Chapter 200 Cerebrospinal Fluid Fistula and Pseudomeningocele after Spine Surgery
Cerebrospinal fluid (CSF) fistulas and pseudomeningoceles are relatively rare complications of spine surgery.1 Although durotomies encountered during spinal surgery are not uncommon, most heal uneventfully after primary suture closure. If a watertight dural closure is not possible, CSF may drain through the surgical tract to form a cutaneous CSF fistula. The presence of CSF leakage requires that immediate measures be taken to stop the leak due to the potential for infection.2–5 CSF leakage contributes to increased perioperative morbidity, prolonged hospitalization, and increased cost of care. These factors are further compounded when additional surgery is needed to manage the leakage.
CSF leakage that occurs after satisfactory wound healing can lead to the development of a pseudomeningocele in the paraspinal tissues. This typically develops slowly into an encapsulated CSF-filled mass that may be confined to the subfascial region or, with greater pressure, may extend through the fascia into the paraspinal tissues. As they enlarge, pseudomeningoceles may contribute to chronic back pain, persistent headache, and, less commonly, nerve root entrapment.6–9
Incidence
The incidence of CSF fistula is relatively rare because most dural tears heal spontaneously and only a small percentage of patients develop symptoms. In a study of 3038 spinal surgeries, the incidence of dural tears during the course of bone removal or during dural sac or root retraction was noted to be 5.9%.10 In Mayfield’s review of 1408 laminectomies, the incidence of CSF fistula requiring reoperation was 0.3% and the incidence of pseudomeningocele was 0.8%.1
The incidence of pseudomeningoceles is difficult to determine because most cases are asymptomatic. Swanson and Fincher reported a 0.068% incidence of pseudomeningocele in a review of 1700 exploratory laminectomies.9 Schumacher et al. reported the incidence of pseudomeningoceles to be less than 0.1% in 3000 patients who had undergone a lumbar discectomy.11 Teplick et al.12 reported a 2% incidence of pseudomeningocele in a series of 400 symptomatic postlaminectomy patients examined with CT. None of these patients required reoperation.
The relatively low incidence of CSF wound complications in these series may be because a majority of the patients underwent uncomplicated laminectomy for discectomy.1,9–12 The incidence is much higher and has not been well reported in patients who have undergone laminectomy for spinal dysraphism or in patients with a history of prior spinal irradiation or surgery. Zide et al. reported that 43% of patients with intramedullary spinal cord neoplasms previously treated with radiation developed a CSF fistula or pseudomeningocele after surgery.13 They also found a high incidence of pseudomeningocele (43%) and CSF fistula (13%) in patients after surgical correction of the tethered spinal cord.14
Shapiro and Scully reviewed 39 patients with CSF fistula after spinal surgery.5 Sixteen leaks occurred after intradural procedures, despite a primary closure or dural patch graft. Of the remaining 23 cases, 19 occurred in lumbar surgeries. In 6 out of 19 lumbar cases (33%), a dural tear was identified and repaired at the time of surgery. In 13 of the lumbar cases (66%), no tear or leak was identified at the time of surgery. A myelogram was performed the day before surgery in 5 out of these 13 cases (38%). Three cases occurred after cervical spine surgery.
Pathophysiology
A dural tear, either occult or recognized, is the initial event that leads to postoperative CSF cutaneous fistula and pseudomeningocele.15 Tears may result from excessive traction or inadvertent disruption during surgical decompression. A myelography needle puncture performed shortly before lumbar surgery can also be the cause of postoperative CSF leaks.5 Durotomies that are recognized and repaired may still result in postoperative CSF leakage due to inadequate closure, particularly of tears that are difficult to access (i.e., ventral and lateral dura). Resection of dural-based tumors may create dural defects that are impossible to close in a watertight manner.
