Chapter 72 Central Serous Chorioretinopathy
Introduction
Central serous chorioretinopathy (CSC) was first described by Albrecht von Graefe as central recurrent retinitis in 1866.1 It is a chorioretinal disorder, incompletely understood, with systemic associations, a multifactorial etiology, as well as a complex pathogenesis. It typically affects young to middle-aged men and is characterized by serous detachment of the neurosensory retina, which is usually located at the posterior pole. It is usually idiopathic but might also be secondary to high levels of endogenous or exogenous corticosteroids. Advances in imaging, particularly in indocyanine green angiography (ICGA) and optical coherence tomography (OCT), have led to a greater understanding of the pathophysiology of CSC. Most cases of CSC are self-limiting, with spontaneous resolution and good visual prognosis. However, some patients may suffer from persistent or recurrent serous macular detachment with subsequent progressive visual loss. A greater understanding of CSC has led many to believe that CSC is not a completely benign condition. Treatments for CSC, in particular the photodynamic therapy (PDT) using lower doses and reduced fluence, and the antivascular endothelial growth factor (anti-VEGF) therapy, are evolving. Although treatment results appear to be promising, more randomized large-scale controlled studies are needed before their treatment roles can be fully delineated.
Pathogenesis, predisposition, and risk factors
The pathophysiology of CSC has yet to be fully elucidated. It has, however, been thought to involve multiple etiologies and mechanisms that ultimately lead to widespread choroidal circulation abnormalities.2 Hyperdynamic choroidal circulation and choroidal vascular hyperpermeability are the main features that are shared among patients with CSC.3 The resultant increase in hydrostatic pressure in the choroid causes breakdown of the retinal pigment epithelial (RPE) barrier with subsequent leakage of fluid from the choroid through defects in RPE cell tight junctions into the subretinal space.4,5 Studies of ICGA of patients with CSC also revealed areas of hypofluorescence, indicating choriocapillary nonperfusion, which might also be one of the mechanisms that lead to choroidal venous dilation and congestion.
Horniker first suggested that psychiatric disturbances were linked to CSC in 1927.6 He described a mechanism in which mental disturbances would lead to retinal angiospasm and subsequent macular exudation. In the subsequent 60 years, however, there was a lack of study in the literature to confirm this link between psychiatric problems and CSC. It was not until 1987 that Yannuzzi’s cross-sectional study demonstrated the association of CSC with Type A behavioral pattern.7 The mechanism was thought to be related to elevation of catecholamine levels, which might trigger vasoconstriction of choroidal vessels by stimulating the sympathetic nervous system and adrenergic receptors.
Exogenous as well as elevated endogenous corticosteroids are well-known predisposing factors for CSC.8 Patients taking systemic steroid for diseases such as autoimmune conditions and after organ transplantation are at risk.4,9,10 CSC has been reported to occur in up to 6% of patients receiving corticosteroids after renal transplant.11 Other routes of exogenous corticosteroids such as intra-articular, intranasal, and topical have also been reported to put patients at risk of CSC.12–14 Diseases associated with an increased endogenous cortisol production such as Cushing’s disease and pregnancy also increase the risk of CSC.15–19 In some cases, CSC can even be the presenting symptom of Cushing syndrome.20 There are a number of proposed mechanisms as to how corticosteroids are related to CSC. Corticosteroids could induce choroidal vasoconstriction by reducing nitric oxide production. Direct increase in the permeability of the blood vessels might also occur, together with RPE cell tight junction damage.21–23 Corticosteroids could reverse the polarity of RPE cells, which causes them to pump ions into the subretinal space. Fluid will then enter the subretinal space by osmosis.24,25
Abnormal coagulation and platelet aggregation have also been proposed to be involved in the pathogenesis of CSC.26 Aqueous sample cytokine analysis showed that eyes with CSC have lower platelet-derived growth factor (PDGF) levels in the aqueous, suggesting that PDGF might play a role in the pathogenesis of CSC.27 The role of VEGF in CSC has been studied and intravitreal anti-VEGF injections have been tried. However, aqueous and plasma samples from patients with CSC showed no elevation in VEGF level when compared with normal controls.27,28
Helicobacter pylori infection has been reported to be associated with CSC and its treatment to hasten the rate of subretinal fluid resolution.29–31 Hypertension, smoking, antibiotic use, antihistamine use, alcohol consumption and allergic respiratory diseases have also been implicated in increasing the risk of CSC.16,32 Obstructive sleep apnea has been postulated to be associated with CSC and its treatment has been reported to lead to the resolution of CSC.33 A case-controlled study showed that patients with CSC have a less chance of glaucoma when compared with controls.34 This might be related to increased choroidal blood supply to the optic nerve but the exact mechanism remains unclear. While there have been isolated case reports of patients developing CSC after rhinoplasty35 and laser in situ keratomileusis,36 their relationship is unclear.
