Chapter 19 Charged Particle Radiotherapy
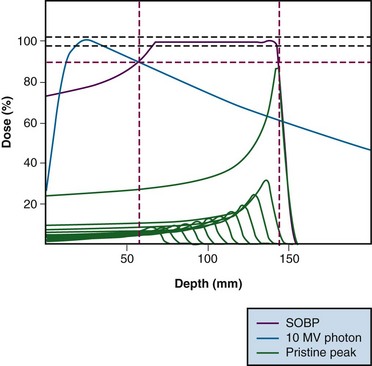
Interest in the use of charged particle radiotherapy has been primarily stimulated by the superior dose distributions that can be achieved with these particles compared with those produced by standard photon therapy techniques. Charged particles deposit energy in tissue through multiple interactions with electrons in the atoms of cells, although a small fraction of energy is also transferred to tissue through collisions with the nuclei of atoms. The energy loss per unit path length is relatively small and constant until near the end of the range where the residual energy is lost over a short distance, resulting in a steep rise in the absorbed dose (energy absorbed per unit mass). This portion of the particle track, where energy is rapidly lost over a short distance, is known as the Bragg peak (Fig. 19-1).
The initial low-dose region in the depth-dose curve, before the Bragg peak, is referred to as the plateau of the dose distribution and is about 30% of the Bragg peak maximum dose. The Bragg peak is too narrow for practical clinical applications. For the irradiation of most tumors, the beam energy is modulated in order to achieve a uniform dose over a significant volume. This is accomplished by superimposing several Bragg peaks of descending energies (ranges) and weights to create a region of uniform dose over the depth of the target; these extended regions of uniform dose are called spread-out Bragg peaks (SOBP) (see Fig. 19-1). Although the beam modulation used to spread out the Bragg peaks does increase the entrance dose, the proton dose distribution is still characterized by a lower-dose region in normal tissue proximal to the tumor, a uniform high-dose region in the tumor, and zero dose beyond the tumor.
Charged particles are generally characterized as having either high or low linear energy transfer (LET), the rate of energy loss by the particle in tissue. The LET influences the biologic impact of the energy deposited in tissue. X and gamma photons, protons, and helium ions are considered to be forms of low LET radiation. Heavier charged particles (e.g., neon ions, carbon ions) are considered to be forms of high LET radiation. There is an initial increase in the relative biologic effectiveness (RBE) with an increase in LET.1 Carbon ions have an RBE of about 3, whereas the recommended RBE of protons is 1.1.2,3 Higher-LET radiation is less influenced by tissue oxygenation and less sensitive to variations in the cell cycle and DNA repair. For particle radiation, the Gray equivalent dose is calculated by multiplying the physical dose administered by the RBE for that particle; the recommended nomenclature for expressing the dose is Gy(RBE) = physical dose in Gy × RBE.3
Development of Proton Beam Radiotherapy
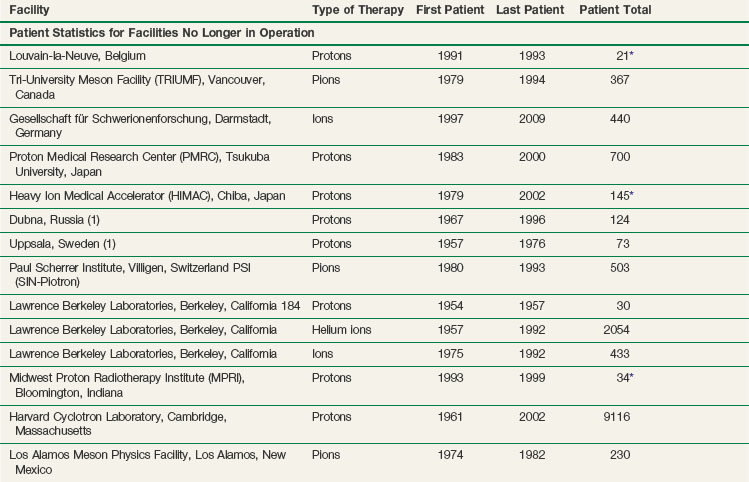
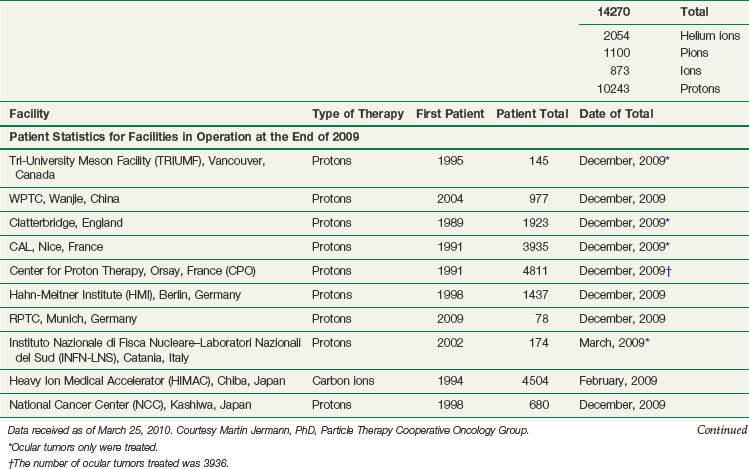
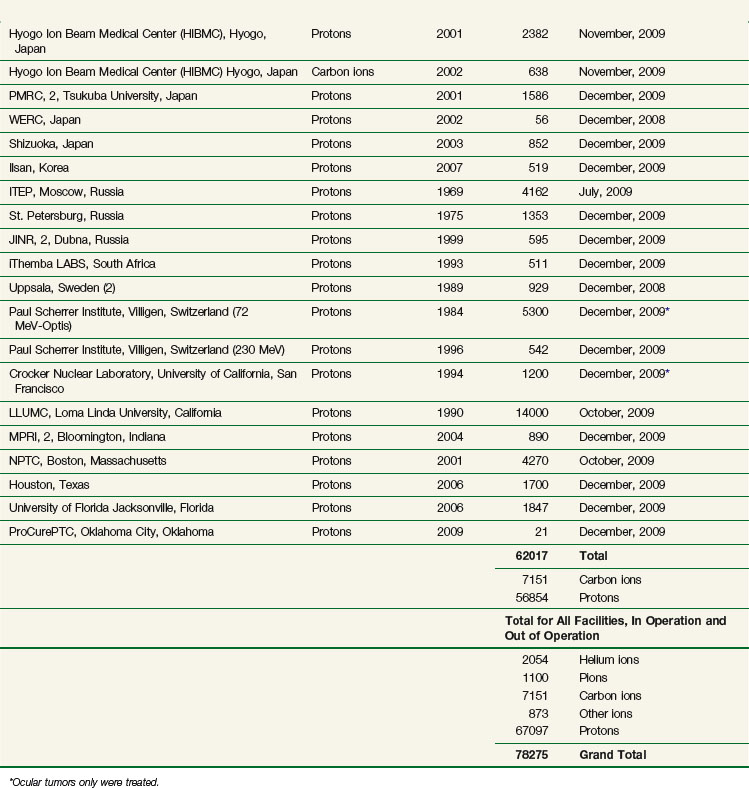
The vast majority of patients receiving charged particle therapy have been treated with protons. As of March 2010, over 67,000 patients had received part or all of their radiotherapy by proton beams.4 Table 19-1 lists currently operational proton beam treatment facilities worldwide. Several more sites are scheduled to begin using proton beam therapy over the next several years.
In 1946, Robert Wilson5 proposed that proton beams would provide superior dose distributions over photons and should be considered for clinical radiotherapy. Initially, patients were being treated at facilities designed and constructed for basic high-energy physics research, and this often meant that treatment delivery was cumbersome. The proton beams were limited to a fixed horizontal position, which meant that the patient had to be moved to align the tumor on the trajectory of the beam. This technique was in contrast to the isocentric capabilities of the modern linear accelerator, which rotates around a point in space and can effectively target any site in the body. In addition, for many of the proton machines, the energy of the beam (which defined the depth of the Bragg peak) was only sufficient to treat superficial lesions (such as those of the eye) or intermediate-depth lesions (such as those at the base of the skull). Because of these technical factors and the interests of the involved physicians, the tumors that initially received the most attention were uveal melanomas in the eye and sarcomas at the base of the skull. The major emphasis in proton therapy clinical research initially was on dose escalation for tumors for which local control with conventional radiotherapy was poor.
Treatment of Specific Cancers with Proton Beam Radiotherapy
Ocular (Uveal) Melanoma
As of December 2002, more than 3000 patients with uveal melanoma had been treated with protons at Massachusetts General Hospital in collaboration with the Massachusetts Eye and Ear Infirmary.6 The 5-year actuarial local control rate was 96% for all sites within the globe, with an 80% survival rate. The probability of eye retention at 5 years was estimated to be 90% for the entire group and 97%, 93%, and 78% for patients with small, intermediate, and large tumors, respectively. Independent risk factors for enucleation were involvement of the ciliary body, tumor height greater than 8 mm, and distance between the posterior tumor edge and the fovea. These results compare favorably with the 5-year local control rates of 93% reported with protons in Nice, France,7 98.9% after 1993 from the Paul Scherrer Institute in Villigen, Switzerland,8 and 96% from the Curie Institute in Orsay, France.9
Because some patients have experienced deteriorating vision after doses of 70 Gy(RBE), a randomized trial of 50 Gy(RBE) versus 70 Gy(RBE) for small and intermediate-sized lesions located within 6 mm of the optic disc or macula was conducted. Interim analysis of 188 patients, with a median follow-up of 60 months, suggested no reduction in either local control or survival rates. No significant improvement in visual outcome or complications has been observed. However, visual field analysis does show a smaller mean defect in the patients randomized to 50 Gy(RBE).10
Egger and colleagues11 reported long-term results of eye retention after treatment of uveal melanoma with proton beam therapy. A total of 2645 patients (2648 eyes) were treated at the Paul Scherrer Institute between 1984 and 1999. The overall eye retention rate at 5, 10, and 15 years after treatment was 89%, 86%, and 83%, respectively. Enucleation was related to large tumor size (mainly, tumor height), male gender, high intraocular pressure, and large degree of retinal detachment at treatment time.
