Chapter 14 Cerebellar Tumors in Adults
Many lesions affect the posterior fossa, and the cerebellum in particular. Some tumors, such as brain stem gliomas, cerebellar pontine angle tumors, fourth ventricle tumors, and pineal area tumors extend into the cerebellum from surrounding areas. Metastatic lesions to the cerebellum are also common. However, tumors intrinsic to the cerebellum are less frequent. Primary cerebellar tumors represent only 3.5% of all primary brain and central nervous system (CNS) tumors.1 In children, infratentorial lesions are more prevalent, comprising 16.6% of CNS tumors, while only 6% of primary CNS tumors are found in the cerebellum of adults.1 In this chapter, we will focus only on intrinsic cerebellar tumors.
Clinical Presentation, Diagnosis, and Preoperative Management
Patients with a cerebellar lesion often present with a headache and signs of cerebellar dysfunction. Clinical signs of these deficits are detected by the presence of dysmetria in finger-to-nose or heel-to-shin testing, and dysdiadochokinesis demonstrated by testing of rapid alternating movements. Patients may also present with ataxic tremor, dysarthria, postural instability, or gait disturbances. The cerebellum demonstrates functional localization; cerebellar signs often correlate with the location of the cerebellar lesion.2 The deep cerebellar nuclei have specific deficits when damaged. The midline fastigial nuclei play a role in postural ataxia, whereas globus, embolliform, and dentate nuclei are important for limb ataxia. Injury to the dentate nuclei can lead to dysarthria and mutism in some patients. Midline lesions, affecting the cerebellar vermis and midline cerebellar nuclei, are more likely to demonstrate truncal instability. Lesions involving the cerebellar hemispheres are more likely to show limb ataxias and associated dysmetria.
Patients with a cerebellar mass may also present with symptoms related to compression. Depending on tumor location, large cerebellar tumors can cause compression of the brain stem, which can cause obstruction of the fourth ventricle and various cranial nerve signs, depending on tumor location. Patients may present with hydrocephalus and increased intracranial pressure. Accordingly, headache, nausea, and vomiting are common presenting symptoms for patients with cerebellar tumors.3,4 When a patient presents with signs and symptoms of obstructive hydrocephalus, such as lethargy or coma, an emergent externalized ventricular catheter should be placed prior to surgical planning. In patients with long-standing ventriculomegaly and minimal clinical symptoms, intravenous steroids may alleviate symptoms and obviate the need for an externalized ventricular catheter.
In adult patients that present with a suspected cerebellar mass, an appropriate work-up should be done to obtain as much information as possible prior to surgery. Neuroimaging typically involves computed tomography (CT) and magnetic resonance imaging (MRI). CT scans have the advantage that they can be done rapidly, and thus help determine immediate treatment upon initial presentation of the patient. MRI gives better visualization of the posterior fossa, and is thus the imaging modality of choice for patients with a suspected cerebellar lesion. The appearance of the lesion in the MRI sequences can help narrow the differential diagnosis of the lesion. Cerebellar tumors can be identified as solid or cystic. Solid components of tumors can be evaluated with T1 and T2 MRI sequences. Most solid components are isointense to gray matter on T1 and T2; however, tumors such as pilocytic astrocytomas can appear isointense to cerebrospinal fluid (CSF) on T2.5,6 For tumors that are highly vascular, such as hemangioblastomas, diffusion-weighted imaging (DWI) will show low signal, and apparent diffusion coefficient (ADC) will show increased signal within the solid vascular portion of the tumor. This imaging pattern contrasts with tumors that are highly cellular, such as medulloblastoma, that will have high DWI and low ADC signal.6 Tumors that have abnormal vessels, such as in Lhermitte-Duclos, or tumors with evidence of prior hemorrhage, will benefit from susceptibility-weighted imaging (SWI) sequences, which are sensitive to venous blood and hemorrhage.7 Cystic portions of tumors are generally hypointense on T1 and hyperintense on T2 due to the liquid component; fluid attenuation inversion recovery (FLAIR) sequences can be helpful in tumors such as hemangioblastomas where the cystic component is different from CSF.6 Tumors with a large percentage of fat-containing substance, such as in cerebellar liponeurocytomas, can be identified by hyperintense streaks on T1, and fat suppression sequences can aid in their diagnosis.8 The pattern of contrast enhancement after the administration of gadolinium is also useful, as some cerebellar tumors enhance strongly and homogeneously, whereas others show heterogeneous enhancement patterns.
Patients, especially adults, with a cerebellar mass should also receive screening scans to evaluate for possible metastatic disease to the cerebellum. Such a workup should include imaging of the chest (via chest x-ray and/or CT scan of the chest) and also CT of the abdomen and pelvis. The treatment of metastatic lesions to the cerebellum is often dictated by a number of factors, including the identity of the tumor of origin, the size, location, and number of metastases. Please refer to the subsequent chapter on metastatic lesions for further details.
Intrinsic Cerebellar Tumors in Adults
Cerebellar Astrocytomas
Gliomas represent 36% of all primary brain and CNS tumors; of these, 3% are located in the cerebellum.1 Gliomas of the cerebellum are frequently astrocytomas. The most common is the pilocytic astrocytoma, which represents between 70% to 90% of cerebellar astrocytomas.3,9 Pilocytic astrocytomas are low-grade, World Health Organization (WHO) classification grade I tumors that represent 5% to 6% of all gliomas.10 In children they are the most common glioma, of which 67% occur in the cerebellum. In adults, the location of pilocytic astrocytomas is evenly distributed between supra- and infra-tentorial lesions.9,11
Cerebellar pilocytic astrocytomas demonstrate consistent imaging features. These tumors are found equally in the cerebellar vermis or hemispheres. They appear as well-circumscribed, unencapsulated lesions that often have cyst formation and a solid mural nodule. The solid component of the tumor is often isointense to CSF on T2 MRI and shows strong contrast enhancement.5 Tumor necrosis is not evident in histopathologic evaluation. Classically, pilocytic astrocytomas show regions with compact bipolar cells that alternate with microcystic, loosely organized regions. Eosinophilic granular bodies and Rosenthal fibers are commonly present.
These tumors generally have a good prognosis after surgical resection with an 85% to 100% 5-year survival rate.1,9,10 The favorable prognosis in these patients has been attributed to the low-grade nature of cerebellar pilocytic astrocytomas and the ability to achieve a gross total resection. It has been documented that surgeons tend to overestimate the degree of tumor resection.12 As most tumor recurrence is related to residual tumor,12,13 it is recommended that within 48 to 72 hours after surgery an MRI, with and without gadolinium, be obtained to determine if gross total resection has occurred. Rarely, malignant transformation of pilocytic astrocytomas can occur.14 Adjuvant therapy, either radiation or chemotherapy, is not indicated for patients with cerebellar pilocytic astrocytomas, as gross total resection alone yields excellent prognosis.9,11 The characteristics of cerebellar astrocytomas are summarized and compared to other intrinsic cerebellar tumors in Table 14-1.
