36 Central Nervous System Dysfunction after Cardiopulmonary Bypass
Overt and subclinical perioperative cerebral injury remains a complex problem. As Ferguson et al1 reported, although overall mortality for patients undergoing coronary artery bypass grafting (CABG) has decreased by 23% during the 1990s, despite a projected risk-adjusted mortality predicting a 33% increase in mortality, the incidence of stroke has remained relatively unchanged.
Age-associated risk for central nervous system injury
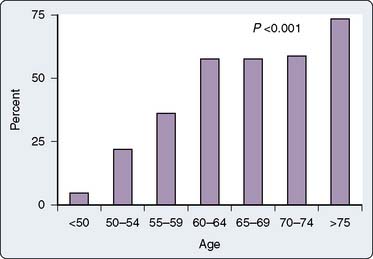
In 1985, Gardner et al2 reported a retrospective review of 3279 patients who underwent CABG between 1974 and 1983. They noted that despite an overall decrease in mortality, there was a corresponding increase in the incidence of stroke over that same decade.2 They also observed a progressive increase in the average age of the patients and noted an increased stroke rate of 7.1% in patients older than 75 years compared with an incidence rate of 0.42% in patients younger than 50 years. Notably, at that time, septuagenarians accounted for only 14.7% of their operative population. Approximately a decade later, Tuman et al3 undertook a prospective study of 2000 patients undergoing CABG and also demonstrated a disproportionately increased risk for neurologic complications as compared with cardiac complications in elderly patients (Figure 36-1). Neurologic events, defined as new sensory, motor, or reflex abnormalities, occurred with an overall frequency rate of 2.8%, having an incidence rate of 0.9% in those patients younger than 65 years, 3.6% in those aged 65 to 74 years, and 8.9% in those patients older than 75 years. Notably, they observed that patients with a neurologic event had a ninefold increase in mortality: 35.7% versus 4.0%.
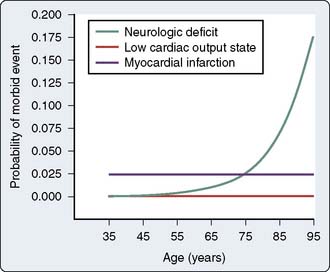
Figure 36-1 Effect of advanced age on the predicted probability of neurologic and cardiac morbidity.
(From Tuman KJ, McCarthy RJ, Najafi H, et al: Differential effects of advanced age on neurologic and cardiac risks of coronary artery operations. J Thorac Cardiovasc Surg 104:1510, 1992.)
Current data confirm a persistent association between increased age and cerebral injury after cardiac surgery.4–14 In a review of 67,764 cardiac surgical patients, of whom 4743 were octogenarians, and who underwent cardiac surgery at 22 centers in the National Cardiovascular Network, Alexander et al12 reported that the incidence of type I cerebral injury, defined by Roach et al14 as stroke, transient ischemic attack (TIA), or coma,13 was 10.2% in patients older than 80, versus 4.2% in patients younger than 80. Importantly, although global mortality for cardiac surgery in octogenarians was greater than in younger patients, the authors reported that when octogenarians without significant comorbidities were considered, their mortality rates were similar to those of younger patients.12 In a more recent review from the Society of Thoracic Surgeons National Adult Cardiac Surgery Database of 774,881 patients undergoing isolated CABG between January 2002 and December 2006, the overall incidence rate of stroke was 1.4%, increasing to 2.3% in patients aged 75 years and older.13 Interestingly, stroke rate was inversely related to body surface area and directly proportional to serum creatinine concentration, as well as presence of valvular heart disease and other comorbidities.13
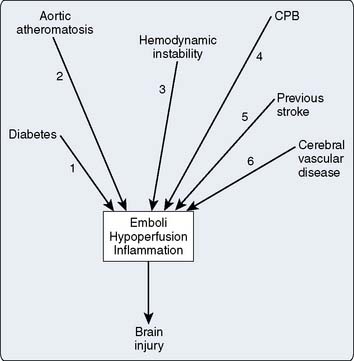
In this respect, in addition to the age-related factor, reports from Europe and North America consistently describe previous cerebrovascular disease, diabetes mellitus, hypertension, peripheral vascular disease, aortic atherosclerosis, and intraoperative and postoperative complications as all being additional factors increasing the incidence of cerebral injury in cardiac surgical patients (Box 36-1). The presence of preoperative comorbidities further increases the age-associated risk for central nervous system (CNS) complications. For example, in a population of 149 patients older than 70 years who also had significant comorbidities including aortic atheroma, diabetes mellitus, and history of previous CNS event, and who underwent cardiac surgery with cardiopulmonary bypass (CPB), Frumento et al8 reported a stroke incidence rate of 16%.
The impact of age-associated cerebral injury in cardiac surgery is becoming more relevant because of the progressive increase in the average age of the general population and, in particular, of the cardiac surgical population.10,11,13–16 As overall survival and quality of life after cardiac surgery continue to improve in elderly patients, advanced age alone is no longer considered a deterrent when evaluating a patient for cardiac surgery.10–12,17 The presence and extent of comorbidities should be considered as being of equal or greater importance than age itself as a risk factor for cerebral injury in cardiac surgical patients.
Central nervous system injury
Roach et al14 classified cerebral injury in two broad categories: type I (focal injury, stupor, or coma at discharge) and type II (deterioration in intellectual function, memory deficit, or seizures).14 A similar classification was adopted by the American College of Cardiology/American Heart Association (ACC/AHA) guidelines for CABG.18 Cerebral injury can also be broadly classified as stroke, TIA, delirium (encephalopathy), or cognitive dysfunction. Perioperative cognitive performance is assessed through the administration of a series of standardized psychometric tests, ideally administered before and after surgery.
The incidence of stroke or type I injury after closed-chamber cardiac procedures is generally considered to be approximately 1% to 4%, increasing to about 8% to 9% in open-chamber (e.g., valvular surgery) or combined/complex procedures. The incidence of cognitive dysfunction (type II) is reported as ranging in incidence rate from 30% to 80% in the early postoperative period.4,18–25 To some extent, there is a difference in the incidence of cerebral injury after cardiac surgery related to the type and complexity of the procedure, such as open chamber, combined valvular, and CABG.4,10,26,27
Overall, the increased length of stay and increased mortality rates associated with any form of cerebral complication in cardiac surgical patients are especially striking findings.15,18,20,21 Despite the relatively greater impact on mortality of stroke as opposed to cognitive dysfunction, type II injury is still associated with a fivefold increase in mortality. In their study, Roach et al14 evaluated 2108 patients undergoing CABG at 24 U.S. institutions and recorded adverse cerebral outcomes in 6.1% of patients overall. Of these, 3.1% experienced type I focal injury, stupor, or coma and had an associated in-hospital mortality rate of 21%, whereas 3.0% of patients experienced deterioration of intellectual function or seizures and had a mortality rate of 10%. In contrast, a significantly lower overall mortality rate of 2% was seen in those patients without adverse cerebral outcomes. In addition, patients with neurologic complications had, on average, a twofold increase in hospital length of stay and a sixfold likelihood of discharge to a nursing home. Independent risk factors were identified for both type I and II cerebral injury. Predictors of both types of cerebral complications included advanced age of older than 70 years and a history or the presence of significant hypertension. Predictors of type I deficits include the presence of proximal aortic atherosclerosis as defined by the surgeon at the time of surgery, a history of prior neurologic disease, use of the intra-aortic balloon pump, diabetes, a history of hypertension, a history of unstable angina, and increasing age. Perioperative hypotension and the use of ventricular venting were also weakly associated with this type of outcome.14
An important caveat that must be borne in mind when interpreting Roach et al’s14 results is that type II injury as identified in their study is not necessarily equivalent to perioperative cognitive dysfunction as demonstrated in other studies. Type II injury was detected on clinical grounds alone rather than on the basis of a deterioration in performance on a predefined series of specific cognitive tests. The latter are a much more sensitive measure of performance and thus detect cognitive dysfunction with a considerably greater frequency, and as such potentially have a much different, although not necessarily benign, implication from the increased mortality associated with type II injury demonstrated by Roach et al.14,28
Using a risk-stratification analysis of this same database, Newman et al29 developed a preoperative index predicting major perioperative neurologic events of which key predictors were age, history of neurologic disease, diabetes, previous CABG, unstable angina, and history of pulmonary disease (Figure 36-2).14,29 The Stroke Risk Index allows neurologic risk to be estimated for each patient, thus enabling the most appropriate perioperative therapy to be used, whether surgical modification, change in perfusion management, applied neuromonitoring, or administration of putative pharmacologic cerebroprotectants. It is also useful as a scale to compare risk indices, and thus the efficacy of different interventions across clinical outcome studies.8,21
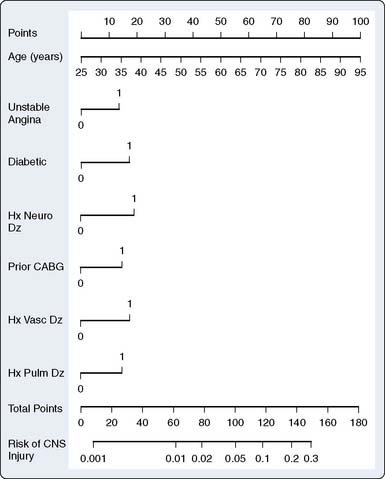
Figure 36-2 Nomogram for computing risk of central nervous system (CNS) injury.
