Central nervous system effects of the inhalation agents
Flow-metabolism coupling is defined as a matching of O2 and glucose delivery to metabolic demand; CBF increases or decreases in concordance with changes in CMR. A misconception about the inhalation anesthetic agents is that, because they increase CBF and decrease CMR, they “uncouple” flow and metabolism. In fact, although increasing concentrations of inhalation anesthetic agents result in a higher CBF for a given CMR, a coupled relationship between these variables persists (Figure 67-1).
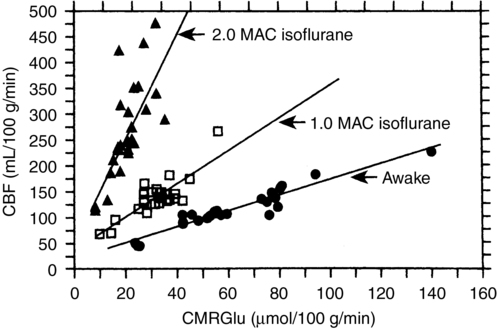
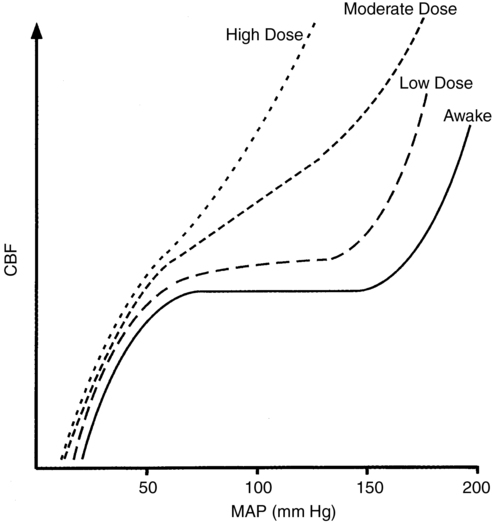
This relationship between CMR and CBF is apparent only if adequate blood pressure is maintained; if blood pressure is allowed to decrease, the increase in CBF will be attenuated or abolished because inhalation anesthesia agents inhibit autoregulation in a dose-dependent fashion (Figure 67-2). However, inhalation anesthetic agents do not inhibit CO2 reactivity and, if anything, may actually exaggerate the response. Thus, in the normal brain, the cerebral vasodilation that occurs in response to an inhalation anesthetic agent can be blunted, abolished, or reversed by decreasing CO2 levels; however, these responses may not apply in the presence of abnormal intracranial anatomy or physiology.
Michenfelder defined critical regional CBF as “that flow below which the majority of subjects develop ipsilateral EEG changes indicative of ischemia within 3 min following carotid occlusion.” Table 67-1 lists the critical CBF rates of various inhalation anesthetic agents.
Table 67-1
Critical Regional Cerebral Blood Flow for Anesthetic Agents in Patients Receiving an Inhalation Anesthetic Agent with N2O
| Agent | Cerebral Blood Flow Rate |
| Isoflurane | 10 mL • 100 g−1 • min−1 |
| Desflurane | ≤10 mL • 100 g−1 • min−1 |
| Sevoflurane | 11.5 mL • 100 g−1 • min−1 |