Not all astrocytomas found in the cerebellum are low-grade pilocytic astrocytomas. The pilomyxoid astrocytoma (PMA) is an astrocytoma that shows similarities to the pilocytic astrocytoma, but histopathology confirms it lacks Rosenthal fibers, has a mucoid matrix, and shows an angiocentric arrangement of tumor cells, making it a distinct entity. Additionally, PMA is more aggressive, defined as WHO grade II, and often shows CSF dissemination at presentation.10,15 The PMA is found most frequently in children; however, cases have been reported in adults, and PMAs have been detected in the cerebellum.16,17 It has been demonstrated that up to 6% of astrocytomas presenting in the cerebellum are WHO grade III, and 17% exhibit the more malignant features of glioblastoma multiforme.3 Radiographic features of these tumors include heterogeneous contrast enhancement and significant edema; leptomeningeal spread is common.18 Because of the infiltrating nature, or the involvement of deep-seated tissues, these tumors are not cured by surgery and recur with higher frequency. For this subset of high-grade cerebellar astrocytomas, additional treatment modalities such as radiation, chemotherapy,19,20 and/or radiosurgery have been performed.21,22
Medulloblastomas
Embryonal tumors, including medulloblastoma, represent 1.5% of primary CNS tumors.1 Medulloblastomas are malignant WHO grade IV tumors of the cerebellum, of which 70% occur in children under the age of 16 years. They are fairly common in children, representing 20% to 30% of all intracranial neoplasms in the pediatric sector, whereas in adults there is an incidence of approximately 0.5 per million.23,24 In adults, medulloblastomas are rarely seen beyond the fifth decade of life. Eighty percent occur in patients aged 21 to 40 years.10 Most childhood medulloblastomas are found in the vermis. With increasing age, there is a progressive involvement of the cerebellar hemispheres, thus the majority of adult medulloblastomas are hemispheric. Additionally, there are radiographic differences in the appearance of childhood and adult medulloblastomas. In children, the classic radiographic appearance is that of a well-defined homogenous tumor that shows marked contrast enhancement and is without cystic or necrotic degeneration. In comparison, adult medulloblastomas are more variable in appearance (see case report 1). They are not as well defined, the degree of contrast enhancement varies, and there are often small cystic or necrotic foci.25,26 Adult patients are also more likely to exhibit the desmoplastic nodular subtype of histopathology. Nodular, reticular free regions of neuronal maturation surrounded by densely packed proliferative cells that produce an intercellular reticulin fiber network characterizes this subtype. It is thought that this desmoplastic subtype may contribute to some of the variety seen on radiographic imaging, but it is unclear whether this subtype affects prognosis.26–28
Despite the differences in childhood and adult medulloblastoma, the recommended treatment regimen is similar. Standard treatment involves gross total resection followed by craniospinal irradiation. Given this treatment, the 5-year overall survival for adult medulloblastomas is 64% to 84%.23,29–31 Many factors influence the outcome of adult patients with medulloblastoma. Approximately 18% to 33% of patients have metastatic disease at presentation including spinal cord, CSF, and extraneural metastases. Metastasis has been shown to be a poor prognostic indicator, as has involvement of the brain stem, involvement of the floor of the fourth ventricle, radiation dose less than 50 Gy, and large-cell variant histopathologic subtype.23,29,32,33 In contrast, patient age less than 20, gross total resection of tumor, and completion of radiation treatment within 48 days are known to be positive prognostic indicators.23,30
Recent studies have explored the role of adjuvant chemotherapy for patients with adult medulloblastoma. The benefit of chemotherapy is still unclear in the adult medulloblastoma population so there are no universal guidelines for administration of chemotherapy. Gross total resection and radiation are the most important factors in preventing recurrence, and primary adjuvant chemotherapy has not been shown to have a significant association with survival.33,34 However, for high-risk patients, it appears that chemotherapy may delay the risk of recurrence by approximately 3 years, and thus, there may be a role for chemotherapy in a subpopulation of adult patients with medulloblastoma.32,35 For patients with widely metastatic medulloblastoma who have failed multiple treatment options, new chemotherapy techniques that target tumor-specific mutations show promise as future treatment modalities.36
Case Report 1: Medulloblastoma
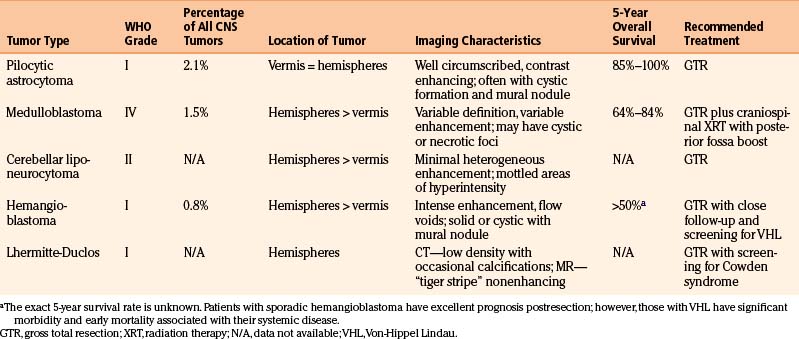
A 22-year-old female, without a significant past medical history, presented to the emergency department with 2 weeks of occipital headache and blurry vision. Ophthalmology evaluation revealed papilledema and dysconjugate gaze. On examination she was sleepy, but arousable, and able to follow simple commands. Head CT revealed a cerebellar lesion with mass effect causing some effacement of the fourth ventricle and hydrocephalus (Fig. 14-1A).
Cerebellar Liponeurocytoma
Cerebellar liponeurocytoma is a rare cerebellar neoplasm found primarily in adults.10 It has previously been named in the literature as lipomatous medulloblastoma, neurolipocytoma, medullocytoma, and lipomatous glioneurocytoma. The now widely accepted name is cerebellar liponeurocytoma. Initially, this entity was thought to hold similarities to medulloblastomas, but the cerebellar liponeurocytoma is distinct. It is found primarily in adults and typically presents in the fifth or sixth decade, which is substantially older than the age of presentation for medulloblastomas. Additionally, cerebellar liponeurocytomas have a low proliferative index, and therefore a favorable prognosis. Although they do not exhibit malignant transformation, they have a recurrence rate of approximately 50%, with a mean time to recurrence of approximately 10 years.37,38 Because of the propensity for recurrence, cerebellar liponeurocytomas are designated as WHO grade II.10
On neuroimaging, cerebellar liponeurocytomas can be difficult to distinguish from other more common cerebellar tumors. They are prevalent in the cerebellar hemispheres as compared to the vermis. MRI T1 imaging usually demonstrates a hypointense lesion with mottled or streaked areas of hyperintensities that correspond to adipose containing areas. These lesions are usually associated with a minimal amount of heterogeneous enhancement, and minimal associated edema.8,10 The main criterion distinguishing this tumor type from others in the cerebellum is the presence of intratumoral areas of fat. Histologically, this is represented by cells showing advanced neuronal differentiation and areas of focal accumulation of mature lipidized tumor.
