Chapter 63A Chemotherapy and radiotherapy for pancreatic and periampullary cancer
Adjuvant, neoadjuvant, and palliative
Adjuvant Chemoradiotherapy
Early studies examining the role of combined external-beam radiotherapy (EBRT) with 5-fluorouracil (5-FU) as a radiosensitizing agent in advanced, unresectable pancreatic adenocarcinoma showed an increased median survival over that of solo EBRT (Douglas et al, 1979; Moertel et al, 1981). Subsequently, combination chemoradiotherapy (CRT) was used in patients who had undergone potentially curative resection for pancreatic adenocarcinoma. The Gastrointestinal Tumor Study Group trial 9173 (Kalser & Ellenberg, 1985) randomized 43 patients to either CRT (40-Gy EBRT, combined with 5-FU and follow-on 5-FU) or no adjuvant treatment after potentially curative pancreatic resection; recruitment was slow and only 43 patients were enrolled after 8 years instead of the intended 150. Median, 2-year, and 5-year survival rates were all increased in the treatment group (Table 63A.1). This trial had several problems, chief among them being poor compliance and inadequate quality assurance, with only 9% of patients completing the intended 2 years of chemotherapy; 32% had violations of the radiation therapy. To increase numbers, another 30 patients were entered into the treatment group without randomization (Douglas et al, 1987). Several subsequent studies have all shown similar advantages (Conlon et al, 1996; Neoptolemos et al, 1998; Yeo et al, 1998), although all were underpowered.
Table 63A.1 Summary of Findings from Major Studies Examining the Effect of Adjuvant Chemoradiotherapy
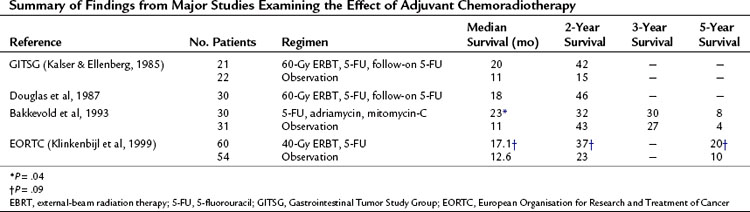
Two studies, however, have countered these results. Bakkevold and colleagues (1993) prospectively randomized 61 patients who had undergone radical pancreatic resections (including 14 patients with periampullary lesions) to adjuvant combination chemotherapy with 5-FU, adriamycin, and mitomycin-C versus observation; no postoperative EBRT was used. Although the median survival and 2-year survival were increased in the treatment groups, the overall long-term cure rate was unaffected (see Table 63A.1).
Later, on behalf of the European Organisation for Research and Treatment of Cancer (EORTC), Klinkenbijl and colleagues (1999) randomized 218 patients following potentially curative resection for pancreatic cancer to CRT (40-Gy EBRT and 5-FU, no follow-on 5-FU) or no adjuvant treatment. This included 114 patients with pancreatic head cancer and 93 with periampullary cancer. Of the patients with pancreatic head cancers, no significant increase in median survival was found between the two groups (see Table 63A.1).
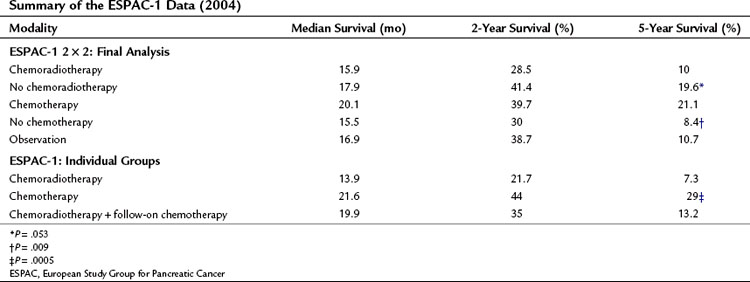
ESPAC-1 has been criticized largely because of confusion over the 2 × 2 trial design; nonetheless, data from both patient datasets are relevant (Neoptolemos et al, 2001, 2004) and are suggestive of the benefit of chemotherapy (Table 63A.2 and Fig. 63A.1), evident in resection margin–positive (R1) as well as resection margin–negative (R0) patients. Two subsequent meta-analyses have confirmed this advantage, suggesting a reduction in risk of death in postresection pancreatic cancers of 25% (hazard ratio [HR], 0.75; 95% confidence interval [CI], 0.64 to 0.90; P = .001) (Stocken et al, 2005) and an advantage of postresectional chemotherapy in patients with an R0 margin over those with an R1 margin (Butturini et al, 2008). Moreover, the reported that survival advantage for adjuvant chemotherapy was maintained when adjusted for quality of life over the 24-month period after resection (Carter et al, 2009).

FIGURE 63A.1 Results from ESPAC-1 2 × 2 factorial design. CRT, chemoradiotherapy; CT, chemotherapy.
(Modified from Neoptolemos JP, et al, 2004: A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 350:1200-1210.)
