J Radiat Oncol Biol Phys. 1996;36:549-556.
Kreisl T.N., Kim L., Moore K., et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27:740-745.
Lin N.U., Carey L.A., Liu M.C., et al. Phase II trial of lapatinib for brain metastases in patients with human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2008;26:1993-1999.
Lin N.U., Dieras V., Paul D., et al. Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin Cancer Res. 2009;15:1452-1459.
Macdonald D.R., Cascino T.L., Schold S.C.Jr., Cairncross J.G. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277-1280.
Muldoon L.L., Soussain C., Jahnke K., et al. Chemotherapy delivery issues in central nervous system malignancy: a reality check. J Clin Oncol. 2007;25:2295-2305.
Packer R.J., Gajjar A., Vezina G., et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24:4202-4208.
Shaw E., Arusell R., Scheithauer B., et al. Prospective randomized trial of low- versus high-dose radiation therapy in adults with supratentorial low-grade glioma: Initial report of a North Central Cancer Treatment Group/Radiation Therapy Oncology Group/Eastern Cooperative Oncology Group study. J Clin Oncol. 2002;20:2267-2276.
Shaw E.G., Wang M., Coons S., et al. Final report of Radiation Therapy Oncology Group (RTOG) protocol 9802: radiation therapy (RT) versus RT + procarbazine, CCNU, and vincristine (PCV) chemotherapy for adult low-grade glioma (LGG). ASCO Meeting Abstracts. 2008;26:2006.
Stupp R., Hegi M.E., Mason W.P., et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459-466.
Stupp R., van den Bent M.J., Hegi M.E. Optimal role of temozolomide in the treatment of malignant gliomas. Curr Neurol Neurosci Rep. 2005;5:198-206.
van den Bent M.J., Afra D., de Witte O., et al. Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet. 2005;366:985-990.
van den Bent M.J., Carpentier A.F., Brandes A.A., et al. Adjuvant procarbazine, lomustine, and vincristine improves progression-free survival but not overall survival in newly diagnosed anaplastic oligodendrogliomas and oligoastrocytomas: a randomized European Organisation for Research and Treatment of Cancer phase III trial. J Clin Oncol. 2006;24:2715-2722.
Vredenburgh J.J., Desjardins A., Herndon J.E.II, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13:1253-1259.
Wen P.Y., Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492-507.
Wen P.Y., Macdonald D.R., Reardon D.A., et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28:1963-1972.
Westphal M., Hilt D.C., Bortey E., et al. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro Oncol. 2003;5:79-88.
Wick W., Hartmann C., Engel C., et al. NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomide. J Clin Oncol. 2009;27:5874-5880.
1. Muldoon L.L., Soussain C., Jahnke K., et al. Chemotherapy delivery issues in central nervous system malignancy: a reality check. J Clin Oncol. 2007;25:2295-2305.
2. Neuwelt E.A. Mechanisms of disease: the blood–brain barrier. Neurosurgery. 2004;54:131-140. discussion 41–42
3. Ehrlich P. Das sauerstoff-Bedurfnis des Organismus: Eine Farbenanalytlsche Studie. Berlin: Hirschwald; 1885.
4. Banks W.A. Physiology and pathology of the blood–brain barrier: implications for microbial pathogenesis, drug delivery and neurodegenerative disorders. J Neurovirol. 1999;5:538-555.
5. Pardridge W.M. Blood–brain barrier biology and methodology. J Neurovirol. 1999;5:556-569.
6. Balis F.M., Lester C.M., Chrousos G.P., et al. Differences in cerebrospinal fluid penetration of corticosteroids: possible relationship to the prevention of meningeal leukemia. J Clin Oncol. 1987;5:202-207.
7. Green S.B., Byar D.P., Walker M.D., et al. Comparisons of carmustine, procarbazine, and high-dose methylprednisolone as additions to surgery and radiotherapy for the treatment of malignant glioma. Cancer Treat Rep. 1983;67:121-132.
8. Watling C.J., Lee D.H., Macdonald D.R., Cairncross J.G. Corticosteroid-induced magnetic resonance imaging changes in patients with recurrent malignant glioma. J Clin Oncol. 1994;12:1886-1889.
9. Macdonald D.R., Cascino T.L., Schold S.C.Jr., Cairncross J.G. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277-1280.
10. Wen P.Y., Macdonald D.R., Reardon D.A., et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28:1963-1972.
11. Riordan J.R., Deuchars K., Kartner N., et al. Amplification of P-glycoprotein genes in multidrug-resistant mammalian cell lines. Nature. 1985;316:817-819.
12. Riordan J.R., Ling V. Genetic and biochemical characterization of multidrug resistance. Pharmacol Ther. 1985;28:51-75.
13. Dean M., Annilo T. Evolution of the ATP-binding cassette (ABC) transporter superfamily in vertebrates. Annu Rev Genomics Hum Genet. 2005;6:123-142.
14. Juliano R.L., Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta. 1976;455:152-162.
15. Cole S.P., Bhardwaj G., Gerlach J.H., et al. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science. 1992;258:1650-1654.
16. Dauchy S., Dutheil F., Weaver R.J., et al. ABC transporters, cytochromes P450 and their main transcription factors: expression at the human blood–brain barrier. J Neurochem. 2008;107:1518-1528.