Sarcomas of the Skull Base and Spine
At the Harvard Cyclotron Laboratory, Massachusetts General Hospital, physicians used a combination of protons and photons to treat patients with tumors of the skull base and cervical spine.12 A total of 169 patients with chordoma and 165 patients with chondrosarcoma were treated. The 10-year local control rate was highest for chondrosarcomas, intermediate for male chordomas, and lowest for female chordomas (94%, 65%, and 42%, respectively). For cervical spine tumors, 10-year local control rates were not significantly different for chordomas and chondrosarcomas (54% and 48%, respectively), nor was there any significant difference in local control rates between males and females. In a Cox multivariate analysis, predictors of local control included gender and equivalent uniform dose, or gender and target volume, or gender and minimum target dose.13 Five-year actuarial rates of endocrinopathy were as follows: 72% for hyperprolactinemia, 30% for hypothyroidism, 29% for hypogonadism, and 19% for hypoadrenalism. The minimum target dose (Dmin) to the pituitary gland was found to be predictive of endocrinopathy: Patients receiving 50 Gy(RBE) or more at Dmin to the pituitary gland had a higher incidence of and greater severity of endocrine dysfunction. Posterior pituitary dysfunction, represented by vasopressin activity with diabetes insipidus, was not observed.14
The French group at Orsay reported on the treatment of patients with skull base tumors, 34 with chordoma and 11 with chondrosarcoma.15 Irradiation was done with a combination of photons and protons, with protons used in one-third of the treatment regimens. The median total dose delivered was 67 Gy(RBE) (range, 60 to 70 Gy[RBE]). With a mean follow-up of 30.5 months (range, 2 to 56 months), the 3-year local control rate for chordomas was 83.1% and for chondrosarcomas was 90%. Three-year overall survival rates were 91% and 90%, respectively.
Between 1998 and 2005, 64 patients with skull base chordomas (42 patients) and chondrosarcomas (22 patients) were treated at the Paul Scherrer Institute with protons using a spot-scanning technique.16 Patients with chordoma received a mean dose of 73.5 Gy(RBE) (range, 67 to 74 Gy[RBE]), and patients with chondrosarcoma received a mean dose of 68.4 Gy(RBE) (range, 63 to 74 Gy[RBE]). With a mean follow-up of 38 months, actuarial 5-year local control rates were 81% and 94% for chordomas and chondrosarcomas, respectively. The actuarial 5-year rate for freedom from high-grade toxicity was 94%.
Torres and associates17 performed a planning study where they compared three-dimensional conformal proton (PR) therapy, intensity-modulated radiotherapy (IMRT) with photons (PH), and combined proton and IMRT photon (PP) irradiation of skull base chordomas to determine the optimal technique. For each of five patients, they generated four treatment plans: (1) an IMRT plan with a 1-mm planning target volume (PH1) for stereotactic treatment; (2) an IMRT plan with a 3-mm planning target volume (PH3) for routine treatment; (3) a PR plan with beam-specific expansion margins on the clinical target volume; and (4) a plan for PP treatment. The mean percentage of planning target volume (%PTV) receiving the prescription dose of 74 Gy(RBE) was highest in the PP plans and lowest in the PH3 plans. The PR plans were the least homogeneous and conformal. The PH3 plans had the highest mean percentage of volume (%V) of brain, brainstem, chiasm, and temporal lobes above the tolerance dose for those organs. The PH1 plans had the lowest brainstem mean %V receiving 67 Gy(RBE) and temporal lobe mean %V receiving 65 Gy(RBE). Global evaluation of the plans based on objective parameters revealed that the PP plans yielded the best target coverage and conformality. This study indicates that there may be dosimetric advantages to using a combination of IMRT and three-dimensional protons, to optimize conformality and minimize integral dose, which may be an important option until intensity-modulated proton therapy is more widely available.
Isacsson and colleagues18 compared conformal radiotherapy treatment plans with combination photon/proton plans for a patient with a cervical Ewing’s sarcoma. The comparison showed small but clear advantages of protons for the boost. At 1% normal tissue complication probability (NPTC) in the spinal cord, the calculated tumor control probability (TPC) was on average 5% higher for the photon/proton boost combination.
Hug and colleagues19 presented results on combined photon/proton treatment of 47 patients with osteogenic and chondrogenic tumors of the axial skeleton. Radiation was delivered postoperatively in 23 patients, preoperatively and postoperatively in 17 patients, and as the sole treatment in 7 patients. Mean radiation doses of 73.9 Gy(RBE), 69.8 Gy(RBE), and 61.8 Gy(RBE) were delivered to group 1 (20 patients with recurrent/primary chordoma or chondrosarcoma), group 2 (15 patients with osteogenic sarcoma), and group 3 (12 patients with giant cell tumors, osteoblastomas, or chondroblastomas), respectively. Five-year actuarial local control and survival rates for patients with chondrosarcoma were 100% and 100% and with chordoma, 53% and 50%. The actuarial 5-year local control rate for patients with osteosarcoma was 59%. The 5-year actuarial local control and survival rates for the group 3 patients were 76% and 87%. Overall, improved local control was noted for patients with primary versus recurrent tumors, those who underwent gross total resection, and those who received target doses of more than 77 Gy(RBE).
Weber and colleagues20 carried out a treatment planning comparison of intensity-modulated photon (IM) and proton therapy (IMPT) for paraspinal sarcomas. Plans for five patients were computed for IM photons (seven coplanar fields) and protons (three coplanar beams). The prescribed dose was 77.4 Gy(RBE) for protons to the gross tumor volume. Surface and center spinal cord dose constraints for all techniques were 64 and 53 Gy(RBE), respectively. Gross tumor volume coverage was optimal and equally homogeneous with both the IM photon and IM proton plans. The median heart, lung, kidney, stomach, and liver mean dose and the dose at the 50% volume level were consistently reduced by a factor of 1.3 to 25 with protons compared with photons. IMPT dose escalation (to 92.9 Gy(RBE) delivered to the gross tumor volume) was possible in all patients without exceeding the normal-tissue dose limits.
At the Francis H. Burr Proton Therapy Center at Massachusetts General Hospital, a phase II study was conducted of combined photon and proton beam radiation therapy, with or without surgical resection, for patients with spinal and paraspinal sarcomas.21 Doses of 77.4 Gy(RBE) at 1.8 Gy(RBE) per day were used for patients with gross residual disease and 70.2 Gy(RBE) for patients with microscopic residual disease. A total of 50 patients (29 with chordoma, 14 with chondrosarcoma, and 7 with other cancers) underwent gross total (25 patients) or subtotal (12 patients) resection or biopsy (13 patients). With a 48-month median follow-up, the 5-year actuarial local control, recurrence-free survival, and overall survival rates were 78%, 63%, and 87%, respectively. Two of 36 patients (5.6%) treated for primary tumors versus 7 of 14 patients (50%) treated for recurrent tumors developed local recurrence (p <.001). The spinal cord center dose was limited to 54 Gy(RBE) and the cord surface dose to 63 Gy(RBE) over a length of 5 cm or less. The cauda equina was constrained to 70.2 Gy(RBE), except for areas in direct contact with tumor, where the dose limit was 77.4 Gy(RBE). Five patients developed late radiation-associated complications; no myelopathy developed, but three grade 3 sacral neuropathies appeared after doses of 77.12 to 77.4 Gy(RBE) had been delivered.
Optic Pathway Glioma
At Loma Linda University, seven children with optic pathway gliomas were treated with proton radiation therapy.22 At a median follow-up of 37 months, all tumors were locally controlled. A reduction in tumor volume was seen in three patients, and tumor volume was stable in the other four. Visual acuity was stable in those that presented with useful vision. Proton plans were compared with photon plans for individual patients. With proton therapy radiation, there was a 47% reduction in the dose to the contralateral optic nerve. There was an 11% reduction in the dose to the chiasm and a 13% reduction in the dose to the pituitary gland. There was also a reduction in the dose to the temporal lobes and frontal lobes.
Astrocytoma
Between 1993 and 1998, 48 patients were treated for nonresectable grade II and III intracranial tumors at the Center for Proton Therapy in Orsay, France.23 Mean tumor doses ranged from 63 to 67 Gy at 1.8 Gy/fraction. With a median follow-up of 18 months, local control rates were 97% (33 of 34 patients) and 43% (6 of 14 patients) for nonparenchymal and parenchymal lesions, respectively.
At the Harvard Cyclotron Laboratory and Massachusetts General Hospital, a phase II study was undertaken by Massachusetts General Hospital researchers to assess whether dose escalation to 90 Gy(RBE) with conformal protons and photons in accelerated fractionation twice a day would improve local tumor control and survival rates.24 A total of 23 patients were enrolled, with ages of 18 to 70 years. Actuarial survival rates at 2 and 3 years were 34% and 18%, respectively. The median survival time was 20 months, with four patients alive 22 to 60 months after diagnosis. All patients developed new areas of gadolinium enhancement during the follow-up period. Histologic examination of tissues obtained at biopsy, resection, or autopsy was conducted in 15 patients. Radiation necrosis only was demonstrated in seven patients, and their survival was significantly longer than patients with recurrent tumor. Tumor regrowth occurred most commonly in areas that received doses of 60 to 70 Gy(RBE) or less; recurrent tumor was found in only one patient in the group that received a dose of 90 Gy(RBE). The authors concluded that attempts to extend local control by enlarging the volume would likely be complicated by a high incidence of radionecrosis.
Benign Meningioma
Between 1981 and 1996, 46 patients with partially resected, biopsied, or recurrent benign meningiomas were treated with combined proton/photon radiation at the Harvard Cyclotron Laboratory/Massachusetts General Hospital.25 The median dose for the tumor was 59 Gy(RBE). Overall survival rates at 5 and 10 years were 93% and 77%, respectively, and recurrence-free rates at 5 and 10 years were 100% and 88%, respectively. Three patients presented with local tumor recurrence at 61, 95, and 125 months. One patient died of focal brain necrosis at 22 months. Neurologic complications, including memory deficits and hearing loss, were also seen. Four patients developed ophthalmologic toxicity. In all of these cases, the maximum dose to the optic structures was more than 58 Gy(RBE). Endocrine abnormalities following treatment were also seen.
Investigators from the Paul Scherrer Institute reported on the treatment of 16 patients with recurrent, residual, or untreated intracranial meningiomas.26 The median prescribed dose was 56 Gy(RBE) (range, 52 to 64 Gy[RBE]) at 1.8 to 2 Gy(RBE) per fraction. Cumulative 3-year local control, progression-free survival, and overall survival rates were 91%, 91%, and 92%, respectively. No patient died of recurrent meningioma. Radiographic follow-up (median, 34 months) revealed an objective response in 3 patients and stable disease in 12 patients. The cumulative 3-year toxicity-free survival rate was 76%. One patient with an optic nerve sheath meningioma presented with sudden visual field deterioration of the ipsilateral eye 30 months after irradiation with 56 Gy(RBE). Another patient with optic nerve encasement by disease developed visual deterioration at 9 months. A third patient developed symptomatic brain necrosis 7 months after treatment. No radiation-induced hypothalamic/pituitary dysfunction was observed.
Paranasal Sinus, Nasal, and Nasopharyngeal Tumors
Mock and associates27 performed a planning comparison study of various photon and proton techniques for the treatment of paranasal sinus carcinoma. In five patients, proton plans were compared with conventional, conformal, and IMRT photon plans. The evaluations analyzed dose-volume histogram findings of the target volumes and organs at risk (i.e., the pituitary gland, optic pathway structures, and brain).