Since the usurpation of 5-FU as the gold standard of chemotherapy for pancreas cancer, current focus has centered on the use of gemcitabine as a radiosensitizer and also on the use of biologic agents. The Radiation Therapy Oncology Group (RTOG) (Regine et al, 2006) conducted a Phase III trial, study 9704, of 538 patients (443 analyzed, with 388 having cancer in the head of the pancreas) based on chemoradiation (50.4-Gy EBRT with 5-FU) combined with either 5-FU or gemcitabine, 3 weeks before CRT and 12 weeks after. Although both the median and 3-year survival rates were increased in the group receiving gemcitabine, these increases were not significant. Analysis of the 388 patients with pancreatic head cancer suggested a 21% reduction in risk of death in the gemcitabine arm (HR, 0.79; 95% CI, 0.63 to 0.99; P = .047). Although this was suggestive of the advantage of gemcitabine, a greater proportion of T3 tumors and higher toxicity were reported in this arm. In response, the EORTC 40013 Phase III trial plans to randomize 538 patients after R0 pancreatic resection to either gemcitabine or two cycles of gemcitabine followed by CRT (gemcitabine and 50.4-Gy EBRT).
Overall, the use of chemoradiation remains controversial, but many U.S. centers continue to support adjuvant chemoradiotherapy as standard treatment (Twombly, 2008).
Adjuvant Chemotherapy
ESPAC-1 has provided the clearest evidence to date that adjuvant chemotherapy is advantageous (see Table 63A.2) when based on regimens of biomodulated 5-FU. The advance made by ESPAC-1 has driven forward the next generation of adjuvant chemotherapy trials, notably ESPAC-3 (v2) and ESPAC-4 in addition to translational run-offs (ESPAC-T plus and ESPAC-4T).
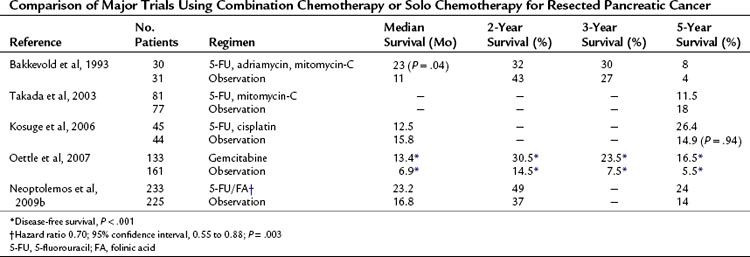
Evidence that emerged during the 1990s showed the usefulness of gemcitabine in pancreatic cancer, initially in the setting of advanced disease (Berlin et al, 2002; Burris et al, 1997; Carmichael et al, 1996; Casper et al, 1994; Hertel et al, 1990; Huang et al, 1991; Rothenberg et al, 1996). Recently, a large, multicenter, Phase III randomized trial of 386 patients was undertaken to determine the influence of adjuvant gemcitabine versus observation following resection of pancreatic cancer on disease-free survival (CONKO-001) (Oettle et al, 2007; Neuhaus et al, 2008). Median disease-free survival for the gemcitabine group was 13.4 months compared with 6.9 months for the observation arm (P < .001); these data are summarized in \xEF”\xBFTable 63A.3\xEF”\xBF\xEF”\xBF\xEF”\xBF. The estimated disease-free survival at 3 and 5 years was 23.5% and 16.5% in the gemcitabine group versus 7.5% and 5.5% in the observation group, respectively. In addition, a significant improvement in median overall survival of 24.2 months was reported with gemcitabine compared with observation alone (20.5 months; P = .02). Estimated overall survival at 3 and 5 years was 34% and 22.5% for gemcitabine patients versus 20.5% and 11.5% for observation patients, respectively. These results were suggestive of prolonged disease-free survival in patients undergoing R0 or R1 resection for pancreatic cancer. These earlier studies formed the basis for the ESPAC-3 study.
ESPAC-3 initially was designed with three arms: six cycles of 5-FU versus six cycles of gemcitabine versus observation, with a recruitment target of 330 patients. With the publication of the ESPAC-1 results, a definite survival advantage for adjuvant chemotherapy after resection of pancreatic ductal adenocarcinoma was apparent over observation alone; the trial design was therefore amended, with the observation arm being dropped. The recruitment targets for the remaining two arms were increased to 515 patients each for a total of 1030 patients with pancreatic ducal adenocarcinoma, and the trial was renamed ESPAC-3 (v2). ESPAC-1 had been underpowered to determine the role of adjuvant therapy in less common malignancies such as bile duct cancers and ampullary tumors; therefore the observation arm of ESPAC-3 was continued for these patients. The trial closed after having recruited 1583 patients, with 1088 having ductal adenocarcinoma. Overall, no difference was found between 5-FU/FA and gemcitabine by treatment (P = .39), treatment effect by R status (P = .56), or by progression-free survival (P = .44). However, significant advantages were found from the use of gemcitabine, notably its significantly reduced side effects and toxicity profiles (Neoptolemos et al, 2009a, 2009b); thus the current recommendation for adjuvant chemotherapy in pancreatic ductal adenocarcinoma is gemcitabine (see Table 63A.3).
Table 63A.3 Comparison of Major Trials Using Combination Chemotherapy or Solo Chemotherapy for Resected Pancreatic Cancer
Currently, interest lies in the use of capecitabine, a prodrug for 5-FU. It is sequentially metabolized into active 5-FU by enzymes highly expressed in both liver and tumor, notably the last enzyme in this cascade, thymidine phosphorylase. This has four potential advantages: 1) systemic side effects are reduced, 2) high concentrations are achieved in the vicinity of the tumor, 3) oral capecitabine has a pharmacokinetic profile similar to that of a continuous systemic infusion 5-FU, and 4) patients tolerate it better than 5-FU. Unfortunately, the effects of solo capecitabine seem minimal: clinical response in 24% and tumor response in 7% (Cartwright et al, 2002). Better responses have been observed when capecitabine and gemcitabine were combined. Eighty-three patients were randomized to either biweekly gemcitabine or biweekly gemcitabine and daily oral capecitabine for a duration of 6 months (Scheithauer et al, 2003). Although no increase was seen in median survival, a 33% increase in clinical benefit response was reported in the combination group.