Similarly to other cerebellar neoplasms, treatment is gross total resection. Cerebellar liponeurocytomas have a tendency for exophytic growth into CSF spaces and gross total resection is not always obtainable. Yet, the role of adjuvant radiotherapy or chemotherapy has not been established. Some authors believe that due to the nature of these tumors to recur, it is feasible to consider adjuvant radiotherapy to the posterior cranial fossa immediately after the initial surgery.39 Yet others suggest that radiation therapy is best reserved for evidence of recurrence.38 Regardless, most are in agreement that due to the nonmalignant nature of these tumors, aggressive radiotherapy, as is standard for medulloblastomas, is not necessary for cerebellar liponeurocytomas.
Hemangioblastomas
Hemangioblastomas represent about 1% of all intracranial tumors,1 but about 8% of posterior fossa tumors in adults. They are slow-growing, highly vascular tumors that are classified as WHO grade I.10 Hemangioblastomas occur most frequently in the cerebellum; however, they can also be found in the brain stem or spinal cord.
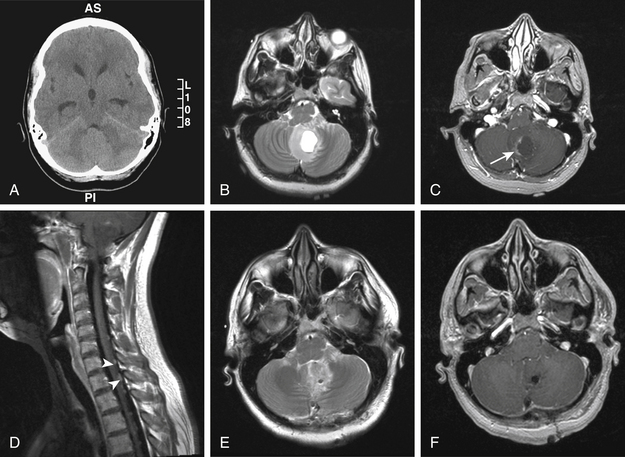
Cerebellar hemangioblastomas are distinct on neuroimaging. Classically, they can be solid, solid with cystic component, or cystic with nodule (see case report 2). They are found more commonly in cerebellar hemispheres as compared to the vermis.40,41 Due to the highly vascular nature of these tumors, flow voids are usually present. In addition, these tumors are heterogeneous in T2, and show intense contrast enhancement.42 Angiography is useful to evaluate the tumor feeding vessels prior to surgical intervention.
Approximately 30% of patients with cerebellar hemangioblastomas have the multisystem cancer syndrome Von-Hippel Lindau (VHL). Patients with VHL develop numerous visceral lesions including renal cell carcinoma, pancreatic islet cell tumors, pheochromocytoma, and papillary cystoadenomas of the epididymis and broad ligament. CNS manifestations include endolymphatic sac tumors and multiple hemangioblastomas. The hemangioblastomas typically occur in the retina, brain stem, cerebellum, spinal cord, and nerve roots. Patients with VHL tend to be younger, and have multiple hemangioblastomas at presentation.41,43–45
Sporadic, compared to VHL-associated cerebellar hemangioblastomas, are not significantly different in location within the cerebellum, solid versus cystic type, or surgical outcomes. Treatment of cerebellar hemangioblastoma involves gross total resection and typically has a favorable prognosis. However, VHL patients have significant morbidity related to their systemic disease, and are more likely to develop additional hemangioblastomas, with an average of one every 2.1 years.43 Thus, the subgroup of patients with VHL must be recognized. Any patient with a cerebellar hemangioblastoma should undergo appropriate screening for VHL. At a minimum, this should include neuroimaging of the neuraxis to look for additional hemangioblastomas. Because patients with VHL often have multiple hemangioblastomas, it is important to have thresholds for treatment of these lesions. Tumors that need resection are generally those that are, or will become, symptomatic. The strongest predictors of symptomatic progression are tumor/cyst growth greater than 112 mm3 per month and tumor/cyst size greater than 69 mm.3,41 Hemangioblastomas in VHL can be asymptomatic, and these patients require long-term follow-up with imaging every 6 to 12 months.
Adjuvant radiotherapy has been considered in some cases of hemangioblastomas. Given that gross total resection gives a favorable prognosis, cases of stereotactic radiosurgery or fractionated external beam radiotherapy are generally preserved for those patients with subtotal resection or tumor recurrence.46 For patients receiving adjuvant stereotactic radiosurgery, better progression-free survival has been associated with smaller tumor volumes, solid tumor type, and margin dose of 15 Gy or more.47
Lhermitte-Duclos Disease
Lhermitte-Duclos disease (LDD) is a rare dysplastic gangliocytoma of the cerebellum that is associated with Cowden syndrome (CS). It is unclear whether LDD is hamartomatous or neoplastic, but if considered neoplastic it is WHO Grade I due to its low proliferative index.10 In support of the neoplastic nature of LDD, a recent case report, in which a patient was initially thought to have cerebellar abnormality on imaging from a head injury, was diagnosed with LDD 5 years later. In this case, the tumor showed a doubling time of approximately 42 months, which is consistent with that of a low-grade tumor.48
CS is an autosomal dominant disorder characterized by multiple hamartomas and high risk for breast, nonmedullary thyroid, and endometrial cancers. Along with mucocutaneous lesions such as trichilemmomas, LDD is a pathognomonic lesion for the diagnosis of CS.49 Virtually all cases of adult LDD are associated with CS. Cowden syndrome is caused by a mutation in the phosphatase and tensin homologue (PTEN) gene found on chromosome ten. Decreased amounts of PTEN have been found in LDD tissue, further supporting the association between CS and LDD.50
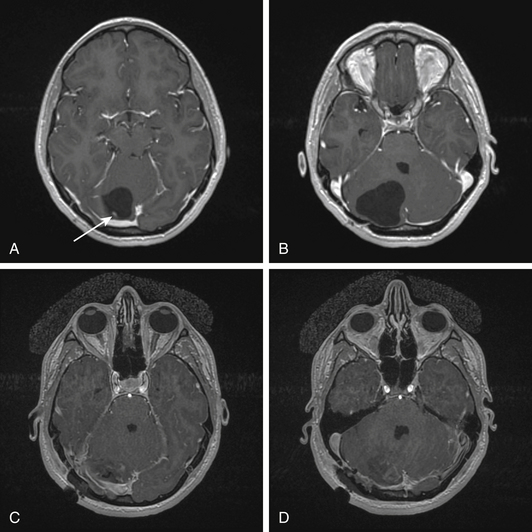
Neuroimaging of LDD is distinct, because it shows a hemispheric cerebellar mass on CT that is hypointense and may have areas of calcification. On MRI, the mass appears as alternating linear bands of hyperintense and isointense areas on T2. This pattern, often referred to as “tiger-stripe,” can also be seen in T1 imaging. The striated appearance correlates with the gross pathology of expanded cerebellar folia. LDD is characterized by having thinned white matter, a widened granule cell layer, and dysplastic molecular layer.7,51 Also apparent on imaging of LDD is a high PET uptake, which does not correlate with tumor proliferative activity. Additionally, MR spectroscopy shows a high lactate peak with decreased choline, N-acetyl aspartate, and creatinine levels. Despite these distinct features, there have been reported cases of adult medulloblastoma mimicking LDD on imaging, thus making intraoperative pathology extremely important, since the management of these two entities is strikingly different.52
Treatment of LDD, as with all cerebellar tumors, is gross total resection. The gross appearance of LDD is usually a pale area with thickened folia, yet the border with surrounding cerebellum is often indistinct (see case report 3). Since LDD is associated with Cowden syndrome, it is important that all patients presenting with LDD be appropriately screened for other systemic cancers, as these are the hallmark of CS and contribute to the morbidity of this disease.53,54
Surgical Approaches to the Cerebellum
Suboccipital Craniotomy