(From Newman MF, Wolman R, Kanchuger M, et al: Multicenter preoperative stroke risk index for patients undergoing coronary artery bypass graft surgery. Multicenter Study of Perioperative Ischemia [McSPI] Research Group. Circulation 49[suppl II]:II-74, 1996.)
Retrospective versus Prospective Neurologic Assessment
The detection of CNS injury depends critically on the methodology used, and retrospective studies have been deemed insensitive by different authors.23,24,30,31 As Sotaniemi31 demonstrated, a retrospective chart review is inadequate as an assessment of the overall incidence of postoperative neurologic dysfunction. In a study of 100 patients in whom a 37% incidence rate of neurologic dysfunction had been diagnosed by careful neurologic examination, the prevalence rate of cerebral abnormalities detected by retrospective analysis of the same patient pool was only 4%. The reasons for the inability of retrospective chart audit to detect the majority of patients with neurologic dysfunction are readily apparent and include incompleteness of records, a reluctance to document apparently minor complications, and most important, an insensitivity to subtle neurologic dysfunction. Many of the types of neurologic impairment now being documented are subclinical and not readily detectable by a standard “foot-of-the-bed” assessment. The timing, thoroughness, and reproducibility (single examiner) of the neurologic examinations, as well as the incorporation of a preoperative assessment for comparison, all determine the sensitivity and accuracy with which postoperative CNS injury can be detected.23,24,30,32
Valvular versus Coronary Artery Bypass Graft Surgery
It appears that increasing the complexity or undertaking open chamber–type procedures increases the risk for CNS injury. Ebert et al33 prospectively studied 42 patients who underwent valve replacement surgery and 42 patients for CABG, with both groups matched post hoc for age, sex, and preoperative cognitive status.
Patients were investigated before surgery, as well as 2 and 7 days after surgery, with a comprehensive neuropsychological and neuropsychiatric assessment. Valve replacement surgery patients exhibited more severe neuropsychological deficits and showed a slower recovery than patients who underwent CABG.33 In Alexander et al’s12 study of 64,467 patients who underwent CABG alone and 3297 patients who underwent CABG in conjunction with aortic valve replacement or CABG in conjunction with mitral valve repair or replacement, the incidence rate of type I cerebral injury in patients younger than 80 years was 4.2% for CABG, 9.1% for CABG with aortic valve replacement, and 11.2% for CABG with mitral valve repair or replacement.12 Notably, the total CPB time was 96 minutes for CABG, 148 minutes for CABG in conjunction with aortic valve replacement, and 161 minutes for CABG with mitral valve repair or replacement. It thus remains unclear whether it is the procedure itself or the prolonged duration of CPB, either acting directly or as a marker of a greater surgical difficulty and thus perioperative hemodynamic instability, that is fundamentally causative.34
Wolman et al20 prospectively studied 273 patients from 24 U.S. medical centers who underwent combined intracardiac surgery and CABG. Included were clinical, historic, specialized testing, neurologic outcome, and autopsy data, and measures of resource utilization. Adverse cerebral outcomes occurred in 16% of patients (43/273), being nearly equally divided between type I cerebral injury (8.4%; 5 cerebral deaths, 16 nonfatal strokes, and 2 new TIAs) and type II cerebral injury (7.3%; 17 new intellectual deteriorations persisting at hospital discharge and 3 newly diagnosed seizures), rates of injury 2- to 3-fold greater than demonstrated after CABG alone by this same group of investigators.20 Associated resource utilization was significantly increased according to type of CNS injury: prolonging median intensive care unit (ICU) stay from 3 days (no adverse cerebral outcome) to 8 days associated with type I injury and from 3 to 6 days in those patients with type II injury. Significant risk factors for type I cerebral injury related primarily to embolic phenomena, including proximal aortic atherosclerosis, intracardiac thrombus, and intermittent clamping of the aorta during surgery. Risk factors for type II cerebral injury included proximal aortic atherosclerosis, as well as a preoperative history of endocarditis, alcohol abuse, perioperative arrhythmia, or poorly controlled hypertension, and the development of a low-output state after CPB.20
CO2 Insufflation during Open-Chamber Procedures
A primary determinant of the number and duration of microgaseous emboli during open-chamber procedures relates to methodologies for removal of intracavitary air. Although needle aspiration and/or aortic root venting are standard techniques for air removal, use of CO2 insufflation, either continuously or immediately before closure of ventriculotomy, has been shown to significantly increase the efficacy of deairing resulting in decreased systemic gaseous emboli.35,36 However, although there has been a general expectation of improvements in neurologic and cognitive outcomes resulting from such CO2 insufflation, it has been surprisingly difficult to demonstrate. In a recent prospective study of 80 patients undergoing valve surgery and randomized to CO2 insufflation versus conventional deairing, although postoperative auditory-evoked potential monitoring did demonstrate shorter P-300 latency in the CO2-insufflated group, there was no detectable difference in clinical outcomes or in the incidence of cognitive dysfunction between groups.37 In a recent review of the role of CO2 insufflation, the authors concluded that although the use of CO2 field flooding has been observed to be associated with a significantly lower count of intracardiac air bubbles and improved survival in two small studies, so far there is no evidence of a sustained reduction of cerebrovascular complications.38
Fewer systemic and cerebral emboli were demonstrated in patients undergoing open-chamber surgery in whom bilateral pleurotomy and passive lung deflation associated with staged perfusion and ventilation of lungs during deairing was used in comparison with those in whom pleural cavities were unopened and dead space ventilation was continued during CPB.39 In this study, CO2 insufflation was not used, and the authors reported that deairing time was significantly shorter in the treatment group.
Neurocognitive Dysfunction Unrelated to Cerebral Microgaseous Emboli
Interestingly, there is some evidence that the incidence of subtle neurologic dysfunction and cognitive abnormalities is similar in all adult patients undergoing surgical coronary artery revascularization, which some studies have related to the duration of CPB.4,12,24,40–43 Increasing age has been repeatedly shown to be one of the major risk factors for stroke after CABG, likely related to the greater prevalence of severe aortic atherosclerosis in the elderly. This suggests that there may be different factors operative in the production of gross neurologic damage than in the genesis of cognitive dysfunction. Whereas calcific or atheromatous macroembolic debris from the ascending aorta or aortic arch appears to be a prime factor in the production of clinical stroke syndromes and it was formerly thought that microembolic elements, either gaseous or particulate, produced cognitive dysfunction, studies from beating-heart surgery in which CPB is avoided, despite a much lower incidence of embolic events, appear to have a relatively similar incidence of cognitive dysfunction to CABG using conventional CPB.44–46
A series of longitudinal studies by Selnes47 and others in patients undergoing off-pump cardiac surgery, as well as those treated medically, have suggested that long-term changes in cognitive function are not specific to CABG or use of CPB and may rather reflect progression of underlying disease.47–50 Other longitudinal studies have, however, demonstrated a greater incidence of cognitive dysfunction in CABG patients in comparison with various nonsurgical control groups,51,52 though the comparability of underlying disease processes between groups remains a significant confound in many such studies.
However, as there is general agreement that the incidence of early postoperative cognitive dysfunction is greater in CABG patients compared with other noncardiac surgical groups, and because correlations have been made between such early postoperative cognitive dysfunction and new ischemic lesions on MRI studies in valve surgery patients,53 and between cerebral oxygen desaturation and early postoperative cognitive dysfunction in CABG patients,54 it does appear as though early postoperative cognitive dysfunction is, in part, reflective of subclinical brain injury; as such, efforts to mitigate against early postoperative cognitive dysfunction are warranted.