The effects of combining chemotherapeutic agents are measured against the solo effects of the current gold standard drugs. Bakkevold and colleagues (1993) randomized patients who had undergone pancreatic resection to FAM (5-FU, adriamycin, and mitomycin-C) or observation. Although a significant increase was reported in median survival (23 vs. 11 months; P = .04), no increase in 3- or 5-year survivals was reported. Excessive toxicity was noted, and only 56% of the treatment group completed the six courses of chemotherapy (see Table 63A.3). Takada and colleagues (2003) enrolled 508 patients with mixed pancreatobiliary cancers (173 with pancreatic cancer) and randomized them to either surgery alone or surgery with combined mitomycin-C and 5-FU. The 5-year survival rates were 18 months and 11.5 months, respectively. Of note was the use of oral 5-FU, which has lower efficacy as a result of first-pass hepatic metabolism when compared with intravenous administration.
Kosuge and colleagues (2006) undertook a multicenter, randomized trial of surgery versus surgery and combination cisplatin and 5-FU. They concluded that although cisplatin and 5-FU were safe and well tolerated, the combination conferred no clear survival benefit over monotherapy (see Table 63A.3). As yet, no convincing evidence shows any benefit of combination chemotherapy over standard monotherapy based on either or both of these agents (Magee et al, 2002), and good evidence shows increased severity of side effects in combined regimens (Ghaneh et al, 1999).
Intraoperative Radiotherapy
The effect of EBRT on the pancreatic bed is limited by the immediacy of radiosensitive structures; the use of intraoperative radiotherapy (IORT) should reduce this effect. Studies that have explored IORT are small and have heterogenous populations, such that conclusions are hard to reach (Coquard et al, 1997; Fossati et al, 1995; Hiraoka et al, 1990; Reni et al, 2001; Shibamoto et al, 1996; Showalter et al, 2009; Zerbi et al, 1994). RTOG gave 20 Gy of IORT with EBRT (a total of 50.4 Gy) to 51 patients with advanced pancreatic cancer. Results showed a disappointing median survival of 9 months (Tepper et al, 1991). Sindelar and Kinsella (1986) similarly showed a median survival of only 12 months in patients treated with surgery or surgery and IORT. There are reports (Valentini et al, 2009), however, of long-term control and survival in operated patients receiving IORT, although such data are contaminated, with 24% of patients receiving preoperative and postoperative EBRT. Nonetheless, preoperative chemotherapy with surgery and IORT gave a 5-year local control of 23.3%, although IORT is unlikely to become mainstream treatment outside specialized centers undertaking this modality because of the limited number of appropriate patients.
Neoadjuvant Chemoradiotherapy, Chemotherapy, And Biologic Treatment
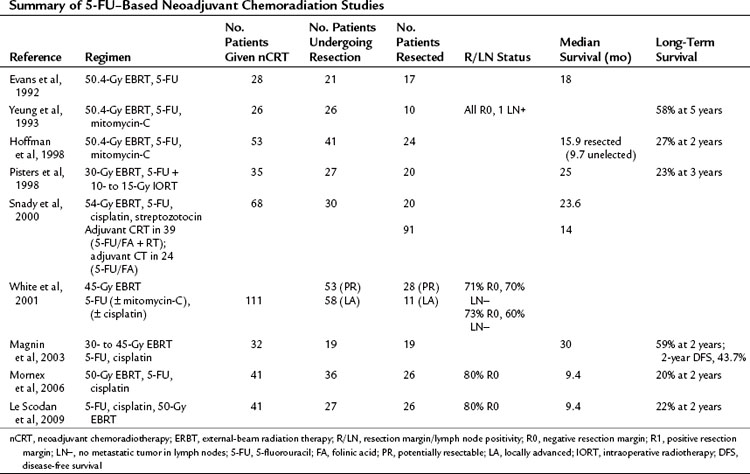
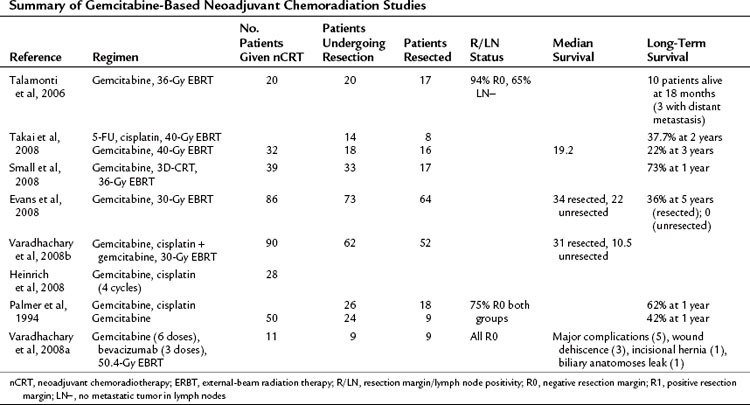
Broadly speaking, medical treatments are either directed at known borderline resectable disease, with the aim of downsizing a tumor in contact with or encasing vascular structures, or to patients with staged resectable disease. The presumption of both is acceptance of the rationale for neoadjuvant chemoradiotherapy (nCRT). Early studies implied the advantage of CRT in advanced pancreatic cancer (Moertel et al, 1969, 1981), hence the extrapolation to neoadjuvant regimens. These can be categorized into studies using 5-FU (Table 63A.4) or gemcitabine (Table 63A.5) as the radiosensitizing agent.