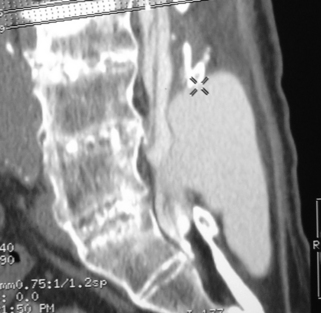
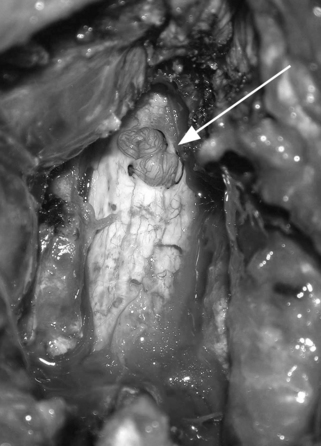
Pseudomeningoceles are caused either by herniation of the arachnoid through a dural tear, which subsequently forms an arachnoid-lined sac filled with CSF,8 or by direct extravasation of CSF into the soft tissues, with eventual development of a fibrous capsule.8,12 CSF pulsations force the fluid into the muscular and superficial subcutaneous tissues (Fig. 200-1). The size, shape, and location of the sac depend on the nature of the soft tissue into which the fluid is forced. In rare cases the capsule may ossify.16 Entrapment of nerve roots in the pseudomeningocele may be a barrier to dural healing6,7 (Fig. 200-2).
The majority of dural tears heal uneventfully after primary repair. Although the most likely cause of a CSF fistula or pseudomeningocele is an inadequate repair at the time of the durotomy, other factors may contribute to persistent CSF leakage. These include factors that delay or prevent healing of the dura mater and overlying soft tissue.15 Dural and wound healing may be compromised by scar tissue, irradiation, localized infection, or foreign body reaction. Systemic factors that impair healing include nutritional deficits, endocrine disorders (e.g., diabetes), chronic disease, and steroid administration.
CSF leakage through a repaired dural tear may be exacerbated by elevated CSF pressure. Cutaneous CSF fistula after myelomeningocele repair is often caused by hydrocephalus (CSF pressure 350–450 mm H2O) and is typically treated with ventricular shunting to correct the abnormal CSF dynamics. Excessive straining can transiently elevate CSF pressure (to >400 mm H2O) and should be avoided in the perioperative period.17 Lumbar intradural pressure is markedly elevated with an erect posture (350–450 mm H2O), compared with supine recordings (70–170 mm H2O).18,19 For this reason, patients at risk for CSF leakage are kept flat in bed in the immediate postoperative period. Less commonly, CSF leakage following a durotomy can be exacerbated by the lowering of paraspinal tissue pressure created by the placement of drains under suction adjacent to the dura.
Diagnosis
The diagnosis of a cutaneous CSF fistula is most often established by inspection of the patient’s wound. A watery discharge is assumed to be CSF, particularly if leakage is augmented by upright posture or Valsalva maneuver or is associated with postural headaches. When headaches occur, they are typically more severe with an erect posture and are relieved in a recumbent position. Headaches are secondary to the reduction of the CSF volume when CSF loss through the fistula exceeds its production. The lowered intracranial pressure induces traction on pain-sensitive structures, such as meninges and blood vessels. In the recumbent position, traction is reduced and the pain is relieved.20 Fever or evidence of meningismus suggests bacterial meningitis. When leakage is profuse and clear at the incision site, the diagnosis is unmistakable. Small and intermittent leaks may be overlooked or misinterpreted, especially if they are mixed with blood. If the leaking fluid produces a clear halo that surrounds a central pink stain on an absorbent surface (e.g., sheets or cotton gauze), the fluid is most likely CSF.
Laboratory analysis of the fluid may be helpful in making a diagnosis. Although determining the glucose content of the fluid has been described as a potentially helpful diagnostic test, it is not consistently reliable in being able to identify CSF.21 A more specific test is immunofixation of β2-transferrin.22 A high proportion of transferrin in CSF exists as a carbohydrate-free isoform (β2-transferrin) that is not present in sweat or serous fluid. Detection of β2-transferrin in such fluids is indicative of CSF leakage. Only a small sample (<1 mL) is required, and no special handling or refrigeration is required.
Pseudomeningoceles may present clinically with localized back pain and postural headaches. Localized nerve root entrapment or adhesions of roots to the dural edges of the pseudomeningocele can produce radicular symptoms.6 Symptoms may occur several weeks to months after surgery. The clinical syndrome in the lumbar region may mimic the symptoms of lumbar disc herniation. Cervical and thoracic pseudomeningoceles may be palpable as boggy masses. Lumbar pseudomeningoceles are usually not palpable on physical examination, but occasionally the collections track into the subcutaneous tissues, producing a noticeable swelling of the wound site.