Circulatory Arrest
Retrograde and Selective Anterograde Cerebral Perfusion
In a nonrandomized study, Reich et al55 performed preoperative and postoperative cognitive testing on 56 patients undergoing HCA, of whom 12 patients underwent retrograde cerebral perfusion (RCP). Memory dysfunction and the overall incidence of cognitive dysfunction had strong associations with RCP even when controlling separately for age and cerebral ischemia time, suggesting worsened outcome with RCP. Okita and colleagues56 separately evaluated 60 patients who were nonrandomized but were sequentially stratified to receive either RCP or selective antegrade cerebral perfusion (ACP) using serial brain imaging, brain isoenzyme measurement, and limited cognitive testing. They also demonstrated that the prevalence of clinically defined transient brain dysfunction was significantly greater in patients with RCP. Svensson and colleagues57 used cognitive testing in a subset of 30 of 139 patients undergoing HCA and prospectively randomized 3 three groups to receive either HCA alone, HCA and RCP, or HCA and selective ACP. Comparison of postoperative mean cognitive test scores showed that the HCA alone group did significantly better than either the RCP or ACP group patients.
Despite its conceptual attractiveness and relative ease of application, RCP has not been demonstrated to result in clinically significant cerebral blood flow (CBF) even under conditions of hypothermia-induced decreased cerebral metabolism. In a primate study comparing HCA alone with HCA combined with RCP, Boeckxstaens and Flameng58 demonstrated that less than 1% of the RCP inflow returned to the aortic arch, and that on histologic analysis, slightly more glial edema was found in the RCP group. Similarly, during HCA in 14 pigs, use of RCP or RCP with inferior vena cava occlusion also resulted in negligible CBFs, and it was similarly observed that less than 13% of retrograde superior vena caval inflow blood returned to the aortic arch with either technique.59
It does appear as though modified RCP may be effective in flushing emboli from the cerebral circulation, though at the cost of some mild cerebral ischemic damage. Juvonen et al60 studied the impact on histologic and behavioral outcome of an interval of RCP with and without inferior vena cava occlusion, versus ACP control, after cerebral arterial embolization in a chronic porcine model. Microsphere recovery from the brain revealed significantly fewer emboli after RCP with inferior vena cava occlusion but demonstrated that significant mild ischemic damage occurred after RCP even in nonembolized animals, but not in the other groups. Behavioral scores by day 7 were considerably lower in all groups after embolization, with no significant differences between groups.
pH Management
The milieu in which HCA is conducted may well also have an important impact on CNS outcomes but has not yet been systematically investigated in adult patients. Although clinical studies and experimental evidence point to a benefit of pH-stat management in infants and children undergoing HCA, it should be noted that neither the clinical studies in pediatric patients61 nor the experimental models using nonatheromatous animals are necessarily relevant to the adult patient who invariably has substantial atheromatous disease within the ascending aorta, often with concomitant extracranial and intracranial involvement. For adults undergoing moderate hypothermic CPB at least, the weight of evidence from CNS outcomes of at least three separate, prospective, randomized, clinical trials supports alpha-stat pH management over pH-stat.62–64 In this context, alpha-stat has also been associated with decreased cerebral embolization65 and preservation of cerebral autoregulation,63,66 factors likely of paramount importance in perioperative CNS injury in adult patients undergoing HCA.
In none of these studies were stroke rate, mortality, or other measures of morbidity influenced by treatment mode, though all were underpowered to detect such outcomes. However, Hagl et al67 retrospectively analyzed outcomes in 717 survivors of ascending aortic and aortic arch surgery. They determined that the method of cerebral protection did not influence the occurrence of stroke, but that ACP did result in a significant reduction in the incidence of temporary neurologic dysfunction, a result not seen after RCP. Directionally similar results demonstrating that antegrade perfusion was associated with significantly lower incidences of temporary neurological complications, earlier extubation, shorter ICU stay, and shorter hospitalization in comparison with patients managed with RCP has been shown by Apostolakis and colleagues.68 Halkos et al69 demonstrated that during proximal aortic surgery, selective ACP was associated with lower mortality, as well as improved resource utilization and fewer pulmonary and renal complications.
It is unlikely that pH management will substantially change the results of RCP versus ACP discussed earlier; however, Harrington et al’s70 study used alpha-stat management, whereas Reich et al’s55 study used pH-stat management, both with similar directional results relatively unfavorable to RCP. Whether pH management will influence the overall incidence of CNS dysfunction after HCA is unknown. A recent meta-analysis has concluded that in the absence of randomized trials, pH-stat management for infants and alpha-stat for adults would appear to be most appropriate strategies for patients undergoing HCA.71 Based on the impact of pH management on CBF, a strong argument could be made to use pH-stat during the cooling phase before circulatory arrest followed by alpha-stat during rewarming, as practiced in some institutions.72
Aortic Atherosclerosis
Atheroembolism from an atheromatous ascending aorta and aortic arch is recognized as a major risk factor in the patient undergoing cardiac surgery and is a widespread problem.73–79 In a study of 298 asymptomatic members from the Framingham cohort aged 60 ± 9 years and of whom 51% were women, subjects underwent thoracoabdominal aortic cardiovascular MRI and demonstrated aortic plaque of 1-mm radial thickness in 38% of the women and 41% of the men.73 The Stroke Prevention: Assessment of Risk in the Community (SPARC) study used transesophageal echocardiography (TEE) in 581 people older than 44.76 Atheroma was identified in 51.3% of patients, of whom 7.6% had severe atheroma (> 4 mm thick, ulcerated or mobile). The prevalence rate of aortic arch atheroma increased with age, such that severe atheroma was seen in more than 20% of patients older than 74.79
Atheroembolism in cardiac surgery has a broad spectrum of clinical presentations, including devastating injuries and death, yet its true incidence is probably underestimated.80–83 Thoracic aorta atheromatosis is associated with coronary artery disease and stroke in the general population.74,75,77–79 In Macleod et al’s74 review, the evidence shows that the risk for stroke is four times greater in patients with severe arch atheroma. Yahia et al77 prospectively studied patients with diagnoses of TIA or stroke using TEE for assessment of aortic atheromatosis. Thoracic aortic atheromas were present in 141 of 237 patients (59%); mild plaque (< 2 mm) was present in 5%, moderate plaque (2 to 4 mm) in 21%, severe plaque (≥ 4 mm) in 33%, and complex plaque in 27%. Plaques were more frequently present in the descending aorta and the arch of the aorta than in the ascending aorta.77 Watanabe et al78 investigated whether thoracic aorta calcification on computed tomography and coronary risk factors had any correlation with obstructive coronary artery disease on angiography. Two hundred twenty-five consecutive patients underwent both thoracic conventional helical computed tomography and coronary angiography. Thoracic aorta calcification was detected in 185 patients; 141 of 225 patients had significant obstructive coronary artery disease. All of the 13 patients without thoracic aorta calcification and no coronary risk factors had no coronary artery disease.78 Overall, it can be seen that atherosclerosis of the ascending aorta is present in 20% to 40% of cardiac surgical patients, the percentage increasing with age (Figure 36-3), and it is an independent risk factor for type I cerebral injury.6–8,84–90
Transesophageal Echocardiography versus Epiaortic Scanning
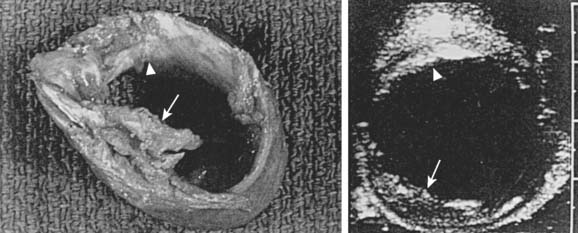
The detection of ascending aorta atheromatosis is a cornerstone of strategies to decrease cerebral injury in cardiac surgery. Despite its widespread utilization, manual palpation of the aorta has a very low sensitivity for this purpose.91,92 The association of severe thoracic aortic plaques (defined as 5-mm-thick focal hyperechogenic zones of the aortic intima and/or lumen irregularities with mobile structures or ulcerations) and coronary artery disease is well established.89 Identifying severe aortic disease has important clinical implications because surgical technique, including surgical procedure and siting of cannulation and anastomotic sites for proximal grafts, may be altered to avoid producing emboli and stroke. Intraoperative epiaortic ultrasound scanning (EAS) has emerged as a most helpful tool for the diagnosis of ascending aortic atherosclerosis and has revealed major insights into the nature and distribution of this disease.