Between 1991 and 1996, 32 patients with carcinomas of the paranasal sinuses were treated with an accelerated photon/proton protocol.28 The stage distribution was T3 in two cases and T4 in 30 cases, and all were stages N0 and M0. Four patients had undergone a gross total resection, and the others had undergone only a biopsy or a subtotal resection. The median follow-up was 2.7 years. The actuarial disease-specific survival rate at 3 years was 62%. There have been 10 deaths, three with intercurrent disease and seven with metastatic disease. The 3-year actuarial local control rate was 89%. Late toxicity has included temporal lobe necrosis in three patients. Three patients have required surgical soft tissue repair.
Truong and colleagues29 performed a retrospective review of 20 patients with locally advanced primary sphenoid sinus malignant tumors treated between 1991 and 2005 with proton radiotherapy to a median dose of 76 Gy(RBE) to determine treatment outcome and prognostic factors. With a median follow-up of 27 months, the 2-year local, regional, and freedom from distant metastasis rates were 86%, 86%, and 50%, respectively. The disease-free and overall survival rates at 2 years were 31% and 53%, respectively. In multivariate analysis, oropharyngeal involvement (p = .005) and anterior cranial fossa invasion (p = .02) were predictive for poor disease-free survival rates. Brain invasion was predictive for decreased overall survival rates (p = .05). No grade 3 or 4 late visual toxicity was reported. Three patients developed chronic nasal symptoms after radiotherapy, consisting of common toxicity criteria (CTC) grade 2 to 3 nasal obstruction secondary to fibrous adhesions. One patient required surgical removal of adhesions to relieve chronic nasal congestion. One patient with symptomatic CTC grade 2 brain toxicity experienced seizures and short-term memory loss. The seizures were controlled with anticonvulsant medications and a short course of steroids. Two patients experienced cerebrospinal fluid leakage after surgery and irradiation. One patient developed a CTC grade 2 cerebrospinal fluid leak from the external auditory canal, secondary to tumor shrinkage and erosion of the petrous temporal bone 5 months after radiotherapy. The patient was offered surgery and declined treatment. One patient developed a CTC grade 5 cerebrospinal fluid leak without evidence of tumor recurrence at 2 months after completion of radiotherapy. The patient underwent four surgical repairs, including transethmoid packing of the ethmoid and sphenoid sinuses and placement of a lumboperitoneal shunt. The patient subsequently died from infectious meningitis. Two patients had endocrinopathies that were medically corrected. The authors concluded that proton radiation therapy results in excellent local control in patients with advanced primary sphenoid sinus malignant disease. Brain invasion and involvement of the oropharynx and anterior cranial fossa were important prognostic factors. Nasal symptoms, brain injury, endocrinopathies, and cerebrospinal fluid leaks, however, may complicate treatment.
Investigators at Massachusetts General Hospital performed a prospective study incorporating chemotherapy, surgery, and combined proton/photon therapy in the treatment of patients with neuroendocrine tumors of the sinonasal tract.30 Nineteen patients with olfactory neuroblastoma or neuroendocrine carcinoma were treated between 1992 and 1998. Patients received chemotherapy with two courses of cisplatin and etoposide followed by high-dose proton/photon radiotherapy to 69.2 Gy(RBE) using 1.6 to 1.8 Gy(RBE) per fraction twice daily in a concomitant boost schedule. Two further courses of chemotherapy were given to responders.
At Loma Linda University, 16 patients with recurrent nasopharyngeal carcinoma were treated with conformal proton irradiation.31 Patients had initially been treated with photon therapy at doses of 50 to 70 Gy. Conformal proton boost radiation was then delivered to bring the total dose to 59 to 70 Gy(RBE). With a mean follow-up of 23 months, the 24-month actuarial overall and locoregional progression-free survival rates were both 50%. No central nervous system complications were observed. An update now includes data on 39 patients Twenty-four-month actuarial overall and locoregional progression-free survival rates were 49% and 70%, respectively. When critical central nervous system structures were re-irradiated, doses were low (0 to 22 Gy[RBE]); one patient experienced grade IV central nervous system side effects. Grade III late head and neck toxicity occurred in seven patients and grade IV toxicity in two patients.
Acoustic Neuroma
Between 1991 and 1999, 30 patients with acoustic neuroma were treated with proton therapy at Loma Linda University.32 Patients with useful hearing before treatment received 54 Gy(RBE) in 30 fractions, and patients without useful hearing received 60 Gy(RBE). Follow-up ranged from 7 to 98 months (median, 34 months), during which no patients demonstrated disease progression on magnetic resonance imaging scans. Eleven patients demonstrated radiographic regression. Of the 13 patients with useful hearing before treatment, 4 (31%) maintained their hearing. No transient or permanent treatment-related trigeminal or facial nerve dysfunction was observed. Investigators are now interested in evaluating a reduction in tumor dose in an attempt to increase hearing preservation rates.
Carcinoma of the Prostate
Investigators at Massachusetts General Hospital completed a phase III trial comparing 67.2 Gy of photons with 75.6 Gy(RBE) of combined photons/protons using a conformal perineal proton boost.33 From 1982 to 1992, 202 patients with stage T3 or T4 prostate cancer received 50.4 Gy by four-field photons. Patients then received either 25.2 Gy(RBE) with conformal protons or a 16.8-Gy photon boost. No differences between the two groups were found in overall survival, total recurrence-free survival, or local recurrence-free survival rates. The local recurrence-free survival rate at 7 years for patients with poorly differentiated tumors (Gleason score of 9 or 10) was 85% for the proton arm and 37% for the photon arm. Rates of grade 1 and 2 rectal bleeding were higher in the proton arm (32% vs. 12%), as were those for urethral stricture (19% vs. 8%). Dose escalation to 75.6 Gy(RBE) by conformal proton boost led to increased late radiation sequelae but not to increased total survival rates in any subgroup. However, there was an improved local recurrence-free survival rate in patients with poorly differentiated tumors.
The Loma Linda University experience of conformal proton therapy for prostate cancer was reported34 for 1255 patients treated from 1991 to 1997. The overall biochemical disease-free survival rate was 73%, and it was 90% in patients with an initial prostate-specific antigen (PSA) level of 4 ng/mL or less; it was 87% in patients with posttreatment PSA nadirs of 0.50 ng/mL or less. Rates dropped with rises in initial and nadir PSA values.
Massachusetts General Hospital and Loma Linda University conducted a phase III randomized dose escalation trial in patients with early-stage prostate cancer.35 Between 1996 and 1999, 393 men with stage T1b to T2b prostate cancer and PSA levels of 15 ng/mL or less were randomly assigned to a total dose of either 70.2 Gy Gy(RBE) or 79.2 Gy(RBE). No patient received androgen suppression therapy with irradiation. Conformal radiation therapy was given in two phases. In phase I, conformal proton beams were used to treat the prostate alone. Either 19.8 Gy(RBE) or 28.8 Gy(RBE) was given in 1.8-Gy(RBE) fractions. In phase II, all men—regardless of trial arm—received 50.4 Gy delivered with photons in 1.8-Gy fractions to the prostate and seminal vesicles. Rates of local failure, biochemical failure, and overall survival were measured as outcomes. With a median follow-up of 8.9 years, men receiving high-dose radiation therapy were significantly less likely to have local failure, with a hazard ratio of 0.57. The 10-year American Society for Therapeutic Radiology and Oncology biochemical failure rates were 32.4% for conventional-dose and 16.7% for high-dose radiation therapy (p <.0001). This difference held when only those with low-risk disease (227 patients; 58% of total) were examined: 28.2% for conventional-dose and 7.1% for high-dose radiation therapy (p <.0001). There was a strong trend in the same direction for the intermediate-risk patients (144 patients; 37% of total; 42.1% vs. 30.4%, p = .06). Eleven percent of patients subsequently required androgen deprivation for recurrence after conventional-dose radiation therapy compared with 6% after high-dose radiation therapy (p = .047). There remains no difference in overall survival rates between the treatment arms (78.4% vs. 83.4%; p = .41). Two percent of patients in both arms experienced late grade 3 or higher genitourinary toxicity, and 1% of patients in the high-dose arm experienced late grade 3 or higher gastrointestinal toxicity. This randomized controlled trial shows superior long-term cancer control for men with localized prostate cancer receiving high-dose versus conventional-dose radiation. This dose escalation was achieved with protons without an increase in grade 3 or higher late urinary or rectal morbidity.
Investigators at the University of Florida evaluated dose distributions between proton plans and intensity-modulated radiotherapy (IMRT) plans.36 Plans using both techniques were generated for 10 consecutive patients. Two beams were used for the proton plans, and five were used for the IMRT plans. All plans were calculated for 78 Gy equivalents (GE).
Gastrointestinal Tumors
Investigators at Tsukuba University in Japan have reported impressive long-term control and survival results in 122 patients with primary hepatocellular carcinoma treated with proton radiotherapy.37 The dose per fraction was 4 Gy(RBE) and the mean total dose was 72 Gy(RBE) (the Tsukuba group did not correct for RBE at that time, so these doses are in physical Gray; hence, the biologically effective dose may have been up to 10% higher). The 7-year local control and survival rates were 94% and 27%, respectively. Proton irradiation did not cause clinically symptomatic changes in liver function. The only notable change observed was a transient increase in liver transaminase levels.
Due to the presence of nearby critical structures, dose limitations are frequently an issue in the treatment of pancreatic cancer. Stripp and associates38 conducted a study comparing proton plans with conventional photon plans for patients with unresectable pancreatic cancer. Dose-volume histograms were generated for the gross tumor volume, clinical target volume, spinal cord, liver, and right and left kidneys. Proton plans used either a two-field or three-field technique. With the clinical target volume and gross tumor volume receiving the same dose from proton and photon plans, all individual proton plans were superior to the photon plans in reduction of normal tissue dose. For the four patients evaluated, the average dose reduction to 50% of the organ at risk was 78% to the spinal cord, 73% to the left kidney, 43% to the right kidney, and 55% to the liver.
Investigators at Massachusetts General Hospital carried out a feasibility study for neoadjuvant hypofractionated proton therapy for pancreatic adenocarcinoma.39 Tumor and normal tissue dosimetry was evaluated for a treatment regimen of 5 Gy(RBE) × 5 fractions. These plans were compared with IMRT plans with conventional fractionation generated for the same nine patients.
Lung Cancer
At Loma Linda University, a prospective study was undertaken to assess the efficacy and toxicity of conformal proton beam radiotherapy for 37 patients with medically inoperable non-small cell lung cancer.40 Eligible patients had clinical stage I to IIIa non-small cell lung cancer and were not candidates for surgical resection either for medical reasons or because of patient refusal. Patients with adequate cardiopulmonary function received 45 Gy to the mediastinum and gross tumor volume with photons with a concurrent proton boost to the gross tumor volume of an additional 28.8 Gy(RBE). Total tumor dose was 73.8 Gy(RBE) given over 5 weeks. Patients with poor cardiopulmonary function received proton beam radiotherapy to the gross tumor volume only, with 51 Gy(RBE) given in 10 fractions over a 2-week period. Follow-up of evaluable patients ranged from 3 to 45 months, with a median of 14 months. Two patients in the proton and photon arm developed pneumonitis that resolved with oral steroids; otherwise, no significant toxicities were encountered. The 2-year actuarial disease-free survival rate for the entire group was 63%; for stage I patients, the 2-year disease-free survival rate was 86% and the local control rate was 87%.