Consideration is given to possible placement of a ventricular catheter for CSF diversion prior to surgery. This depends on the size of the tumor, its mass effect, the status of the fourth ventricle, and the need for CSF diversion for brain relaxation intraoperatively or during the postoperative period. If indicated, an external ventricular catheter may be placed on the operating table after induction of anesthesia via an occipital burr hole. The burr hole is placed 3 to 4 cm from the midline and 6 to 7 cm above the inion. The catheter is then inserted with a trajectory parallel to the skull base, aiming for the middle of the forehead. In adults, an insertion length of approximately 10 to 12 cm will reach the foramen of Monro. Sometimes, as a precaution, a burr hole can be drilled without placement of a catheter, allowing the ability to place a ventricular catheter in an acute situation.
If stereotactic navigation is employed, registration and verification of accuracy can be done prior to draping and prepping the patient. One advantage of frameless stereotactic navigation is that it may guide the surgeon’s ability to minimize the size of skin incision and bony removal, particularly for small lesions. Frameless stereotaxy can also be incorporated into intraoperative MRI, if available. Intraoperative MRI can be useful to detect residual tumor, and has been shown to increase the extent of tumor resection from 83% to 98%.55,56 However, it is necessary that appropriate precautions are taken by the anesthesia team and operating room staff to ensure that the patient can be monitored safely during the surgical procedure.57
The size of the craniotomy or craniectomy is often dictated by the size and location of the tumor. When making burr holes there are many important considerations. Anatomically, the suboccipital space is just below the transverse sinus, in the midline lies the confluence of sinuses, and far laterally the transverse-sigmoid sinus junction is encountered. It is advantageous to avoid making a burr hole directly over the sinuses. Traditionally, the superior nuchal line has been used to estimate the intracranial course of the transverse sinus, and the inion has been used as a marker for the torcular herophili. Yet, neither superficial landmark is always reliable in marking the location of the sinuses. The insertion point of the semispinalis capitus muscles has been shown to be a better landmark for identification of the medial transverse sinus.58 Stereotactic navigation can aid in mapping out the location of the sinuses, and some studies suggest that a faster craniotomy is possible, and that complications such as venous air embolism are decreased with navigation-assisted surgery.59
Once the craniotomy is open, the dura is incised in a Y-shaped fashion. The midline incision of the dura will usually cut into the occipital sinus with fairly extensive hemorrhage. This can be controlled by cutting the dura and then employing surgical clips on either side of the midline sinus prior to the final dural cut. The suboccipital surface of the cerebellum will then be facing the surgeon. If indicated, the arachnoid covering the cisterna magna can be incised allowing for CSF drainage and brain relaxation. Sometimes, a shortened ventriculostomy catheter can be sutured to the dura and left in the cisterna magna, in order to allow for CSF drainage during the operation, particularly with paramedian or lateral approaches where the inferior lobe or tonsil of the cerebellum can block continuous drainage of the cisterna magna. This catheter is then removed at the conclusion of the intracranial part of the surgery, before dural closure. Careful dissection in this area is necessary, as branches of the posterior inferior cerebellar artery course along this surface. Stereotactic navigation as well as intraoperative ultrasound can be used to aid in location of tumors that are not seen readily at the surface. A small corticectomy parallel to the cerebellar folia is often necessary to reach tumors that do not reach the pial surface. Once the tumor is reached, it is carefully dissected from the surrounding normal tissue and removed with a combination of aspiration, cautery, and microdissection experiments. Ultrasonic aspiration is helpful for debulking large tumors necessitating piecemeal removal. When deciding on a method of resection, it is important to bear in mind that some studies suggest that piecemeal removal of tumors is associated with increased risk of recurrence, and thus en bloc resection may be preferable, particularly for metastatic lesions.60,61
Certain tumor types require specific resection techniques to ensure the best resection. Cerebellar pilocytic astrocytomas have a fleshy appearance, are well demarcated from surrounding cerebellar tissue, and often have a cystic component with a mural nodule. The cyst should be aspirated, and the mural nodule should be removed. The cyst wall need not be removed, as it is usually not a separate capsule, but instead displaced portions of normal brain tissue. Medulloblastomas tend to be friable, thus careful dissection and prudent hemostasis are necessary. Cerebellar liponeurocytomas are yellowish on gross appearance due to the high content of fat cells. This distinct coloration aids in the total resection of this tumor type. Hemangioblastomas are often red in appearance due to their vascularity, and often benefit from preop angiography, which allows the surgeon to identify the main feeding arteries. Careful attention must be made to cauterize feeding vessels as the tumor is removed.44 In cases of cystic hemangioblastoma, the cyst wall should be evaluated for signs of tumor, as failure to remove intramural tumor has been associated with recurrence.62 In cases of Lhermitte-Duclos, the indistinct border between abnormal and normal cerebellum is challenging. Care must be taken to avoid resection of normal cerebellar tissue since too wide of a surgical excision has been shown to be associated with lingering cerebellar signs.54
Meticulous hemostasis is needed in the postoperative tumor cavity prior to closure, especially in the posterior fossa. Bipolar electrocautery, with different sizes of cottonoids, cotton balls, and other hemostatic products are essential. The tumor cavity can be also lined with a single layer of oxidized cellulose or other hemostatic product; however, care must be taken that such product is not able to float away in the postoperative period, potentially causing problems with normal flow of CSF pathways. In cases of doubt, it is preferable not to leave such products. Closure of the surgical site involves a watertight dural closure. If the native dura is not amenable to this, nuchal ligament harvested during exposure, pericranium from the occipital area or a dural substitute can be used. The patient should be given a Valsalva maneuver to test for CSF leakage. The surgical wound is closed in multiple layers to help prevent formation of a pseudomeningocoele.