Clinical features
Demographics
CSC predominately affects males, with a male to female ratio of approximately 6 : 1.37 The age of onset is usually between 30 and 50 years but patients with chronic CSC might continue to suffer from the disease even though they are advanced in age. However, if a patient over 50 years of age presents a clinical appearance of CSC, one should be suspicious of differential diagnoses such as age-related macular degeneration (AMD) and polypoidal choroidal vasculopathy (PCV).38 CSC appears to have a low incidence in blacks when compared to whites and Asians, but it might behave more aggressively in blacks.39–41 At presentation, involvement is usually unilateral. However bilateral involvement is common in chronic cases and cases related to excessive endogenous or exogenous corticosteroids.
Signs
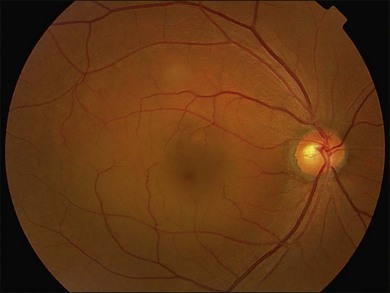
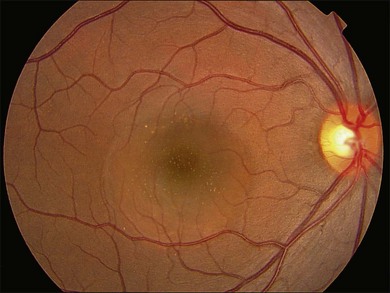
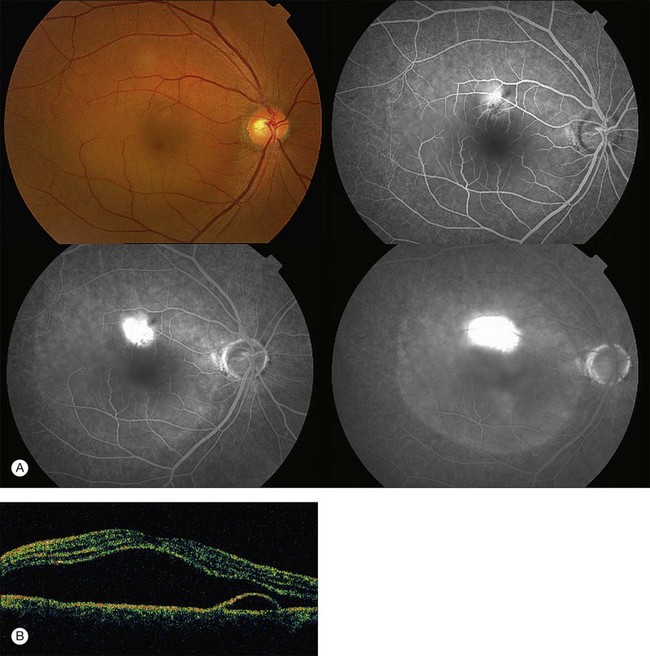
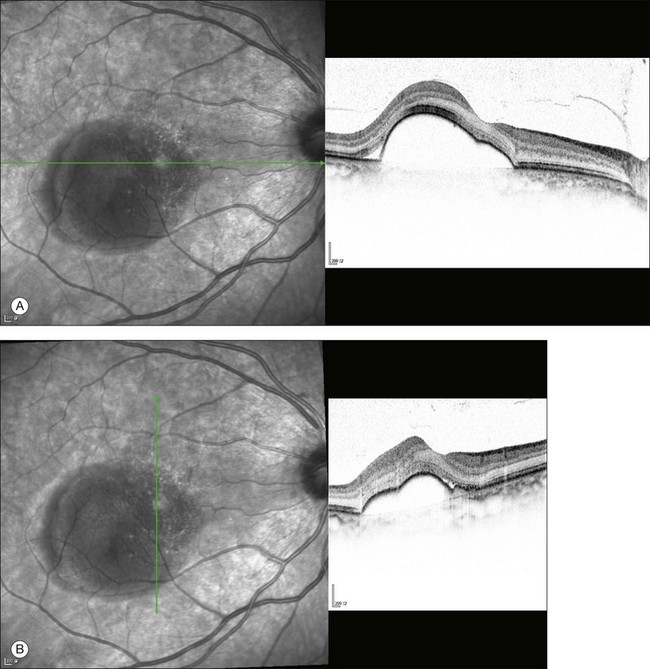
Fundus examination typically shows a well-demarcated oval-shaped area of neurosensory retinal detachment in the posterior pole (Fig. 72.1). Serous pigment epithelium detachment (PED) can also occur together or independently. The diagnosis is usually obvious from examination with indirect ophthalmoscopy. However, in cases with minimal subretinal fluid or small PED, slit-lamp biomicroscopy with fundus contact lens might be useful. In these cases, the loss of the normal foveal reflex might provide a good hint. Yellow dots are frequently observed on the posterior surface of the detached retina and are postulated to be associated with phagocytosis of shed photoreceptor outer segments (Fig. 72.2).42 Yellowish discoloration of the fovea is often seen and is caused by increased visibility of retinal xanthophyll.4,17,39,43 The subretinal fluid is usually transparent and colorless, but occasionally it can also appear cloudy.39,44 Fibrin can form in the subretinal and sub-RPE spaces and cause the subretinal fluid to become opaque. The fibrin usually dissolves spontaneously but rarely it could cause fibrosis and lead to permanent drop in vision. In chronic recurrent cases, RPE change and atrophy might develop. Patients with CSC can also present bullous neurosensory detachments,10,45–48 which are usually located inferiorly as the subretinal fluid drains down from the macula by gravity. In chronic cases, an atrophic RPE tract connecting the macula to the inferior detachment might be seen. Other complications of chronic CSC include secondary choroidal neovascularization (CNV) formation, cystoid macular edema, subretinal lipid deposition, and choriocapillaris atrophy.49
Investigations
Fluorescein angiography
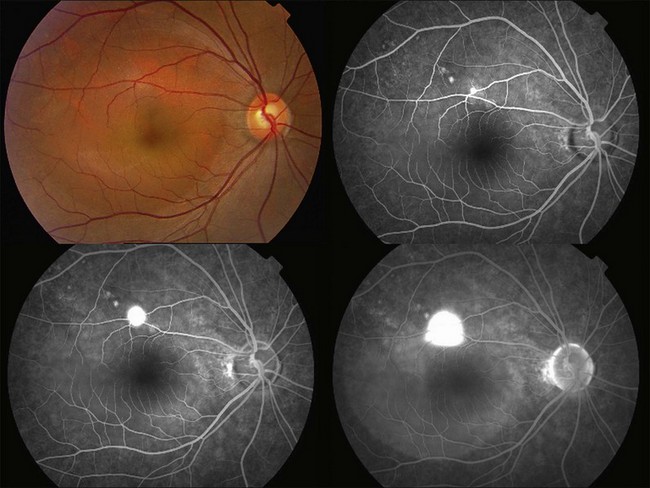
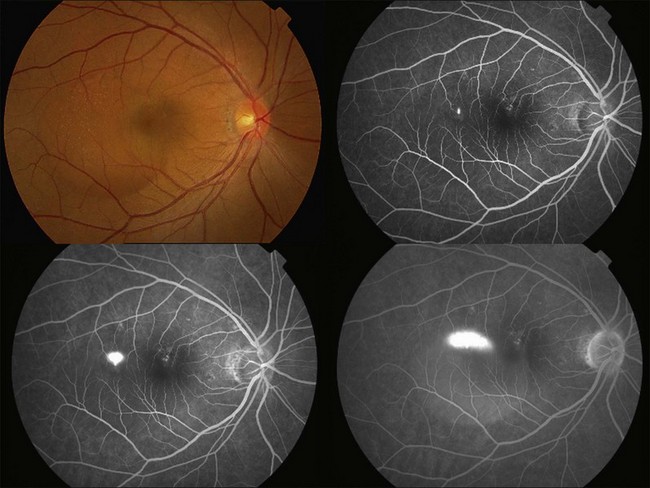
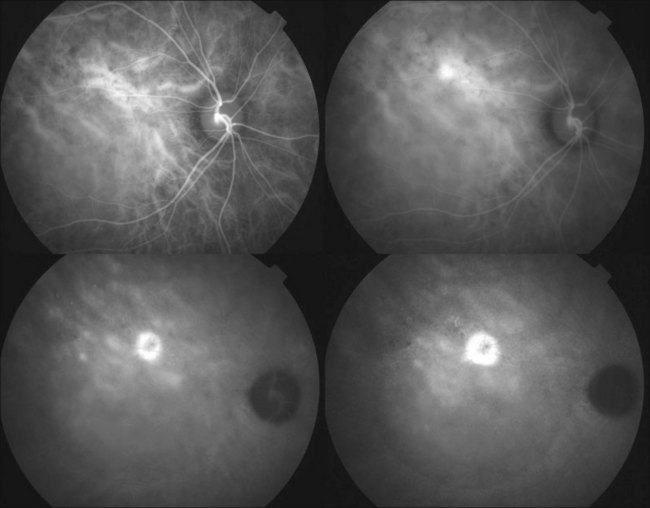
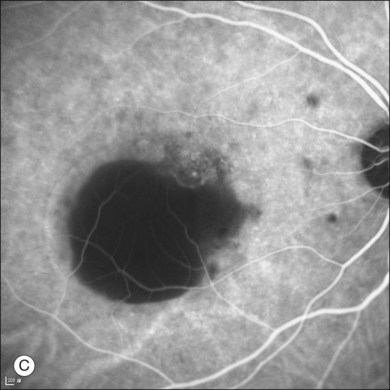
Fluorescein angiography (FA) in acute CSC typically shows one of two different types of leakage patterns: ink blot or smoke stack. In the former, the leakage starts as a pin point in the early phase and then concentrically diffuses out in the late phase and appears like an ink blot (Fig. 72.3). In the smoke stack appearance, the leakage again starts as a pin point in the early phase, but it gradually tracks upward and then expands to form a mushroom cloud or umbrella-like appearance (Fig. 72.4). Smoke stack appearance is less common and only appears in about 10–15% of patients with acute CSC.50 It is caused by an increased protein concentration in the subretinal fluid. In cases in which there is PED, the FA appearance would be the pooling of dye in the sub-RPE space (Fig. 72.5). Chronic CSC might show an RPE window defect due to RPE atrophy. Multifocal CSC would show multiple sites of leakage. FA is also useful in differentiating CSC from other diagnoses such as choroidal neovascularization (CNV) and Vogt–Koyanagi–Harada disease.
Indocyanine green angiography
ICGA is one of the most important investigations in CSC because it demonstrates the choroidal vascular abnormalities and can act as a guide to treatments such as photodynamic therapy. In CSC, delay in choroidal filling is usually present. Typical features include abnormally dilated choroidal vasculature in the early phase and choroidal hyperpermeability in the late phase (Fig. 72.6).51–54 The area of choroidal vascular abnormality is usually much more widespread than the leakage point on FA and is commonly present in the fellow eye as well. Punctate hyperfluorescent spots are often seen in the mid-phase.55 In the later phases of ICGA, the dye usually leaks into the deeper layers of the choroid to produce hyperfluorescent patches.39 Hypofluorescent areas can also be seen on ICGA. They represent areas of choriocapillary nonperfusion and this might be one of the mechanisms that lead to choroidal venous dilation and congestion.