From November 2001 to July 2008, 55 medically inoperable patients with stage I non–small cell lung cancer were treated with proton beam therapy at Tsukuba University in Japan.41 A total of 58 tumors (30 at stage T1, 28 at stage T2) were treated. The median age of study participants was 77 years (range, 52 to 86 years). A total dose of 66 GyE in 10 fractions was given to peripherally located tumors and 72.6 GyE in 22 fractions to centrally located tumors. The rates (95% confidence interval) of overall survival and progression-free survival for all patients and of local control of all tumors at 2 years were 97.8% (93.6% to 102%), 88.7% (77.9% to 99.5%), and 97% (91.1% to 102.8%), respectively. There was no statistically significant difference in the progression-free rate between T1 and T2 tumors (p = .87). Two patients (3.6%) had deterioration in pulmonary function, and two patients (3.6%) had grade 3 pneumonitis. The researchers concluded that proton beam therapy was effective and well tolerated in medically inoperable patients with stage I non-small cell lung cancer.
The M.D. Anderson group carried out a treatment planning study on 10 patients with stage IIIB non-small cell lung cancer.42 Dose-volume histograms were generated for plans using intensity-modulated proton therapy (IMPT), intensity-modulated radiation therapy (IMRT), and passive scattering proton therapy (PSPT). The IMRT cases were planned to 60 to 63 Gy, and the proton cases were planned to 74 Gy. The possibility of increasing the total tumor dose with IMPT for each patient without exceeding the dose volume constraints (maximum tolerated dose) was also investigated. Compared with IMRT, IMPT showed improved tissue sparing for virtually every parameter measured, even with dose escalation from 63 Gy to 83.5 Gy, with a mean maximum tolerated dose of 74 Gy. IMPT also showed dosimetric advantages for those cases with complicated tumor anatomies. A randomized phase II multi-institutional study comparing three-dimensional conformal protons with three-dimensional conformal photon therapy or photon IMRT is currently being conducted by the M.D. Anderson Cancer Center and Massachusetts General Hospital.
Pediatric Cancers
Investigators in Switzerland looked at the potential influence of improved dose distribution with proton beams compared with conventional or intensity-modulated (IM) x-ray beams on the incidence of treatment-induced secondary cancers in children.43 Two children, one with parameningeal rhabdomyosarcoma and a second with medulloblastoma, were used as models for this study. After defining the target and critical structures, treatment plans were calculated and optimized, four for the rhabdomyosarcoma case (conventional x-ray, IM x-rays, three-dimensional conformal protons, and IM protons) and three for the irradiation of the spinal axis in medulloblastoma (conventional x-ray, IM x-rays, three-dimensional conformal protons). The secondary cancer incidence was estimated using a model by the International Commission on Radiologic Protection. This model allowed estimation of absolute risks of secondary cancer for each treatment plan based on dose-volume distributions for nontarget organs. Proton beams reduced the expected incidence of radiation-induced secondary cancers for the rhabdomyosarcoma patient by a factor equal to or greater than 2, and for the medulloblastoma cases a factor of 8 to 15 when compared with either IM or conventional x-ray plans. This study underscores the concern with using radiation therapy in the treatment of pediatric cancers. It is the goal of clinicians to not only eradicate the primary tumor but also to minimize the risk of radiation-induced malignant tumors over the lifetime of these patients.
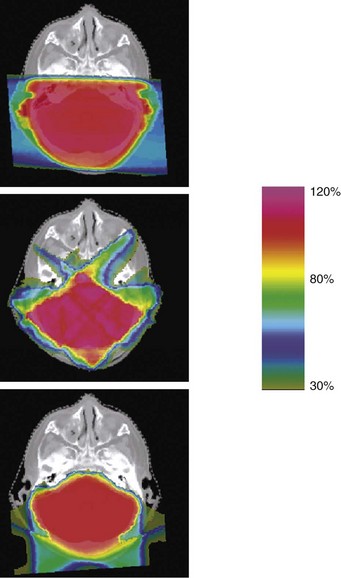
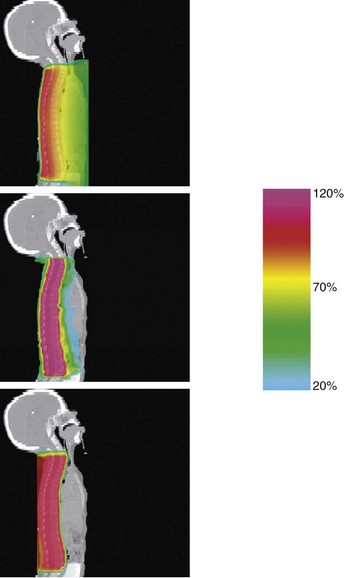
In a second Massachusetts General Hospital study, treatment plans were compared from standard three-dimensional conformal photon therapy to intensity-modulated x rays (IMRT) and three-dimensional conformal protons for craniospinal axis irradiation and posterior fossa boost in a patient with medulloblastoma.44 Substantial normal tissue sparing was realized with IMRT and proton irradiation of the posterior fossa and spinal axis (Figs. 19-2 and 19-3). The dose to 90% of the cochlea was reduced from 101% of the prescribed posterior fossa boost dose from conventional x-rays to 33% and 2% from IMRT and protons, respectively. The dose to 50% of the heart volume was reduced from 72% for photons to 30% for IMRT and 0.5% for protons.
Merchant and coworkers45 modeled the dose characteristics of both to critical normal tissue volumes using data from patients with four types of childhood brain tumors. Three-dimensional imaging and treatment planning data, including targeted tumor and normal tissue contours, were acquired for 40 patients, 10 each with optic pathway glioma, craniopharyngioma, infratentorial ependymoma, and medulloblastoma. Dose-volume data were collected for the entire brain, temporal lobes, cochlea, and hypothalamus from each patient. The data were averaged and compared based on treatment modality (protons vs. photons) using dose-cognitive effects models. Outcomes were estimated over 5 years. The results suggested that relatively small critical normal tissue volumes such as the cochlea and hypothalamus may be spared from radiation exposure when they are not adjacent to the primary tumor volume. It was found that larger normal tissue volumes such as the supratentorial brain or temporal lobes receive less of the low and intermediate doses. When applied to longitudinal models of radiation dose-cognitive effects, these differences resulted in clinically significant higher IQ scores for patients with medulloblastoma and craniopharyngioma and higher academic reading scores in patients with optic pathway glioma. Extreme differences between proton and photon dose distributions precluded meaningful comparison of protons and photons for patients with infratentorial ependymoma. The authors concluded that differences in the overall dose distributions, as indicated by modeling changes in cognitive function, showed that a reduction in the lower-dose volumes or mean dose with protons would have long-term, clinical advantages for children with medulloblastoma, craniopharyngioma, and optic pathway glioma.
Loma Linda University investigators evaluated the safety and efficacy of proton beam irradiation in the treatment of pediatric patients with intracranial low-grade astrocytoma.46 Between 1991 and 1997, 27 patients underwent fractionated proton radiation therapy for progression of recurrent low-grade astrocytoma. Patients were 2 to 18 years old. Twenty-five of the 27 patients (92%) were treated for progressive, unresectable, or residual disease following subtotal resection. The mean target dose was 55.2 Gy(RBE) (range, 50.4 to 63 Gy[RBE]) and the fraction size was 1.8 Gy(RBE). At a mean follow-up period of 3.3 years (range, 0 to 6.8 years), 6 of 27 patients experienced local failure within the irradiated field and 4 of 27 had died. Local control and survival rates were 87% and 93%, respectively, for centrally located tumors, 71% and 86% for hemispheric tumors, and 60% and 60% for tumors of the brainstem. All children with local control maintained their performance status except one, who developed moyamoya disease. All six patients with optic pathway tumors and useful vision maintained or improved their visual status.
Four pediatric patients presenting with aggressive giant cell tumors of the skull base were treated with a combination of proton and photon beam irradiation at Massachusetts General Hospital.47 Three female patients and one male patient (ages 10 to 15 years) had undergone prior extensive surgical resection(s) and were treated for either primary or recurrent disease. Gross residual tumor was evident in three patients and microscopic disease suspected in one patient. Combined proton and photon radiation therapy was based on three-dimensional planning. Target doses of 57.6 to 61.2 Gy(RBE) were given in daily fractions of 1.8 Gy(RBE). At observation times between 3.1 and 5.8 years, all four patients were alive and well and remained locally controlled without evidence of recurrent disease. Except for one patient with partial pituitary insufficiency following radiotherapy for recurrent sellar disease, no late effects attributable to radiation therapy have thus far been observed.
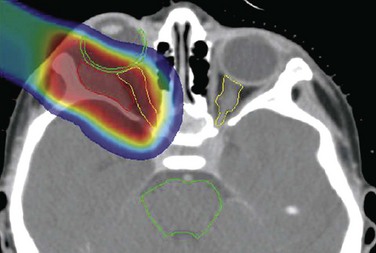
Retroperitoneal tumors, such as neuroblastoma, can be quite difficult to treat, given the proximity of the spinal cord, liver, and kidneys. In a comparison of proton and photon plans for a patient treated for neuroblastoma, a significant reduction in normal tissue exposure was achieved using protons.48 Likewise, protons offered a preferable dose distribution to photons in two patients treated for orbital rhabdomyosarcoma.49 Dose-volume histograms were obtained for target and nontarget regions, including the lens, bony orbit, pituitary gland, optic chiasm, optic nerves, lacrimal gland, and ipsilateral frontal and temporal lobes. Doses to 90%, 50%, and 5% of lens volume were kept at less than 1%, less than 2%, and less than 8%, respectively. At a mean follow-up of 3 years, visual acuity for both patients was excellent and there was no evidence of cataract formation. Furthermore, pituitary function was normal; cosmetically, only mild enophthalmos was noticeable. The steep dose gradient beyond the orbit minimized irradiation of normal brain parenchyma, with sparing of the pituitary gland (Fig. 19-4).