17. Gerson S.L., Berger S.J., Varnes M.E., Donovan C. Combined depletion of O6-alkylguanine-DNA alkyltransferase and glutathione to modulate nitrosourea resistance in breast cancer. Biochem Pharmacol. 1994;48:543-548.
18. Hegi M.E., Diserens A.C., Gorlia T., et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997-1003.
19. Friedman H.S., Pluda J., Quinn J.A., et al. Phase I trial of carmustine plus O6-benzylguanine for patients with recurrent or progressive malignant glioma. J Clin Oncol. 2000;18:3522-3528.
20. Quinn J.A., Desjardins A., Weingart J., et al. Phase I trial of temozolomide plus O6-benzylguanine for patients with recurrent or progressive malignant glioma. J Clin Oncol. 2005;23:7178-7187.
21. Masutani M., Nakagama H., Sugimura T. Poly(ADP-ribosyl)ation in relation to cancer and autoimmune disease. Cell Mol Life Sci. 2005;62:769-783.
22. Tentori L., Portarena I., Torino F., et al. Poly(ADP-ribose) polymerase inhibitor increases growth inhibition and reduces G(2)/M cell accumulation induced by temozolomide in malignant glioma cells. Glia. 2002;40:44-54.
23. Curtin N.J., Wang L.Z., Yiakouvaki A., et al. Novel poly(ADP-ribose) polymerase-1 inhibitor, AG14361, restores sensitivity to temozolomide in mismatch repair-deficient cells. Clin Cancer Res. 2004;10:881-889.
24. Fenstermacher J.D., Cowles A.L. Theoretic limitations of intracarotid infusions in brain tumor chemotherapy. Cancer Treat Rep. 1977;61:519-526.
25. Hiesiger E.M., Green S.B., Shapiro W.R., et al. Results of a randomized trial comparing intra-arterial cisplatin and intravenous PCNU for the treatment of primary brain tumors in adults: Brain Tumor Cooperative Group trial 8420A. J Neurooncol. 1995;25:143-154.
26. Shapiro W.R., Green S.B., Burger P.C., et al. A randomized comparison of intra-arterial versus intravenous BCNU, with or without intravenous 5-fluorouracil, for newly diagnosed patients with malignant glioma. J Neurosurg. 1992;76:772-781.
27. Ommaya A.K. Subcutaneous reservoir and pump for sterile access to ventricular cerebrospinal fluid. Lancet. 1963;2:983-984.
28. Chamberlain M.C. Meningeal metastases. Neurology. 1995;45:1238-1239.
29. Zlokovic B.V., Apuzzo M.L. Strategies to circumvent vascular barriers of the central nervous system. Neurosurgery. 1998;43:877-878.
30. Kroll R.A., Pagel M.A., Muldoon L.L., et al. Improving drug delivery to intracerebral tumor and surrounding brain in a rodent model: a comparison of osmotic versus bradykinin modification of the blood–brain and/or blood-tumor barriers. Neurosurgery. 1998;43:879-886. discussion 86–89
31. Neuwelt E.A., Howieson J., Frenkel E.P., et al. Therapeutic efficacy of multiagent chemotherapy with drug delivery enhancement by blood–brain barrier modification in glioblastoma. Neurosurgery. 1986;19:573-582.
32. Kobrinsky N.L., Packer R.J., Boyett J.M., et al. Etoposide with or without mannitol for the treatment of recurrent or primarily unresponsive brain tumors: a Children’s Cancer Group Study, CCG-9881. J Neurooncol. 1999;45:47-54.
33. Durando X., Lemaire J.J., Tortochaux J., et al. High-dose BCNU followed by autologous hematopoietic stem cell transplantation in supratentorial high-grade malignant gliomas: a retrospective analysis of 114 patients. Bone Marrow Transplant. 2003;31:559-564.
34. Mbidde E.K., Selby P.J., Perren T.J., et al. High dose BCNU chemotherapy with autologous bone marrow transplantation and full dose radiotherapy for grade IV astrocytoma. Br J Cancer. 1988;58:779-782.
35. Westphal M., Hilt D.C., Bortey E., et al. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro Oncol. 2003;5:79-88.
36. Westphal M., Ram Z., Riddle V., et al. Gliadel wafer in initial surgery for malignant glioma: long-term follow-up of a multicenter controlled trial. Acta Neurochir (Wien). 2006;148:269-275. discussion 75
37. Bidros D.S., Liu J.K., Vogelbaum M.A. Future of convection-enhanced delivery in the treatment of brain tumors. Future Oncol. 2010;6:117-125.
38. Bobo R.H., Laske D.W., Akbasak A., et al. Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci U S A. 1994;91:2076-2080.
39. Bidros D.S., Vogelbaum M.A. Novel drug delivery strategies in neuro-oncology. Neurotherapeutics. 2009;6:539-546.
40. Kunwar S., Chang S., Westphal M., et al. Phase III randomized trial of CED of IL13-PE38QQR vs. Gliadel wafers for recurrent glioblastoma. Neuro Oncol. 2010;12:871-881.
41. Claus E.B., Black P.M. Survival rates and patterns of care for patients diagnosed with supratentorial low-grade gliomas: data from the SEER program, 1973-2001. Cancer. 2006;106:1358-1363.