Optical coherence tomography
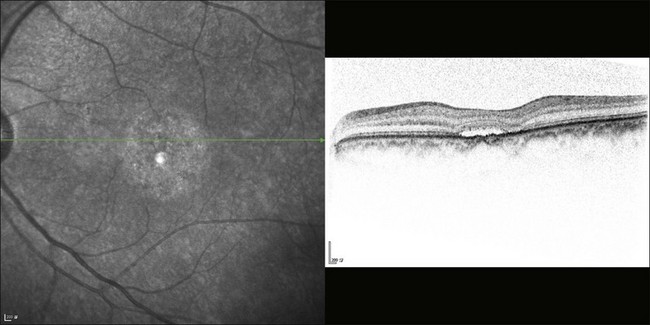
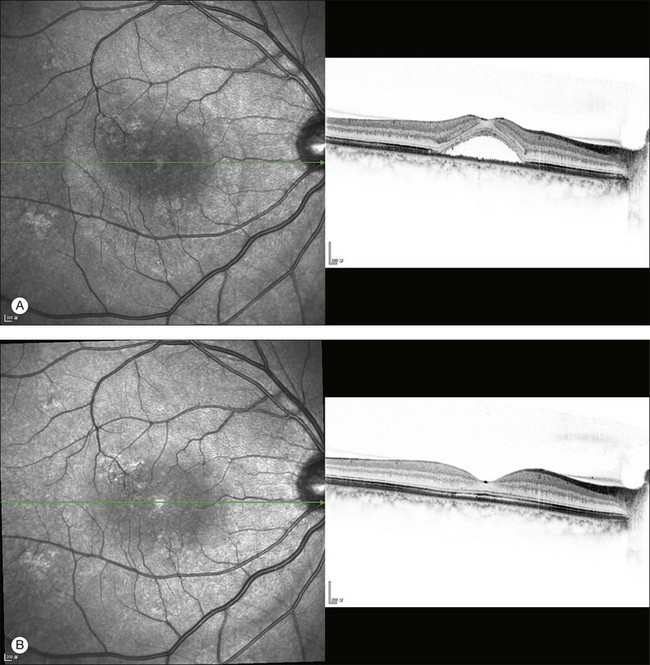
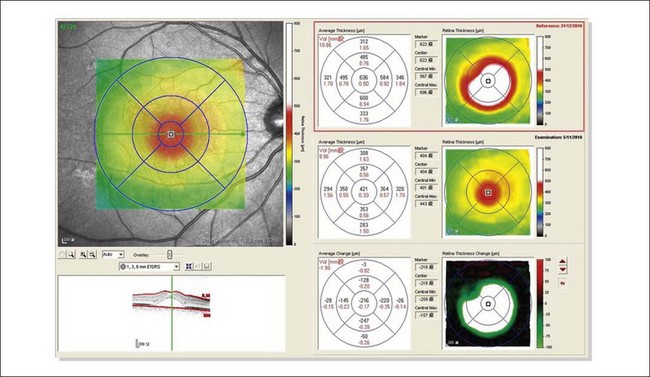
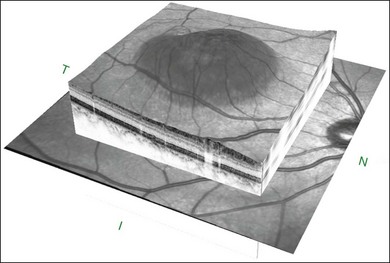
The availability of OCT has vastly enhanced the anatomical assessment and understanding of CSC by providing cross-sectional imaging of the macula. OCT demonstrates beautifully the presence of subretinal fluid or PED and helps in differentiation between the two.56,57 Subretinal yellow dots observed clinically, which typically show high reflectivity, can also be seen.42 Outer nuclear layer OCT is especially useful in detecting shallow subretinal fluid and small PED, which might be difficult to identify clinically (Fig. 72.7). OCT might be able to demonstrate lesions in the fellow asymptomatic eye, such as RPE bumps and small PED.58 Serial scans can be used to assess disease progression and treatment response (Fig. 72.8). Quantitative measurements and thickness maps can be generated and are useful for documentation as well as research purposes (Fig. 72.9). The software can also render three-dimensional images to allow better structural visualization and understanding (Fig. 72.10). Choroidal vascular hyperpermeability on ICGA is associated with an increase in subfoveal choroidal thickness on OCT.59,60 With the introduction of enhanced depth imaging, many researchers consider that increased choroidal thickening is a hallmark of CSC. OCT can also offer valuable prognostic information. Cystoid degeneration and disruption of the outer photoreceptor layer and the inner/outer-segment junction have been reported to be associated with poor visual outcomes.61 OCT can also assist in differentiating CSC from other diagnosis such as CNV and PCV by detecting lesions in the subretinal space (Fig. 72.11). Another reported distinguishing feature between PCV and CSC is that eyes with PCV might show thinning of the photoreceptor outer segments on OCT.62
Fundus autofluorescence
Fundus autofluorescence (FAF) is an adjunctive tool for the assessment of CSC. During the acute phase of the disease, FAF typically shows hypofluorescence at the leakage point and over the area of neurosensory detachment due to blockage by subretinal fluid.63 The subretinal yellow dots observed clinically might demonstrate hyperfluorescence.42 In chronic-recurrent CSC, hyperfluorescence is common in areas of residual neurosensory detachment. Therefore, FAF might give additional information on whether the disease is acute or chronic.64 Moreover, the pattern of FAF has been shown to correlate with visual acuity.65 After the resolution of subretinal fluid, areas of hyperfluorescence might become visible due to release of fluorophore materials into the subretinal space. Photopigment density has been studied with autofluorescence densitometry. It was found to be decreased in eyes with CSC and showed a delayed recovery after the resolution of subretinal fluid.66
Multifocal electroretinography
Multifocal electroretinography (mfERG) is useful in evaluating macular function in CSC. First- and second-order kernel mfERG response amplitudes have been shown to be reduced in patients with CSC.67,68 Reductions in response amplitudes appear to be localized in the center for the first-order kernel mfERG but predominately affect the more peripheral retina for the second-order kernel mfERG. These suggest that while outer retinal dysfunction is localized in the center, inner retinal dysfunction might be more widespread. Unlike OCT, mfERG response amplitudes were found to correlate with visual acuity.67,69 Therefore mfERG and OCT can complement each other in the functional and anatomical assessments of CSC respectively.
Microperimetry
Microperimetry is useful in the assessment of macular sensitivity in patients with CSC. Macular sensitivity is reduced in both the central and paracentral areas in the active phase of the disease when there is subretinal fluid.50 It has been shown to improve after the resolution of subretinal fluid with or without treatment.70–72 However, more often than not there are residual focal areas with reduced sensitivity that correspond to RPE irregularities or defects of the inner/outer-segment junction on OCT.73,74 Moreover, macular sensitivity was demonstrated to correlate with central macular thickness on OCT, which suggested the existence of structural and functional correlation.75
Natural history
The disease is usually self-limiting and 90% of the cases will show spontaneous recovery within a few months without significant visual loss.37,76,77 However, some patients may develop chronic or recurrent diseases that lead to areas of RPE atrophy or hypertrophy with visual loss. Poor visual acuity on presentation and a prolonged duration of serous macular detachment appear to be associated with poor visual outcomes.78 Up to 50% of patients might develop recurrence.79–82 Recurrence can develop at any time and CSC recurs in about 50% of the patients within the first year. A history of psychiatric illness is associated with a higher rate of recurrence.82 A small proportion of patients develop severe irreversible visual loss due to, for example, RPE atrophy, CNV development (in up to 6% of patients), and transformation into polypoidal choroidal vasculopathy (PCV) with exudation and hemorrhage. Adaptive optics scanning laser ophthalmoscopy was able to demonstrate reduced cone densities in eyes with resolved CSC.83 Even patients whose visual acuity has recovered to baseline might be left with residual symptoms such as metamorphopsia, scotoma, and reduced contrast sensitivity. Therefore, CSC should not be considered a benign disease.
Differential diagnosis
The diagnosis of CSC is usually clear and straightforward from clinical examination and is confirmed by FA, ICGA, and OCT. However, several diseases can mimic CSC and it is important to keep in mind the following differential diagnoses (Table 72.1).
Table 72.1 Important differential diagnoses of central serous chorioretinopathy and their differentiating features
| Differential diagnosis | Differentiating features |
|---|---|
| Optic disc pit | Presence of optic disc pit, absence of leakage on FA |
| AMD | Older age group, CNV on FA |
| PCV | ICGA shows polyps and branching vascular network |
| Inflammatory and infectious diseases | Systemic features and bilateral involvement in VKH; ultrasonic T-sign in posterior scleritis |
| Autoimmune and vascular disorders | Systemic features are usually evident |
| Intraocular tumors | Ultrasound is useful in the detection and differentiation between different types of tumors |
FA, fluorescein angiography; AMD, age-related macular degeneration; CNV, choroidal neovascularization; PCV, polypoidal choroidal vasculopathy; ICGA, indocyanine green angiography; VKH, Vogt–Koyanagi–Harada disease..