Other Surgical Approaches to the Posterior Fossa
A modification to the suboccipital craniotomy, the supracerebellar infratentorial approach can be used to resect anterior cerebellar tumors, especially those at the posterior incisura space and within the culmen of the vermis. For this approach, the skin incision is made approximately 2 cm superior to the inion and extends to the posterior cervical region. A midline or U-shaped incision can be used. The craniotomy should extend from just above the torcular herophili superiorly, to the foramen magnum inferiorly. Ideally, the craniotomy allows the inferior border of the superior sagittal sinus, and the medial edges of the transverse sinuses to be clearly viewed. The dura is opened in a Y- or U-shaped fashion. Bridging veins from the cerebellum to the tentorium are cauterized. Gentle retraction on the tentorium allows the tentorial surface of the cerebellum to come into view facilitating resection of lesions on this surface. This approach accesses lesions at the cerebellar-brain stem junction, and is a traditional approach used to resect pineal lesions.63,64 Adjustment of this approach to a paramedian or lateral craniotomy allows progressively further anterior lesions to be reached.65
The occipital transtentorial approach is another alternative when resecting anterosuperior cerebellar lesions. In this approach, the patient is prone and a curved or a U-shaped skin incision is made over the confluence of sinuses. Craniotomy location is dictated by exact tumor location, but in general, burr holes are placed adjacent to the venous sinuses. Upon removal of the bone flap, a curved dural incision is made and the occipital pole is retracted rostrally with caution not to injure any nearby bridging veins. The tentorium is then visualized with the tentorial surface of the cerebellum lying beneath. Care is taken with manipulation of this surface, as it can lead to significant transient bradycardia in some patients. An incision in the tentorium is made parallel to the straight sinus, and then peaked to allow access to the anterosuperior cerebellar tumor. For this approach, intraoperative drainage of CSF by incision of cisternal arachnoid membranes is often useful to promote brain relaxation. With the use of image guidance and sometimes endoscopy, this approach has been shown to be a safe and effective approach for anterosuperior cerebellar lesions.66 The disadvantages of this approach are that the tumor is at a far working distance, and significant retraction of the occipital pole may be necessary to reach the tumor edge.
An additional tactic to tumors in this region is the posterior subtemporal transtentorial approach. For this method the patient is positioned supine with the head turned to the side. An extraventricular catheter or lumbar drain is usually placed prior to incision to allow adequate drainage of CSF during the case. A U-shaped incision is made in the parietotemporal region beginning approximately 5 mm anterior to the tragus and ending 10 mm posterior to the mastoid notch. A temporal craniotomy is made, and the dura is opened in a U-shaped fashion. At this point, the vein of Labbe is encountered and precautions are made to avoid injury to this vessel. The temporal lobe is gently retracted. Patient positioning that allows gravity to gently elevate the temporal lobe, CSF diversion, and patient diuresis can facilitate the necessary retraction of temporal lobe. Once the temporal lobe is sufficiently elevated from the floor of the temporal fossa, the tentorium is encountered and incised, with careful adherence to the course of the trochlear nerve. The tentorium is tacked up, and the tumor can be reached and resected. The advantages of this approach are the short working distance and avoidance of unnecessary damage to the cerebellum.67,68 However, disadvantages include the significant risks encountered such as potential injury to the vein of Labbe and the risk of temporal lobe edema or injury from retraction.
In cases where a cerebellar lesion extends to the anterior inferomedial region, a far lateral approach can be used. In this approach, an inverted L- or J-shaped incision is made. This incision begins approximately 5 cm below the inion, extends superiorly to the superior nuchal line and laterally to the mastoid, staying just in front of the posterior border of the sternocleidomastoid muscle. The muscular anatomy is important for this approach, as is the vascular anatomy, because after craniectomy the vertebral artery and its branches are exposed.69 Careful dissection of the vertebral artery is done, as this allows better mobilization of the artery. If desired, the occipital condyle can be drilled with attention to the anatomy of the underlying hypoglossal canal. The amount of condyle that is drilled depends on surgeon’s preference and degree of exposure needed; however, with greater than 50% of unilateral occipital condyle resection, hypermobility is noted, thus atlanto-occipital fusion should be considered.70,71 With the far lateral approach, lesions that extend to the skull base and craniocervical junction can be resected.
There are numerous surgical approaches to the posterior fossa and the cerebellum, and none have been shown to be superior.72–74 Ultimately, choice of approach to tumors in this region is dictated by surgeon’s experience and preference.
Postsurgical Complications and Management
Surgery in the posterior fossa has been reported to have a complication rate as high as 32% in a series of 500 patients,75 although with modern techniques this rate may have become much lower. Of surgeries exclusive to resection of cerebellar tumors, complications included cerebellar edema (5%), hydrocephalus (5%), cerebellar hematomas (3%), and cerebellar mutism (1%).75
CSF leaks are the most common surgical complication in the posterior fossa. They can be minimized with watertight dural closure. Dural closure is best when using native dura or harvested autologous material. A dural substitute can be used as on onlay or suturable material when a watertight closure cannot otherwise be obtained. However, recent studies suggest that these dural substitutes may increase complication rates. A suturable bovine matrix dural substitute was associated with a 50% risk of complications, such as CSF leak, aseptic meningitis, hydrocephalus, and symptomatic pseudomeningocoele, compared to 18% of cases where no dural substitute was used.76 Ideally, surgeons should strive to achieve primary dural closure whenever possible.
The need for permanent CSF diversion is associated with CSF leak and hydrocephalus. Approximately 35% of children that have undergone posterior fossa surgery require either ventriculoperitoneal shunting or endoscopic third ventriculostomy.77,78 This requirement has been associated with younger age and midline tumors in children, but factors predicting need for CSF diversion in adults have not been established.
Cerebellar mutism is a rare postoperative complication seen with resection of cerebellar tumors. This complication occurs in about 1% of cases, and is observed more commonly in children, although cases have been reported in adults. Cerebellar mutism is considered a severe form of dysarthria; it manifests as hesitant slow speech, or frank mutism. In all documented cases it is transient and associated with vermal tumors.79,80 Cerebellar mutism is believed to be related to post surgical edema or ischemia involving the dentate nucleus or pathways of the dentatorubrothalamic tract.
Abel T.W., Baker S.J., Fraser M.M., et al. Lhermitte-Duclos disease: a report of 31 cases with immunohistochemical analysis of the PTEN/AKT/mTOR pathway. J Neuropathol Exp Neurol. 2005;64:341-349.