42. Smith J.S., Chang E.F., Lamborn K.R., et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol. 2008;26:1338-1345.
43. Karim A.B., Maat B., Hatlevoll R., et al. A randomized trial on dose-response in radiation therapy of low-grade cerebral glioma: European Organization for Research and Treatment of Cancer (EORTC) Study 22844. Int J Radiat Oncol Biol Phys. 1996;36:549-556.
44. Shaw E., Arusell R., Scheithauer B., et al. Prospective randomized trial of low- versus high-dose radiation therapy in adults with supratentorial low-grade glioma: initial report of a North Central Cancer Treatment Group/Radiation Therapy Oncology Group/Eastern Cooperative Oncology Group study. J Clin Oncol. 2002;20:2267-2276.
45. van den Bent M.J., Afra D., de Witte O., et al. Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet. 2005;366:985-990.
46. Shaw E.G., Wang M., Coons S., et al. Final report of Radiation Therapy Oncology Group (RTOG) protocol 9802: radiation therapy (RT) versus RT + procarbazine, CCNU, and vincristine (PCV) chemotherapy for adult low-grade glioma (LGG). ASCO Meeting Abstracts. 2008;26:2006.
47. Kaloshi G., Benouaich-Amiel A., Diakite F., et al. Temozolomide for low-grade gliomas: predictive impact of 1p/19q loss on response and outcome. Neurology. 2007;68:1831-1836.
48. Everhard S., Kaloshi G., Criniere E., et al. MGMT methylation: a marker of response to temozolomide in low-grade gliomas. Ann Neurol. 2006;60:740-743.
49. Gladson C.L., Prayson R.A., Liu W.M. The pathobiology of glioma tumors. Annu Rev Pathol. 2010;5:33-50.
50. Walker M.D., Alexander E.Jr., Hunt W.E., et al. Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas. A cooperative clinical trial. J Neurosurg. 1978;49:333-343.
51. Walker M.D., Strike T.A., Sheline G.E. An analysis of dose–effect relationship in the radiotherapy of malignant gliomas. Int J Radiat Oncol Biol Phys. 1979;5:1725-1731.
52. Nelson D.F., Diener-West M., Horton J., et al. Combined modality approach to treatment of malignant gliomas—re-evaluation of RTOG 7401/ECOG 1374 with long-term follow-up: a joint study of the Radiation Therapy Oncology Group and the Eastern Cooperative Oncology Group. NCI Monogr. 1988:279-284.
53. Chang C.H., Horton J., Schoenfeld D., et al. Comparison of postoperative radiotherapy and combined postoperative radiotherapy and chemotherapy in the multidisciplinary management of malignant gliomas. A joint Radiation Therapy Oncology Group and Eastern Cooperative Oncology Group study. Cancer. 1983;52:997-1007.
54. Wallner K.E., Galicich J.H., Krol G., et al. Patterns of failure following treatment for glioblastoma multiforme and anaplastic astrocytoma. Int J Radiat Oncol Biol Phys. 1989;16:1405-1409.
55. Liang B.C., Thornton A.F.Jr., Sandler H.M., Greenberg H.S. Malignant astrocytomas: focal tumor recurrence after focal external beam radiation therapy. J Neurosurg. 1991;75:559-563.
56. Hochberg F.H., Pruitt A. Assumptions in the radiotherapy of glioblastoma. Neurology. 1980;30:907-911.
57. Pirzkall A., McKnight T.R., Graves E.E., et al. MR-spectroscopy guided target delineation for high-grade gliomas. Int J Radiat Oncol Biol Phys. 2001;50:915-928.
58. Grosu A.L., Weber W.A., Riedel E., et al. L-(methyl-11C) methionine positron emission tomography for target delineation in resected high-grade gliomas before radiotherapy. Int J Radiat Oncol Biol Phys. 2005;63:64-74.
59. Narayana A., Yamada J., Berry S., et al. Intensity-modulated radiotherapy in high-grade gliomas: clinical and dosimetric results. Int J Radiat Oncol Biol Phys. 2006;64:892-897.
60. Coughlin C., Scott C., Langer C., et al. Phase II P. two-arm RTOG trial (94-11) of bischloroethyl-nitrosourea plus accelerated hyperfractionated radiotherapy (64.0 or 70.4 Gy) based on tumor volume (> 20 or < or = 20 cm(2), respectively) in the treatment of newly-diagnosed radiosurgery-ineligible glioblastoma multiforme patients. Int J Radiat Oncol Biol Phys. 2000;48:1351-1358.
61. Stupp R., van den Bent M.J., Hegi M.E. Optimal role of temozolomide in the treatment of malignant gliomas. Curr Neurol Neurosci Rep. 2005;5:198-206.
62. Stupp R., Hegi M.E., Mason W.P., et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459-466.
63. Ahluwalia M.S., Gladson C.L. Progress on antiangiogenic therapy for patients with malignant glioma. J Oncol. 2010;2010:689018.
64. Lai A., Tran A., Nghiemphu P.L., et al. Phase II study of bevacizumab plus temozolomide during and after radiation therapy for patients with newly diagnosed glioblastoma multiforme. J Clin Oncol. 2010;29(20):142-148.