Palliative Chemotherapy
Until the landmark study by Burris and colleagues (1997), 5-FU had been considered the gold standard of palliative chemotherapy despite its poor response rates, but gemcitabine has subsequently been shown to be more beneficial than 5-FU when used as monotherapy in advanced pancreatic cancer (Carmichael et al, 1996; Casper et al, 1994; Hertel et al, 1990; Huang et al, 1991). Better response rates, longer median and 1-year survival, and enhanced clinical benefit response were achieved. The cornerstone study exploring these advantages (Burris et al, 1997) randomized patients with advanced pancreatic cancer to receive either 5-FU or gemcitabine. Those receiving gemcitabine had a modest but significant increase in median survival (5.56 vs. 4.41 months; P = .0025) and improved clinical benefit (23.8% vs. 4.8%; P = .0022). A follow-on study by the same group showed advantage for patients with advanced pancreatic cancer previously treated with 5-FU in whom there had been disease progression. The patients were subsequently given gemcitabine (Rothenberg et al, 1996), and a 27% clinical benefit response was achieved for a median of 14 weeks, with an overall median survival of 3.85 months. The Eastern Cooperative Oncology Group randomized 322 patients with advanced pancreatic cancer to receive solo gemcitabine or gemcitabine followed by 5-FU (Berlin et al, 2002). This regimen achieved a nonsignificant rise in median survival, although it did show a significantly improved progression-free survival for gemcitabine plus 5-FU (P = .022).
Overall, combining 5-FU with gemcitabine has little to offer over monotherapy with gemcitabine (Berlin et al, 2002; Crown et al, 1991; DeCaprio et al, 1991; Di Costanzo et al, 2005; Hansen et al, 1988; Maisey et al, 2002; Riess et al, 2005). Alternately, combining gemcitabine with platinum compounds has a better overall response rate and to some extent better median and progression-free survival (Alberts et al, 2003; Cascinu et al, 2003; Colucci et al, 2002; Heinemann et al, 2000, 2006; Philip et al, 2001; Poplin et al, 2006); however, it is associated with more toxicity side effects. The same is true for most other cytotoxic agents.
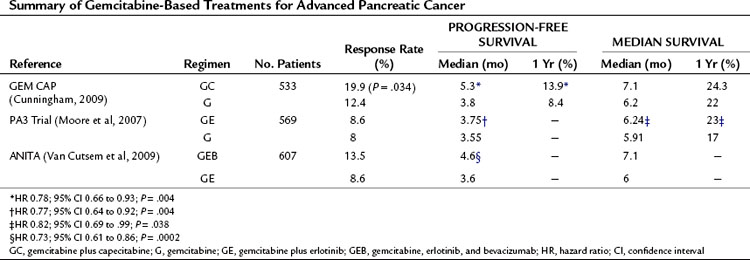
Combination of gemcitabine with capecitabine has shown promise in Phase II (Scheithauer et al, 2003) and Phase III (Herrmann et al, 2007) trials. The largest Phase III trial to date (Cunningham et al, 2009) has recently reported and is summarized in Table 63A.6. In this study, 533 patients were randomized to either gemcitabine (G) or gemcitabine and capecitabine (GC). Those receiving GC had significantly better objective response rates (19.1% GC vs. 12.4 G; P = 0.034) and progression-free survival (HR, 0.78; 95% CI, 0.66 to 0.93). Although not reaching significance, a trend toward increased overall survival was reported for GC. Median survival and 1-year survival for GC and G were 7.1 months versus 6.2 months and 24.3% versus 22%, respectively. This study also includes a meta-analysis of this report and directly comparable Phase II and III trials (Herrmann et al, 2007; Scheithauer et al, 2003), all of which show a significant survival benefit from gemcitabine combined with capecitabine (HR, 0.86; 95% CI 0.75 to 0.98; P = .02). The initial excitement regarding combining gemcitabine with biologic agents—such as erlotinib, an epidermal growth-factor receptor tyrosine kinase inhibitor, or bevacizumab, monoclonal antibody to vascular endothelial growth factor A—have not shown the expected promise in recent phase III trials and appear no better than combination gemcitabine and capeciabine (see Table 63A.6).
Palliative Radiotherapy
Much of the poor reputation of palliative radiotherapy may be unjustified in the modern era. This is because of a recent dramatic shift in radiotherapy delivery techniques from fractional or low doses (e.g., 2 Gy over 6 weeks with a radiation holiday of 2 weeks in the middle) to a continuous-course fraction with a higher overall dose (e.g., 50.4 Gy compared with 40 Gy in fractionated low-dose EBRT). This has the advantage of faster completion of treatment and better overall local control (Lim et al, 2003; Regine et al, 2006). Radiation field size has decreased with the use of intensity-modulated radiation therapy (IMRT), in which smaller fields are targeted by “pencil” beams to reduce the traditional scatter effect to surrounding tissues (Bai et al, 2003; Ben-Josef et al, 2004; Crane et al, 2001; Landry et al, 2002; Milano et al, 2004). This has the added advantage of potentially reducing side effects of combination agents.