Djaiani et al90 performed TEE and EAS to assess the severity of aortic atherosclerosis in the ascending aorta and the aortic arch. Patients were allocated to either low- or high-risk groups according to aorta intimal thickness. Transcranial Doppler (TCD) was used to monitor the middle cerebral artery. Diffusion-weighted MRI was performed 3 to 7 days after surgery. The NEECHAM Confusion Scale was used for assessment and monitoring patient consciousness level. In the high-risk group (intimal thickness > 2 mm), confusion was present in six (16%) patients versus five (7%) patients in the low-risk group, and there was a threefold increase in median embolic count, 223.5 versus 70.0. Diffusion-weighted MRI-detected brain lesions were present only in patients from the high-risk group, 61.5% versus 0%. There was significant correlation between the NEECHAM scores and embolic count in the high-risk group.87 Multiple studies have documented that most of the significant atherosclerotic lesions in the ascending aorta are missed by intraoperative palpation by the surgeon, and intraoperative echocardiographic studies of the aorta have been recommended22,86,88,90–96 (Figure 36-4). However, the ability of TEE to reliably detect all ascending aorta and aortic arch lesions is limited.
The high acoustic reflectance attributable to the air-tissue interface resulting from overlying right main bronchus and trachea limits TEE assessment of the upper ascending aorta where cannulation is generally undertaken.91,92,96,97 Intraoperative EAS has emerged as a most helpful tool for the diagnosis of ascending aortic atherosclerosis and has revealed major insights into the nature and distribution of this disease. Konstadt et al93,97 investigated 81 patients (57 male and 24 female; aged 32 to 88 years, mean age, 64 years) scheduled for elective cardiac surgery. A comprehensive examination of the entire thoracic aorta in both the longitudinal and transverse planes was performed by biplane TEE. In both echocardiographic examinations, the presence and location of protruding plaques and intimal thickening greater than 3 mm were recorded. Fourteen (17%) of the 81 patients had significant atherosclerotic disease of the ascending aorta as diagnosed by EAS echocardiography. The sensitivity of TEE was 100%, the specificity was 60%, the positive predictive value was 34%, and the negative predictive value was 100%. According to the authors, if the complete biplane TEE examination is negative for plaque, it is highly unlikely that there is significant plaque in the ascending aorta. If the TEE examination is positive for plaque, there is a 34% chance that there is significant disease of the ascending aorta, and EAS should be considered. TEE is a sensitive but only mildly specific method of determining whether ascending aortic atherosclerosis is present.93–97
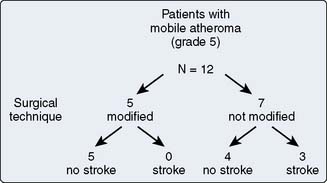
The standard for aortic assessment before instrumentation continues to be visual inspection and palpation by the surgeon, despite the fact that this has been shown to identify atheromatous disease in only 25% to 50% of patients, and even then to significantly underestimate its severity.91,92,98–100 Identification of ascending aorta atheromatous disease would prompt the surgical team for strategies to either modify, decrease, or avoid aortic manipulation. Management strategies for the diseased ascending aorta range from minimally invasive aortic “no-touch” techniques (NTTs) to maximally invasive procedures, including ascending aorta replacement or extensive aortic debridement under deep HCA.101 A recent review has outlined specific techniques for EAS, as well as steps to be used by the surgical team to mitigate aortic atheroemboli.102 Operative modifications in CABG include avoidance of aortic cross-clamping, alternative sites of aortic cross-clamping, and avoidance of proximal anastomoses by usage of all arterial conduit or Y-grafts. A decreased incidence of stroke and CNS dysfunction has been associated with this approach (Figure 36-5).103
“No-Touch” Technique
Avoidance of instrumentation of the ascending aorta in patients with severe aortic atheromatosis has been advocated. Leacche et al85 retrospectively reviewed data from 640 off-pump CABG (OPCAB) patients and identified 84 patients in whom they adopted an NTT. In these patients, revascularization was performed with single or bilateral internal thoracic arteries and by connecting additional coronary grafts (saphenous vein, radial artery) in a T or Y configuration. The right gastroepiploic artery was used as a conduit in two patients, whereas the brachiocephalic artery was used as an alternative inflow site for arterial cannulation in three reoperations. Age, sex, risk factors, functional class, and history of congestive heart failure were comparable in the two groups. In the NTT group, the frequencies were greater for severe atherosclerosis of the aorta (13% vs. 0%), carotid disease (25% vs. 16%), and history of previous cerebrovascular accidents (17% vs. 8%). In the NTT group, weak trends toward a lower incidence of postoperative delirium (8% vs. 15%; P = 0.12), a lower incidence rate of stroke (0% vs. 1%; P = 0.85), and a shorter ICU stay (P = 0.07) were observed.85 In a review of 1993 beating-heart surgery patients, Calafiore et al104 observed that in patients with evidence of peripheral vascular disease, use of aortic partial occlusion clamp was associated with a similar stroke rate as in patients in whom conventional CPB was used. They concluded that in patients with extracoronary vasculopathy, aortic manipulation must be avoided to reduce the incidence of stroke. Gaspar et al96 used EAS and TEE in 22 patients considered to be at high risk for stroke in whom severe aortic atheroma (maximum aortic wall thickness > 5 mm or mobile plaque) was detected, and with the use of aortic NTT and beating-heart surgery, no strokes occurred.
Royse et al91 performed screening of the aorta for atheroma before aortic manipulation and used an exclusive Y-graft revascularization technique, which has no aortic coronary anastomoses. Aortic atheroma was detected using EAS and TEE. In the control group, aortic atheroma was assessed by manual palpation, whereas TCD of the right middle cerebral artery was used to detect cerebral microemboli. Neuropsychological dysfunction was assessed using a battery of 10 psychometric tests, and they demonstrated that at 60 days after surgery, dysfunction in the control group was 38.1%, whereas in the TEE/Y-graft group, it was reduced to 3.8%. Microemboli detected by TCD during periods of aortic manipulation were greater for those with late dysfunction (5.2 ± 3.0 compared with 0.5 ± 0.2), consistent with an embolic cause for cognitive dysfunction.91
Neuropsychological dysfunction
Compared with stroke, cognitive dysfunction (neurocognitive dysfunction [NCD]) is a considerably more frequent sequela of cardiac surgery and has been demonstrated in up to 80% of patients early after surgery.24,105–107 The pathogenesis of cognitive dysfunction after cardiac surgery is still uncertain. Variables that have been postulated to explain the development of postoperative neurocognitive decline include advanced age, concomitant cerebrovascular disease, and severity of cardiovascular disease, as well as progression of underlying disease. Various intraoperative factors such as embolization, cerebral hypoperfusion or hypoxia, activation of inflammatory processes, aortic cross-clamp or CPB time, and low mean arterial pressure (MAP) and cerebral venous hypertension have all been implicated. In many instances, subtle signs of neuropsychological dysfunction are detectable only with sophisticated cognitive testing strategies, although depression and personality changes may be noted by family members. It should be recognized that formalized cognitive testing is reproducible and quantifiable and represents an objective outcome measure; as such, it can act as a benchmark to assess various therapeutic interventions (e.g., the efficacy of putative cerebroprotectants, equipment modifications, pH management strategies). In addition, a number of studies have made correlations between early postoperative cognitive dysfunction and intraoperative cerebral oxygen desaturation, as well as new ischemic lesions on MRI.53,54 Assessment of early cognitive dysfunction can be used to discriminate between various intraoperative treatment modalities (e.g., pH management, use of cell saver, epiaortic scanning).
However, whether early postoperative cognitive dysfunction represents permanent neurologic damage remains controversial.48 In a long-term follow-up study of 97 patients having undergone CABG an average of 39 months earlier, Murkin et al106 demonstrated an incidence rate of neuropsychological dysfunction of 22%, and 18% of patients exhibited abnormal neurologic findings. The overall incidence rate of combined neurobehavioral dysfunction was 35%, similar to the incidence at 2 to 3 months after surgery in this same group.62 Knipp et al105 prospectively investigated cerebral injury early and 3 months after CABG. Patients were studied at three points in time: before operation, early before discharge, and 3 months after operation using a well-validated battery of 13 standardized psychometric tests. Neurocognitive assessment early after surgery disclosed a significant decline in performance in 4 of the 11 psychometric tests compared with the preoperative status, whereas at the 3-month follow-up examination, 3 of the 4 tests disclosed early cognitive decline, and both depression and mood scores had returned to their baseline values. However, the test score for verbal learning ability remained significantly lower at 3 months.105 Newman et al52 sought to determine the course of cognitive change 5 years after CABG and the effect of perioperative decline on long-term cognitive function. They performed neurocognitive tests in 261 patients who underwent CABG; tests were administered before surgery (at baseline), before discharge, and 6 weeks, 6 months, and 5 years after CABG. Among the patients studied, the incidence rate of cognitive decline was 53% at discharge, 36% at 6 weeks, 24% at 6 months, and 42% at 5 years. Cognitive function at discharge was a significant predictor of long-term function. Their results confirmed the relatively high prevalence and persistence of cognitive decline after CABG and suggested a pattern of early improvement followed by a later decline that is predicted by the presence of early postoperative cognitive decline.52 What is interesting is the apparent lack of association between cognitive dysfunction and aortic atherosclerosis, at least in one study. Of 162 CABG patients who had a perioperative neurocognitive evaluation and evaluable intraoperative TEE images, no significant relation was found between cognitive dysfunction and atheroma burden in the ascending arch or descending aorta, suggesting that aortic atherosclerosis may not be the primary factor in the pathogenesis of post-CABG cognitive changes.108
In the systematic review and meta-analysis by van Dijk et al,24 data from six highly comparable studies were pooled and demonstrated an incidence of cognitive deficit, defined as a decrease of at least 1 standard deviation in at least 2 of 9 or 10 neuropsychological tests, of 22.5% (95% confidence interval, 18.7 to 26.4) in CABG patients at 2 months after surgery. In a prospective study of 316 CABG patients, Murkin et al62 reported a perioperative stroke rate of 2.8% and demonstrated that 33% of 239 patients assessed 2 months after surgery evidenced cognitive dysfunction, and that 45% experienced either neurologic or cognitive dysfunction, in comparison with their preoperative performance.