Ammerman J.M., Lonser R.R., Dambrosia J., et al. Long-term natural history of hemangioblastomas in patients with von Hippel-Lindau disease: implications for treatment. J Neurosurg. 2006;105:248-255.
Ammerman J.M., Lonser R.R., Oldfield E.H. Posterior subtemporal transtentorial approach to intraparenchymal lesions of the anteromedial region of the superior cerebellum. J Neurosurg. 2005;103:783-788.
Ammirati M., Bernardo A., Musumeci A., et al. Comparison of different infratentorial-supracerebellar approaches to the posterior and middle incisural space: a cadaveric study. J Neurosurg. 2002;97:922-928.
Bonneville F., Savatovsky J., Chiras J. Imaging of cerebellopontine angle lesions: an update. Part 2: intra-axial lesions, skull base lesions that may invade the CPA region, and non-enhancing extra-axial lesions. Eur Radiol. 2007;17:2908-2920.
Central Brain Tumor Registry of the United States. Primary Brain Tumors in the United States, Statistical Report 2000-2004. CBTRUS; 2008.
Conway J.E., Chou D., Clatterbuck R.E., et al. Hemangioblastomas of the central nervous system in von Hippel-Lindau syndrome and sporadic disease. Neurosurgery. 2001;48:55-62. discussion 62-63
Dubey A., Sung W., Shaya M., et al. Complications of posterior cranial fossa surgery—an institutional experience of 500 patients. Surg Neurol. 2009;72:369-375.
Hirschl R.A., Wilson J., Miller B., et al. The predictive value of low-field strength magnetic resonance imaging for intraoperative residual tumor detection. Clinical article. J Neurosurg. 2009;111:252-257.
Jackson T.R., Regine W.F., Wilson D., et al. Cerebellar liponeurocytoma. Case report and review of the literature. J Neurosurg. 2001;95:700-703.
Jagannathan J., Lonser R.R., Smith R., et al. Surgical management of cerebellar hemangioblastomas in patients with von Hippel-Lindau disease. J Neurosurg. 2008;108:210-222.
Kunschner L.J., Kuttesch J., Hess K., et al. Survival and recurrence factors in adult medulloblastoma: the M.D. Anderson Cancer Center experience from 1978 to 1998. Neuro-oncology. 2001;3:167-173.
Lai R. Survival of patients with adult medulloblastoma: a population-based study. Cancer. 2008;112:1568-1574.
Lanzino G., Paolini S., Spetzler R.F. Far-lateral approach to the craniocervical junction. Neurosurgery. 2005;57:367-371. discussion 367-371
Linscott L.L., Osborn A.G., Blaser S., et al. Pilomyxoid astrocytoma: expanding the imaging spectrum. AJNR Am J Neuroradiol. 2008;29:1861-1866.
Louis D.N., Ohgaki H., Wiestler O.D., et al. WHO classification of tumours of the central nervous system. France: international Agency for Research on Cancer Lyon; 2007.
Meltzer C.C., Smirniotopoulos J.G., Jones R.V. The striated cerebellum: an MR imaging sign in Lhermitte-Duclos disease (dysplastic gangliocytoma). Radiology. 1995;194:699-703.
Morreale V.M., Ebersold M.J., Quast L.M., et al. Cerebellar astrocytoma: experience with 54 cases surgically treated at the Mayo Clinic, Rochester, Minnesota, from 1978 to 1990. J Neurosurg. 1997;87:257-261.
Moskowitz S.I., Liu J., Krishnaney A.A. Postoperative complications associated with dural substitutes in suboccipital craniotomies. Neurosurgery. 2009;64:28-33.
Patel A.J., Suki D., Hatiboglu M.A., et al. Factors influencing the risk of local recurrence after resection of a single brain metastasis. J Neurosurg. 2009. doi:10.3171/2009.11.JNS09659
Riffaud L., Saikali S., Leray E., et al. Survival and prognostic factors in a series of adults with medulloblastomas. J Neurosurg. 2009;111:478-487.
Robinson S., Cohen A.R. Cowden disease and Lhermitte-Duclos disease: an update. Case report and review of the literature. Neurosurg Focus. 2006;20:E6.
Sherman J.H., Sheehan J.P., Elias W.J., et al. Cerebellar mutism in adults after posterior fossa surgery: a report of 2 cases. Surg Neurol. 2005;63:476-479.
Shirane R., Kumabe T., Yoshida Y., et al. Surgical treatment of posterior fossa tumors via the occipital transtentorial approach: evaluation of operative safety and results in 14 patients with anterosuperior cerebellar tumors. J Neurosurg. 2001;94:927-935.
1. Central Brain Tumor Registry of the United States. Primary Brain Tumors in the United States, Statistical Report 2000-2004. CBTRUS; 2008.
2. Schoch B., Dimitrova A., Gizewski E.R., et al. Functional localization in the human cerebellum based on voxelwise statistical analysis: a study of 90 patients. Neuroimage. 2006;30:36-51.
3. Morreale V.M., Ebersold M.J., Quast L.M., et al. Cerebellar astrocytoma: experience with 54 cases surgically treated at the Mayo Clinic, Rochester, Minnesota, from 1978 to 1990. J Neurosurg. 1997;87:257-261.
4. Ilgren E.B., Stiller C.A. Cerebellar astrocytomas. Clinical characteristics and prognostic indices. J Neurooncol. 1987;4:293-308.
5. Arai K., Sato N., Aoki J., et al. MR signal of the solid portion of pilocytic astrocytoma on T2-weighted images: is it useful for differentiation from medulloblastoma? Neuroradiology. 2006;48:233-237.
6. Bonneville F., Savatovsky J., Chiras J. Imaging of cerebellopontine angle lesions: an update. Part 2: intra-axial lesions, skull base lesions that may invade the CPA region, and non-enhancing extra-axial lesions. Eur Radiol. 2007;17:2908-2920.
7. Thomas B., Krishnamoorthy T., Radhakrishnan V.V., et al. Advanced MR imaging in Lhermitte-Duclos disease: moving closer to pathology and pathophysiology. Neuroradiology. 2007;49:733-738.
8. Alkadhi H., Keller M., Brandner S., et al. Neuroimaging of cerebellar liponeurocytoma. Case report. J Neurosurg. 2001;95:324-331.
9. Burkhard C., Di Patre P., Schüler D., et al. A population-based study of the incidence and survival rates in patients with pilocytic astrocytoma. J Neurosurg. 2003;98:1170-1174.
10. Louis D.N., Ohgaki H., Wiestler O.D., et al. WHO classification of tumours of the central nervous system. France: International Agency for Research on Cancer Lyon; 2007.
11. Bell D., Chitnavis B.P., Al-Sarraj S., et al. Pilocytic astrocytoma of the adult—clinical features, radiological features and management. Br J Neurosurg. 2004;18:613-616.