Stereotactic radiotherapy (CyberKnife; Accuray, Sunnyvale, CA) uses focused radiotherapy with controlled organ movement via implanted metallic markers. Unfortunately, both Phase I (25 Gy, 15 patients) (Koong et al, 2004) and Phase II trials (45-Gy IMRT with a 25 Gy stereotactic boost, 19 patients) (Koong et al, 2005) have failed to show any great effect, with median survivals of 11 and 7.6 months, respectively. Median time to progression with sole 25-Gy stereotactic radiation was 2 months. Despite the lack of evidence for this technique, which involves a specialized setup, it is gaining some popularity as a result of recent high-profile cases.
Palliative Chemoradiotherapy
Palliative radiotherapy has been used in conjunction with chemotherapeutic agents, mainly 5-FU, which allows radiosensitization of tissues. Early work by Moertel and colleagues (1969) suggested a survival advantage for those patients given CRT (35 to 40-Gy EBRT + 5-FU) over those given only EBRT (35 to 40 Gy), with a median survival of 10.4 versus 6.3 months and a 1-year survival of 22% versus 6.3%, respectively. Several small, historic trials have addressed the role of 5-FU and EBRT and show median survival times of 10 to 15 months (Gunderson et al, 1987; Haycox et al, 1998; Ikeda et al, 2001), although these studies may be subject to patient selection bias. The larger GITSG trial (Moertel et al, 1981) randomized 194 patients with advanced pancreatic cancer to either ERBT (60 Gy), ERBT + 5-FU (40 Gy), or EBRT + 5-FU (60 Gy). Concomitant EBRT with 5-FU increased median survival from 23 to 42 weeks. Unfortunately, none of these trials had a control arm. A smaller, follow-on GITSG trial (1988) of 48 advanced pancreatic cancer patients randomized to either 5-FU plus 54-Gy EBRT with follow-on streptozotocin, mitomycin-C, and 5-FU (SMF) versus SMF only. The combination regimen was seen to increase median survival from 32 weeks in the SMF group to 42 weeks in the combination SMF group.
Contrary to this, the Eastern Cooperative Oncology Group (Klaassen et al, 1985) randomized 91 patients to either 5-FU plus 40-Gy EBRT versus 5-FU alone and found no difference in survival in either arm. At present, there seems to be little difference between chemotherapy based on 5-FU and the radiosensitizer EBRT in the treatment of advanced pancreatic cancer (Haycox et al, 1998). A recent multicenter, randomized, Phase II trial by Wilkowski and colleagues (2009) failed to elicit any advantage of CRT regimens. In this trial, 95 patients were randomized to either 5-FU with 50-Gy EBRT (RT–5-FU arm); gemcitabine and cisplatin with 50-Gy EBRT (RT-GC arm), or gemcitabine and cisplatin with 50-Gy EBRT with follow-on gemcitabine and cisplatin (RT-GC + GC arm). Median survivals of 9.6, 9.3, and 7.3 months for RT–5-FU, RT-GC, and RT-GC + GC, respectively, with corresponding progression-free survival at 4, 5.6, and 6 months. The authors conclude that such CRT is no better than solo gemcitabine.
Alberts SR, et al. Gemcitabine and oxaliplatin for metastatic pancreatic adenocarcinoma: a North Central Cancer Treatment Group phase II study. Ann Oncol. 2003;14:580-585.
Bai YR, et al. Intensity modulated radiation therapy and chemotherapy for locally advanced pancreatic cancer: results of feasibility study. World J Gastroenterol. 2003;9:2561-2564.
Bakkevold KE, et al. Adjuvant combination chemotherapy (AMF) following radical resection of carcinoma of the pancreas and papilla of Vater—results of a controlled, prospective, randomised multicentre study. Eur J Cancer. 1993;5:698-703.
Ben-Josef E, et al. Intensity-modulated radiotherapy (IMRT) and concurrent capecitabine for pancreatic cancer. Int J Oncol Biol Phys. 2004;59:454-459.
Berlin JD, et al. Phase III study of gemcitabine in combination with fluorouracil versus gemcitabine alone in patients with advanced pancreatic carcinoma: Eastern Cooperative Oncology Group Trial E2297. J Clin Oncol. 2002;20:3270-3275.
Burris HA3rd, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreatic cancer: a randomised trial. J Clin Oncol. 1997;15:2403-2413.
Butturini G, et al. Influence of resection margins and treatment on survival in patients with pancreatic cancer: meta-analysis of randomized controlled trials. Arch Surg. 2008;143:75-83.
Carmichael J, et al. Phase II study of gemcitabine in patients with advanced pancreatic cancer. Br J Cancer. 1996;73:101-105.
Carter R, et al. Longitudinal quality of life data can provide insights on the impact of adjuvant treatment for pancreatic cancer: subset analysis of the ESPAC-1 data. Int J Cancer. 2009;124:2960-2965.
Cartwright TH, et al. Phase II study of oral capecitabine in patients with advanced metastatic pancreatic cancer. J Clin Oncol. 2002;20:160-164.