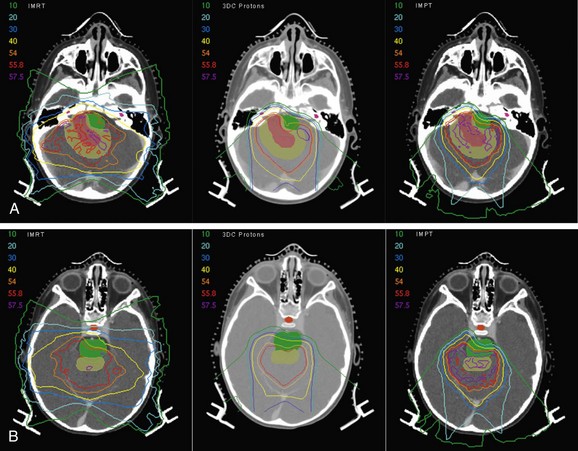
The Massachusetts General Hospital group recently reported clinical outcomes for 17 pediatric patients treated with proton irradiation for intracranial ependymoma from 2000 to 2006.50 All patients were treated using the three-dimensional conformal passive scanning technique. The median prescribed dose was 55.8 Gy(RBE) (range, 52.2 to 59.4 Gy[RBE]). At a median follow-up of 26 months, local control, progression-free survival, and overall survival rates were 86%, 80%, and 89%, respectively. Additionally, the investigators carried out a treatment planning study, comparing several three-dimensional proton plans with IMRT and IMPT plans. Adequate tumor coverage was achieved with all techniques, but substantial normal tissue sparing was seen with proton plans compared with IMRT. Use of IMPT allowed for additional sparing of critical structures. For the infratentorial plan, the mean dose received by the temporal lobes was 16 Gy with IMRT, 4 Gy(RBE) with protons, and 2 Gy(RBE) with IMPT. For the same case, the left cochlea received a dose of 37 Gy with IMRT, 2 Gy(RBE) with protons, and less than 0.1 Gy(RBE) with IMPT (Fig. 19-5).
Breast Cancer
Some patients with breast cancer have anatomic configurations that make it difficult to adequately treat the breast while sparing the underlying lung and heart. A treatment planning exercise was undertaken comparing standard photon therapy with IMRT and proton therapy in the treatment of breast cancer.51 Using CT data from a breast cancer patient, treatment plans were computed for the different treatment techniques. A dose of 50 Gy was prescribed to the target volume consisting of the involved breast and the internal mammary, supraclavicular, and axillary nodes. Comparison of plans revealed worse dose heterogeneity for the photon plan versus the other two plans. Lung dose-volume histograms for the photon and IMRT plans were comparable, whereas the proton plan showed the best sparing over all dose levels. Mean doses to the ipsilateral lung for the three plans were 17 Gy, 15 Gy, and 13 Gy, for the photon, IMRT, and proton plans, respectively. For the heart, the IMRT plan delivered the highest mean dose (16 Gy), reflecting the extra dose delivered through this organ to spare the lungs. This was reduced somewhat by the standard plan (15 Gy), with the best sparing being provided by the proton plan (6 Gy). When the IMRT plan was reoptimized with an increased precedence to the normal tissues, the mean doses to all neighboring organs at risk could be reduced but only at the cost of substantial target dose heterogeneity. Only the two-field, energy-modulated proton plan had the potential to preserve target dose homogeneity while simultaneously minimizing the dose delivered to the lungs, heart, and contralateral breast.
Techniques of Proton Beam Delivery
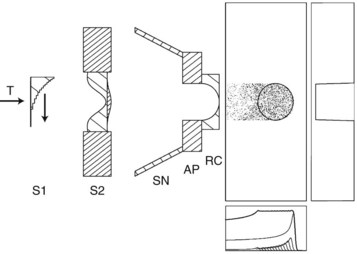
Currently, most proton facilities use passive scattering for beam delivery. With this technique, a spatially uniform dose distribution is achieved by scattering and degrading the primary proton beam in a set of distributed absorbers. Passive systems use either single or double scattering foils. The foil is made of high-Z material such as lead, and effectively scatters the beam while keeping energy loss to a minimum. Generally speaking, double scatter systems are preferred because larger, more uniform beams can be generated (Fig. 19-6).
Beam shaping is accomplished with the use of some devices that are patient- and field-specific and some that are not. Range modulation refers to the concept that pristine Bragg peaks must be spread out to be clinically useful. In the passive scatter system this is done with either a propeller-shaped modulator (Fig. 19-7) or a ridge filter, which is not patient-specific but may be range-specific, with a small library of range modulators available to address the different range modulator options that might be clinically desired. The Bragg peaks are spread out by placing these devices, which have variable thicknesses in the path of a given beam. The thicker the modulator, the more the beam is shifted in range.
Additional beam conformality is achieved with the use of patient-specific devices (Fig. 19-8). Brass apertures are formulated to be used for a specific field for a given patient. Apertures are equivalent to the blocks used in conventional radiation therapy. In January 2010, the University of Pennsylvania Health System received approval from the Food and Drug Administration (FDA) to use multileaf collimators in their new proton facility.
Although proton therapy has the potential to spare more healthy tissue than x-ray therapy, by virtue of physical qualities of the Bragg peak, there is a concern for increased neutron production. Neutrons can be generated whenever high-energy protons are slowed down by nuclear interactions. Such events can take place inside or outside of the patient. There is a certain amount of neutron production that takes place from proton interactions within the patient, and this is unavoidable. But with the passive scatter system, neutrons are primarily produced from proton interactions with scattering material and collimators in the accelerator. Hall52 determined that this external neutron dose was more than 100 times the internal dose. Others argue that Hall’s technical assumptions and calculations reflect the worst case scenario of treatment of a small target with a large amount of brass in the aperture, and still other investigators have measured substantially lower levels of neutrons, because the number of neutrons generated will be a function of the specific beam line as well as the beam-modifying hardware upstream from the patient.53
Chung and colleagues54 quantified the risk of a second malignant tumor associated with the use of proton therapy compared with photon radiation therapy. In this retrospective study, 1450 patients treated with protons at the Harvard Cyclotron Laboratory from 1974 to 2001 were matched with similar patients from the Surveillance Epidemiology and End Results (SEER) Program database. With a median follow-up of more than 6 years, a second cancer developed in 6.4% of proton patients and 12.8% of photon patients (adjusted hazard ratio of 1.87 to 3.98, p <.0001). Although the magnitude of neutron production and its clinical significance in contributing to radiation-induced malignant disease have been hotly debated, the issue remains a concern. Active beam scanning will likely markedly reduce external neutron contamination in the beam, and for this reason, the technique will be preferred over the passively scattered beam technique.
Active Scanning
The next step in the evolution of proton beam delivery is active (spot or pencil beam) scanning. With passive scanning, it is difficult to obtain large, uniform fields without significant energy loss, even with double scattering systems. In 1980, investigators in Japan first described a spot scanning system.55 The technique was further refined by the group at the Paul Scherrer Institute in Switzerland.56 With this technique, the beam is not spread out or scattered; rather, a narrow pencil beam is precisely steered through the treatment volume by magnets in the nozzle of the accelerator. One of the benefits of this system is that there is no field-specific or patient-specific hardware; therefore, the time and cost of fabrication are avoided. Furthermore, the speed of the treatment is increased because these heavy devices do not need to be changed for each treatment field. Most importantly, neutron production is greatly diminished with the absence of this hardware.
These pencil beams deposit the dose layer by layer, with the distal edge treated first and the more superficial layers treated thereafter (Fig. 19-9). Active scanning allows for good conformation of the proximal edge of the treatment volume, which is typically not the case with passive scanning. This ability to conform the dose to the proximal tumor volume may further be enhanced with the use of intensity-modulated proton therapy (IMPT). This excellent dose-shaping ability of IMPT makes it the best technique for the treatment of irregularly shaped tumors.
Cost Comparisons for Proton Beam Radiotherapy
All of the clinical and treatment planning results that have been reported indicate that proton beams offer a significant potential for improvements in clinical outcomes for cancer patients over a broad range of disease sites. There is hope that prospective clinical trials that have begun in the new hospital-based proton therapy facilities will establish the magnitude of these improvements. It will be important for physicians and patients and even society in general to have some sense of the cost of these benefits, to be able to place them in an appropriate context and allow comparison with other medical interventions. Goitein and Jermann57 performed cost comparisons between proton radiation therapy and technically sophisticated photon radiation therapy in an effort to define the relative costs of the technologies. The expense of proton therapy per patient is expected to decrease as more facilities are built and greater numbers of patients are treated. The adoption of hypofractionation and beam scanning, as well as technical advances including smaller proton facilities, is expected to further reduce the cost. At the present time, when neither costs nor benefits have been adequately determined, it is not possible to carry out a reliable cost/benefit analysis. Goitein and Jermann57 were able to estimate that the relative cost of proton beam radiation therapy compared with intensity-modulated photon beam radiation therapy was in the range of 2.4 in 2003 but might come down to 1.7 to 2.1 over time. Studies that have assessed cost-effectiveness suggest that protons will be cost-effective for pediatric malignant disease because of the reduction in late effects associated with the 50% to 60% reduction in integral dose associated with protons.58
Protons are estimated to be cost-effective for some adult tumors such as left-sided breast cancer.59 Konski and associates,60 however, evaluated proton radiotherapy for prostate cancer and concluded that, even when based on the unproven assumption that protons will permit a 10-Gy escalation of prostate dose compared with IMRT photons, proton beam therapy was not cost-effective for most patients with prostate cancer using the commonly accepted standard of $50,000 per quality-adjusted life-year.
Carbon Ion Radiotherapy
Carbon ions have a slight physical advantage over protons in that they will have a narrower penumbra over protons, particularly for deep-seated tumors.61 On the other hand, carbon ions will also produce some spallation products deep to the Bragg peak. Whether the higher RBE and differential effect on hypoxic cells of carbon ions will translate into a clinical advantage remains to be determined, particularly since the clinical use of carbon has generally been with larger fraction sizes where there will be less RBE difference between carbon ions and protons.
The Heavy Ion Medical Accelerator (HIMAC) in Chiba, Japan, began clinical studies in 1994. Kamada and colleagues62 reported the results of a phase I/II study evaluating the tolerance for and effectiveness of carbon ion radiotherapy in patients with unresectable bone and soft tissue sarcomas. Fifty-seven patients with 64 sites of bone and soft tissue sarcomas not suited for resection received carbon ion therapy. Tumors involved the spine or paraspinous soft tissues in 19 patients, pelvis in 32 patients, and extremities in 6 patients. The total dose ranged from 52.8 to 73.6 carbon GyE and was administered in 16 fractions over 4 weeks (3.3 to 4.6 GyE/fraction). Escalation was then halted at this level. Seventeen of the patients treated with the highest total dose of 73.6 GyE experienced RTOG grade 3 acute skin reactions. No other severe acute reactions (grade >3) were observed in this series. The overall local control rates were 88% and 73% at 1 and 3 years of follow-up, respectively. The 1- and 3-year overall survival rates were 82% and 46%, respectively. It is important to continue close follow-up of these patients to ensure that the large dose fractions are not associated with late injury of normal tissue.
Raster scanned carbon ion radiation therapy at the Gesellschaft für Schwerionenforschung in Darmstadt, Germany, where it has been used to treat patients since 1997. Between November 1998 and July 2005, a total of 96 patients with chordomas of the skull base were treated with carbon ion radiation therapy.63 All patients had gross residual tumors. The median total dose was 60 Gy(RBE) (range, 60 to 70 Gy[RBE]), delivered in 20 fractions within 3 weeks. The mean follow-up was 31 months (range, 3 to 91 months). Fifteen patients developed local recurrences after carbon ion radiation therapy. The actuarial local control rates were 80.6% and 70% at 3 and 5 years, respectively. Target doses in excess of 60 Gy(RBE) and primary tumor status were associated with higher local control rates. Overall survival rates were 91.8% and 88.5% at 3 and 5 years, respectively. Grade 3 toxicity was seen in 4% of patients and consisted of fat necrosis and optic neuropathy.