One important confounder in many of the earlier studies is the absence of a nonsurgical control cohort with similar comorbidities also followed longitudinally with cognitive testing. Several more recent studies have demonstrated similar incidences of later cognitive dysfunction whether patients underwent CABG, off-pump surgery, percutaneous coronary interventions (PCIs), or were managed medically.47,48 These results strongly imply that underlying comorbidities and progression of atherosclerotic disease are the most relevant factors in late postoperative cognitive dysfunction rather than cardiac surgery per se.
The mid- and long-term impact of cognitive dysfunction on quality of life after cardiac surgery has been addressed by different studies.28,109,110 Ahlgren et al109 prospectively evaluated neurocognitive function and driving performance after CABG in 27 patients who underwent neuropsychological examination involving 12 cognitive tests, including a standardized on-road driving test and a test in an advanced driving simulator before and 4 to 6 weeks after surgery. Twenty patients who underwent PCIs under local anesthesia served as a control group. After surgery, 48% of patients in the CABG group showed cognitive decline, whereas significantly fewer patients in the PCI group, only 10%, showed cognitive decline after intervention. Of particular relevance to functional quality of life, patients demonstrating cognitive decline also tended to drop in the on-road driving scores to a larger extent than did patients without a cognitive decline.109 Di Carlo et al110 administered a series of cognitive tests before and 6 months after the operation to 110 patients (mean age, 64.1 years; 70.9% male sex) undergoing cardiac surgery. The degree of the impairment was determined by two independent neuropsychologists in relation to its impact on everyday life activities. At 6-month assessment, 10 patients (9.1%) were ranked as having severe deterioration, 22 (20%) as having mild or moderate deterioration, and 78 (70.9%) as unchanged or improved.110 At 5-year follow-up, Newman et al28 also found a significant correlation between cognitive function and quality of life in patients after cardiac surgery. Lower overall cognitive function scores at 5 years were associated with lower general health and a less productive working status.
Preoperative Cognitive Function
One of the earliest prospective reports of neurobehavioral sequelae of cardiac surgery appeared in 1954, and it focused on the acute and chronic stress responses manifested as psychobehavioral syndromes in patients undergoing valvular surgery.111 As Tufo and others112,113 noted, this led to a tendency for some investigators to focus on postoperative emotional reactions, regarding them as primarily psychiatric syndromes, rather than systematically investigating cardiac patients for neurologic deficits. Millar et al114 examined the effect of preexisting cognitive impairment on cognitive outcome in 81 patients undergoing CABG. Patients performed the Stroop Neuropsychological Screening Test and other psychometric assessments before and at 6 days and 6 months after CABG. Those with preexisting cognitive deficits were significantly more likely to display impairment at 6-day and 6-month follow-ups than were those without preexisting deficits, possibly reflecting underlying intrinsic pathology rather than specific intraoperative events. Rankin et al115 used a 1-hour neuropsychological battery administered before surgery to 43 patients before prospective randomization to either CABG or OPCAB and again to 34 of those patients 2 to 3 months after surgery by an examiner blind to surgical condition. Neuropsychological status did not change 2.5 months after surgery between OPCAB or CABG groups. However, both groups showed dramatic presurgical cognitive deficits in multiple domains, particularly verbal memory and psychomotor speed. This corroborates previous research suggesting that patients requiring CABG may evidence significant presurgical cognitive deficits as a result of existing vascular disease. In Di Carlo et al’s110 study of 110 patients undergoing cardiac surgery, the previous level of education was protective against cognitive decline (odds ratio per year of increment, 0.53; 95% confidence interval, 0.31 to 0.90). It is speculative whether this suggests that greater education reflects greater intellectual capacity, and thus a better ability to compensate for perioperative stresses.
Neuropsychological Test Selection
Research suggests that among the tests most appropriate under these circumstances are tests of attention/concentration, psychomotor speed, motor dexterity, and verbal learning. Research as to the behavioral consequences of hypoxia (and other conditions associated with more diffuse brain damage) suggests these domains are likely to be compromised.116,117 This presumption has also been supported by research to date examining behavioral consequences of CPB. Frequently, the Grooved Pegboard Test (motor dexterity), various subtests of the Wechsler Adult Intelligence Scale-Revised (Digit Symbol [psychomotor speed]), some of the seven subtests of the Wechsler Memory Scale (Mental Control [Attention], Digit Span [concentration], Paired Associates Verbal Learning [verbal learning]), as well as the Halstead Reitan Trail Making Test (Trails A and B), have been used in whole or in part for the assessment of cognitive impairment after CPB.28,33,40,52,62,91,105,109,110,114,118–123
Methodologic Issues in Neurobehavioral Assessment
The Statement of Consensus on Assessment of Neurobehavioral Outcomes after Cardiac Surgery encouraged a more standardized and comparable methodology in assessment of cognitive injury, identifying several key issues of concern in perioperative cognitive testing.32
Mechanisms of brain injury
Determining which factor or, more likely, which combination of factors is responsible for postoperative neurologic or behavioral dysfunction in patients undergoing cardiac surgery using CPB is problematic (Box 36-2). From the few studies in which a surgical control group has been used, it appears that elements inherent to CPB are causative, particularly in dysfunction occurring in the immediate postoperative period.62,123 How much of this dysfunction is as a direct result of exposure to CABG and CPB or occurs as a result of underlying comorbid disease, such as aortic and cerebrovascular atherosclerosis, hypertension, and diabetes, which predispose such patients to CNS dysfunction as a result of nonspecific “stress” associated with major surgery independent of CABG, is an area of active ongoing investigation. Based on postmortem studies, as well as correlative analyses of intraoperative events with neurologic outcomes, two primary mechanisms appear to be responsible for brain injury in otherwise uncomplicated cardiac operations: cerebral hypoperfusion and cerebral emboli.
Intraoperative cerebral embolization of particulate and microgaseous elements has been demonstrated to have a significant role in the genesis of cerebral events in postoperative cardiac surgical patients.42,81,107,124–131 Increasing attention is also being paid to the role of perioperative hypoperfusion, particularly in patients with intracranial and extracranial atherosclerosis, and to the effect of inflammatory processes triggered during exposure to surgery and CPB.27,36,100
In a prospective study of 151 consecutive Japanese patients (115 men and 36 women ranging in age from 41 to 82 years) scheduled for CABG, carotid and intracranial arteries were examined for occlusive lesions with magnetic resonance angiography.137 Cervical carotid artery stenoses of more than 50% narrowing were detected in 16.6% of the subjects, and intracranial artery stenoses of more than 50% narrowing were detected in 21.2% of the subjects.137 In a similar study of 201 Korean patients presenting for CABG, more than 50% had evidence of either extracranial or intracranial atherosclerotic disease, whereas 13% of patients had evidence of both.135 In this series, 25.4% of patients had single or multiple postoperative CNS complications, and intracranial atherosclerotic disease was found to have a strong independent association with the development of CNS complications. The presence of both extracranial and intracranial atherosclerotic disease was even more strongly associated with adverse perioperative CNS outcomes than was intracranial atherosclerotic disease alone.138
In those studies in which a control group (subjected to a noncardiac procedure) was used, the incidence of both new neurologic signs and cognitive dysfunction was significantly greater in the patients undergoing CABG in the first several postoperative days compared with the surgical cohort.62,79
Cognitive dysfunction after noncardiac surgery can also be persistent on long-term follow-up, although to a variable extent compared with the incidence reported for cardiac surgery (see earlier). Abildstrom et al139 reported that 1% of patients showed persistent cognitive dysfunction 2 years after major noncardiac surgery. Several studies have also demonstrated the release of markers of cerebral injury and demonstrated a lesser but significant incidence of cognitive dysfunction after noncardiac surgery.140,141 The occurrence of cerebral emboli during noncardiac surgery has also been demonstrated.141–144 Accordingly, it appears as though some of the same mechanisms operative in CABG may also be culpable. Edmonds et al142 recorded TCD signals from the middle cerebral artery in 23 patients undergoing total hip arthroplasty and demonstrated that, in 8 of 20 patients, there were embolic signals ranging in number from 1 to 200. In all eight of these patients, signals were recorded during impaction of a cemented component or after relocation of the hip. In 2 patients, there were 150 and 200 embolic signals; mild respiratory symptoms developed in both of these patients, and one patient also became overtly agitated during a flurry of emboli.