Polypoidal choroidal vasculopathy
PCV is characterized by the development of abnormal polypoidal dilations arising from the inner choroidal circulation, which causes recurrent episodes of exudative maculopathy with serous or hemorrhagic PED. When patients present PCV with simply serous PED or serous macular detachment, it might be mistaken as CSC. Differential diagnosis can be made by ICGA, which demonstrates branching vascular networks and polyps.84 OCT can also help to differentiate between the two entities. OCT is able to demonstrate polyps in PCV whereas in CSC, the subretinal space should be clean.
Treatment
Observation in most cases
Since CSC has a favorable natural history, observation has been considered as an appropriate first-line approach. As high levels of endogenous or exogenous corticosteroids have been implicated as an etiology for CSC, it is important to check whether CSC patients have such problems by asking if they have had exposure to nasal sprays, intra-articular injections or other covert sources of corticosteroids. In case of high levels of endogenous and exogenous corticosteroids, correction to normal levels, when possible, can lead to resolution of detachment in 90% of the cases.85 Lifestyle modification and other forms of psychosocial therapies may help in some CSC patients who are prone to psychological stress.37,86 However, with a greater understanding of pathophysiology and the natural course of CSC, there are increasing doubts about the proclaimed “benign” nature of CSC.37,86 Foveal attenuation, cystoid macular degeneration, and damage of the foveal photoreceptor layer have been proposed to be the pathological changes contributing to irreversible visual loss in CSC.37,86
There has been ongoing debate regarding when active treatment should be offered to patients with CSC. It is important to note that photoreceptor atrophy in the fovea occurs as early as 4 months after onset of symptoms.77 Therefore, it has been recommended that if symptoms persist for more than 3 months, further active treatments should be considered.37,86 On the other hand, there are data showing that although some treatments may speed up visual recovery, no treatment has been shown to improve the final visual acuity of patients with acute CSC.87,88 Therefore, we have to refrain from tipping the balance in favor of either observation or treatment. Treatments with known risks or side-effects should be used cautiously in acute CSC. Observation with vigilance is usually the mainstay of treatment for the good visual prognosis group: patients with good presenting visual acuity and duration of symptom presentation of less than 3 months. In the wake of increasing reports of permanent visual quality derangements including metamorphopsia, paracentral or central scotoma observed in patients having been managed conservatively by observation and reassurance,37,86 the indication for active and timely treatment, however, may be much stronger in some susceptible individuals who suffer from chronic CSC, i.e., symptoms presenting for at least six months or indication of serous macular elevation associated with RPE atrophic areas and subtle leaks or ill-defined staining on FA.39,89 The debate as to the best timing for treating CSC continues and further research is needed.
Treatment for selected cases
In the past few years, an array of new treatment modalities for CSC had emerged. Based on the best scientific evidence available in the literature, the efficacy and safety of these evolving treatment modalities should, however, be interpreted with caution as most of these studies have limitations like selection bias, lack of randomization, a small sample size, and short follow-up duration.37,86
Safety-enhanced photodynamic therapy in selected cases
PDT with verteporfin
Following the success of TAP and VIP studies in the management of age-related macular degeneration, expanded indication of PDT has allowed attempted use of PDT to tackle some CSC cases.90,91 Subsequent studies have confirmed efficacy and favorable visual outcomes of PDT to treat CSC in a majority of cases, especially if it is used under guidance of indocyanine green angiography (ICGA).92–97 The proposed mechanisms of PDT are choriocapillaris narrowing, choroidal hypoperfusion, reduction of choroidal exudation and choroidal vascular remodeling.96,98,99 However, the use of PDT is not without complications, especially with conventional verteporfin dosage, as well as fluence and large laser spot size, as RPE atrophy, choriocapillaris ischemia, and secondary CNV have been reported.94,96 The use of mfERG has further revealed that PDT might cause transient impairment in retinal function after treatment.100,101
Conventional PDT with normal dosage and fluence
Conventional or standard PDT for treatment of neovascular age-related macular degeneration was performed using the dose of 6 mg/m2 infusion of verteporfin (Visudyne, Novartis AG, Bülach, Switzerland). Infusion of verteporfin was performed over 15 minutes, followed by delivery of laser at 692 nm 15 minutes after commencement of the infusion. A total light energy of 50 J/cm2, light dose rate of 600 mW/cm2, and photosensitization time of 83 seconds was delivered to the targeted area. Pilot studies showed normalization in calibers of the dilated and congested choroidal vasculature, and a decrease in the extravascular leakage in cases treated by PDT.96 The effect of the vascular modulation persisted and was sustained for years. With alterations in blood flow and decreased leakages in the choroidal vessels, the corresponding fluorescence leakages also stopped in the fluorescein angiography coupled with subjective and objective visual improvements in most of the patients who had received treatment for CSC. The possible development of CNV, post-treatment visual loss, and potential choroidal ischemia may have limited the widespread application of standard dosage PDT treatment for CSC patients.
Safety-enhanced PDT with reduced verteporfin dosage
In order to enhance the efficacy of PDT in treating CSC while minimizing its side-effects, various ways such as reducing the dosage of verteporfin and shortening the interval between infusion and laser application have been tried. As demonstrated by a previous study, the maximal concentration of verteporfin within the choroidal circulation is achieved 10 minutes after the infusion, when the concentration at the retinal outer segment is still relatively low.102 Shortening the interval between the infusion and laser application might, therefore, minimize any collateral damage to adjacent retinal structures. Reducing the dosage of verteporfin infusion by half may lower the risk of retinal-choroidal complications without compromising the vascular remodeling ability of PDT as photochemical response in the choroid is dose–response-dependent.103 Safety-enhanced PDT was performed using half the normal dose of verteporfin at 3 mg/m2. Infusion of verteporfin was performed over 8 minutes, followed by a delivery of laser at 692 nm 10 minutes afterwards. A total light energy of 50 J/cm2 was delivered within 83 seconds.104,105 The treatment efficacy of this half-dose PDT was not affected while the well-proven safety issues such as impairment of retinal function associated with full-dose PDT were alleviated by the use of the half-dose regimen, as reflected by prospective studies worldwide.72,104,105 In a study of 48 eyes from 48 patients with chronic central serous chorioretinopathy, 40 (83.3%) eyes had complete resolution of serous detachment 3 months afterwards, and 43 (89.6%) eyes, 12 months afterwards. The mean improvement in visual acuity was 1.6 lines and 45 (95.8%) eyes had stable or improved vision. Eyes without PED had significantly greater visual improvement, compared with eyes with PED. Interestingly, patients with CSC for 6 months or less, or younger than 45 years of age, were more likely to gain vision by 2 or more lines after treatment.105 In a randomized controlled trial involving 63 patients with acute symptomatic CSC for the duration of less than 3 months, 39 patients were randomized into the verteporfin group and 19 patients were enrolled into the placebo group. Thirty-seven (94.9%) eyes in the verteporfin group, compared with 11 (57.9%) eyes in the placebo group, showed absence of macular subretinal fluid 12 months afterwards. All the 39 (100%) eyes treated with verteporfin had stable or improved vision, compared with 15 (78.9%) eyes in the placebo group. No ocular or systemic adverse event was encountered in all the treated patients.87 The success of safety-enhanced PDT to manage CSC has given birth to more than 10 prospective, retrospective, and/or interventional case series, with or without randomization, so far.37,86 In spite of the encouraging results and reports on the safety of half-dose PDT in the management of CSC, it is prudent to individualize management plans owing to the absence of a randomized controlled trial of a large sample size to document treatment adversity, and sporadic observations of transient impairment of multifocal ERG response with the use of half-dose PDT.105,106
Safety-enhanced PDT with reduced laser fluence
Drug dosage and the timing of laser application are not the only parameters that can be modified to enhance the safety of PDT. Similarly, other parameters like laser fluence and infusion time can also be changed. For instance, improved efficacy and safety profiles of low fluence PDT have been documented in the management of chronic CSC.107–110 It should, however, be noted that a lack of quality randomized controlled trials to discern either short-term or long-term safety, coupled with reports of severe complications like RPE rip/tear, is sufficient to remind clinicians of the importance of caution and individualization when managing CSC by reduced laser fluence PDT.111
Historical thermal (argon) laser photocoagulation and micropulsed diode laser
The use of thermal laser photocoagulation in the management of CSC probably stems from the observation that a successful laser photocoagulation of a fluorescein-detectable pigment epithelial leak may accelerate resolution of the associated neurosensory detachment.37,76,77,112,113 This initially promising treatment method has gone out of favor in recent years because of significant adverse effects such as permanent scotoma, enlargement of RPE scar, secondary laser-induced CNV formation, and, rarely but sinisterly, inadvertent foveal photocoagulation, especially if the leaking point is subfoveal, or parafoveal, or when more than one pigment epithelial leakage is identified.114–117 Therefore, in the event of multiple leaking points or subfoveal leaking, safety-enhanced PDT may be considered as a viable alternative. Thermal laser photocoagulation may still have a role in the management of CSC with a discrete, solitary extrafoveal leaking point. However, it should be emphasized that thermal laser photocoagulation may not affect the final functional outcomes and the rate of recurrence.80 This may be due to the fact that zonal hyperperfusion and hyperpermeability of the choriocapillaris, the presumptive primary alternation in CSC, are not amenable to laser photocoagulation therapy. Piccolino et al. studied 145 patients with CSC by ICGA and areas of choroidal leakage attributable to hyperpermeability of choriocapillaris were identified in 98.6% of the patients in association with active or resolved CSC.118 Some areas of choroidal hyperpermeability and leakage did not show any significant change after a mean follow-up period of 10 months. These changes in the choroidal level persisted even after resolution of fluorescein leakage and subretinal fluid.103 As a result, recurrence of the fluorescein leakage point may still occur over areas of choroidal hyperpermeability despite thermal laser treatment.