12. Dirven C.M., Mooij J.J., Molenaar W.M. Cerebellar pilocytic astrocytoma: a treatment protocol based upon analysis of 73 cases and a review of the literature. Childs Nerv Syst. 1997;13:17-23.
13. National Comprehensive Cancer Network. The NCCN Clinical Practice Guidelines in Oncology: Central Nervous System Cancers, vol. 2. Fort Washington, PA: NCCN; 2009.
14. Ellis J., Waziri A., Balmaceda C., et al. Rapid recurrence and malignant transformation of pilocytic astrocytoma in adult patients. J Neuro-Oncol. 2009;95:377-382.
15. Komotar R.J., Mocco J., Jones J.E., et al. Pilomyxoid astrocytoma: diagnosis, prognosis, and management. Neurosurg Focus. 2005;18:E7.
16. Komotar R.J., Mocco J., Zacharia B.E., et al. Astrocytoma with pilomyxoid features presenting in an adult. Neuropathology. 2006;26:89-93.
17. Linscott L.L., Osborn A.G., Blaser S., et al. Pilomyxoid astrocytoma: expanding the imaging spectrum. AJNR Am J Neuroradiol. 2008;29:1861-1866.
18. Endo H., Kumabe T., Jokura H., et al. Leptomeningeal dissemination of cerebellar malignant astrocytomas. J Neurooncol. 2003;63:191-199.
19. Stupp R., Mason W.P., van den Bent M.J., et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987-996.
20. Weber D.C., Miller R.C., Villà S., et al. Outcome and prognostic factors in cerebellar glioblastoma multiforme in adults: a retrospective study from the Rare Cancer Network. Int J Radiat Oncol Biol Phys. 2006;66:179-186.
21. Boëthius J., Ulfarsson E., Rähn T., et al. Gamma knife radiosurgery for pilocytic astrocytomas. J Neurosurg. 2002;97:677-680.
22. Kano H., Kondziolka D., Niranjan A., et al. Stereotactic radiosurgery for pilocytic astrocytomas part 1: Outcomes in adult patients. J Neurooncol. 2009;95:211-218.
23. Lai R. Survival of patients with adult medulloblastoma: a population-based study. Cancer. 2008;112:1568-1574.
24. Smee R.I., Williams J.R. Medulloblastomas-primitive neuroectodermal tumours in the adult population. J Med Imaging Radiat Oncol. 2008;52:72-76.
25. Bourgouin P.M., Tampieri D., Grahovac S.Z., et al. CT and MR imaging findings in adults with cerebellar medulloblastoma: comparison with findings in children. AJR Am J Roentgenol. 1992;159:609-612.
26. Malheiros S.M.F., Carrete H., Stávale J.N., et al. MRI of medulloblastoma in adults. Neuroradiology. 2003;45:463-467.
27. Sarkar C., Pramanik P., Karak A.K., et al. Are childhood and adult medulloblastomas different? A comparative study of clinicopathological features, proliferation index and apoptotic index. J Neurooncol. 2002;59:49-61.
28. Giordana M.T., Cavalla P., Dutto A., et al. Is medulloblastoma the same tumor in children and adults? J Neurooncol. 1997;35:169-176.
29. Padovani L., Sunyach M., Perol D., et al. Common strategy for adult and pediatric medulloblastoma: a multicenter series of 253 adults. Int J Radiat Oncol Biol Phys. 2007;68:433-440.
30. Chan A.W., Tarbell N.J., Black P.M., et al. Adult medulloblastoma: prognostic factors and patterns of relapse. Neurosurgery. 2000;47:623-631. discussion 631-632
31. Kunschner L.J., Kuttesch J., Hess K., et al. Survival and recurrence factors in adult medulloblastoma: the M.D. Anderson Cancer Center experience from 1978 to 1998. Neuro-oncology. 2001;3:167-173.
32. Riffaud L., Saikali S., Leray E., et al. Survival and prognostic factors in a series of adults with medulloblastomas. J Neurosurg. 2009;111:478-487.
33. Herrlinger U., Steinbrecher A., Rieger J., et al. Adult medulloblastoma: prognostic factors and response to therapy at diagnosis and at relapse. J Neurol. 2005;252:291-299.
34. Malheiros S.M.F., Franco C.M.R., Stávale J.N., et al. Medulloblastoma in adults: a series from Brazil. J Neurooncol. 2002;60:247-253.
35. Brandes A.A., Franceschi E., Tosoni A., et al. Long-term results of a prospective study on the treatment of medulloblastoma in adults. Cancer. 2007;110:2035-2041.
36. Rudin C.M., Hann C.L., Laterra J., et al. Treatment of medulloblastoma with hedgehog pathway inhibitor GDC-0449. N Engl J Med. 2009;361:1173-1178.
37. Jenkinson M.D., Bosma J.J.D., Du Plessis D., et al. Cerebellar liponeurocytoma with an unusually aggressive clinical course: case report. Neurosurgery. 2003;53:1425-1427. discussion 1428
38. Patel N., Fallah A., Provias J., et al. Cerebellar liponeurocytoma. Can J Surg. 2009;52:E117-E119.
39. Jackson T.R., Regine W.F., Wilson D., et al. Cerebellar liponeurocytoma. Case report and review of the literature. J Neurosurg. 2001;95:700-703.
40. Rachinger J., Buslei R., Prell J., et al. Solid haemangioblastomas of the CNS: a review of 17 consecutive cases. Neurosurg Rev. 2009;32:37-47. discussion 47-48
41. Ammerman J.M., Lonser R.R., Dambrosia J., et al. Long-term natural history of hemangioblastomas in patients with von Hippel-Lindau disease: implications for treatment. J Neurosurg. 2006;105:248-255.
42. Quadery F.A., Okamoto K. Diffusion-weighted MRI of haemangioblastomas and other cerebellar tumours. Neuroradiology. 2003;45:212-219.
43. Conway J.E., Chou D., Clatterbuck R.E., et al. Hemangioblastomas of the central nervous system in von Hippel-Lindau syndrome and sporadic disease. Neurosurgery. 2001;48:55-62. discussion 62-63
44. Jagannathan J., Lonser R.R., Smith R., et al. Surgical management of cerebellar hemangioblastomas in patients with von Hippel-Lindau disease. J Neurosurg. 2008;108:210-222.
45. Hasselblatt M., Jeibmann A., Gerss J., et al. Cellular and reticular variants of haemangioblastoma revisited: a clinicopathologic study of 88 cases. Neuropathol Appl Neurobiol. 2005;31:618-622.
46. Koh E., Nichol A., Millar B., et al. Role of fractionated external beam radiotherapy in hemangioblastoma of the central nervous system. Int J Radiat Oncol Biol Phys. 2007;69:1521-1526.