Cascinu S, et al. Weekly gemcitabine and cisplatin chemotherapy: a well-tolerated but ineffective chemotherapeutic regimen in advanced pancreatic cancer patients: a report from the Italian Group for the Study of Digestive Tract Cancer (GISCAD). Ann Oncol. 2003;14:205-208.
Casper ES, et al. Phase II trial of gemcitabine (2,2’-difluorodeoxycytidine) in patients with adenocarcinoma of the pancreas. Invest New Drugs. 1994;12:29-34.
Colucci G, et al. Gemcitabine alone or with cisplatin for the treatment of patients with locally advanced and/or metastatic pancreatic carcinoma: a prospective, randomized phase III study of the Gruppo Oncologia dell’Italia Meridionale. Cancer. 2002;94:902-910.
Conlon KC, et al. Long-term survival after curative resection for pancreatic ductal adenocarcinoma: clinicopathological analysis of 5-year survivors. Ann Surg. 1996;223:273-279.
Coquard R, et al. Intraoperative radiotherapy in resected pancreatic cancer: feasibility and results. Radiother Oncol. 1997;44:271-275.
Crane CH, et al. Phase I study of concomitant gemcitabine and IMRT for patients with unresectable adenocarcinoma of the pancreatic head. Int J Gastrointest Cancer. 2001;30:123-132.
Crown J, et al. Lack of efficacy of high-dose leucovorin and fluorouracil in patients with advanced pancreatic adenocarcinoma. J Clin Oncol. 1991;9:1682-1686.
Cunningham D, et al. Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2009;27:5513-5518.
DeCaprio JA, et al. Fluorouracil and high-dose leucovorin in previously untreated patients with advanced adenocarcinoma of the pancreas: results of a phase II trial. J Clin Oncol. 1991;9:2128-2133.
Di Costanzo F, et al. Gemcitabine with or without continuous infusion 5-FU in advanced pancreatic cancer: a randomised phase II trial of the Italian Oncology Group for Clinical Research (GOIRC). Br J Cancer. 2005;93:185-189.
Douglas HO, Gastrointestinal Tumor Study Group. Further evidence of effective adjuvant combined radiation and chemotherapy following curative resection of pancreatic cancer. Cancer. 1987;59:2006-2010.
Evans DB, et al. Preoperative chemoradiation and pancreaticoduodenectomy for adenocarcinoma of the pancreas. Arch Surg. 1992;127:1335-1339.
Evans DB, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:3496-3502.
Fossati V, et al. The role of intraoperative therapy by electron beam and combination of adjuvant chemotherapy and external radiotherapy in carcinoma of the pancreas. Tumori. 1995;81:23-31.
Gastrointestinal Tumor Study Group. Multi-institutional comparative trial of radiation therapy alone and in combination with 5-flurouracil for locally unresectable pancreatic carcinoma. Ann Surg. 1979;189:205-210.
Gastrointestinal Tumor Study Group. Treatment of locally unresectable carcinoma of the pancreas: comparison of combined modality therapy (chemotherapy plus radiotherapy) to chemotherapy alone. J Natl Cancer Inst. 1988;80:751-755.
Ghaneh P, et al. Adjuvant treatment for pancreatic cancer. World J Surg. 1999;23:937-945.
Gunderson LL, et al. Intraoperative and external beam irradiation ± 5-FU for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 1987;13:319-329.
Hansen R, et al. Continuous 5-fluorouracil (5FU) infusion in carcinoma of the pancreas: a phase II study. Am J Med Sci. 1988;295:91-93.
Haycox A, et al. Review article: current treatment and optimal patient management in pancreatic cancer. Aliment Pharmacol Ther. 1998;12:949-964.
Heinemann V, et al. Gemcitabine and cisplatin in the treatment of advanced or metastatic pancreatic cancer. Ann Oncol. 2000;11:1399-1403.
Heinemann V, et al. Randomized phase III trial of gemcitabine plus cisplatin compared with gemcitabine alone in advanced pancreatic cancer. J Clin Oncol. 2006;24:3946-3952.
Heinrich S, et al. Prospective phase II trial of neoadjuvant chemotherapy with gemcitabine and cisplatin for resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:2526-2531.
Herrmann R, et al. Gemcitabine plus capecitabine compared with gemcitabine alone in advanced pancreatic cancer: a randomized, multicenter, phase III trial of the Swiss Group for Clinical Cancer Research and the Central European Cooperative Oncology Group. J Clin Oncol. 2007;25:2212-2217.
Hertel LW, et al. Evaluation of the antitumour activity of gemcitabine (2′,2′-diflluoro-2′-deoxycytidine). Cancer Res. 1990;50:4417-4422.
Hiraoka T, et al. Combination of intraoperative radiation with resection of cancer of the pancreas. Int J Pancreatol. 1990;7:201-207.
Hoffman JP, et al. Phase II trial of preoperative radiation therapy and chemotherapy for patients with localized, resectable adenocarcinoma of the pancreas: an Eastern Cooperative Oncology Group study. J Clin Oncol. 1998;16:317-323.
Huang P, et al. Action of 2′,2′-difluorodeoxycitadine on DNA synthesis. Cancer Res. 1991;51:6110-6117.