Kato and associates64 from Chiba, Japan, recently reported the results of a carbon ion dose escalation study for 24 patients with hepatocellular carcinoma. Fifteen fractions were delivered over 5 weeks, and total doses ranged from 49.5 to 79.5 GyE. During a median follow-up of 71 months (range, 63 to 83 months), no severe adverse effects or treatment-related deaths occurred. The overall tumor response rate was 71%. The local control and overall survival rates were 92% and 92%, 81% and 50%, and 81% and 25% at 1, 3, and 5 years, respectively.
Hypofractionated carbon ion therapy has also been used for other tumors, including uveal melanoma, non–small cell lung cancer, head and neck mucosal melanoma, adenoid cystic carcinoma, squamous cell carcinoma, prostate carcinoma, and renal cell carcinoma.61 The new charged particle facility in Heidelberg, Germany, can treat with either carbon ions or protons. Because of the greater complexity and cost of carbon ions compared with protons, researchers hope that randomized studies can be done at this facility to compare these two charged particles.
Neon Ion Radiotherapy
High-LET charged particle therapy with neon ions was studied in the treatment of malignant glioblastoma of the brain because of its increased biologic potential for destruction of radioresistant tumors. At the University of California, San Francisco, and Lawrence Berkeley Laboratories, 15 patients with glioblastoma multiforme were entered into a randomized protocol comparing two dose levels of neon ion irradiation.65 Patients received either 20 Gy or 25 Gy in 4 weeks. However, there was no significant difference in overall survival time (13 to 14 months). Furthermore, an optimal dose level was not identified. Neon ions are not in current clinical use.
π-Meson Radiotherapy
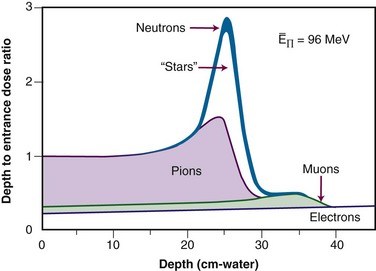
Sub-atomic particles called π-mesons provide the nuclear binding force between nuclei. These particles are produced when protons (600+ MeV) affect a target. Three types of mesons are produced: a neutral form, a positive form, and a negative form. It is the negative form, π−, that is used for radiotherapy. When the π− slows down, it is “captured” by a nucleus, causing it to “explode” in a “star” event, producing neutrons and charged nuclear fragments having high-LET properties66 (Fig. 19-10).
Negative π-mesons (pions) were used to treat 228 patients at the Los Alamos Meson Physics Facility between 1974 and 1981. One hundred twenty-nine patients received pion therapy only. All patients had locally advanced disease, and a number of different sites were treated. Local control was achieved in 86% of patients with prostate cancer, in 26% with head and neck cancers, and in none with pancreatic cancer. A steep rise in the complication rate was seen beyond a dose level of 3750 cGy.67
At TRIUMF (Vancouver, British Columbia), 81 patients with high-grade gliomas were randomized to either a 33- to 34.5-Gy pion dose given over 3 weeks or a 60-Gy photon dose given over 6 weeks. The median survival time was 10 months for both arms, and there was no significant difference in time to progression, radiation toxicity, performance status, or quality of life assessments.68 TRF was also the site for a phase III trial comparing pion therapy with photon therapy in the treatment of locally advanced prostate cancer. At a follow-up of 3.75 years, there was no difference in local control or survival rates. There was significantly more acute grade 3 to 4 bladder toxicity for the pion group; however, no difference in late effects was seen.67 π-Mesons are also no longer in clinical use.
Helium Radiotherapy
A retrospective study was done at the University of California, San Francisco, and Lawrence Berkeley Laboratories to evaluate the use of helium ions in the treatment of uveal melanomas.69 Ten years after helium ion radiation, 208 of the 218 eyes irradiated had local tumor control (95.4%). At 10 years, 46 eyes (22%) had been enucleated; the majority resulting from anterior ocular segment complications. At 10 years, 51 patients (23%) had died of metastatic melanoma. The best corrected visual acuity after irradiation was greater than 20/40 in 21 of 93 eyes (23%) of patients who were alive and who had retained their eyes 10 or more years after treatment. Visual acuity was related to height of tumor and location near the nerve or fovea.
A study was completed comparing iodine-125 (125I) plaques to helium ions by the group at the University of California, San Francisco, and Lawrence Berkeley Laboratories.70 Patients with melanomas less than 10 mm in height and less than 15 mm in diameter were randomized by the research group to receive either 70 Gy(RBE) in five treatments with helium ions or 70 Gy to the tumor apex with 125I plaque brachytherapy. Local control was 100% in those patients treated with helium ions versus 87% in those patients treated with 125I plaques.
Schoenthaler and colleagues71 reported on the use of charged particle irradiation for sacral chordomas. At Lawrence Berkeley Laboratories, 14 patients with sacral chordomas were treated with charged particles of helium and neon. All patients were treated postoperatively; 10 had gross disease. The median dose was 7565 cGy and the median follow-up was 5 years. The Kaplan-Meier survival rate at 5 years was 85%, and the overall 5-year local control rate was 55%. A trend in improved local control at 5 years was seen in patients treated with neon ions compared with patients treated with helium ions (62% vs. 34%), in patients following complete resection versus patients with gross residual tumor (75% vs. 40%), and in patients who had treatment courses less than 73 days (61% vs. 21%). No patient developed neurologic sequelae or pain syndromes. One previously irradiated patient required colostomy; one patient had delayed wound healing following a negative postradiation biopsy; and one patient developed a second malignant tumor. There were no genitourinary complications.
Conclusions
As discussed, the main benefit of protons over conventional photon beam radiotherapy is a reduction in integral dose. With intensity modulation, dose conformalities with protons and photons are comparable (assuming a small enough proton pencil beam diameter). It remains to be determined how much clinical benefit this reduction in integral dose achieves for patients. From planning studies, the greatest benefit is projected for larger targets in younger patients, where studies project a reduction in second cancers72 and other late effects to render protons cost-effective.58 With minimization of the normal tissue dose, protons may allow for better tolerance of combined chemotherapy and radiation therapy regimens; indeed, many of the trials of pediatric proton radiation therapy employ chemotherapy in the same way that it has been commonly employed in pediatric protocol-directed therapy for solid tumors.73 For smaller targets in older patients, such as prostate cancer, the cost-effectiveness of protons has been questioned,60 and randomized studies of protons versus IMRT have been proposed.
1 Demizu Y, Kagawa K, Ejima Y. Cell biological basis for combination radiotherapy using heavy-ion beams and high energy X-rays. Radiother Oncol. 2004;74:207.
2 Koike S, Ando K, Oohira C, et al. Relative biological effectiveness of 290 MeV/u carbon ions for the growth delay of a radioresistant murine fibrosarcoma. J Radiat Res (Tokyo). 2002;43(3):247-255.
3 DeLuca PMJr, Wambersie A, Whitmore G. Prescribing, recording, and reporting proton-beam therapy. J ICRU. 2007;7:1-210.
4 Particle Therapy Cooperative Oncology Group website, www.ptcog.com.
8 Egger E, Schalenbourg A, Zografos L, et al. Maximizing local tumor control and survival after proton beam radiotherapy of uveal melanoma. Int J Radiat Oncol Biol Phys. 2001;51(1):138-147.
9 Dendale R, Lumbroso-Le Rouic L, Noel G, et al. Proton beam radiotherapy for uveal melanoma. Results of Curie Institut-Orsay Proton Therapy Center (ICPO). Int J Radiat Oncol Biol Phys. 2006;65:780-787.
10 Gragoudas ES, Lane AM, Regan S, et al. A randomized controlled trial of varying radiation doses in the treatment of choroidal melanoma. Arch Ophthalmol. 2000;118(6):773-778.
11 Egger E, Zografos L, Schalenbourg A, et al. Eye retention after proton beam radiotherapy for uveal melanoma. Int J Radiat Oncol Biol Phys. 2003;55(4):867-880.
14 Pai HH, Thornton A, Katznelson L, et al. Hypothalamic/pituitary function following high-dose conformal radiotherapy to the base of skull. Demonstration of a dose-effect relationship using dose-volume histogram analysis. Int J Radiat Oncol Biol Phys. 2001;49(4):1079-1092.
15 Noel G, Habrand JL, Mammar H, et al. Combination of photon and proton radiation therapy for chordomas and chondrosarcomas of the skull base. The Centre de Protontherapie D’Orsay experience. Int J Radiat Oncol Biol Phys. 2001;51(2):392-398.
16 Ares C, Hug EB, Lomax AJ, et al. Effectiveness and safety of spot scanning proton radiation therapy for chordomas and chondrosarcomas of the skull base. First long-term report. Int J Radiat Oncol Biol Phys. 2009;75:1111-1118.
17 Torres MA, Chang EL, Mahajan A, et al. Optimal treatment planning for skull base chordoma. Photons, protons, or a combination of both? Int J Radiat Oncol Biol Phys. 2009;74:1033-1039.
20 Weber DC, Trofimov AV, Delaney TF, Bortfeld T. A treatment planning comparison of intensity modulated photon and proton therapy for paraspinal sarcomas. Int J Radiat Oncol Biol Phys. 2004;58(5):1596-1606.
21 DeLaney TF, Liebsch NJ, Pedlow FX, et al. Phase II study of high dose photon/proton radiotherapy in the management of spine sarcomas. Int J Radiat Oncol Biol Phys. 2009;74(3):732-739.
25 Wenkel E, Thornton AF, Finkelstein D, et al. Benign meningioma: partially resected, biopsied, and recurrent intracranial tumors treated with combined proton and photon radiotherapy. Int J Radiat Oncol Biol Phys. 2000;48(5):1363-1370.
26 Weber DC, Lomax AJ, Rutz HP. Spot-scanning proton radiation therapy for recurrent, residual or untreated intracranial meningiomas. Radiother Oncol. 2004;71(3):251-258.
27 Mock U, Georg D, Bogner J, et al. Treatment planning comparison of conventional, 3D conformal, and intensity-modulated photon (IMRT) and proton therapy for paranasal sinus carcinoma. Int J Radiat Oncol Biol Phys. 2004;58(1):147-154.
29 Truong MT, Kamat UR, Liebsch NJ, et al. Proton radiation therapy for primary sphenoid sinus malignancies. Treatment outcome and prognostic factors. Head Neck. 2009;31:1297-1308.
30 Fitzek MM, Thornton AF, Varvares M, et al. Neuroendocrine tumors of the sinonasal tract. Results of a prospective study incorporating chemotherapy, surgery, and combined proton-photon radiotherapy. Cancer. 2002;94(10):2623-2634.
32 Bush DA, McAllister CJ, Loredo LN, et al. Fractionated proton beam radiotherapy for acoustic neuroma. Neurosurgery. 2002;50(2):270-273.