47. Kano H., Niranjan A., Mongia S., et al. The role of stereotactic radiosurgery for intracranial hemangioblastomas. Neurosurgery. 2008;63:443-450. discussion 450-451
48. Goto Y., Hashimoto N., Okita Y., et al. A surgically treated case of Lhermitte-Duclos disease with a precise natural history and high uptake of FDG on PET. J Neurooncol. 2010;97:445-450.
49. National Comprehensive Cancer Network. The NCCN Clinical Practice Guidelines in Oncology: Genetic/Familial High-Risk Assessment: Breast and Ovarian, vol. 1. Fort Washington, PA: NCCN; 2009.
50. Abel T.W., Baker S.J., Fraser M.M., et al. Lhermitte-Duclos disease: a report of 31 cases with immunohistochemical analysis of the PTEN/AKT/mTOR pathway. J Neuropathol Exp Neurol. 2005;64:341-349.
51. Meltzer C.C., Smirniotopoulos J.G., Jones R.V. The striated cerebellum: an MR imaging sign in Lhermitte-Duclos disease (dysplastic gangliocytoma). Radiology. 1995;194:699-703.
52. Douglas-Akinwande A.C., Payner T.D., Hattab E.M. Medulloblastoma mimicking Lhermitte-Duclos disease on MRI and CT. Clin Neurol Neurosurg. 2009;111:536-539.
53. Robinson S., Cohen A.R. Cowden disease and Lhermitte-Duclos disease: an update. Case report and review of the literature. Neurosurg Focus. 2006;20:E6.
54. Prestor B. Dysplastic gangliocytoma of the cerebellum (Lhermitte-Duclos disease). J Clin Neurosci. 2006;13:877-881.
55. Hatiboglu M.A., Weinberg J.S., Suki D., et al. Impact of intraoperative high-field magnetic resonance imaging guidance on glioma surgery: a prospective volumetric analysis. Neurosurgery. 2009;64:1073-1081. discussion 1081
56. Hirschl R.A., Wilson J., Miller B., et al. The predictive value of low-field strength magnetic resonance imaging for intraoperative residual tumor detection. Clinical article. J Neurosurg. 2009;111:252-257.
57. Barua E., Johnston J., Fujii J., et al. Anesthesia for brain tumor resection using intraoperative magnetic resonance imaging (iMRI) with the Polestar N-20 system: experience and challenges. J Clin Anesth. 2009;21:371-376.
58. Tubbs R.S., Salter G., Oakes W.J. Superficial surgical landmarks for the transverse sinus and torcular herophili. J Neurosurg. 2000;93:279-281.
59. Gharabaghi A., Rosahl S.K., Feigl G.C., et al. Image-guided lateral suboccipital approach: Part 2—impact on complication rates and operation times. Neurosurgery. 2008;62:24-29. discussion 29
60. Patel A.J., Suki D., Hatiboglu M.A., et al. Factors influencing the risk of local recurrence after resection of a single brain metastasis. J Neurosurg. 2009. doi:10.3171/2009.11.JNS09659
61. Suki D., Abouassi H., Patel A.J., et al. Comparative risk of leptomeningeal disease after resection or stereotactic radiosurgery for solid tumor metastasis to the posterior fossa. J Neurosurg. 2008;108:248-257.
62. Bishop F.S., Liu J.K., Chin S.S., et al. Recurrent cerebellar hemangioblastoma with enhancing tumor in the cyst wall: case report. Neurosurgery. 2008;62:E1378-E1379. discussion E1379
63. Stein B.M. The infratentorial supracerebellar approach to pineal lesions. J Neurosurg. 1971;35:197-202.
64. Rhoton A.L. Tentorial incisura. Neurosurgery. 2000;47:S131-S153.
65. Ammirati M., Bernardo A., Musumeci A., et al. Comparison of different infratentorial-supracerebellar approaches to the posterior and middle incisural space: a cadaveric study. J Neurosurg. 2002;97:922-928.
66. Shirane R., Kumabe T., Yoshida Y., et al. Surgical treatment of posterior fossa tumors via the occipital transtentorial approach: evaluation of operative safety and results in 14 patients with anterosuperior cerebellar tumors. J Neurosurg. 2001;94:927-935.
67. Ammerman J.M., Lonser R.R., Oldfield E.H. Posterior subtemporal transtentorial approach to intraparenchymal lesions of the anteromedial region of the superior cerebellum. J Neurosurg. 2005;103:783-788.
68. Beppu T., Hirooka R., Fujiwara S., et al. Choice of posterior subtemporal transtentorial approach for tumor resection in deep anteromedial superior cerebellum. Neurol Med Chir (Tokyo). 2009;49:42-46.
69. Rhoton A.L. The far-lateral approach and its transcondylar, supracondylar, and paracondylar extensions. Neurosurgery. 2000;47:S195-S209.
70. Lanzino G., Paolini S., Spetzler R.F. Far-lateral approach to the craniocervical junction. Neurosurgery. 2005;57:367-371. discussion 367-371
71. Vishteh A.G., Crawford N.R., Melton M.S., et al. Stability of the craniovertebral junction after unilateral occipital condyle resection: a biomechanical study. J Neurosurg. 1999;90:91-98.
72. Heros R.C. Transtentorial approach. J Neurosurg. 2005;103:776-777. discussion 777
73. Spetzler R.F. Subtemporal transtentorial approach. J Neurosurg. 2006;104:854. author reply 855-856
74. Pompili A., Pace A., Occhipinti E. Subtemporal trantentorial approach. J Neurosurg. 2006;104:854-855. author reply 855-856
75. Dubey A., Sung W., Shaya M., et al. Complications of posterior cranial fossa surgery—an institutional experience of 500 patients. Surg Neurol. 2009;72:369-375.
76. Moskowitz S.I., Liu J., Krishnaney A.A. Postoperative complications associated with dural substitutes in suboccipital craniotomies. Neurosurgery. 2009;64(suppl 3):28-33.
77. Santos de Oliveira R., Barros Jucá C.E., Valera E.T., et al. Hydrocephalus in posterior fossa tumors in children. Are there factors that determine a need for permanent cerebrospinal fluid diversion? Childs Nerv Syst. 2008;24:1397-1403.
78. Culley D.J., Berger M.S., Shaw D., et al. An analysis of factors determining the need for ventriculoperitoneal shunts after posterior fossa tumor surgery in children. Neurosurgery. 1994;34:402-407. discussion 407-408
79. Erşahin Y., Mutluer S., Cağli S., et al. Cerebellar mutism: report of seven cases and review of the literature. Neurosurgery. 1996;38:60-65. discussion 66
80. Sherman J.H., Sheehan J.P., Elias W.J., et al. Cerebellar mutism in adults after posterior fossa surgery: a report of 2 cases. Surg Neurol. 2005;63:476-479.