There has been a revival of interest in recent years in using laser, or micropulse diode laser, instead of the conventional argon laser photocoagulation, to treat CSC.119–123 The diode laser with micropulsed emission enables subthreshold therapy without a visible burn endpoint. This may theoretically curtail the risk of structural and functional retinal damage while retaining the therapeutic efficacy of conventional laser treatment. A small case series has demonstrated the beneficial effect of using indocyanine green (ICG) dye-enhanced subthreshold diode-laser micropulse (SDM) photocoagulation.123 Nevertheless, there are concerns over the efficacy and safety of micropulse diode laser in the face of more than one leaking point, which is more commonly encountered in chronic CSC. Randomized controlled trials involving comparison between micropulse diode laser and placebo or control groups are warranted in order to fully substantiate the observed treatment efficacy and safety of micropulse diode laser in the management of CSC.
Transpupillary thermotherapy
Several small case series have shown promising results of transpupillary thermotherapy (TTT) in treating chronic CSC cases and suggested that it may be a viable treatment alternative.124–127 Future studies involving comparison of TTT with either standard treatment like PDT or placebo are warranted.
Intravitreal anti-VEGF therapy with or without adjuvant PDT
In the era of intravitreal pharmacology and therapeutics for macular diseases, it is not surprising that we should use intravitreal anti-VEGF to treat CSC. Several studies have demonstrated anatomic and functional improvement following either intravitreal bevacizumab or intravitreal ranibizumab injections.110,128–133 These findings suggest that VEGF may be involved in fluid leakage in patients who have suffered from chronic CSC, despite the failure to detect changes in VEGF and interleukine-8 concentrations in aqueous humor and plasma from CSC patients after intravitreal anti-VEGF injections.28 It has been suggested that combined PDT and intravitreal anti-VEGF has a beneficial role to play for CSC patients in a small case series but long-term efficacy and safety remain issues that warrant further studies.134
Anticorticosteroid treatment
Anticorticosteroid treatment was first suggested by Jampol et al.135 This was based on the association of endogenous hypercortisolism with the development of CSC. The use of antiglucocorticoid agents including RU486 (mifepristone) and ketoconazole has not yet yielded significant results. The interest in using an anticorticosteroid to treat CSC has been revived since Packo and coworkers reported an interesting observation at the 2010 American Society of Retina Specialists Annual Meeting. They found that rifampin, a semisynthetic antituberculosis antibiotic with DNA-dependent RNA polymerase inhibitory effect, had caused resolution of macular edema and subretinal fluid in a CSC patient suspected initially to have tuberculosis. It is believed that the collateral anticorticosteroid or endogenous steroid production inhibition of rifampin may contribute to its inadvertent therapeutic efficacy in CSC. Since then, Packo et al. have used rifampin in several other patients with CSC and good results with resolution of the fluid were seen within 1–4 weeks.136 Further research in using corticosteroid antagonists in treatment of CSC is warranted.
Management of special variants of CSC
Bullous CSC and its putative management
Bullous CSC is an uncommon form of CSC associated with a large amount of subretinal fluid and notably poor visual prognosis because of its frequent recurrence even after initial complete regression.137 Traditional management options including observation or thermal laser photocoagulation, however, have dubious treatment benefits as the outcome of thermal laser photocoagulation is similar to the natural course of the disease in terms of disease duration and final visual acuity.137 Ng et al. have reported the beneficial treatment effect of half-dose verteporfin PDT in the management of a case of bullous CSC with complete resolution of subretinal fluid and bullous retina detachment three months afterwards.138
Other potential but still exploratory systemic therapies
Systemic acetazolamide
The use of systemic acetazolamide to treat CSC came from sporadic cases and series reports claiming resolution of neurosensory retinal detachmentt after several weeks of systemic acetazolamide therapy.139 Although acetazolamide might shorten the duration of symptoms it does not alter the final visual outcomes and the recurrence rate.140 Prior to a good clinical trial involving systemic acetazolamide for CSC, its role remains elusive.
Antiadrenergic blockage and beta-blockers
Tatham et al. have reported two successful trials of using oral propanolol 40 mg twice a day in two CSC patients.141 It is, however, unknown whether the resolution of neurosensory retinal detachment was due to the proposed therapeutic effect of a beta-blocker or a spontaneous recovery.
Aspirin, finasteride, anti-Helicobacter pylori treatment
The usefulness of low-dose aspirin (100 mg per day orally for 1 month, followed by 100 mg on alternate days for 5 months) to manage CSC was identified in a prospective study comprising 109 study subjects and 89 historic control subjects.142 More rapid visual rehabilitation with fewer recurrences in the treated group was observed. The effectiveness of treatment with aspirin may be accounted for by its antiplatelet aggregation and profibinolytic effect against the postulated multifocal vascular occlusive disease of choriocapillaris in CSC (under the influence of plasminogen activator inhibitor-1, PAI-1).142 A small pilot case series consisting of five chronic CSC patients who were put on finasteride, an inhibitor of dihydrotestosterone synthesis, has reported anatomical improvement and drug safety despite a lack of concomitant visual improvement.143 Another prospective study involving 25 H. pylori-positive CSC patients treated with the standard H. pylori eradication regiment (metronidazole and amoxicillin 500 mg three times a day for 2 weeks and omeprazole once a day for 6 weeks) has demonstrated an expedition of subretinal fluid reabsorption, compared with the 25 controls.144 No effect on final visual outcomes or recurrence rate was noted.144 Similar to other new treatment modalities, the aforementioned new systemic treatments for CSC may require well-designed randomized controlled trials to document their long-term efficacy and safety.
1 Von Graefe A. Ueber central recidivierende retinitis. Graefes Arch Clin Exp Ophthalmol. 1866;12:211–215.
2 Spaide RF, Goldbaum M, Wong DWK, et al. Serous detachment of the retina. Retina. 2003;23:820–846.
3 Guyer DR, Yannuzzi LA, Slakter JS, et al. Digital indocyanine green videoangiography of central serous chorioretinopathy. Arch Ophthalmol. 1994;112:1057–1062.
4 Gass JD. Stereoscopic atlas of macular diseases. St Louis: CV Mosby; 1987.
5 Pryds A, Sander B, Larsen M. Characterization of subretinal fluid leakage in central serous chorioretinopathy. Invest Ophthalmol Vis Sci. 2010;51:5853–5857.
6 Horniker. Su di unaforma di retinite centrale di origine vasoneurotica. Ann Ottalmol. 1927;55:578–600. 830–40, 665–63
7 Yannuzzi LA. Type-A behavior and central serous chorioretinopathy. Retina. 1987;7:111–131.
8 Zakir SM, Shukla M, Simi ZU, et al. Serum cortisol and testosterone levels in idiopathic central serous chorioretinopathy. Indian J Ophthalmol. 2009;57:419–422.
9 Friberg TR, Eller AW. Serous retinal detachment resembling central serous chorioretinopathy following organ transplantation. Graefes Arch Clin Exp Ophthalmol. 1990;228:305–309.
10 Gass JD, Little H. Bilateral bullous exudative retinal detachment complicating idiopathic central serous chorioretinopathy during systemic corticosteroid therapy. Ophthalmology. 1995;102:737–747.
11 Lee CS, Kang EC, Lee KS, et al. Central serous chorioretinopathy after renal transplantation. Retina. 2011;31:1896–1903.