Ikeda M, et al. Prognostic factors in patients with locally advanced pancreatic carcinoma receiving chemoradiotherapy. Cancer. 2001;91:490-495.
Kalser MH, Ellenberg SS. Pancreatic cancer: adjuvant combined radiation and chemotherapy following curative resection. Arch Surg. 1985;120:899-903.
Klaassen DJ, et al. Treatment of locally unresectable cancer of the stomach and pancreas: a randomized comparison of 5-fluorouracil alone with radiation plus concurrent and maintenance 5-fluorouracil: an Eastern Cooperative Oncology Group study. J Clin Oncol. 1985;3:373-378.
Klinkenbijl JH, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampulliary region: a phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg. 1999;230:776-784.
Knaebel HP, et al. Phase III trial of postoperative cisplatin, interferon alpha-2b, and 5-FU combined with external radiation treatment versus 5-FU alone for patients with resected pancreatic adenocarcinoma—CapRI: study protocol [ISRCTN62866759]. BMC Cancer. 2005;5:37.
Koong AC, et al. Phase I study of stereotactic radiosurgery in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2004;58:1017-1021.
Koong AC, et al. Phase II study to assess the efficacy of conventionally fractionated radiotherapy followed by a stereotactic radiosurgery boost in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2005;63:320-323.
Kosuge T, et al. A multicenter randomized controlled trial to evaluate the effect of adjuvant cisplatin and 5-fluorouracil therapy after curative resection in cases of pancreatic cancer. Jpn J Clin Oncol. 2006;36:159-165.
Landry JC, et al. Treatment of pancreatic cancer tumors with intensity-modulated radiation therapy (IMRT) using the volume at risk approach (VARA): employing dose-volume histogram (DVH) and normal tissue complication probability (NTCP) to evaluate small bowel toxicity. Med Dosim. 2002;27:121-129.
Le Scodan R, et al. Preoperative chemoradiation in potentially resectable pancreatic adenocarcinoma: feasibility, treatment effect evaluation and prognostic factors, analysis of the SFRO-FFCD 9704 trial and literature review. Ann Oncol. 2009;20:1387-1396.
Lim JE, et al. Prognostic factors following curative resection for pancreatic adenocarcinoma: a population-based, linked database analysis of 396 patients. Ann Surg. 2003;237:74-85.
Magee CJ, et al. The role of adjuvant therapy for pancreatic cancer. Expert Opin Investig Drugs. 2002;11:87-107.
Magnin V, et al. Neoadjuvant preoperative chemoradiation in patients with pancreatic cancer. Int J Radiat Oncol Biol Phys. 2003;55:1300-1304.
Maisey N, et al. Multicenter randomized phase III trial comparing protracted venous infusion (PVI) fluorouracil (5-FU) with PVI 5-FU plus mitomycin in inoperable pancreatic cancer. J Clin Oncol. 2002;20:3130-3136.
Milano MT, et al. Intensity-modulated radiotherapy in treatment of pancreatic and bile duct malignancies: toxicity and clinical outcome. Int J Radiat Oncol Biol Phys. 2004;59:445-453.
Moertel CG, et al. Combined 5-fluorouracil and supervoltage radiation therapy of locally unresectable gastrointestinal cancer. Lancet. 1969;25:865-867.
Moertel CG, et al. Therapy of locally unresectable pancreatic carcinoma: a randomised comparison of high dose (6000 rads) radiation alone, moderate dose radiation (4000 rads + 5-FU) and high dose radiation + 5-FU. Cancer. 1981;48:1705-1710.
Moore MJ, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960-1966.
Mornex F, et al. Feasibility of preoperative combined radiation therapy and chemotherapy with 5-fluorouracil and cisplatin in potentially resectable pancreatic adenocarcinoma: the French SFRO-FFCD 97-04 Phase II trial. Int J Radiat Oncol Biol Phys. 2006;65:1471-1478.
Neoptolemos JP, et al. Adjuvant radiotherapy and follow-on chemotherapy in patients with pancreatic cancer: results of the UK Pancreatic Cancer Study Group (UKPACA-1). Gastrointestinal Cancer. 1998;2:235-245.
Neoptolemos JP, et al. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet. 2001;358(9293):1576-1585.
Neoptolemos JP, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200-1210.
Neoptolemos J, et al. ESPAC-3 (v2): a multicenter, international, open label, randomised controlled phase III trial of adjuvant 5-fluorouracil/folinic acid (5-FU/FA) versus gemcitabine (GEM) in patients with resected pancreatic ductal adenocarcinoma. J Clin Oncol. 2009:185. 27
Neoptolemos JP, et al. Adjuvant 5-fluorouracil and folinic acid vs observation for pancreatic cancer: composite data from the ESPAC-1 and -3 (v1) trials. Br J Cancer. 2009;100:246-250.
Neuhaus P, et al. CONKO-001: Final results of the randomized, prospective, multicenter phase III trial of adjuvant chemotherapy with gemcitabine versus observation in patients with resected pancreatic cancer (PC). Oxford, UK: Oxford University Press; 2008. 45-46
Oettle H, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297:267-277.
Palmer KR, et al. Chemotherapy prolongs survival in inoperable pancreatic carcinoma. Br J Surg. 1994;81:882-885.
Philip PA, et al. Phase II study of gemcitabine and cisplatin in the treatment of patients with advanced pancreatic carcinoma. Cancer. 2001;92:569-577.