There is also evidence of a greater stroke rate in cardiac surgery from the recently published SYNTAX trial in which 1800 patients with three-vessel or left mainstem coronary artery disease were randomized to PCI or conventional CABG surgery.145 This study demonstrated no difference in mortality at 1 year but a significantly (P = 0.002) lower incidence of primary composite end point of major adverse cardiac or cerebrovascular event in CABG (12.4%) versus PCI (17.8%) patients. However, although the overall outcome should argue strongly in favor of CAB surgery, the stroke rate was significantly greater in CAB (2.2%) than PCI (0.6%) patients.
Neuropathologic Studies
In an early series from 1962 to 1970 that examined 206 patients dying after cardiac surgery or CABG and a group of 110 patients dying after non-CPB vascular surgery, Aguilar et al146 reported that there was a high correlation between the use of CPB and the incidence of brain lesions. They reported that the most significant abnormalities found, in both severity and frequency of occurrence, were emboli in small cerebral vessels; acute petechial, perivascular, and focal subarachnoid hemorrhages; and acute ischemic neuronal damage (Box 36-3). They noted the virtual disappearance from the brain of nonfat emboli such as fibrin, platelet aggregates, polarizable crystalline material, xanthomatous debris, striated muscle, and calcium in cases examined after the introduction of arterial line filtration, whereas they reported that cerebral embolization of such debris was commonly observed in patients dying after CPB before the introduction of arterial line filtration.146 Other early studies showed that measures taken to decrease the duration of CPB, as well as the introduction of arterial line filtration and filtration of the cardiotomy suction return, decreased overt neurologic dysfunction.124,125,147
A review of autopsy findings from 221 patients dying after CABG or valve surgery between 1982 and 1989 reported a direct correlation among age, severe atherosclerosis of the ascending aorta, and presence of atheroemboli. Atheroemboli were significantly more common in patients who underwent CABG versus valvular surgery, and there was a high correlation of atheroemboli with severe atherosclerosis of the ascending aorta, being present in 37.4% of patients with severe disease of the aorta versus only 2% of those without. Of all patients who had evidence of atheroemboli, 95.8% had severe atherosclerosis of the ascending aorta.80 Doty et al81 reviewed the records of 49,377 autopsy cases and surgical specimens from the Johns Hopkins Hospital between 1973 and 1995. Three hundred twenty-seven patients (0.7%) had an identifiable atheroembolism on histologic examination. Of these patients, 29 (0.2%) had undergone a cardiac surgical procedure within 30 days of autopsy or surgical resection. Six of the 29 patients (21%) had atheroembolism to the heart, 7 patients (24%) had embolism to the CNS, 19 patients (66%) had embolism to the gastrointestinal tract, 14 patients (48%) had embolism to one or both kidneys, and 5 patients (17%) had embolism to a lower extremity. Sixteen patients (55%) had atheroembolism in two or more areas. In six patients (21%), death was directly attributable to atheroembolism, including intraoperative cardiac failure from coronary embolism (three), massive stroke (two), and extensive gastrointestinal embolization (one).81
In a neuropathologic study of brains from 262 patients dying after having undergone CABG, valve replacement, or heart transplantation surgery, 49% of cases demonstrated evidence of circulatory disturbances identified as macrohemorrhages and microhemorrhages, infarcts, subarachnoid hemorrhages, or hypoxemic brain damage.148 The infarcts were caused by local arteriosclerosis of cerebral arteries, fat emboli, arterial emboli from operative sites, or foreign body emboli. These authors concluded that histologically overt microemboli did not play a major role in their findings, and that nonfatal white matter microhemorrhages were found with varying frequency, especially after valve operations. These observations are not inconsistent with the apparent lack of correlation seen in several beating-heart surgery studies between differing incidences of TCD-detected cerebral emboli and cognitive dysfunction.44,45
Watershed Infarctions
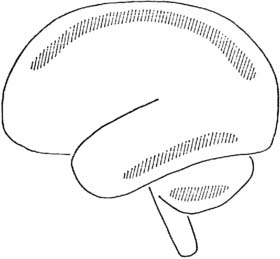
Watershed, or boundary zone, infarcts are ischemic lesions that are situated along borderzones between the territories of two major cerebral arteries (e.g., the middle and posterior, or the anterior and middle cerebral arteries) where terminal arteriolar anastomoses exist149–152 (Figure 36-6). In a series reported by Malone et al,152 a correlation was made between the presence of intraoperative electroencephalographic (EEG) abnormalities (virtual or complete electrical silence) usually seen in conjunction with sustained hypotensive episodes and neuropathologic lesions found at necropsy. In all nine patients with clinical evidence of brain damage, cortical boundary zone (watershed) lesions were observed in the parieto-occipital areas. They suggest that this location is the most sensitive area for placement of recording EEG electrodes because it is where minimal boundary zone ischemic lesions occurred in the absence of other lesions, and it is also where ischemic lesions were found in their maximal severity and extent.
A profound reduction in systemic blood pressure is the most frequent cause of watershed infarcts. These areas are thought to be more susceptible to ischemia resulting from hypotension because of their critical dependence on a single blood supply. Wityk et al153 studied the pattern of ischemic changes on diffusion- and perfusion-weighted MRI in 14 patients with neurologic complications after cardiac surgery, of whom 8 patients presented with encephalopathy, which was associated with focal neurologic deficits in 4, 4 with focal deficits alone, and 2 with either fluctuating symptoms or TIAs. Acute ischemic lesions were classified as having a territorial, watershed, or lacunar pattern of infarction. Patients with multiple territorial infarcts in differing vascular distributions that were not explained by occlusive vascular lesions were classified as having multiple emboli. Acute infarcts were found in 10 of 14 patients by diffusion-weighted imaging. Among patients with encephalopathy, seven of eight had patterns of infarction suggestive of multiple emboli, including three of four patients with no focal neurologic deficits. Several patients had combined watershed and multiple embolic patterns of ischemia. Diffusion-weighted MRI findings were abnormal in two of four patients, showing diffusion–perfusion mismatch; both patients had either fluctuating deficits or TIAs, and their conditions improved with blood pressure increase.153
By the same rationale, however, these areas are also highly susceptible to ischemia because of end-artery embolization, and it is also recognized that although severe hypotension is the most common cause, showers of microemboli may lodge preferentially in these areas and cause infarcts in the underlying brain.153–156 As such, although they are commonly due to profoundly hypotensive episodes, watershed lesions are not pathognomonic of a hypotensive episode and may be the result of cerebral emboli. Embolization and hypoperfusion acting together play a synergistic role and either cause or magnify the brain damage of cardiac surgical patients. The negative influence of hemodynamic instability and hypoxia has been demonstrated by several authors, showing improved outcomes by an early and aggressive recognition and correction of hypoperfusion.5,27,40,157–159
Cerebral Perfusion Pressure
Intraoperative hypotension during cardiac surgery has been related to postoperative neurologic dysfunction.4,5,21,27,42,160 Ridderstolpe et al27 published a retrospective study of 3282 patients of mean age of 65.6 years who underwent cardiac surgery in the period from July 1996 through June 2000. Cerebral complications occurred in 107 patients (3.3%). Of these, 60 (1.8%) were early, 33 (1.0%) were delayed, and in 14 (0.4%) patients the onset was unknown. Predictors of early cerebral complications were older age, preoperative hypertension, aortic aneurysm surgery, prolonged CPB time, hypotension at CPB completion and soon after CPB, and postoperative arrhythmia and supraventricular tachyarrhythmia. Predictors of delayed cerebral complications were female sex, diabetes, previous cerebrovascular disease, combined valve and CABG, postoperative supraventricular tachyarrhythmia, and prolonged ventilator support. Early cerebral complications seemed to be more serious, with more permanent deficits and a greater overall mortality (35.0% vs. 18.2%). The results of this study suggest that aggressive antiarrhythmic treatment and blood pressure control may improve the cerebral outcome after cardiac surgery.27