65. Vredenburgh J.J., Desjardins A., Reardon D.A., et al. Addition of bevacizumab to standard radiation therapy and daily temozolomide is associated with minimal toxicity in newly diagnosed glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2011. Apr 29 [Epub ahead of print]
66. Chamberlain M.C., Glantz M.J., Chalmers L., et al. Early necrosis following concurrent Temodar and radiotherapy in patients with glioblastoma. J Neurooncol. 2007;82:81-83.
67. de Wit M.C., de Bruin H.G., Eijkenboom W., et al. Immediate post-radiotherapy changes in malignant glioma can mimic tumor progression. Neurology. 2004;63:535-537.
68. Taal W., Brandsma D., de Bruin H.G., et al. Incidence of early pseudo-progression in a cohort of malignant glioma patients treated with chemoirradiation with temozolomide. Cancer. 2008;113:405-410.
69. Brandes A.A., Franceschi E., Tosoni A., et al. MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol. 2008;26:2192-2197.
70. Hanahan D., Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353-364.
71. Friedman H.S., Prados M.D., Wen P.Y., et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733-4740.
72. Kreisl T.N., Kim L., Moore K., et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27:740-745.
73. Pope W.B., Lai A., Nghiemphu P. MRI in patients with high-grade gliomas treated with bevacizumab and chemotherapy. Neurology. 2006;66:1258-1260.
74. Brem H., Piantadosi S., Burger P.C., et al. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The Polymer-Brain Tumor Treatment Group. Lancet. 1995;345:1008-1012.
75. Wick W., Platten M., Weller M. New (alternative) temozolomide regimens for the treatment of glioma. Neuro Oncol. 2009;11:69-79.
76. Spiro T.P., Liu L., Majka S. Temozolomide: the effect of once- and twice-a-day dosing on tumor tissue levels of the DNA repair protein O(6)-alkylguanine-DNA-alkyltransferase. Clin Cancer Res. 2001;7:2309-2317.
77. Kurzen H., Schmitt S., Naher H., Mohler T. Inhibition of angiogenesis by non-toxic doses of temozolomide. Anticancer Drugs. 2003;14:515-522.
78. Wick A., Felsberg J., Steinbach J.P., et al. Efficacy and tolerability of temozolomide in an alternating weekly regimen in patients with recurrent glioma. J Clin Oncol. 2007;25:3357-3361.
79. Brandes A.A., Tosoni A., Amista P., et al. How effective is BCNU in recurrent glioblastoma in the modern era? A phase II trial. Neurology. 2004;63:1281-1284.
80. Fabrini M.G., Silvano G., Lolli I., et al. A multi-institutional phase II study on second-line Fotemustine chemotherapy in recurrent glioblastoma. J Neurooncol. 2009;92:79-86.
81. Schmidt F., Fischer J., Herrlinger U. PCV chemotherapy for recurrent glioblastoma. Neurology. 2006;66:587-589.
82. Francesconi A.B., Dupre S., Matos M., et al. Carboplatin and etoposide combined with bevacizumab for the treatment of recurrent glioblastoma multiforme. J Clin Neurosci. 2010;17:970-974.
83. de Groot J.F., Gilbert M.R., Aldape K., et al. Phase II study of carboplatin and erlotinib (Tarceva, OSI-774) in patients with recurrent glioblastoma. J Neurooncol. 2008;90:89-97.
84. Puduvalli V.K., Giglio P., Groves M.D., et al. Phase II trial of irinotecan and thalidomide in adults with recurrent glioblastoma multiforme. Neuro Oncol. 2008;10:216-222.
85. Brandes A.A., Tosoni A., Basso U., et al. Second-line chemotherapy with irinotecan plus carmustine in glioblastoma recurrent or progressive after first-line temozolomide chemotherapy: a phase II study of the Gruppo Italiano Cooperativo di Neuro-Oncologia (GICNO). J Clin Oncol. 2004;22:4779-4786.
86. Batchelor T., Mulholland P., Neyns B., et al. The efficacy of cediranib as monotherapy and in combination with lomustine compared to lomustine alone in patients with recurrent glioblastoma: a phase III randomized study. Neuro-Oncology. 2010;12:iv69-iv78.
87. Wen P.Y., Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492-507.
88. Batchelor T.T., Duda D.G., di Tomaso E., et al. Phase II study of cediranib, an oral pan-vascular endothelial growth factor receptor tyrosine kinase inhibitor, in patients with recurrent glioblastoma. J Clin Oncol. 2010;28:2817-2823.
89. Batchelor T.T., Duda D.G., di Tomaso E., et al. Normalization of tumor vasculature and alleviation of edema by AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor in glioblastoma patients. International Journal of Radiation. 2007;69(3):S50-S51.
90. Ackermann M., Carvajal I.M., Morse B.A., et al. Adnectin CT-322 inhibits tumor growth and affects microvascular architecture and function in Colo205 tumor xenografts. Int J Oncol. 2011;38:71-80.
91. Dineen S.P., Sullivan L.A., Beck A.W., et al. The Adnectin CT-322 is a novel VEGF receptor 2 inhibitor that decreases tumor burden in an orthotopic mouse model of pancreatic cancer. BMC Cancer. 2008;8:352.
92. Mamluk R., Carvajal I.M., Morse B.A., et al. Anti-tumor effect of CT-322 as an adnectin inhibitor of vascular endothelial growth factor receptor-2. MAbs. 2010:2.