Imaging Studies
MRI and CT will adequately localize the CSF fistula tract or pseudomeningocele.7 MRI is the study of choice because of its superior imaging of soft tissue compared with CT. To best define the fistula tract for operative planning, iopamidol is injected into the subarachnoid space, followed by CT scanning. Suspected pleural CSF fistula23–25 or slow and intermittent leaks, such as those occurring after a lumbar puncture, are often best evaluated with radionuclide myelography.23,26
Conservative Management
Cerebrospinal Fluid Fistula
Several options are available for the initial treatment of postoperative CSF fistula. Successful treatment has been reported with simple measures, including bedrest, oversewing of the wound,19 closed subarachnoid drainage,2–527 and percutaneous injection of an epidural blood patch.28 Others have recommended reoperation for repair of the dura mater as an initial treatment.21
A trial of CSF diversion through a subarachnoid drain can predictably stop most CSF cutaneous fistulas that occur through small durotomies. After percutaneous insertion of the subarachnoid drain, CSF is drained (120–360 mL/day) for 3 to 5 days. Spinal headaches occur in approximately 60% of patients,1,3,5 and treatment consists of IV hydration, adjustment of the rate of drainage, and medication, particularly caffeine. The risk of infection is approximately 10% and includes meningitis (2.5%), discitis (5%), and wound infection (2.5%).5 Transient lumbar nerve root irritation occurs in 24% of patients and resolves after drain removal. Catheter blockage has been reported in up to 10% of patients,2 but this is less common with the relatively recent development of Teflon or silicone catheters.5 CSF diversion is successful at alleviating the cutaneous fistula in 90% to 100% of cases.3–5
A percutaneous blood patch, commonly used for headaches following lumbar punctures,29 has also been reported to be effective in treating postoperative CSF fistula.28 Advocates for the procedure cite a theoretically smaller risk of infection and earlier mobilization compared with a trial of CSF diversion. Approximately 10 to 25 mL of fresh autologous blood is injected into the epidural space near the dural puncture or laminectomy site.28 Injected blood stops the leak by forming an occlusive clot over the dural breach and increasing extradural tissue pressure.
The use of prophylactic antibiotics for patients with cutaneous fistula is controversial. Most of the literature reviewing the use of prophylactic antibiotics for CSF fistula pertains to rhinorrhea and otorrhea. The evidence does not appear to favor their administration.30 If antibiotics are administered, a broad-spectrum antibiotic is recommended.31
Pseudomeningocele
Symptomatic pseudomeningoceles may be observed for progression or, in many cases, spontaneous resolution of symptoms. Those that are associated with subcutaneous swelling at the wound site may be managed with a compressive dressing, liposuction garments, or abdominal binders. Children and infants can be fitted for Jobst garments.13,14,32 Serial aspirations of the pseudomeningocele may also be helpful, although this may increase the potential for infection. A 3- to 5-day period of subarachnoid drainage is another reasonable treatment option to consider.
Surgical Management
Indications
Some patients may have impaired CSF absorption with elevated intradural pressures that will compromise any attempts at direct surgical repair of a durotomy. These patients may be considered for a ventricular shunt before any attempted surgical repair. Shunts have also been successfully used to treat leaks in which wound exploration and dural repair may be complicated (e.g., pleural fistulas)24 and fistulas at the occipitocervical junction.27 Postlaminectomy cutaneous fistulas have also been successfully managed with the insertion of a lumboperitoneal shunt. This option has been promoted as the initial management option in preference to a trial of temporary CSF diversion.33 The rationale is that lumboperitoneal shunting minimizes the risk of infection and does not require prolonged bedrest.
