24 Cerebral Bypass in the Treatment of ACA Aneurysms
Introduction
Cerebral revascularization has had a tumultuous history with the nadir being just after the publication of the Extracranial/Intracranial Bypass Study Group (1985)1 Currently, cerebral revascularization has gained momentum for specific disease processes: basal occlusive disease (MMD), giant or fusiform aneurysms unamenable to clip ligation or endovascular technology, and symptomatic intracranial and/or extracranial atherosclerotic occlusive disease. In 1963, Woringer and Kunlin performed the first external to intracranial internal carotid artery bypass utilizing a saphenous vein graft.2 Yasargil is credited with advancement of cerebral revascularization techniques with the incorporation of microinstruments, bipolar technology, as well as the microscope.3 At present, conduits available for bypass include scalp branches such as the superficial temporal artery (STA) and occipital artery (OA), and interposition grafts such as reverse saphenous vein (SV) and radial artery (RA). More recently, intracranial vessels such as the anterior temporal artery, the posterior inferior cerebellar artery (PICA) or vessels in close proximity to each other such as the pericallosal (A2–4) segments of the anterior cerebral artery (ACA) have been used to allow for in situ bypass.
While controversy still exists regarding cerebral revascularization for occlusive disease—and indeed at the time of this writing the Carotid Occlusion Surgery Study (COSS) is still ongoing—its role in the treatment of giant/fusiform aneurysms is well documented.4,5 In some instances, it is simply impossible to exclude an aneurismal segment of vessel without sacrificing the parent vessel. In addition, dissection of a large aneurysm may require a prolonged period of temporary flow arrest, which would require a cerebral bypass for flow augmentation and prevention of ischemic complications. In such cases, cerebral bypass provides temporary or permanent flow arrest of the parent vessel harboring the aneurysm. Current bypass techniques utilize temporary occlusion of the recipient and donor vessels; however, the ELANA (excimer-laser assisted nonocclusive anastomosis) technique popularized in Europe will allow for cerebral bypass without temporary occlusion.6
Aneurysms in the anterior circulation unamenable for simple clip ligation or coiling procedures include giant aneurysms, fusiform aneurysms, and dissecting aneurysms. While clip reconstruction could be performed in some, the possibility of parent or branch vessel occlusion or injury and the time required for surgical dissection lend way to utilization of a cerebral bypass so that the aneurismal segment may be safely sacrificed. Coiling of giant aneurysms is generally not practical because (1) it is rarely a durable form of treatment, and (2) endovascular treatment of distal ACA segment aneurysms is not feasible at present if a stent is required, as stents in use today are not approved for vessels <2.5 mm. However, cerebral bypass may be utilized with coil embolization to deconstruct a vessel in order to minimize cerebral retraction.7
Methods
There are multiple options for bypasses within the anterior circulation, and these include both IC-IC and EC-IC bypasses. The workhorse bypasses include in situ pericallosal side-to-side grafts and interposition grafts using arterial or venous donor vessels as follows: graft to distal A1, A1 to contralateral A1, A1 to ipsilateral or contralateral A2, A2 to A2, or A3 to A3.8
An anastomosis performed on any portion of the ACA is difficult as there is great depth in addition to a small working area. Yokoh et al. performed autopsy based studies to better define bypass techniques within the anterior circulation.9 A1 mobility is approximately 3 mm unless the perforators are sacrificed purposefully to increase mobility. In comparison to the A1 segment, the A2 and A3 segments have greater mobility and their surgical exposure affords a wider access to these pericallosal vessels, thus rendering them somewhat less difficult for cerebral bypass. When bypassing in the region of the A2 and A3, the recurrent artery of Heubner (RAH), orbitofrontal artery (OfA), and frontopolar (FpA) arteries may need to be reimplanted utilizing an end-to-side technique. In their study, Yokoh et al. showed that each of the perforating vessels mentioned above measured 1 mm in diameter except the RAH, which averaged at 0.7 mm.