12 Hurvitz AP, Hodapp KL, Jadgchew J, et al. Central serous chorioretinopathy resulting in altered vision and color perception after glenohumeral corticosteroid injection. Orthopedics. 2009;32:600.
13 Haimovici R, Gragoudas ES, Duker JS, et al. Central serous chorioretinopathy associated with inhaled or intranasal corticosteroids. Ophthalmology. 1997;104:1653–1660.
14 Fernandez C, Mendoza AJ, Arevalo JF. Central serous chorioretinopathy associated with topical dermal corticosteroids. Retina. 2004;24:471–474.
15 Garg SP, Dada T, Talwar D, et al. Endogenous cortisol profile in patients with central serous chorioretinopathy. Br J Ophthalmol. 1997;81:962–964.
16 Haimovici R, Koh S, Gagnon DR, et al. Risk factors for central serous chorioretinopathy: a case–control study. Ophthalmology. 2004;111:244–249.
17 Gass JD. Central serous chorioretinopathy and white subretinal exudation during pregnancy. Arch Ophthalmol. 1991;109:677–681.
18 Fastenberg DM, Ober RR. Central serous choroidopathy in pregnancy. Arch Ophthalmol. 1983;101:1055–1058.
19 Quillen DA, Gass DM, Brod RD, et al. Central serous chorioretinopathy in women. Ophthalmology. 1996;103:72–79.
20 Iannetti L, Spinucci G, Pesci FR, et al. Central serous chorioretinopathy as a presenting symptom of endogenous Cushing syndrome: a case report. Eur J Ophthalmol. 2011;21:661–664.
21 Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 1992;267:1244–1252.
22 Smith TJ. Dexamethasone regulation of glycosaminoglycan synthesis in cultured human skin fibroblasts. Similar effects of glucocorticoid and thyroid hormones. J Clin Invest. 1984;74:2157–2163.
23 Pratt WB, Aronow L. The effect of glucocorticoids on protein and nucleic acid synthesis in mouse fibroblasts growing in vitro. J Biol Chem. 1966;241:5244–5250.
24 Bastl CP. Regulation of cation transport by low doses of glucocorticoids in in vivo adrenalectomized rat colon. J Clin Invest. 1987;80:348–356.
25 Sandle GI, McGlone F. Acute effects of dexamethasone on cation transport in colonic epithelium. Gut. 1987;28:701–706.
26 Caccavale A, Romanazzi F, Imparato M, et al. Central serous chorioretinopathy: a pathogenetic model. Clin Ophthalmol. 2011;5:239–243.
27 Shin MC, Lim JW. Concentration of cytokines in the aqueous humor of patients with central serous chorioretinopathy. Retina. 2011;31:1937–1943.
28 Lim JW, Kim MU, Shin MC. Aqueous humor and plasma levels of vascular endothelial growth factor and interleukin-8 in patients with central serous chorioretinopathy. Retina. 2010;30:1465–1471.
29 Rahbani-Nobar MB, Javadzadeh A, Ghojazadeh L, et al. The effect of Helicobacter pylori treatment on remission of idiopathic central serous chorioretinopathy. Mol Vis. 2011;17:99–103.
30 Cotticelli L, Borrelli M, D’Alessio AC, et al. Central serous chorioretinopathy and Helicobacter pylori. Eur J Ophthalmol. 2006;16:274–278.
31 Misiuk-Hojlo M, Michalowska M, Turno-Krecicka A. Helicobacter pylori –a risk factor for the developement of the central serous chorioretinopathy. Klin Oczna. 2009;111:30–32.
32 Tittl MK, Spaide RF, Wong D, et al. Systemic findings associated with central serous chorioretinopathy. Am J Ophthalmol. 1999;128:63–68.
33 Jain AK, Kaines A, Schwartz S. Bilateral central serous chorioretinopathy resolving rapidly with treatment for obstructive sleep apnea. Graefes Arch Clin Exp Ophthalmol. 2010;248:1037–1039.
34 Imamura Y, Fujiwara T, Spaide RF. Frequency of glaucoma in central serous chorioretinopathy: a case-control study. Retina. 2010;30:267–270.
35 Moschos MM, Droutsas K, Margetis I. Central serous chorioretinopathy after rhinoplasty. Case Report Ophthalmol. 2010;1:90–93.
36 Peponis VG, Chalkiadakis SE, Nikas SD, et al. Bilateral central serous retinopathy following laser in situ keratomileusis for myopia. J Cataract Refract Surg. 2011;37:778–780.
37 Ross A, Ross AH, Mohamed Q. Review and update of central serous chorioretinopathy. Curr Opin Ophthalmol. 2011;22(3):166–173.
38 Leibowitz HM, Krueger DE, Maunder LR, et al. The Framingham Eye Study monograph: An ophthalmological and epidemiological study of cataract, glaucoma, diabetic retinopathy, macular degeneration, and visual acuity in a general population of 2631 adults, 1973–1975. Surv Ophthalmol. 1980;24(Suppl):335–610.
39 Spaide RF, Campeas L, Haas A, et al. Central serous chorioretinopathy in younger and older adults. Ophthalmology. 1996;103:2070–2079. discussion 9–80
40 Fukunaga K. [Central chorioretinopathy with disharmony of the autonomous nerve system.]. Nippon Ganka Gakkai Zasshi. 1969;73:1468–1477.
41 Desai UR, Alhalel AA, Campen TJ, et al. Central serous chorioretinopathy in African Americans. J Natl Med Assoc. 2003;95:553–559.
42 Maruko I, Iida T, Ojima A, et al. Subretinal dot-like precipitates and yellow material in central serous chorioretinopathy. Retina. 2011;31:759–765.
43 Ie D, Yannuzzi LA, Spaide RF, et al. Subretinal exudative deposits in central serous chorioretinopathy. Br J Ophthalmol. 1993;77:349–353.
44 Gass JD. Stereoscopic atlas of macular diseases: diagnosis and treatment, 4th ed. St Louis: Mosby; 1997.
45 Yannuzzi LA, Shakin JL, Fisher YL, et al. Peripheral retinal detachments and retinal pigment epithelial atrophic tracts secondary to central serous pigment epitheliopathy. Ophthalmology. 1984;91:1554–1572.
46 Cohen D, Gaudric A, Coscas G, et al. [Diffuse retinal epitheliopathy and central serous chorioretinopathy.]. J Fr Ophtalmol. 1983;6:339–349.
47 Gass JD. Bullous retinal detachment. An unusual manifestation of idiopathic central serous choroidopathy. Am J Ophthalmol. 1973;75:810–821.
48 Nadel AJ, Turan MI, Coles RS. Central serous retinopathy. A generalized disease of the pigment epithelium. Mod Probl Ophthalmol. 1979;20:76–88.
49 Schatz H, Osterloh MD, McDonald HR, et al. Development of retinal vascular leakage and cystoid macular oedema secondary to central serous chorioretinopathy. Br J Ophthalmol. 1993;77:744–746.
50 Bujarborua D, Nagpal PN, Deka M. Smokestack leak in central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2010;248:339–351.
51 Prunte C, Flammer J. Choroidal capillary and venous congestion in central serous chorioretinopathy. Am J Ophthalmol. 1996;121:26–34.
52 Spaide RF, Hall L, Haas A, et al. Indocyanine green videoangiography of older patients with central serous chorioretinopathy. Retina.. 1996;16:203–213.
53 Iida T, Kishi S, Hagimura N, et al. Persistent and bilateral choroidal vascular abnormalities in central serous chorioretinopathy. Retina. 1999;19:508–512.
54 Iida T, Spaide RF, Haas A, et al. Leopard-spot pattern of yellowish subretinal deposits in central serous chorioretinopathy. Arch Ophthalmol. 2002;120:37–42.
55 Tsujikawa A, Ojima Y, Yamashiro K, et al. Punctate hyperfluorescent spots associated with central serous chorioretinopathy as seen on indocyanine green angiography. Retina. 2010;30:801–809.
56 Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science. 1991;254:1178–1181.
57 Hee MR, Puliafito CA, Wong C, et al. Optical coherence tomography of central serous chorioretinopathy. Am J Ophthalmol. 1995;120:65–74.
58 Gupta P, Gupta V, Dogra MR, et al. Morphological changes in the retinal pigment epithelium on spectral-domain OCT in the unaffected eyes with idiopathic central serous chorioretinopathy. Int Ophthalmol. 2010;30:175–181.