Surgical Principles
In most cases of persistent CSF leakage, a successful outcome can be achieved by accurately identifying the dural opening, obtaining a satisfactory watertight closure, and performing a sufficient reapproximation of the three layers of the surgical wound. In some complex cases, this approach may not be adequate. Novel wound closure techniques have been developed from experience with wound complications after resection of intramedullary spinal cord neoplasms in patients with a history of multiple surgeries and postoperative radiation and in children following tethered cord surgery.13,14 In some complex cases, plastic surgery consultation for the use of myofascial flaps and other innovative wound closure techniques may be necessary to successfully obliterate the leakage of CSF.13,14,32
Dural Closure
In addition to primary suture repair and tissue patch application, the use of sealant materials for dural repair has been investigated. Jankowitz et al. evaluated the use of fibrin glue in the repair of incidental durotomies following lumbar laminectomies.34 Fibrin glue was not found to have any benefit compared to primary closure alone in preventing percutaneous CSF leaks. However, Shaffrey et al. noted a 90% success rate with the use of fibrin glue as an adjunct to dural closure.35
Polyethylene glycol hydrogel (PEG) glue (DuraSeal Spine Sealant, Covidien, Bedford, MA) is a synthetic, biocompatible material. Unlike fibrin glue, it is nonbiologic, eliminating any risk of disease transmission. Flexible, absorbable, and adherent to tissue, it has been approved by the U.S. Food and Drug Administration for use as a dural sealant. It is sprayed directly onto the primary suture line and surrounding dura after satisfactory suture closure. Due to a potential for swelling of the hydrogel material, it should be used on dura that is exposed in the surgical field rather than on dura in confined areas (i.e., intraforaminal).36
Than et al. compared PEG glue to fibrin glue for dural repair following posterior fossa surgery. A statistically significant reduction in CSF leaks was found when PEG glue was used compared to fibrin glue.37 A prospective multicenter study found that PEG glue had a higher success rate of watertight closure at the time of initial dural repair when compared with standard treatment (more sutures, fibrin glue). However, no statistically significant reduction in postoperative CSF leaks was observed.38
The use of collagen matrix overlays (DuraGen Dural Graft matrix, Integra LifeSciences, Plainsboro, NJ) to facilitate dural repair has also been investigated. This bovine-derived material is biodegradable and serves as a scaffold for the regrowth of natural tissue. It is typically placed over the primary suture line without the need to suture it into place. The material is safe and effective and has been demonstrated to be a satisfactory adjunct for dural repair.5,39
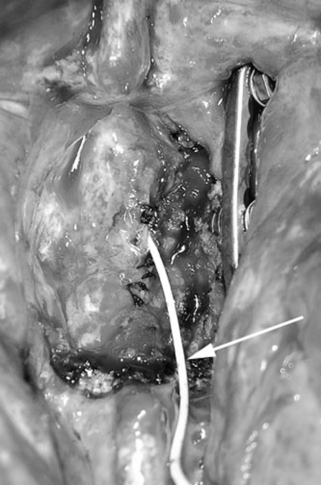
When CSF leakage persists despite all of the primary closure and dural sealant measures attempted, a subarachnoid catheter can be placed intraoperatively through the partially closed durotomy and brought out through a separate stab incision (Fig. 200-3). After catheter placement, dural sealant is placed over the dura around the catheter. The wound is closed and CSF is drained for several days. When sufficient wound healing has occurred, the drain can be removed. The dural sealant placed around the catheter’s site of dural entry helps prevent any further leakage. If CSF continues to leak through the drainage tract, it can be managed with one or two sutures to close the tract.
Wound Closure
Once a watertight dural closure or pseudomeningocele repair has been achieved, wound closure is performed using a standard multilayered technique. Nonabsorbable sutures are used to obtain a tight fascial closure. The fascia may be reinforced with a second embricated running suture closure over the first suture line. In cases of irradiated or scarred tissue it may be necessary to adequately mobilize the fascia and paraspinal muscles before attempted closure. If sufficient fascial closure cannot be achieved, it may be necessary to obtain plastic surgery assistance to create a myofascial flap for adequate coverage. The subcutaneous fat and dermis are then closed in single or multiple layers depending on the depth of the wound. The skin is then closed with sutures as opposed to staples, using either an interrupted mattress or running technique.
The use of tissue expanders to create adequate soft tissue coverage has been described as another alternative for assistance with complex wound closures.40 Deflated silicone elastomer (Silastic) tissue expander reservoirs are inserted into the paraspinal region through a small incision several weeks before the anticipated spinal surgery. Periodic expansion is performed by injections of sterile saline solution into the expander reservoir. The expanders, removed at the time of definitive spine surgery, provide sufficient midline soft tissue coverage without undue tension.
The presence of CSF in the drainage fluid does not necessarily call for immediate drain removal.41 In this setting, CSF diversion from the wound provides more time for sufficient wound healing. Once the drain is removed, the drainage tract typically collapses. Any persistent CSF drainage through the tract can be managed by placement of a single suture as opposed to the multiple sutures frequently necessary to manage CSF leakage through a surgical wound.
Hadani M., Findler G., Knoler N., et al. Entrapped lumbar nerve root in pseudomeningocele after laminectomy: report of three cases. Neurosurgery. 1986;19:405-407.