End-to-end anastomosis is possible between the A1, A2, or A3 segments if the cut surfaces are <5 mm apart.9 Distal ACA side-to-side anastomosis protects the pericallosal distribution from ischemic injury and can be performed with approximately 1 cm of width space.9,10 Grafts utilizing RA, STA, and or vein can be used to bypass to the distal ACA in order to prevent ischemia with parent vessel occlusion.
Anterior circulation bypass scenarios
Scenario I: Diseased A1 Segment
A giant fusiform aneurysm involving the A1 segment could be treated with exclusion of the diseased segment and bypass of the diseased A1. If angiography reveals that the contralateral A1 is dominant and supplies bilateral A2s, then clip ligation of the aneurismal A1 should be performed without any revascularization unless the RAH emanates from the distal A1.11 In this case, the RAH can be reimplanted onto proximal A2 in an end-to-side anastomosis. When the contralateral A1 is hypoplastic, further flow augmentation is needed within the A2 or A3. This can be achieved with an A3-A3 side-to-side anastomosis or a STA-RA graft to the ipsilateral A2.
Cerebral bypass technique
Both the recipient and donor vessels are highlighted with a marking pen in the region to be anastomosed to enhance visualization. End-to-end or end-to-side anastomoses are performed with 8-0 to 10-0 nylon suture (Deme Tech, Miami, FL) in an interrupted fashion, while side-to-side anastomosis are performed in a running fashion. When the anastomosis is nearly complete, we use the technique described by Dacey, which employs unclipping of a temporarily clipped side branch on the donor vessel to back-bleed and rid the anastomosis of any air or debris.12 Indocyanine green (ICG) angiography is then performed to evaluate the patency of the bypass. Tamponade with fibrillary collagen (avitene) rather than bipolar cautery should be used to obtain hemostasis of any slow oozing at the anastomosis. Rarely since the advent of ICG angiography do we perform intraoperative catheter cerebral angiography.
Illustrative case
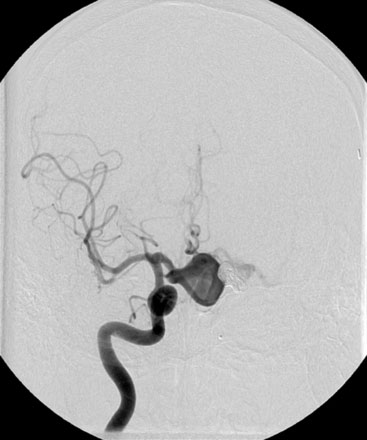
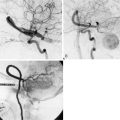
After a motor vehicle accident in 2004, the patient underwent evaluation with a computer tomographic angiography (CTA) of the head that revealed continued filling of the left ICA terminus aneurysm as well as development of a large anterior communicating (ACOM) aneurysm. These findings were corroborated by a diagnostic cerebral angiogram (Figure 24–1). A 10-month follow-up angiogram revealed enlargement of the ACOM aneurysm as well as persistence of the left ICA terminus aneurysm. The patient underwent coil embolization of the left ICA terminus aneurysm without any complications. Eight months later the ICA terminus aneurysm had significant recurrence as well as further enlargement of the ACOM aneurysm.
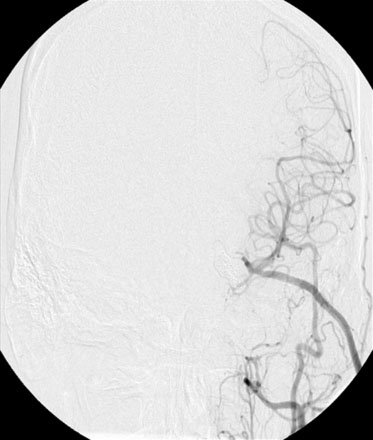
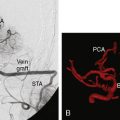
With the presence of an enlarging ACOM aneurysm and recurrent ICA terminus aneurysm, the decision was made to proceed with clip ligation and cerebral bypass. Since the left cerebral circulation was dependent on the ACOM complex, an ECA to M3 segment bypass was performed utilizing a radial artery graft (Figure 24–2). Twenty-four hours after the procedure, cerebral angiography revealed a thrombosed left ICA terminus aneurysm.