59 Maruko I, Iida T, Sugano Y, et al. Subfoveal choroidal thickness in fellow eyes of patients with central serous chorioretinopathy. Retina. 2011;31(8):1603–1608.
60 Imamura Y, Fujiwara T, Margolis R, et al. Enhanced depth imaging optical coherence tomography of the choroid in central serous chorioretinopathy. Retina. 2009;29:1469–1473.
61 Kim YY, Flaxel CJ. Factors influencing the visual acuity of chronic central serous chorioretinopathy. Korean J Ophthalmol. 2011;25:90–97.
62 Ooto S, Tsujikawa A, Mori S, et al. Thickness of photoreceptor layers in polypoidal choroidal vasculopathy and central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2010;248:1077–1086.
63 Dinc UA, Tatlipinar S, Yenerel M, et al. Fundus autofluorescence in acute and chronic central serous chorioretinopathy. Clin Exp Optom. 2011;94:452–457.
64 Framme C, Walter A, Gabler B, et al. Fundus autofluorescence in acute and chronic-recurrent central serous chorioretinopathy. Acta Ophthalmol Scand. 2005;83:161–167.
65 Imamura Y, Fujiwara T, Spaide RF. Fundus autofluorescence and visual acuity in central serous chorioretinopathy. Ophthalmology. 2011;118:700–705.
66 Ojima A, Iida T, Sekiryu T, et al. Photopigments in central serous chorioretinopathy. Am J Ophthalmol. 2011;151:940–952. e1
67 Lai TY, Lai RY, Ngai JW, et al. First- and second-order kernel multifocal electroretinography abnormalities in acute central serous chorioretinopathy. Doc Ophthalmol. 2008;116:29–40.
68 Shimada Y, Imai D, Ota Y, et al. Retinal adaptability loss in serous retinal detachment with central serous chorioretinopathy. Invest Ophthalmol Vis Sci. 2010;51:3210–3215.
69 Yip YWY, Ngai JWS, Fok ACT, et al. Correlation between functional and anatomical assessments by multifocal electroretinography and optical coherence tomography in central serous chorioretinopathy. Doc Ophthalmol. 2010;120:193–200.
70 Reibaldi M, Boscia F, Avitabile T, et al. Functional retinal changes measured by microperimetry in standard-fluence vs low-fluence photodynamic therapy in chronic central serous chorioretinopathy. Am J Ophthalmol. 2011;151:953–960.
71 Senturk F, Karacorlu M, Ozdemir H, et al. Microperimetric changes after photodynamic therapy for central serous chorioretinopathy. Am J Ophthalmol. 2011;151:303–309.
72 Fujita K, Yuzawa M, Mori R. Retinal sensitivity after photodynamic therapy with half-dose verteporfin for chronic central serous: short-term results. Retina. 2011;31:772–778.
73 Ojima Y, Tsujikawa A, Hangai M, et al. Retinal sensitivity measured with the micro perimeter 1 after resolution of central serous chorioretinopathy. Am J Ophthalmol. 2008;146:77–84.
74 Kim S-W, Oh J, Huh K. Correlations among various functional and morphological tests in resolved central serous chorioretinopathy. Br J Ophthalmol. 2011. PMID 21617156 Epub ahead of print May 26
75 Dinc UA, Yenerel M, Tatlipinar S, et al. Correlation of retinal sensitivity and retinal thickness in central serous chorioretinopathy. Ophthalmologica. 2010;224:2–9.
76 Aggio FB, Roisman L, Melo GB, et al. Clinical factors related to visual outcome in central serous chorioretinopathy. Retina. 2010;30:1128–1134.
77 Gass JD. Pathogenesis of disciform detachment of the neuroepithelium. Am J Ophthalmol. 1967;63(Suppl):1–139.
78 Ficker L, Vafidis G, While A, et al. Long-term follow-up of a prospective trial of argon laser photocoagulation in the treatment of central serous retinopathy. Br J Ophthalmol. 1988;72:829–834.
79 Yap EY, Robertson DM. The long-term outcome of central serous chorioretinopathy. Arch Ophthalmol. 1996;114:689–692.
80 Fok AC, Chan PP, Lam DS, et al. Risk factors for recurrence of serous macular detachment in untreated patients with central serous chorioretinopathy. Ophthalmic Res. 2011;46:160–163.
81 Ooto S, Hangai M, Sakamoto A, et al. High-resolution imaging of resolved central serous chorioretinopathy using adaptive optics scanning laser ophthalmoscopy. Ophthalmology. 2010;117:1800–1809. 1809.e1–2
82 Spaide RF, Yannuzzi LA, Slakter JS, et al. Indocyanine green videoangiography of idiopathic polypoidal choroidal vasculopathy. Retina. 1995;15:100–110.
83 Sharma T, Shah N, Rao M, et al. Visual outcome after discontinuation of corticosteroids in atypical severe central serous chorioretinopathy. Ophthalmology. 2004;111:1708–1714.
84 Gemenetzi M, De Salvo G, Lotery AJ. Central serous chorioretinopathy: update on pathogenesis and treatments. Eye. 2010;24:1743–1756.
85 Yannuzzi LA. Central serous chorioretinopathy: a personal perspective. Am J Ophthalmol. 2010;149:361–363.
86 Wang MS, Sander B, Larsen M. Retinal atrophy in idiopathic central serous chorioretinopathy. Am J Ophthalmol. 2002;133:787–793.
87 Chan WM, Lai TY, Lai RY, et al. Half-dose verteporfin photodynamic therapy for acute central serous chorioretinopathy: one-year results of a randomized controlled trial. Ophthalmology. 2008;115:1756–1765.
88 Lim JW, Ryu SJ, Shin MC. The effect of intravitreal bevacizumab in patients with acute central serous chorioretinopathy. Korean J Ophthalmol. 2010;24:155–158.
89 Yannuzzi LA, Slakter JS, Kaufman SR, et al. Laser treatment of diffuse retinal pigment epitheliopathy. Eur J Ophthalmol. 1992;2:103–114.
90 Chan WM, Lai TY, Tano Y, et al. Photodynamic therapy in macular diseases of Asian populations: when East meets West. Jpn J Ophthalmol. 2006;50:161–169.
91 Chan WM, Lim TH, Pece A, et al. Verteporfin PDT for non-standard indications – a review of current literature. Graefes Arch Clin Exp Ophthalmol. 2010;248:613–626.
92 Battaglia Parodi M, Da Pozzo S, Ravalico G. Photodynamic therapy in chronic central serous chorioretinopathy. Retina. 2003;23:235–237.
93 Canakis C, Livir-Rallatos C, Panayiotis Z, et al. Ocular photodynamic therapy for serous macular detachment in the diffuse retinal pigment epitheliopathy variant of idiopathic central serous chorioretinopathy. Am J Ophthalmol. 2003;136:750–752.
94 Cardillo Piccolino F, Eandi CM, Ventre L, et al. Photodynamic therapy for chronic central serous chorioretinopathy. Retina. 2003;23:752–763.
95 Yannuzzi LA, Slakter JS, Gross NE, et al. Indocyanine green angiography-guided photodynamic therapy for treatment of chronic central serous chorioretinopathy: a pilot study. Retina. 2003;23:288–298.
96 Chan WM, Lam DS, Lai TY, et al. Choroidal vascular remodelling in central serous chorioretinopathy after indocyanine green guided photodynamic therapy with verteporfin: a novel treatment at the primary disease level. Br J Ophthalmol. 2003;87:1453–1458.
97 Taban M, Boyer DS, Thomas EL, et al. Chronic central serous chorioretinopathy: photodynamic therapy. Am J Ophthalmol. 2004;137:1073–1080.
98 Schlotzer-Schrehardt U, Viestenz A, Naumann GO, et al. Dose-related structural effects of photodynamic therapy on choroidal and retinal structures of human eyes. Graefes Arch Clin Exp Ophthalmol. 2002;240:748–757.
99 Schmidt-Erfurth U, Laqua H, Schlotzer-Schrehard U, et al. Histopathological changes following photodynamic therapy in human eyes. Arch Ophthalmol. 2002;120:835–844.
100 Lai TY, Chan WM, Lam DS. Transient reduction in retinal function revealed by multifocal electroretinogram following photodynamic therapy. Am J Ophthalmol. 2004;137:826–833.
101 Tzekov R, Lin T, Zhang KM, et al. Ocular changes after photodynamic therapy. Invest Ophthalmol Vis Sci. 2006;47:377–385.