Kim K.D., Wright N.M. Polyethylene glycol hydrogel spinal sealant (Durseal Spinal Sealant) as an adjunct to sutured dural repair in the spine: results of a prospective, multicenter, randomized control study. Spine (Phila Pa 1976). 2011;36(23):1906-1912.
Kitchel S., Eismont F., Green B. Closed subarachnoid drainage for management of cerebrospinal fluid leakage after an operation on the spine. J Bone Joint Surg [Am]. 1989;71:984-987.
McCallum J., Maroon J., Jannetta P. Treatment of postoperative cerebrospinal fluid fistulas by subarachnoid drainage. J Neurosurg. 1975;42:434-437.
Schumacher H.-W., Wassman H., Podlinski C. Pseudomeningocele of the lumbar spine. Surg Neurol. 1988;29:77-78.
Waisman M., Schweppe Y. Postoperative cerebrospinal fluid leakage after lumbar spine operations: conservative treatment. Spine (Phila Pa 1976). 1991;16:52-53.
1. Mayfield F.H. Complications of laminectomy. Clin Neurosurg. 1975;42:434-439.
2. Findler G., Sahar A., Beller A. Continuous lumbar drainage of cerebrospinal fluid in neurosurgical patients. Surg Neurol. 1977;8:455-457.
3. Kitchel S., Eismont F., Green B. Closed subarachnoid drainage for management of cerebrospinal fluid leakage after an operation on the spine. J Bone Joint Surg [Am]. 1989;71:984-987.
4. McCallum J., Maroon J., Jannetta P. Treatment of postoperative cerebrospinal fluid fistulas by subarachnoid drainage. J Neurosurg. 1975;42:434-437.
5. Shapiro S.A., Scully T. Closed continuous drainage of cerebrospinal fluid via a lumbar subarachnoid catheter for treatment or prevention of cranial/spinal cerebrospinal fluid fistula. Neurosurgery. 1992;30:241-245.
6. Hadani M., Findler G., Knoler N., et al. Entrapped lumbar nerve root in pseudomeningocele after laminectomy: report of three cases. Neurosurgery. 1986;19:405-407.
7. Lee K.S., Hardy I. Postlaminectomy lumbar pseudomeningocele: report of four cases. Neurosurgery. 1992;30:111-114.
8. Miller P.R., Elder F.W. Meningeal pseudocysts (meningocele spurious) following laminectomy. Report of ten cases. J Bone Joint Surg [Am]. 1968;50:268-276.
9. Swanson H.S., Fincher E.F. Extradural arachnoidal cysts of traumatic origin. J Neurosurg. 1947;4:530-538.
10. Oppel F., Schramm J., Schirmer M., et al. Results and complicated course after surgery for lumbar disc herniation. Adv Neurosurg. 1977;4:36-46.
11. Schumacher H-W., Wassman H., Podlinski C. Pseudomeningocele of the lumbar spine. Surg Neurol. 1988;29:77-78.
12. Teplick J.G., Peyster R.G., Teplick S.K., et al. CT identification of postlaminectomy pseudomeningocele. Am J Roentgenol. 1983;4:179-182.
13. Zide B.M., Wisoff J.H., Epstein F.J. Closure of extensive and complicated laminectomy wounds. J Neurosurg. 1987;67:59-64.
14. Zide B.M., Epstein F.J., Wisoff J. Optimal wound closure after tethered cord corrections. J Neurosurg. 1991;74:673-676.
15. Nash C.L., Kaufman B., Frankel V.H. Postsurgical meningeal pseudocysts of the lumbar spine. Clin Orthop Relat Res. 1971;75:167-177.
16. Tsuji H., Handa N., Handa O., et al. Postlaminectomy ossified extradural pseudocysts. J Neurosurg. 1990;73:785-787.
17. Sakikawa Y., Kobayashi H., Nomura Y. Changes in cerebrospinal fluid pressure in daily life. Ann Otol Rhinol Laryngol. 1994;103:959-963.
18. Von Storch T.J.C., Carmichael E.A., Banks T.E. Factors producing lumbar cerebrospinal fluid pressure in man in the erect posture. Arch Neurol Psychiatry. 1937;38:1158.
19. Waisman M., Schweppe Y. Postoperative cerebrospinal fluid leakage after lumbar spine operations: conservative treatment. Spine (Phila Pa 1976). 1991;16:52-53.