34 Slater JD, Rossi CJJr, Yonemoto LT, et al. Proton therapy for prostate cancer. The initial Loma Linda University experience. Int J Radiat Oncol Biol Phys. 2004;59(2):348-352.
35 Zietman AL, Bae K, Slater JD, et al. Randomized trial comparing conventional-dose with high-dose conformal radiation therapy in early-stage adenocarcinoma of the prostate. Long-term results from Proton Radiation Oncology Group/American College of Radiology 95-09. J Clin Oncol. 2010;28:1106-1111.
36 Chera BS, Vargas C, Morris CG, et al. Dosimetric study of pelvic proton radiotherapy for high-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2009;75:994-1002.
38 Hsiung-Stripp DC, McDonough J, Masters HM, et al. Comparative treatment planning between proton and x-ray therapy in pancreatic cancer. Med Dosim. 2001;26(3):255-259.
39 Kozak KR, Kachnic LA, Adams J, et al. Dosimetric feasibility of hypofractionated proton radiotherapy for neoadjuvant pancreatic cancer treatment. Int J Radiat Oncol Biol Phys. 2007;68:1557-1566.
41 Nakayama H, Sugahara S, Tokita M, et al. Proton beam therapy for patients with medically inoperable stage I non–small-cell lung cancer at the University of Tsukuba. Int J Radiat Oncol Biol Phys. 2010;78(2):467-471.
42 Chang JY, Zhang X, Wang X, et al. Significant reduction of normal tissue dose by proton radiotherapy compared with three-dimensional conformal or intensity-modulated radiation therapy in stage I or stage III non-small-cell lung cancer. Int J Radiation Oncology Biol Phys. 2006;65:1087-1096.
43 Miralbell R, Lomax A, Cella L, Schneider U. Potential reduction of the incidence of radiation-induced second cancers by using proton beams in the treatment of pediatric tumors. Int J Radiat Oncol Biol Phys. 2002;54(3):824-829.
44 St Clair WH, Adams JA, Bues M, et al. Advantage of protons compared to conventional X-ray or IMRT in the treatment of a pediatric patient with medulloblastoma. Int J Radiat Oncol Biol Phys. 2004;58(3):727-734.
45 Merchant TE, Hua CH, Shukla H, et al. Proton versus photon radiotherapy for common pediatric brain tumors. Comparison of models of dose characteristics and their relationship to cognitive function. Pediatr Blood Cancer. 2008;51:110-117.
46 Hug EB, Muenter MW, Archambeau JO, et al. Conformal proton radiation therapy for pediatric low-grade astrocytomas. Strahlenther Onkol. 2002;178(1):10-17.
47 Hug EB, Muenter MW, Adams JA, et al. 3-D-conformal radiation therapy for pediatric giant cell tumors of the skull base. Strahlenther Onkol. 2002;178(5):239-244.
48 Hug EB, Nevinny-Stickel M, Fuss M, et al. Conformal proton radiation treatment for retroperitoneal neuroblastoma. Introduction of a novel technique. Med Pediatr Oncol. 2001;37(1):36-41.
49 Hug EB, Adams J, Fitzek M, et al. Fractionated, three-dimensional, planning-assisted proton-radiation therapy for orbital rhabdomyosarcoma. A novel technique. Int J Radiat Oncol Biol Phys. 2000;47(4):979-984.
50 MacDonald SM, Safai S, Trofimov A, et al. Proton radiotherapy for childhood ependymoma. Initial clinical outcomes and dose comparisons. Int J Radiat Oncol Biol Phys. 2008;71(4):979-986.
51 Fogliata A, Bolsi A, Cozzi L. Critical appraisal of treatment techniques based on conventional photon beams, intensity modulated photon beams and proton beams for therapy of intact breast. Radiother Oncol. 2002;62(2):137-145.
52 Hall EJ. Intensity-modulated radiation therapy, protons, and the risk of second cancers. Int J Radiat Oncol Biol Phys. 2006;65(1):1-7.
53 Paganetti H, Bortfeld T, Delaney TF. Neutron dose in proton radiation therapy. In regard to Eric J. Hall (Int J Radiat Oncol Biol Phys 65:1-7, 2006). Int J Radiat Oncol Biol Phys. 2006;66(5):1594-1595.
54 Chung CS, Keating N, Yock T, Tarbell N. Comparative analysis of second malignancy risk in patients treated with proton therapy versus conventional photon therapy. Int J Radiat Oncol Biol Phys. 2008;72(1):S8.
57 Goitein M, Jermann M. The relative costs of proton and X-ray radiation therapy. Clin Oncol (R Coll Radiol). 2003;15(1):S37-S50.
58 Lundkvist J, Ekman M, Ericsson SR, et al. Cost-effectiveness of proton radiation in the treatment of childhood medulloblastoma. Cancer. 2005;103(4):793-801.
59 Lundkvist J, Ekman M, Ericsson SR, et al. Economic evaluation of proton radiation therapy in the treatment of breast cancer. Radiother Oncol. 2005;75(2):179-185.
60 Konski A, Speier W, Hanlon A, et al. Is proton beam therapy cost effective in the treatment of adenocarcinoma of the prostate? J Clin Oncol. 2007;25(24):3603-3608.
61 Schulz-Ertner D, Tsujii H. Particle radiation therapy using proton and heavier ion beams. J Clin Oncol. 2007;25(8):953-964.
62 Kamada T, Tsujii H, Tsuji H, et al. Efficacy and safety of carbon ion radiotherapy in bone and soft tissue sarcomas. J Clin Oncol. 2002;20(22):4466-4471.
63 Schulz-Ertner D, Karger CP, Feuerhake A, et al. Effectiveness of carbon ion radiotherapy in the treatment of skull-base chordomas. Int J Radiat Oncol Biol Phys. 2007;68(2):449-457.
64 Kato H, Tsujii H, Miyamoto T. Results of the first prospective study of carbon ion radiotherapy for hepatocellular carcinoma with liver cirrhosis. Int J Radiat Oncol Biol Phys. 2004;59(5):1468-1476.
66 Thornton AF, Laramore GE. Particle radiation therapy. In: Gunderson LL, Tepper JE, editors. Clinical radiation oncology. Philadelphia: Churchill Livingstone; 2000:225-234.
72 Miralbell R, Lomax A, Cella L, Schneider U. Potential reduction of the incidence of radiation-induced second cancers by using proton beams in the treatment of pediatric tumors. Int J Radiat Oncol Biol Phys. 2002;54(3):824-829.
1 Demizu Y, Kagawa K, Ejima Y. Cell biological basis for combination radiotherapy using heavy-ion beams and high energy X-rays. Radiother Oncol. 2004;74:207.
2 Koike S, Ando K, Oohira C, et al. Relative biological effectiveness of 290 MeV/u carbon ions for the growth delay of a radioresistant murine fibrosarcoma. J Radiat Res (Tokyo). 2002;43(3):247-255.
3 DeLuca PMJr, Wambersie A, Whitmore G. Prescribing, recording, and reporting proton-beam therapy. J ICRU. 2007;7:1-210.
4 Particle Therapy Cooperative Oncology Group website, www.ptcog.com.
5 Wilson RR. Radiological uses of fast protons. Radiology. 1946;47:487.
6 Munzenrider JE. Proton therapy for uveal melanomas and other eye lesions. Srahlenther Onkol. 1999;175(Suppl 2):68.
7 Courdi A, Caujolle JP, Grange JD, et al. Results of proton therapy of uveal melanomas treated in Nice. Int J Radiat Oncol Biol Phys. 1999;45(1):5-11.
8 Egger E, Schalenbourg A, Zografos L, et al. Maximizing local tumor control and survival after proton beam radiotherapy of uveal melanoma. Int J Radiat Oncol Biol Phys. 2001;51(1):138-147.
9 Dendale R, Lumbroso-Le Rouic L, Noel G, et al. Proton beam radiotherapy for uveal melanoma. Results of Curie Institut-Orsay Proton Therapy Center (ICPO). Int J Radiat Oncol Biol Phys. 2006;65:780-787.
10 Gragoudas ES, Lane AM, Regan S, et al. A randomized controlled trial of varying radiation doses in the treatment of choroidal melanoma. Arch Ophthalmol. 2000;118(6):773-778.
11 Egger E, Zografos L, Schalenbourg A, et al. Eye retention after proton beam radiotherapy for uveal melanoma. Int J Radiat Oncol Biol Phys. 2003;55(4):867-880.
12 Munzenrider JE, Liebsch NJ. Proton therapy for tumors of the skull base. Strahlenther Onkol. 1999;175(Suppl 2):57-63.
13 Terahara A, Niemierko A, Goitein M, et al. Analysis of the relationship between tumor dose inhomogeneity and local control in patients with skull base chordoma. Int J Radiat Oncol Biol Phys. 1999;45(2):351-358.
14 Pai HH, Thornton A, Katznelson L, et al. Hypothalamic/pituitary function following high-dose conformal radiotherapy to the base of skull. Demonstration of a dose-effect relationship using dose-volume histogram analysis. Int J Radiat Oncol Biol Phys. 2001;49(4):1079-1092.
15 Noel G, Habrand JL, Mammar H, et al. Combination of photon and proton radiation therapy for chordomas and chondrosarcomas of the skull base. The Centre de Protontherapie D’Orsay experience. Int J Radiat Oncol Biol Phys. 2001;51(2):392-398.
16 Ares C, Hug EB, Lomax AJ, et al. Effectiveness and safety of spot scanning proton radiation therapy for chordomas and chondrosarcomas of the skull base. First long-term report. Int J Radiat Oncol Biol Phys. 2009;75:1111-1118.
17 Torres MA, Chang EL, Mahajan A, et al. Optimal treatment planning for skull base chordoma. Photons, protons, or a combination of both? Int J Radiat Oncol Biol Phys. 2009;74:1033-1039.
18 Isacsson U, Hagberg H, Johansson KA, et al. Potential advantages of protons over conventional radiation beams for paraspinal tumours. Radiother Oncol. 1997;45(1):63-70.
19 Hug EB, Fitzek MM, Liebsch NJ. Locally challenging osteo- and chondrogenic tumors of the axial skeleton. Results of combined proton and photon radiation therapy using three-dimensional treatment planning. Int J Radiat Oncol Biol Phys. 1995;31(3):467.
20 Weber DC, Trofimov AV, Delaney TF, Bortfeld T. A treatment planning comparison of intensity modulated photon and proton therapy for paraspinal sarcomas. Int J Radiat Oncol Biol Phys. 2004;58(5):1596-1606.
21 DeLaney TF, Liebsch NJ, Pedlow FX, et al. Phase II study of high dose photon/proton radiotherapy in the management of spine sarcomas. Int J Radiat Oncol Biol Phys. 2009;74(3):732-739.
22 Fuss M, Hug EB, Schaefer RA, et al. Proton radiation therapy (PRT) for pediatric optic pathway gliomas. Comparison with 3D planned conventional photons and a standard photon technique. Int J Radiat Oncol Biol Phys. 1999;45(5):1117-1126.