93. Rich J.N., Reardon D.A., Peery T., et al. Phase II trial of gefitinib in recurrent glioblastoma. J Clin Oncol. 2004;22:133-142.
94. van den Bent M.J., Brandes A.A., Rampling R., et al. Randomized phase II trial of erlotinib versus temozolomide or carmustine in recurrent glioblastoma: EORTC brain tumor group study 26034. J Clin Oncol. 2009;27:1268-1274.
95. Chang S.M., Wen P., Cloughesy T., et al. Phase II study of CCI-779 in patients with recurrent glioblastoma multiforme. Invest New Drugs. 2005;23:357-361.
96. Galanis E., Buckner J.C., Maurer M.J., et al. Phase II trial of temsirolimus (CCI-779) in recurrent glioblastoma multiforme: a North Central Cancer Treatment Group Study. J Clin Oncol. 2005;23:5294-5304.
97. Cloughesy T.F., Wen P.Y., Robins H.I., et al. Phase II trial of tipifarnib in patients with recurrent malignant glioma either receiving or not receiving enzyme-inducing antiepileptic drugs: a North American Brain Tumor Consortium Study. J Clin Oncol. 2006;24:3651-3656.
98. Gilbert M, Gaupp P, Liu V. A phase I study of temozolomide (TMZ) and the farnesyltransferase inhibitor (FTI), lonafarnib (Sarazar, SCH66336) in recurrent glioblastoma. In: Proceedings of the American Society of Clinical Oncology Meeting, Atlanta, GA; 2006.
99. Wen P.Y., Yung W.K., Lamborn K.R., et al. Phase I/II study of imatinib mesylate for recurrent malignant gliomas: North American Brain Tumor Consortium Study 99-08. Clin Cancer Res. 2006;12:4899-4907.
100. Reardon D.A., Desjardins A., Vredenburgh J.J., et al. Safety and pharmacokinetics of dose-intensive imatinib mesylate plus temozolomide: Phase 1 trial in adults with malignant glioma. Neuro Oncol. 2008;10:330-340.
101. Ahluwalia M.S., Groot J., Liu W.M., Gladson C.L. Targeting SRC in glioblastoma tumors and brain metastases: rationale and preclinical studies. Cancer Lett. 2010;298:139-149.
102. Graff J.R., McNulty A.M., Hanna K.R., et al. The protein kinase C beta-selective inhibitor, enzastaurin (LY317615.HCI), suppresses signaling through the AKT pathway, induces apoptosis, and suppresses growth of human colon cancer and glioblastoma xenografts. Cancer Res. 2005;65:7462-7469.
103. Iwamoto F.M., Reiner A.S., Panageas K.S. Patterns of care in elderly glioblastoma patients. Ann Neurol. 2008;64:628-634.
104. Keime-Guibert F., Chinot O., Taillandier L., et al. Radiotherapy for glioblastoma in the elderly. N Engl J Med. 2007;356:1527-1535.
105. Roa W., Brasher P.M., Bauman G., et al. Abbreviated course of radiation therapy in older patients with glioblastoma multiforme: a prospective randomized clinical trial. J Clin Oncol. 2004;22:1583-1588.
106. Glantz M., Chamberlain M., Liu Q. Temozolomide as an alternative to irradiation for elderly patients with newly diagnosed malignant gliomas. Cancer. 2003;97:2262-2266.
107. Malmstrom A., Gronberg B.H., Stupp R., et al. Glioblastoma (GBM) in elderly patients: a randomized phase III trial comparing survival in patients treated with 6-week radiotherapy (RT) versus hypofractionated RT over 2 weeks versus temozolomide single-agent chemotherapy (TMZ). ASCO Meeting Abstracts. 28, 2010. LBA2002
108. Wick W., Engel C., Combs S.E., et al. NOA-08 randomized phase III trial of 1-week-on/1-week-off temozolomide versus involved-field radiotherapy in elderly (older than age 65) patients with newly diagnosed anaplastic astrocytoma or glioblastoma (Methusalem). ASCO Meeting Abstracts. 28, 2010. LBA2001
109. Pérez-Larraya J.G., Honnorat J., Chinot O., et al. A phase II trial of temozolomide in elderly patients with glioblastoma and poor performance status (KPS<70): Preliminary results of the ANOCEF “TAG” trial. Neuro-Oncology. 2010;12:iv69-iv78.
110. Brandes A.A., Nicolardi L., Tosoni A., et al. Survival following adjuvant PCV or temozolomide for anaplastic astrocytoma. Neuro-Oncology. 2006;8:253-260.
111. Wick W., Hartmann C., Engel C., et al. NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomide. J Clin Oncol. 2009;27:5874-5880.
112. Yung W.K., Prados M.D., Yaya-Tur R., et al. Multicenter phase II trial of temozolomide in patients with anaplastic astrocytoma or anaplastic oligoastrocytoma at first relapse. Temodal Brain Tumor Group. J Clin Oncol. 1999;17:2762-2771.
113. Vredenburgh J.J., Desjardins A., Herndon J.E.2nd, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13:1253-1259.
114. Desjardins A., Reardon D.A., Herndon J.E.II, et al. Bevacizumab plus irinotecan in recurrent WHO grade 3 malignant gliomas. Clin Cancer Res. 2008;14:7068-7073.