Pisters PW, et al. Rapid-fractionation preoperative chemoradiation, pancreaticoduodenectomy, and intraoperative radiation therapy for resectable pancreatic adenocarcinoma. J Clin Oncol. 1998;16:3843-3850.
Poplin E, et al. Phase III trials of gemcitabine (30 minute infusion) versus gemcitabine (fixed dose rate infusion [FDR]) versus gemcitabine + oxaliplatin (GEMOX) in patients with advanced pancreatic cancer (E6201) [abstract]. J Clin Oncol. 24, 2006. LBA4004
Regine WF, et al. A phase III study of adjuvant pre and post chemoradiation 5FU vs. gemcitabine for resected pancreatic adenocarcinoma. J Clin Oncol. 24(18S), 2006. 4007
Reni M, et al. Effect on local control and survival of electron beam intraoperative irradiation for resectable pancreatic adenocarcinoma. Int J Radiat Oncol Biol Phys. 2001;50:651-658.
Riess H, et al. A randomised, prospective, multicentre, phase III trial of gemcitabine, 5-fluorouracil (5FU), folinic acid vs. gemcitabine alone in patients with advanced pancreatic cancer. J Clin Oncol. 2005;23:310s.
Rothenberg ML, et al. A phase II trial of gemcitabine in patients with 5-FU–refractory pancreas cancer. Ann Oncol. 1996;7:347-353.
Scheithauer W, et al. Biweekly highdose gemcitabine alone or in combination with capecitebine in patients with metastatic pancreatic adenocarcinoma: a randomised phase II trial. Ann Oncol. 2003;14:97-104.
Shibamoto Y, et al. High-dose intraoperative radiotherapy for irresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 1996;34:57.
Showalter TN, et al. Does intraoperative radiation therapy improve local tumor control in patients undergoing pancreaticoduodenectomy for pancreatic adenocarcinoma? A propensity score analysis. Ann Surg Oncol. 2009;16:2116-2122.
Sindelar W, Kinsella T. Randomised trial of intraoperative radio-therapy in resected carcinoma of the pancreas. Int J Radiat Oncol Biol Phys. 1986;12:148.
Small WJr, et al. Full-dose gemcitabine with concurrent radiation therapy in patients with nonmetastatic pancreatic cancer: a multicenter phase II trial. J Clin Oncol. 2008;26:942-947.
Snady H, et al. Survival advantage of combined chemoradiotherapy compared with resection as the initial treatment of patients with regional pancreatic carcinoma: an outcomes trial. Cancer. 2000;89:314-327.
Stocken DD, et al. Meta-analysis of randomised adjuvant therapy trials for pancreatic cancer. Br J Cancer. 2005;92:1372-1381.
Takada T, et al. Is postoperative adjuvant chemotherapy useful for gallbladder carcinoma? A phase III multicenter prospective randomized controlled trial in patients with resected pancreaticobiliary carcinoma. Cancer Treat Rev. 2003;29:135-137.
Takai S, et al. Neoadjuvant chemoradiation in patients with potentially resectable pancreatic cancer. Pancreas. 2008;36:e26-e32.
Talamonti MS, et al. A multi-institutional phase II trial of preoperative full-dose gemcitabine and concurrent radiation for patients with potentially resectable pancreatic carcinoma. Ann Surg Oncol. 2006;13:150-158.
Tepper JE, et al. Intraoperative radiation therapy of pancreatic carcinoma: a report of RTOG-8505. Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys. 1991;21:1145-1149.
Twombly R. Adjuvant chemoradiation for pancreatic cancer: few good data, much debate. J Natl Cancer Inst. 2008;100(23):1670-1671.
Valentini V, et al. Intra-operative radiotherapy (IORT) in pancreatic cancer: joint analysis of the ISIORT-Europe experience. Radiother Oncol. 2009;91:54-59.
Van Cutsem E, et al. Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J Clin Oncol. 2009;27:2231-2237.
Varadhachary GR, et al. Preoperative gemcitabine (gem) and bevacizumab (bev)-based chemoradiation for resectable pancreatic adenocarcinoma [abstract]. J Clin Oncol. 2008;26:4630.
Varadhachary GR, et al. Preoperative gemcitabine and cisplatin followed by gemcitabine-based chemoradiation for resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:3487-3495.
White RR, et al. Neoadjuvant chemoradiation for localized adenocarcinoma of the pancreas. Ann Surg Oncol. 2001;8:758-765.
Wilkowski R, et al. Chemoradiotherapy with concurrent gemcitabine and cisplatin with or without sequential chemotherapy with gemcitabine/cisplatin vs chemoradiotherapy with concurrent 5-fluorouracil in patients with locally advanced pancreatic cancer: a multi-centre randomised phase II study. Br J Cancer. 2009;101:1853-1859.
Yeo CJ, et al. Pancreaticoduodenectomy for pancreatic adenocarcinoma: postoperative adjuvant chemoradiation improves survival: a prospective, single-institution experience. Ann Surg. 1998;225:621.
Yeung RS, et al. Neoadjuvant chemoradiation in pancreatic and duodenal carcinoma: a Phase II study. Cancer. 1993;72:2124-2233.
Zerbi A, et al. Intraoperative radiation therapy adjuvant to resection in the treatment of pancreatic cancer. Cancer. 1994;73:2930-2935.