EEG patterns consistent with ischemia—increased slow wave activity, diffuse slowing of EEG activity—have been reported to occur during CPB episodes thought to be associated with cerebral hypoperfusion.161–163 Episodes of flow reduction during normothermia frequently produced ischemic changes, whereas similar decreases during stable hypothermia were not associated with EEG changes.162 Ischemic EEG changes are also frequently seen in association with reductions in perfusion flow rate during the initiation of CPB161,162 (see Chapters 16, 28, and 29). Using computerized EEG to quantitate episodes of low-frequency power as an index of cerebrocortical ischemia in 96 patients undergoing CABG, a correlation was made among episodes of hypotension, focal increases in low-frequency EEG power, and the occurrence of postoperative disorientation.164
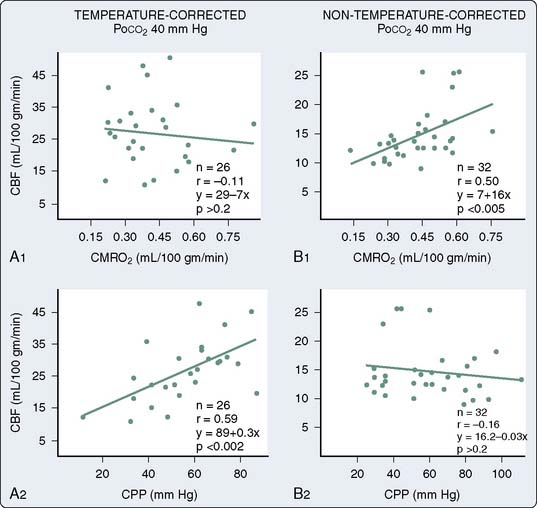
During the transition to CPB, the brain is particularly vulnerable to ischemia, inasmuch as cerebral metabolic rate for oxygen (CMRO2) is apparently unchanged, yet the brain is initially perfused with an asanguineous prime, and even after equilibration during established CPB, hematocrit is generally maintained at a range between 20% and 30%. As a result, any further decreases in cerebral perfusion, in the absence of concomitant decreases in CMRO2, are poorly tolerated. During hypothermic conditions, there is a profound decrease in CMRO2, exceeding 50% for a 10° C reduction in temperature.66 Without need to postulate an extension of the lower limit of cerebral autoregulation, it is clear that under anesthesia, and particularly during hypothermic CPB, CBF is maintained at very low levels of cerebral perfusion pressure. As discussed later, this was initially reported by Govier et al165 and further explored by Murkin et al66 and Prough et al.166 As the average age and extent of disease in patients presenting for CABG continue to increase, however, the number of patients with concomitant cerebrovascular disease, and thus potentially deranged cerebral autoregulation, presents an increasingly important group.
Using radioisotope techniques for measurement of CBF, and incorporating a jugular venous catheter for calculation of CMRO2, it was determined that there is a profound reduction in CMRO2 during hypothermic CPB, and that CBF is decreased proportionately and will autoregulate down to a cerebral perfusion pressure of 20 mm Hg, in the presence of alpha-stat pH management.66 Low arterial pressure during the hypothermic phase of CPB is thus unlikely to result in cerebral ischemia in the absence of cerebrovascular disease. In a study of high versus low arterial pressure management during CPB in 248 patients undergoing CABG, however, an apparently lower rate of postoperative complications was reported by Gold et al167 for those patients in the high-pressure group. Although specific CNS morbidity, cognitive and functional status outcomes, and mortality did not differ significantly between groups, the overall complication rate for combined cardiac and neurologic complications was significantly lower in the high-pressure group. This is not inconsistent with data demonstrating a high incidence of cerebrovascular disease in coronary revascularization patients.108,109
Cerebral Venous Obstruction
It should also be appreciated that during CPB, cerebral venous hypertension can result from partial obstruction of the superior vena cava (Figure 36-7), particularly in the presence of a single two-stage venous cannula, and may cause cerebral edema, as well as produce a disproportionate decline in cerebral perfusion pressure relative to arterial pressure.168 In a study by Avraamides,169 surgical dislocation of the heart during CPB produced increases in proximal superior vena cava pressure and resulted in significant decreases in CBF velocity as measured with TCD, despite stable arterial pressure and pump flow rates. This strongly suggests that cerebral venous hypertension, as can occur during CPB with myocardial dislocation and impaired drainage of superior vena cava, may result in cerebral ischemia if unrecognized and untreated. It is feasible that such unrecognized cerebral venous hypertension has resulted in some of the postoperative neurologic syndromes that have been reported.170
Although the association between hypotension during CPB and cerebral dysfunction remains contentious, there is some evidence that certain subsets of patients may be at particular risk. Newman et al171 used preoperative and postoperative cognitive testing to assess the effects of MAP and rate of rewarming on cognitive decline in 237 patients. They demonstrated significant interactions between cognitive decline and MAP less than 50 mm Hg on one measure of cognitive performance and between rate of rewarming and age on another. They concluded that although MAP and rewarming were not primary determinants of cognitive decline, hypotension and rapid rewarming contributed significantly to cognitive dysfunction in the elderly. Again, because elderly patients comprise an increasing segment of the cardiac surgical population, these aspects are becoming increasingly important clinical management issues.
Hemodynamic Instability
The interaction of emboli, perfusion pressure, and the particular conditions of the regional cerebral circulation (e.g., preexisting cerebral intravascular lesions) will determine the final expression of brain damage in the cardiac surgical patient. In a retrospective study, Ganushchak et al172 tested the hypothesis that combinations of hemodynamic events from apparently normal CPB procedures are related to the development of postoperative neurologic complications and affect the impact of common clinical risk factors. A multivariate statistical procedure (cluster analysis) was applied to a data set of automatically recorded perfusions from 1395 patients who underwent CABG. The following five parameters emerged for cluster analysis: MAP, dispersion of MAP, dispersion of systemic vascular resistance, dispersion of arterial pulse pressure, and the maximum value of mixed venous saturation. Using these parameters, they found four clusters that were significantly different by CPB performance (first cluster, 389 patients; second cluster, 431 patients; third cluster and fourth cluster, 229 patients each). Patients in the fourth cluster had the greatest dispersion of MAP, that is, greatest instability on CPB. The frequency of postoperative neurologic complications was 0.3% in the first cluster and increased to 3.9% in the fourth cluster. Importantly, the impact of common clinical risk factors for postoperative neurologic complications was affected by the performance of the CPB procedure. For example, the frequency of neurologic complications among patients with cerebrovascular disease in their medical history was 22% in the fourth cluster, whereas it was zero in the second cluster. Patients who underwent CPB procedures with large fluctuations in hemodynamic parameters showed a particularly increased risk for the development of postoperative neurologic complications.172
Cerebral Emboli and Outcome
Two different types of cerebral emboli appear to occur during CPB composed of solid or gaseous matter, such as macroemboli (e.g., atherosclerotic debris) and microemboli (e.g., microgaseous bubbles, microparticulate matter). Overt and focal neurologic damage likely reflects the occurrence of cerebral macroemboli (e.g., calcific and atheromatous debris generated during valve tissue removal or instrumentation of an atheromatous aorta), whereas less focal neurologic dysfunction has been ascribed to cerebral microemboli.14 Microemboli appear to have some role in diffuse, subtle neurologic and cognitive disturbances, whereas macroemboli likely produce clinically apparent catastrophic strokes. Whatever the nature of the cerebral insult, however, it seems that coexistent inflammatory processes can exacerbate the magnitude of injury.
In a study to assess the impact of surgical manipulation of the aorta and correlations with postoperative stroke, Ura et al173 performed EAS before aortic cannulation and after decannulation in 472 patients undergoing cardiac surgery with CPB. A new lesion in the ascending aortal intima was identified in 16 patients (3.4%) after aortic decannulation, of whom 10 patients sustained neurologic complications. New lesions were severe, with mobile lesions or disruption of the intima in 10 patients. Six of the severe lesions were related to aortic clamping and the other four to aortic cannulation. Three patients in this group had postoperative stroke. The incidence rate of new lesions was directly related to extent of aortic atheroma, being 11.8% if the atheroma was approximately 3 to 4 mm thick and as high as 33.3% if the atheroma was greater than 4 mm, but only 0.8% when it was less than 3 mm.173 Again, this underscores the need to reliably detect and ultimately avoid disruption of aortic atherosclerotic plaque.