115. Mork S.J., Lindegaard K.F., Halvorsen T.B., et al. Oligodendroglioma: incidence and biological behavior in a defined population. J Neurosurg. 1985;63:881-889.
116. Cairncross J.G., Macdonald D.R. Successful chemotherapy for recurrent malignant oligodendroglioma. Ann Neurol. 1988;23:360-364.
117. Cairncross J.G., Ueki K., Zlatescu M.C., et al. Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst. 1998;90:1473-1479.
118. Brandes A.A., Tosoni A., Vastola F., et al. Efficacy and feasibility of standard procarbazine, lomustine, and vincristine chemotherapy in anaplastic oligodendroglioma and oligoastrocytoma recurrent after radiotherapy. A Phase II study. Cancer. 2004;101:2079-2085.
119. Soffietti R., Ruda R., Bradac G.B., Schiffer D. PCV chemotherapy for recurrent oligodendrogliomas and oligoastrocytomas. Neurosurgery. 1998;43:1066-1073.
120. Buckner J.C., Gesme D.Jr., O’Fallon J.R., et al. Phase II trial of procarbazine, lomustine, and vincristine as initial therapy for patients with low-grade oligodendroglioma or oligoastrocytoma: efficacy and associations with chromosomal abnormalities. J Clin Oncol. 2003;21:251-255.
121. Cairncross G., Berkey B., Shaw E., et al. Phase III trial of chemotherapy plus radiotherapy compared with radiotherapy alone for pure and mixed anaplastic oligodendroglioma: Intergroup Radiation Therapy Oncology Group Trial 9402. J Clin Oncol. 2006;24:2707-2714.
122. van den Bent M.J., Carpentier A.F., Brandes A.A., et al. Adjuvant procarbazine, lomustine, and vincristine improves progression-free survival but not overall survival in newly diagnosed anaplastic oligodendrogliomas and oligoastrocytomas: a randomized European Organisation for Research and Treatment of Cancer phase III trial. J Clin Oncol. 2006;24:2715-2722.
123. McNeil D.E., Cote T.R., Clegg L., Rorke L.B. Incidence and trends in pediatric malignancies medulloblastoma/primitive neuroectodermal tumor: a SEER update. Surveillance Epidemiology and End Results. Med Pediatr Oncol. 2002;39:190-194.
124. Carrie C., Muracciole X., Gomez F., et al. Conformal radiotherapy, reduced boost volume, hyperfractionated radiotherapy, and online quality control in standard-risk medulloblastoma without chemotherapy: results of the French M-SFOP 98 protocol. Int J Radiat Oncol Biol Phys. 2005;63:711-716.
125. Friedman H.S., Oakes W.J. The chemotherapy of posterior fossa tumors in childhood. J Neurooncol. 1987;5:217-229.
126. Weiss H.D., Walker M.D., Wiernik P.H. Neurotoxicity of commonly used antineoplastic agents (second of two parts). N Engl J Med. 1974;291:127-133.
127. Weiss H.D., Walker M.D., Wiernik P.H. Neurotoxicity of commonly used antineoplastic agents (first of two parts). N Engl J Med. 1974;291:75-81.
128. Packer R.J., Gajjar A., Vezina G., et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24:4202-4208.
129. Brandes A.A., Franceschi E., Tosoni A., et al. Efficacy of tailored treatment for high- and low-risk medulloblastoma in adults: a large prospective phase II trial. ASCO Meeting Abstracts. 2010;28:2003.
130. Dunkel I.J., Boyett J.M., Yates A., et al. High-dose carboplatin, thiotepa, and etoposide with autologous stem-cell rescue for patients with recurrent medulloblastoma. Children’s Cancer Group. J Clin Oncol. 1998;16:222-228.
131. Guruangan S., Dunkel I.J., Goldman S., et al. Myeloablative chemotherapy with autologous bone marrow rescue in young children with recurrent malignant brain tumors. J Clin Oncol. 1998;16:2486-2493.
132. Wen P.Y., Quant E., Drappatz J. Medical therapies for meningiomas. J Neurooncol. 2010;99:365-378.
133. Loven D., Hardoff R., Sever Z.B., et al. Non-resectable slow-growing meningiomas treated by hydroxyurea. J Neurooncol. 2004;67:221-226.
134. Newton H.B., Scott S.R., Volpi C. Hydroxyurea chemotherapy for meningiomas: enlarged cohort with extended follow-up. Br J Neurosurg. 2004;18:495-499.
135. Chamberlain M.C., Glantz M.J., Fadul C.E. Recurrent meningioma: salvage therapy with long-acting somatostatin analogue. Neurology. 2007;69:969-973.
136. Schrell U.M., Rittig M.G., Anders M., et al. Hydroxyurea for treatment of unresectable and recurrent meningiomas. I. Inhibition of primary human meningioma cells in culture and in meningioma transplants by induction of the apoptotic pathway. J Neurosurg. 1997;86:845-852.
137. Rosenthal M.A., Ashley D.L., Cher L. Treatment of high risk or recurrent meningiomas with hydroxyurea. J Clin Neurosci. 2002;9:156-158.
138. Grunberg S.M., Weiss M.H., Spitz I.M., et al. Treatment of unresectable meningiomas with the antiprogesterone agent mifepristone. J Neurosurg. 1991;74:861-866.