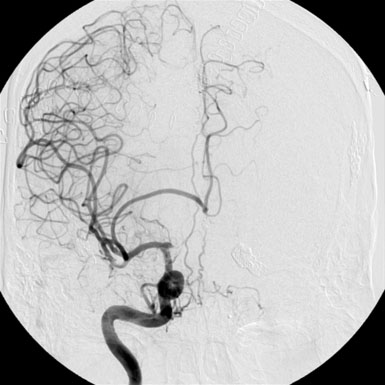
Since the ACOM aneurysm was a fusiform aneurysm, clip ligation of the neck was not possible; instead, the patient required cerebral bypass and trapping of the aneurysm. First an A3-A3 side-to-side anastomosis was performed and then a radial graft was utilized to perform a right-M1 to left-A3 bypass (Figure 24–3). The right A1 was clipped distally and the A2s were not clipped to allow backfilling of perforating vessels arising from the A2. Subsequent angiography revealed thrombosis of the left ICA terminus and ACOM aneurysms. The patient tolerated these three separate procedures and made a remarkable recovery, returning to his daily life.
1 The International Cooperative Study of Extracranial/Intracranial Arterial Anastomosis (EC/IC Bypass Study): methodology and entry characteristics. The EC/IC Bypass Study group. Stroke. 1985;16:397-406.
2 Woringer E., Kunlin J. [Anastomosis between the Common Carotid and the Intracranial Carotid or the Sylvian Artery by a Graft, Using the Suspended Suture Technique]. Neurochirurgie. 1963;200:181-188.
3 Hayden M.G., et al. The evolution of cerebral revascularization surgery. Neurosurg Focus. 2009;26:E17.
4 Cote R. [International cooperative study of extracranial artery bypass: development report]. Union Med Can. 1984;113:242-244.
5 Sanai N., Zador A., Lawton M.T. Bypass surgery for complex brain aneurysms: an assessment of intracranial-intracranial bypass. Neurosurgery. 2009;65:670-683. discussion 683
6 Streefkerk H.J., et al. The ELANA technique: constructing a high flow bypass using a non-occlusive anastomosis on the ICA and a conventional anastomosis on the SCA in the treatment of a fusiform giant basilar trunk aneurysm. Acta Neurochir (Wien). 2004;146:1009-1019. discussion 1019
7 Kim L.J., et al. Combined surgical and endovascular treatment of a recurrent A3-A3 junction aneurysm unsuitable for stand-alone clip ligation or coil occlusion. Technical note. Neurosurg Focus. 2005;18:E6.
8 Kashimura H., et al. Trapping and vascular reconstruction for ruptured fusiform aneurysm in the proximal A1 segment of the anterior cerebral artery. Neurol Med Chir (Tokyo). 2006;46:340-343.
9 Yokoh A., et al. Anterior cerebral artery reconstruction. Neurosurgery. 1986;19:26-35.
10 Kim K., et al. Giant intracranial aneurysm of the anterior communicating artery treated by direct surgery using A3-A3 side-to-side anastomosis and A3-RA graft-STA anastomosis. Acta Neurochir (Wien). 2006;148:353-357. discussion 357
11 Lownie S.P., et al. Clinical presentation and management of giant anterior communicating artery region aneurysms. J Neurosurg. 2000;92:267-277.
12 Dacey R.G., et al. Use of a side branch in a saphenous vein interposition graft for high-flow extracranial-intracranial bypass procedure. J Neurosurg. 2005;103:186-187.