20. Gass H., Goldstein A.S., Ruskin R., Leopold N.A. Chronic postmyelogram headache. Arch Neurol. 1971;25:168.
21. Eismont F.J., Wiesel S.W., Rothman R.H. Treatment of dural tears associated with spinal surgery. J Bone Joint Surg [Am]. 1981;63:1132-1136.
22. Ryall R.G., Peacock M.K., Simpson D.A. Usefulness of β2-transferrin assay in the detection of cerebrospinal fluid leaks following head injury. J Neurosurg. 1992;77:737-739.
23. Hofstetter K.R., Bjelland J.C., Patton D.D., et al. Detection of bronchopleural-subarachnoid fistula by radionuclide myelography, case report. J Nucl Med. 1977;18:981.
24. Katz S.S., Savitz M.H., Osei C., Harris L. Successful treatment by lumboperitoneal shunting of a spinal subclavicular fistula following thoracotomy. Neurosurgery. 1982;11:795-796.
25. Larson A.M., Graham M.M. Demonstration of pleural cerebrospinal fluid leak by In-111 DTPA radionuclide myelography. Clin Nucl Med. 1992;17:754-755.
26. Primeau M., Carrier L., Milette P.C., et al. Spinal cerebrospinal fluid leak demonstrated by radioisotopic cisternography. Clin Nucl Med. 1988;13:701-703.
27. Chumas P.D., Kulkarni A.V., Drake J.M., et al. Lumboperitoneal shunting: a retrospective study in the pediatric population. Neurosurgery. 1993;32:376-382.
28. Maycock N.F., Van Essen J., Pfitzner J. Post-laminectomy cerebrospinal fluid fistula treated with epidural blood patch. Spine (Phila Pa 1976). 1994;19:2223-2225.
29. Gormley J.B. Treatment of postspinal headache. Anesthesiology. 1960;21:565-566.
30. Eljamel M.S. Antibiotic prophylaxis in unrepaired CSF fistulae. Br J Neurosurg. 1993;7:501-505.
31. Price D.J., Sleigh J.D. Control of infection due to Klebsiella aerogenes in a neurosurgical unit by withdrawal of all antibiotics. Lancet. 1970;2:1213-1215.
32. Zide B.M. How to reduce the morbidity of wound closure following extensive and complicated laminectomy and tethered cord surgery. Pediatr Neurosurg. 1992;18:157-166.
33. Aoki N. Correspondence: closed continuous drainage of cerebrospinal fluid via lumbar subarachnoid catheter for treatment or prevention of cranial/spinal cerebrospinal fluid fistula. Neurosurgery. 1992;31:381.
34. Jankowitz B.T., Atteberry D.S., Gerszten P.C., et al. Effect of fibrin glue on the prevention of persistent cerebral spinal fluid leakage after incidental durotomy during lumbar spinal surgery. Eur Spine J. 2009;18:1169-1174.
35. Shaffrey C.I., Spotnitz W.D., Shaffrey M.E., Jane J.A. Neurosurgical applications of fibrin glue: augmentation of dural closure in 134 patients. Neurosurgery. 1990;26:207-210.
36. Epstein N.M. Dural repair with four spinal sealants: focused review of the manufacturer’s inserts and the current literature. Spine J. 2010;10:1065-1068.
37. Than K.D., Baird C.J., Olivi A. Polyethylene glycol hydrogel dural sealant may reduce incisional cerebrospinal fluid leak after posterior fossa surgery. Neurosurgery. 2008;63(1 Suppl 1):ONS182-186.
38. Kim K.D., Wright N.M. Polyethylene glycol hydrogel spinal sealant (Durseal Spinal Sealant) as an adjunct to sutured dural repair in the spine: results of a prospective, multicenter, randomized control study. Spine (Phila Pa 1976). 2011;36(23):1906-1912.
39. Narotam P.K., José S., Nathoo N., et al. Collagen matrix (DuraGen) in dural repair: analysis of a new modified technique. Spine (Phila Pa 1976). 2004;29:2861-2867.
40. Paonessa K.J., Zide B., Errico T., Engler G.L. Using tissue expanders in spinal surgery for deficient soft tissue or postirradiation cases. Spine (Phila Pa 1976). 1991;16(Suppl):S324-S327.
41. Hughes S.A., Ozgur B.M., German M., Taylor W.R. Prolonged Jackson-Pratt drainage in the management of lumbar cerebrospinal fluid leaks. Surg Neurol. 2006;65:410-415.