23 Habrand JL, Haie-Meder C, Rey A, et al. [Radiotherapy using a combination of photons and protons for locally aggressive intracranial tumors. Preliminary results of protocol CPO 94-C1]. Cancer Radiother. 1999;3(6):480-488.
24 Fitzek MM, Thornton AF, Rabinov JD, et al. Accelerated fractionated proton/photon irradiation to 90 cobalt gray equivalent for glioblastoma multiforme. Results of a phase II prospective trial. J Neurosurg. 1999;91(2):251-260.
25 Wenkel E, Thornton AF, Finkelstein D, et al. Benign meningioma: partially resected, biopsied, and recurrent intracranial tumors treated with combined proton and photon radiotherapy. Int J Radiat Oncol Biol Phys. 2000;48(5):1363-1370.
26 Weber DC, Lomax AJ, Rutz HP. Spot-scanning proton radiation therapy for recurrent, residual or untreated intracranial meningiomas. Radiother Oncol. 2004;71(3):251-258.
27 Mock U, Georg D, Bogner J, et al. Treatment planning comparison of conventional, 3D conformal, and intensity-modulated photon (IMRT) and proton therapy for paranasal sinus carcinoma. Int J Radiat Oncol Biol Phys. 2004;58(1):147-154.
28 Thornton AF, Fitzek MM, Varvarres M, et al. Accelerated, hyperfractionated photon/proton irradiation for advanced paranasal sinus cancer. Results of a prospective phase I-II study. Int J Radiat Oncol Biol Phys. 1998;42:222.
29 Truong MT, Kamat UR, Liebsch NJ, et al. Proton radiation therapy for primary sphenoid sinus malignancies. Treatment outcome and prognostic factors. Head Neck. 2009;31:1297-1308.
30 Fitzek MM, Thornton AF, Varvares M, et al. Neuroendocrine tumors of the sinonasal tract. Results of a prospective study incorporating chemotherapy, surgery, and combined proton-photon radiotherapy. Cancer. 2002;94(10):2623-2634.
31 Lin R, Slater JD, Yonemoto LT, et al. Nasopharyngeal carcinoma. Repeat treatment with conformal proton therapy—dose-volume histogram analysis. Radiology. 1999;213(2):489-494.
32 Bush DA, McAllister CJ, Loredo LN, et al. Fractionated proton beam radiotherapy for acoustic neuroma. Neurosurgery. 2002;50(2):270-273.
33 Shipley WU, Verhey LJ, Muzenrider JE, et al. Advanced prostate cancer. Results of a randomized comparative trial of high dose irradiation boosting with conformal protons compared with conventional dose irradiation using protons alone. Int J Radiat Oncol Biol Phys. 1995;32:3-12.
34 Slater JD, Rossi CJJr, Yonemoto LT, et al. Proton therapy for prostate cancer. The initial Loma Linda University experience. Int J Radiat Oncol Biol Phys. 2004;59(2):348-352.
35 Zietman AL, Bae K, Slater JD, et al. Randomized trial comparing conventional-dose with high-dose conformal radiation therapy in early-stage adenocarcinoma of the prostate. Long-term results from Proton Radiation Oncology Group/American College of Radiology 95-09. J Clin Oncol. 2010;28:1106-1111.
36 Chera BS, Vargas C, Morris CG, et al. Dosimetric study of pelvic proton radiotherapy for high-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2009;75:994-1002.
37 Matsuzaki Y, Osuga T, Chiba T, et al. New, effective treatment using proton irradiation for unresectable hepatocellular carcinoma. Intern Med. 1995;34:302-307.
38 Hsiung-Stripp DC, McDonough J, Masters HM, et al. Comparative treatment planning between proton and X-ray therapy in pancreatic cancer. Med Dosim. 2001;26(3):255-259.
39 Kozak KR, Kachnic LA, Adams J, et al. Dosimetric feasibility of hypofractionated proton radiotherapy for neoadjuvant pancreatic cancer treatment. Int J Radiat Oncol Biol Phys. 2007;68:1557-1566.
40 Bush DA, Slater JD, Bonnet R, et al. Proton-beam radiotherapy for early-stage lung cancer. Chest. 1999;116(5):1313-1319.
41 Nakayama H, Sugahara S, Tokita M, et al. Proton beam therapy for patients with medically inoperable stage I non-small-cell lung cancer at the University of Tsukuba. Int J Radiat Oncol Biol Phys. 2010;78(2):467-471.
42 Chang JY, Zhang X, Wang X, et al. Significant reduction of normal tissue dose by proton radiotherapy compared with three-dimensional conformal or intensity-modulated radiation therapy in stage I or stage III non-small-cell lung cancer. Int J Radiation Oncology Biol Phys. 2006;65:1087-1096.
43 Miralbell R, Lomax A, Cella L, Schneider U. Potential reduction of the incidence of radiation-induced second cancers by using proton beams in the treatment of pediatric tumors. Int J Radiat Oncol Biol Phys. 2002;54(3):824-829.
44 St Clair WH, Adams JA, Bues M, et al. Advantage of protons compared to conventional X-ray or IMRT in the treatment of a pediatric patient with medulloblastoma. Int J Radiat Oncol Biol Phys. 2004;58(3):727-734.
45 Merchant TE, Hua CH, Shukla H, et al. Proton versus photon radiotherapy for common pediatric brain tumors. Comparison of models of dose characteristics and their relationship to cognitive function. Pediatr Blood Cancer. 2008;51:110-117.
46 Hug EB, Muenter MW, Archambeau JO, et al. Conformal proton radiation therapy for pediatric low-grade astrocytomas. Strahlenther Onkol. 2002;178(1):10-17.
47 Hug EB, Muenter MW, Adams JA, et al. 3-D-conformal radiation therapy for pediatric giant cell tumors of the skull base. Strahlenther Onkol. 2002;178(5):239-244.
48 Hug EB, Nevinny-Stickel M, Fuss M, et al. Conformal proton radiation treatment for retroperitoneal neuroblastoma. Introduction of a novel technique. Med Pediatr Oncol. 2001;37(1):36-41.
49 Hug EB, Adams J, Fitzek M, et al. Fractionated, three-dimensional, planning-assisted proton-radiation therapy for orbital rhabdomyosarcoma. A novel technique. Int J Radiat Oncol Biol Phys. 2000;47(4):979-984.
50 MacDonald SM, Safai S, Trofimov A, et al. Proton radiotherapy for childhood ependymoma. Initial clinical outcomes and dose comparisons. Int J Radiat Oncol Biol Phys. 2008;71(4):979-986.
51 Fogliata A, Bolsi A, Cozzi L. Critical appraisal of treatment techniques based on conventional photon beams, intensity modulated photon beams and proton beams for therapy of intact breast. Radiother Oncol. 2002;62(2):137-145.
52 Hall EJ. Intensity-modulated radiation therapy, protons, and the risk of second cancers. Int J Radiat Oncol Biol Phys. 2006;65(1):1-7.
53 Paganetti H, Bortfeld T, Delaney TF. Neutron dose in proton radiation therapy. In regard to Eric J. Hall (Int J Radiat Oncol Biol Phys 65:1-7, 2006). Int J Radiat Oncol Biol Phys. 2006;66(5):1594-1595.
54 Chung CS, Keating N, Yock T, Tarbell N. Comparative analysis of second malignancy risk in patients treated with proton therapy versus conventional photon therapy. Int J Radiat Oncol Biol Phys. 2008;72(1):S8.
55 Kanai T, Kawachi K, Kumamoto Y, et al. Spot scanning system for proton radiotherapy. Med Phys. 1980;7(4):365-369.
56 Blattmann H, Coray A, Pedroni E, Greiner R. Spot scanning for 250 MeV protons. Strahlenther Onkol. 1990;166(1):45-48.
57 Goitein M, Jermann M. The relative costs of proton and X-ray radiation therapy. Clin Oncol (R Coll Radiol). 2003;15(1):S37-S50.
58 Lundkvist J, Ekman M, Ericsson SR, et al. Cost-effectiveness of proton radiation in the treatment of childhood medulloblastoma. Cancer. 2005;103(4):793-801.
59 Lundkvist J, Ekman M, Ericsson SR, et al. Economic evaluation of proton radiation therapy in the treatment of breast cancer. Radiother Oncol. 2005;75(2):179-185.
60 Konski A, Speier W, Hanlon A, et al. Is proton beam therapy cost effective in the treatment of adenocarcinoma of the prostate? J Clin Oncol. 2007;25(24):3603-3608.
61 Schulz-Ertner D, Tsujii H. Particle radiation therapy using proton and heavier ion beams. J Clin Oncol. 2007;25(8):953-964.
62 Kamada T, Tsujii H, Tsuji H, et al. Efficacy and safety of carbon ion radiotherapy in bone and soft tissue sarcomas. J Clin Oncol. 2002;20(22):4466-4471.
63 Schulz-Ertner D, Karger CP, Feuerhake A, et al. Effectiveness of carbon ion radiotherapy in the treatment of skull-base chordomas. Int J Radiat Oncol Biol Phys. 2007;68(2):449-457.
64 Kato H, Tsujii H, Miyamoto T. Results of the first prospective study of carbon ion radiotherapy for hepatocellular carcinoma with liver cirrhosis. Int J Radiat Oncol Biol Phys. 2004;59(5):1468-1476.
65 Castro JR, Phillips TL, Prados M, et al. Neon heavy charged particle radiotherapy of glioblastoma of the brain. Int J Radiat Oncol Biol Phys. 1997;38(2):257-261.
66 Thornton AF, Laramore GE. Particle radiation therapy. In: Gunderson LL, Tepper JE, editors. Clinical radiation oncology. Philadelphia: Churchill Livingstone; 2000:225-234.
67 von Essen CF, Bagshaw MA, Bush SE, et al. Long-term results of pion therapy at Los Alamos. Int J Radiat Oncol Biol Phys. 1987;13(9):1389-1398.
68 Pickles T, Goodman GB, Rheaume DE, et al. Pion radiation for high grade astrocytoma. Results of a randomized study. Int J Radiat Oncol Biol Phys. 1997;37(3):491-497.
69 Char DH, Kroll SM, Castro J. Ten-year follow-up of helium ion therapy for uveal melanoma. Am J Ophthalmol. 1998;125(1):81-89.
70 Char DH, Quivey JM, Castro JR, et al. Helium ions versus iodine 125 brachytherapy in the management of uveal melanoma. A prospective, randomized, dynamically balanced trial. Ophthalmology. 1993;100:1547.
71 Schoenthalar R, Castro JR, Petti PL. Charged particle irradiation of sacral cordomas. Int J Radiat Oncol Biol Phys. 1993;26:291-298.
72 Miralbell R, Lomax A, Cella L, Schneider U. Potential reduction of the incidence of radiation-induced second cancers by using proton beams in the treatment of pediatric tumors. Int J Radiat Oncol Biol Phys. 2002;54(3):824-829.