139. Grunberg S., Rankin C., Townsend J., et al. Phase III double-blind randomized placebo-controlled study of mifepristone (RU) for the treatment of unresectable meningioma. Proc Am Soc Clin Oncol. 2001, 2001.
140. Raizer J.J., Grimm S., Chandler J., et al. A phase II trial of PTK787/ZK 222584 (PTK787) in recurrent or progressive meningiomas. Neuro-Oncology. 2010:12.
141. Kaley T.J., Schiff D., Karimi S., et al. Phase II trial of sunitinib (SU011248) for recurrent meningioma. Neuro-Oncology. 2010;12:iv69-iv78.
142. Mikkelsen T., Anderson J., Doyle T.J., et al. Phase I/II dose escalation trial of concurrent temozolomide and whole brain radiation therapy for multiple brain metastasis. J Neurooncol. 2010;100:241-247.
143. Jacot W., Gerlotto-Borne M.C., Thezenas S., et al. Carmustine and methotrexate in combination after whole brain radiation therapy in breast cancer patients presenting with brain metastases: a retrospective study. BMC Cancer. 2010;10:257.
144. Siegelmann-Danieli N., Stein M., Bar-Ziv J. Complete response of brain metastases originating in breast cancer to capecitabine therapy. Isr Med Assoc J. 2003;5:833-834.
145. Yamada R., Takumi C., Hiraoka N. [A case of brain metastasis of small cell lung cancer improved with nogitecan hydrochloride (topotecan) after prophylactic irradiation.]. Gan To Kagaku Ryoho. 2004;31:1071-1073.
146. Gluck S., Castrellon A. Lapatinib plus capecitabine resolved human epidermal growth factor receptor 2-positive brain metastases. Am J Ther. 2009;16:585-590.
147. Fujita A., Fukuoka S., Takabatake H. Combination chemotherapy of cisplatin, ifosfamide, and irinotecan with rhG-CSF support in patients with brain metastases from non-small cell lung cancer. Oncology. 2000;59:291-295.
148. Bafaloukos D., Tsoutsos D., Fountzilas G., et al. The effect of temozolomide-based chemotherapy in patients with cerebral metastases from melanoma. Melanoma Res. 2004;14:289-294.
149. Ebert B.L., Niemierko E., Shaffer K., Salgia R. Use of temozolomide with other cytotoxic chemotherapy in the treatment of patients with recurrent brain metastases from lung cancer. Oncologist. 2003;8:69-75.
150. Wong E.T., Berkenblit A. The role of topotecan in the treatment of brain metastases. Oncologist. 2004;9:68-79.
151. Ceresoli G.L., Cappuzzo F., Gregorc V., et al. Gefitinib in patients with brain metastases from non–small-cell lung cancer: a prospective trial. Ann Oncol. 2004;15:1042-1047.
152. Kim J.E., Lee D.H., Choi Y., et al. Epidermal growth factor receptor tyrosine kinase inhibitors as a first-line therapy for never-smokers with adenocarcinoma of the lung having asymptomatic synchronous brain metastasis. Lung Cancer. 2009;65:351-354.
153. Grossi F., Scolaro T., Tixi L. The role of systemic chemotherapy in the treatment of brain metastases from small-cell lung cancer. Crit Rev Oncol Hematol. 2001;37:61-67.
154. Lee J.S., Murphy W.K., Glisson B.S. Primary chemotherapy of brain metastasis in small-cell lung cancer. J Clin Oncol. 1989;7:916-922.
155. Chen G., Huynh M., Chen A. Chemotherapy for brain metastases in small-cell lung cancer. Clin Lung Cancer. 2008;9:35-38.
156. Postmus P.E., Haaxma-Reiche H., Smit E.F., et al. Treatment of brain metastases of small-cell lung cancer: comparing teniposide and teniposide with whole-brain radiotherapy—a phase III study of the European Organization for the Research and Treatment of Cancer Lung Cancer Cooperative Group. J Clin Oncol. 2000;18:3400-3408.
157. Rivera E., Meyers C., Groves M., et al. Phase I study of capecitabine in combination with temozolomide in the treatment of patients with brain metastases from breast carcinoma. Cancer. 2006;107:1348-1354.
158. Trudeau M.E., Crump M., Charpentier D., et al. Temozolomide in metastatic breast cancer (MBC): a phase II trial of the National Cancer Institute of Canada–Clinical Trials Group (NCIC-CTG). Ann Oncol. 2006;17:952-956.
159. Franciosi V., Cocconi G., Michiara M., et al. Front-line chemotherapy with cisplatin and etoposide for patients with brain metastases from breast carcinoma, nonsmall cell lung carcinoma, or malignant melanoma: a prospective study. Cancer. 1999;85:1599-1605.
160. Rosner D., Nemoto T., Lane W.W. Chemotherapy induces regression of brain metastases in breast carcinoma. Cancer. 1986;58:832-839.
161. Lin N.U., Dieras V., Paul D., et al. Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin Cancer Res. 2009;15:1452-1459.