102 Haimovici R, Kramer M, Miller JW, et al. Localization of lipoprotein-delivered benzoporphyrin derivative in the rabbit eye. Curr Eye Res. 1997;16:83–90.
103 Maruko I, Iida T, Sugano Y, et al. Subfoveal choroidal thickness after treatment of central serous chorioretinopathy. Ophthalmology. 2010;117:1792–1799.
104 Lai TY, Chan WM, Li H, et al. Safety enhanced photodynamic therapy with half dose verteporfin for chronic central serous chorioretinopathy: a short term pilot study. Br J Ophthalmol. 2006;90:869–874.
105 Chan WM, Lai TY, Lai RY, et al. Safety enhanced photodynamic therapy for chronic central serous chorioretinopathy: one-year results of a prospective study. Retina. 2008;28:85–93.
106 Wu ZH, Lai RY, Yip YW, et al. Improvement in multifocal electroretinography after half-dose verteporfin photodynamic therapy for central serous chorioretinopathy: a randomized placebo-controlled trial. Retina. 2011. PMID 21386761 Epub ahead of print March 3
107 Reibaldi M, Cardascia N, Longo A, et al. Standard-fluence versus low-fluence photodynamic therapy in chronic central serous chorioretinopathy: a nonrandomized clinical trial. Am J Ophthalmol. 2010;149:307–315.
108 Reibaldi M, Boscia F, Avitabile T, et al. Functional retinal changes measured by microperimetry in standard-fluence vs low-fluence photodynamic therapy in chronic central serous chorioretinopathy. Am J Ophthalmol. 2011;151:953–960.
109 Shin JY, Woo SJ, Yu HG, et al. Comparison of efficacy and safety between half-fluence and full-fluence photodynamic therapy for chronic central serous chorioretinopathy. Retina. 2011;31:119–126.
110 Bae SH, Heo JW, Kim C, et al. Randomized pilot study of low-fluence photodynamic therapy versus intravitreal ranibizumab for chronic central serous chorioretinopathy. Am J Ophthalmol. 2011;152:784–792.
111 Kim SW, Oh J, Oh IK, et al. Retinal pigment epithelial tear after half fluence PDT for serous pigment epithelial detachment in central serous chorioretinopathy. Ophthalmic Surg Lasers Imaging. 2009;40:300–303.
112 Ascaso FJ, Rojo M, Minguez E, et al. Diagnostic and therapeutic challenges. Retina. 2011;31:616–622.
113 Burumcek E, Mudun A, Karacorlu S, et al. Laser photocoagulation for persistent central serous chorioretinopathy: results of long term follow-up. Ophthalmology. 1997;104:616–622.
114 Robertson DM, Ilstrup D. Direct, indirect, and sham laser photocoagulation in the management of central serous chorioretinopathy. Am J Ophthalmol.. 1983;95:457–466.
115 Gartner J. Long-term follow-up of an ophthalmologist’s central serous retinopathy,photocoagulated by sungazing. Doc Ophthalmol. 1987;66:19–33.
116 Lim JI. Iatrogenic choroidal neovascularization. Surv Ophthalmol. 1999;44:95–111.
117 Lim JW, Kang SW, Kim YT, et al. Comparative study of patients with central serous chorioretinopathy undergoing focal laser photocoagulation or photodynamic therapy. Br J Ophthalmol. 2011;95:514–517.
118 Piccolino FC, Borgia L, Zinicola E, et al. Indocyanine green angiographic findings in central serous chorioretinopathy. Eye. 1995;9:324–332.
119 Sivaprasad S, Elagonz M, McHugh D, et al. Micropulsed diode laser therapy: evolution and clinical applications. Surv Ophthalmol. 2010;55:516–530.
120 Gupta B, Elagouz M, McHugh D, et al. Micropulse diode laser photocoagulation for central serous chorioretinopathy. Clin Exp Ophthalmol. 2009;37:801–805.
121 Chen SN, Hwang JF, Tseng LF, et al. Subthreshold diode micropulse photocoagulation for the treatment of chronic central serous chorioretinopathy with juxtafoveal leakage. Ophthalmology. 2008;115:2229–2234.
122 Lanzetta P, Furlan F, Morgante L, et al. Nonvisible subthreshold micropulse diode laser (810 nm) treatment of central serous chorioretinopathy. A pilot study. Eur J Ophthalmol. 2008;18:934–940.
123 Ricci F, Missiroli F, Regine F, et al. Indocycnine green enhanced subthreshold diode-laser micropulse photocoagulation treatment of chronic central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2009;247:597–607.
124 Hussain N, Khanna R, Hussain A, et al. Transpupillary thermotherapy for chronic central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2006;244:1045–1051.
125 Shukla D, Kolluru C, Vignesh TP, et al. Transpupillary thermotherapy for subfoveal leaks in central serous chorioretinopathy. Eye. 2008;22:100–106.
126 Sharma T, Parikh SD. Transpupillary thermotherapy for juxtafoveal leak in central serous chorioretinopathy. Ophthalmic Surg Lasers Imaging. 2010;9:1–3.
127 Giudice GL, de Belvis V, Tavolato M, et al. Large-spot subthreshold transpupillary thermotherapy for chronic serous macular detachment. Clin Ophthalmol. 2011;5:355–360.
128 Schaal KB, Hoeh AE, Scheuerle A, et al. Intravitreal bevacizumab for treatment of chronic central serous chorioretinopathy. Eur J Ophthalmol. 2009;19:613–617.
129 Lim SJ, Roh MI, Kwon OW. Intravitreal bevacizumab injection for central serous chorioretinopathy. Retina. 2010;30:100–106.
130 Artunay O, Yuzbasioglu E, Rasier R, et al. Intravitreal bevacizumab in treatment of idiopathic persistent central serous chorioretinopathy: a prospective, controlled clinical study. Curr Eye Res. 2010;35:91–98.
131 Inoue M, Kadonosono K, Watanabe Y, et al. Results of one-year follow-up examinations after intravitreal bevacizumab administration for chronic central serous chorioretinopathy. Ophthalmologica. 2011;225:37–40.
132 Lim JW, Kim MU. The efficacy of intravitreal bevacizumab for idiopathic central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2011;249:969–974.
133 Symeonidis C, Kaprinis K, Manthos K, et al. Central serous chorioretinopathy with subretinal deposition of fibrin-like material and its prompt response to ranibizumab injections. Case Report Ophthalmol. 2011;2:59–64.
134 Arevalo JF, Espinoza JV. Single-session combined photodynamic therapy with verteporfin and intravitreal anti-vascular endothelial growth factor therapy for chronic central serous chorioretinopathy: a pilot study at 12-month follow-up. Graefes Arch Clin Exp Ophthalmol. 2011;249:1159–1166.
135 Jampol LM, Weinreb R, Yannuzzi L. Involvement of corticosteroids and catecholamines in the pathogenesis of central serous chorioretinopathy: a rationale for new treatment strategies. Ophthalmology. 2002;109:1765–1766.
136 Khorram D. The Retina Blog: Rifampin for central serous chorioretinopathy [August 2010; cited 2011, July 29]. Available from [Internet] http://theretinablog.com/2010/08/30/rifampin-for-central-serous-chorioretinopathy
137 Otsuka S, Ohba N, Nakao K. A long-term follow up study of severe variant of central serous chorioretinopathy. Retina. 2002;22:25–32.
138 Ng WW, Wu ZH, Lai TY. Half-dose verteporfin photodynamic therapy for bullous variant of central serous chorioretinopathy: a case report. J Med Case Reports. 2011;5:208.
139 Gonzalez C. [Serous retinal detachment. Value of acetazolamide.]. J Fr Ophtalmol. 1992;15:529–536.
140 Pikkel J, Beiran I, Ophir A, et al. Acetazolamide for central serous retinopathy. Ophthalmology. 2002;109:1723–1725.
141 Tatham A, Macfarlane A. The use of propranolol to treat central serous chorioretinopathy: an evaluation by serial OCT. J Ocul Pharmacol Ther. 2006;22:145–149.
142 Caccavale A, Romanazzi F, Imparato M, et al. Low-dose aspirin as treatment for central serous chorioretinopathy. Clin Ophthalmol. 2010;4:899–903.
143 Forooghian F, Meleth AD, Cukras C, et al. Finasteride for chronic central serous chorioretinopathy. Retina. 2011;31:766–771.
144 Rahbani-Nobar MB, Javadzadeh A, Ghojazadeh L, et al. The effect of Helicobacter pylori treatment on remission of idiopathic central serous chorioretinopathy. Mol Vis. 2011;17:99–103.