162. Hodi F.S., O’Day S.J., McDermott D.F., et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;19;363(8):711-723.
163. Lawrence D.P., Hamid O., McDermott D.F., et al. Phase II trial of ipilimumab monotherapy in melanoma patients with brain metastases. J Clin Oncol. 2010. ASCO Annual Meeting Proceedings. Vol 28, No 15 suppl (May 20 Supplement), 2010:8523.
164. Lin N.U., Carey L.A., Liu M.C., et al. Phase II trial of lapatinib for brain metastases in patients with human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2008;26:1993-1999.
165. Agarwala S.S., Kirkwood J.M., Gore M., et al. Temozolomide for the treatment of brain metastases associated with metastatic melanoma: a phase II study. J Clin Oncol. 2004;22:2101-2107.
166. Schadendorf D., Hauschild A., Ugurel S., et al. Dose-intensified bi-weekly temozolomide in patients with asymptomatic brain metastases from malignant melanoma: a phase II DeCOG/ADO study. Ann Oncol. 2006;17:1592-1597.
167. Mornex F., Thomas L., Mohr P., et al. A prospective randomized multicentre phase III trial of fotemustine plus whole brain irradiation versus fotemustine alone in cerebral metastases of malignant melanoma. Melanoma Res. 2003;13:97-103.
168. DeAngelis L., Posner J. Neurologic Complications of Cancer. (Contemporary Neurology Series.). New York: Oxford University Press, 2009.
169. Chamberlain M.C. Carcinomatous meningitis. Arch Neurol. 1997;54:16-17.
170. Chamberlain M.C. Neoplastic meningitis. Oncologist. 2008;13:967-977.
171. Chang E.L., Maor M.H. Standard and novel radiotherapeutic approaches to neoplastic meningitis. Curr Oncol Rep. 2003;5:24-28.
172. Chamberlain M.C. Neoplastic meningitis. Curr Neurol Neurosci Rep. 2008;8:249-258.
173. Glantz M.J., Jaeckle K.A., Chamberlain M.C., et al. A randomized controlled trial comparing intrathecal sustained-release cytarabine (DepoCyt) to intrathecal methotrexate in patients with neoplastic meningitis from solid tumors. Clin Cancer Res. 1999;5:3394-3402.
174. Chamberlain M.C., Dirr L. Involved-field radiotherapy and intra-Ommaya methotrexate/cytarabine in patients with AIDS-related lymphomatous meningitis. J Clin Oncol. 1993;11:1978-1984.
175. Glantz M.J., LaFollette S., Jaeckle K.A., et al. Randomized trial of a slow-release versus a standard formulation of cytarabine for the intrathecal treatment of lymphomatous meningitis. J Clin Oncol. 1999;17:3110-3116.
176. Ferrario C., Davidson A., Bouganim N. Intrathecal trastuzumab and thiotepa for leptomeningeal spread of breast cancer. Ann Oncol. 2009;20:792-795.
177. Cortot A.B., Geriniere L., Robinet G., et al. Phase II trial of temozolomide and cisplatin followed by whole brain radiotherapy in non–small-cell lung cancer patients with brain metastases: a GLOT-GFPC study. Ann Oncol. 2006;17:1412-1417.
178. Chiu C.H., Tsai C.M., Chen Y.M. Gefitinib is active in patients with brain metastases from non-small cell lung cancer and response is related to skin toxicity. Lung Cancer. 2005;47:129-138.
179. Bernardo G., Cuzzoni Q., Strada M.R., et al. First-line chemotherapy with vinorelbine, gemcitabine, and carboplatin in the treatment of brain metastases from non–small-cell lung cancer: a phase II study. Cancer Invest. 2002;20:293-302.
180. Robinet G., Thomas P., Breton J.L., et al. Results of a phase III study of early versus delayed whole brain radiotherapy with concurrent cisplatin and vinorelbine combination in inoperable brain metastasis of non–small-cell lung cancer: Groupe Francais de Pneumo-Cancerologie (GFPC) Protocol 95-1. Ann Oncol. 2001;12:59-67.
181. Postmus P.E., Smit E.F., Haaxma-Reiche H., et al. Teniposide for brain metastases of small-cell lung cancer: a phase II study. European Organization for Research and Treatment of Cancer Lung Cancer Cooperative Group. J Clin Oncol. 1995;13:660-665.
182. Korfel A., Oehm C., von Pawel J., et al. Response to topotecan of symptomatic brain metastases of small-cell lung cancer also after whole-brain irradiation: a multicentre phase II study. Eur J Cancer. 2002;38:1724-1729.
183. Oberhoff C., Kieback D.G., Wurstlein R., et al. Topotecan chemotherapy in patients with breast cancer and brain metastases: results of a pilot study. Onkologie. 2001;24:256-260.
184. Murphy C., Nulsen B., Rump M., Seidman A.D. Phase II trial of patupilone in patient with breast cancer metastases progressing or recurring after whole brain radiotherapy. Paper presented at ASCO Breast Cancer Symposium. 2009.
185. Boogerd W., Dalesio O., Bais E.M., van der Sande J.J. Response of brain metastases from breast cancer to systemic chemotherapy. Cancer. 1992;69:972-980.
186. Atkins M.B., Sosman J.A., Agarwala S., et al. Temozolomide, thalidomide, and whole brain radiation therapy for patients with brain metastasis from metastatic melanoma: a phase II Cytokine Working Group study. Cancer. 2008;113:2139-2145.