Sylivris et al174 studied 41 consecutive patients undergoing CABG with TCD monitoring and preoperative and postoperative MRI brain scans. A subgroup of 32 patients underwent neuropsychological testing the day before and 5 to 6 days after the operation, of whom 27 had TCD data. Among the subgroup of patients with reliable TCD data and neuropsychological studies, early neuropsychological deficit after CABG was found in 17 (63%) of the 27 patients. On univariate analysis, the time duration on CPB, total microembolic load during bypass, and microembolic rates during bypass were all significantly greater in the group with neuropsychological decline. Actual rates of emboli detected per minute were greatest during release of the aortic cross-clamp. Five patients had strokes, of which four had a significant decline in neuropsychological functioning. Unlike the association between microembolic signals (MESs) during bypass and neuropsychological deficits, there was no relation between these factors and radiologic evidence of cerebral infarction. Not inconsistent with the findings of Ura et al173 described earlier, there was a significantly greater microembolic load at cannulation in patients with cerebral infarction, temporally suggestive of particulate emboli, which was not apparent in comparison with patients with neuropsychological deficits alone.174
A study with a newer generation of TCD, which uses two different frequencies for insonation and purportedly discriminates between gaseous and particulate emboli, compared the number and nature of intraoperative microemboli in patients undergoing on-pump and off-pump cardiac surgery procedures in 45 patients (15 OPCAB, 15 on-pump CABG, and 15 open cardiac procedures).128 They demonstrated significantly fewer emboli in the OPCAB versus on-pump CABG and open procedure groups, averaging 40 (28 to 80), 275 (199 to 472), and 860 (393 to 1321) emboli, respectively (P < 0.01). Twelve percent of microemboli in the OPCAB group were defined as solid compared with 28% and 22% in the on-pump CABG and open procedure groups, respectively. In the on-pump groups, 24% of microemboli occurred during CPB, and 56% occurred during aortic manipulation for cannulation, decannulation, application, and removal of cross clamp or side clamp, again underscoring the importance of minimizing aortic instrumentation.44
Gaseous emboli are not innocuous, however. It has been demonstrated that the effects of air emboli on the cerebral vasculature not only are due to bubble entrapment with direct blockage of cerebral vessels but represent the effects that such bubbles have on vascular endothelial cells.175 Ultrastructural examinations of pial vessels in rats exposed to cerebral air emboli demonstrated severe injury to endothelial plasmalemma, leading to loss of cellular integrity and endothelial cell swelling.176 Such endothelial damage produces disruptions of vasoreactivity, as has been observed in cat pial vessels exposed to air emboli. In these capillary beds, the endothelial layer demonstrated ultrastructural abnormalities that included degradation of intercellular junctions, flattening of nuclei, and crenation of the plasmalemma. Air embolism also produces changes in blood elements leading to formation of a proteinaceous capsule around the bubbles, marked dilation of pial vessels, platelet sequestration, and damage to endothelial cells.177–179 Air-induced mechanical trauma to the endothelium causes basement membrane disruption, thrombin production, release of P-selectin from intracellular vesicles, synthesis of platelet-activating factor, and a reperfusion-like injury with perturbations in inflammation and thrombotic processes. These phenomena likely impair nitric oxide production, causing alterations in cerebral microvascular regulation.180–182
In Moody et al’s126 study, four of five patients dying after CPB, two patients dying after proximal aortography, and six dogs placed on CPB were all found to have small cerebral capillary and arteriolar dilations (SCADs), consistent with sites of gas bubbles or fat emboli (Figure 36-8). These microvascular anomalies were only found in conjunction with utilization of CPB or proximal aortic instrumentation. In a subsequent series of elegant studies by this same group, use of colored microspheres was able to “time-lock” the development of SCADs to the period associated with CPB183 (Figure 36-9). In further studies from this group, Challa et al184 identified SCADs in thick colloidin sections of the brains of eight patients who died after cardiac surgery supported with a membrane oxygenator and in two dogs that underwent CPB with a bubble oxygenator. In SCADs of the eight patients who had cardiac surgery, both aluminum and silicone values were higher, and silicone values were also high in the two dogs in which a bubble oxygenator was used. Their results indicated that contamination with aluminum and silicone occurred during cardiac surgery assisted by CPB, and that switching to membrane oxygenators from bubble oxygenators for CPB may have reduced silicone contamination of blood.184 Kincaid et al185 used a cell saver to process the cardiotomy blood in dogs that underwent hypothermic CPB. The brain tissue from two groups of dogs (group I, cardiotomy suction blood reinfused through arterial line filter; group II, cardiotomy suction blood collected and processed in a cell saver) was examined for the presence of SCADs. Mean SCAD density in the cell-saver group was less than the arterial filter group (11 ± 3 vs. 24 ± 5; P = 0.02). The authors concluded that using a cell saver to scavenge shed blood during CPB decreases cerebral lipid microembolization.185
More recently, two separate randomized, prospective studies in cardiac surgical patients have assessed the impact of cell-saver usage on cognitive dysfunction after cardiac surgery.186,187 A series of 226 patients older than 60 years undergoing CABG surgery were randomly allocated to either cell-saver or control groups.186 Anesthesia and surgical management were standardized. Epiaortic scanning of the proximal thoracic aorta was performed in all patients, and TCD was used to measure cerebral embolic rates. Standardized neuropsychological testing was conducted 1 week before and 6 weeks after surgery. Cognitive dysfunction was present in 6% of patients in the cell-saver group and 15% of patients in the control group 6 weeks after surgery (P = 0.038). However, significantly (P = 0.018) more patients in the cell-saver group required transfusion of fresh frozen plasma (25%) versus the control group (12%). In a remarkably similar study from Rubens et al,187 patients undergoing coronary and/or aortic valve surgery using CPB were randomized to receive unprocessed blood (control, n = 134) or cardiotomy blood that had been processed by centrifugal washing and lipid filtration (treatment, n = 132). The treatment group received more intraoperative red blood cell transfusions (0.23 ± 0.69 vs. 0.08 ± 0.34 units; P = 0.004), and both red blood cell and non–red blood cell blood product use was greater in the treatment group. Postoperative bleeding was greater in the treatment group. Patients also underwent neuropsychometric testing before surgery and at 5 days and 3 months after surgery. There was no difference in the incidence of postoperative cognitive dysfunction in the two groups (relative risk: 1.16, 95% CI: 0.86 to 1.57 at 5 days after surgery; relative risk: 1.05, 95% CI: 0.58 to 1.90 at 3 months). Similarly, there was no difference in the quality of life, nor was there a difference in the number of emboli detected in the two groups. These authors concluded that processing of cardiotomy blood before reinfusion results in greater blood product use with greater postoperative bleeding in patients undergoing cardiac surgery, and that there was no clinical evidence of any neurologic benefit with this approach in terms of postoperative cognitive function. In summary, both these studies showed an increase in utilization of allogeneic blood products and perioperative blood loss as a consequence of routine cell-saver usage, with either no or minor improvements in incidence of postoperative cognitive decline. In view of the variable impact on NCD demonstrated in these studies and the detrimental impact of perioperative allogeneic transfusion,188 routine usage of cell saver for processing of cardiotomy suction blood is probably unwarranted.
Cerebral blood flow
In the mid-1960s, Wollman et al189 used changes in the arterial and jugular venous oxygen content differences (A-Vdo2) to estimate changes in CBF during alterations of MAP and arterial carbon dioxide tensions (Paco2) in patients undergoing CPB. They observed a direct correlation between Paco2 and A-Vdo2 (CBF), but no relation between A-Vdo2 and MAP. Although the concepts of alpha-stat and pH-stat pH management had not been formulated at that time, these authors recommended maintaining temperature-corrected Paco2 between 30 and 40 mm Hg during hypothermic CPB (see Chapter 28).
In 1968, a Japanese investigator, using the recently developed technique of radioisotope clearance, measured CBF and CMRO2 during CPB.190 In a series of 40 patients, krypton-85 clearance, with concomitant cannulation of the superior jugular bulb, was used to measure CBF and calculate CMRO2 during CPB. The influence of nonpulsatile CPB on the cerebral vasculature was also directly observed using retinal photomicrography. Although critical data such as esophageal temperatures and hematocrits were not reported, the observed 35% decrease in CBF with institution of CPB, 63% decrease in CMRO2 during hypothermia, 23% decrease in CMRO2 during rewarming and retinal venous engorgement during rewarming are consistent with subsequent research findings. This report apparently also marked the first observations in humans of retinal microembolism occurring during CPB, consistent with but markedly preceding the reports of Blauth and colleagues.127
Few subsequent radioisotope CBF studies during CPB in humans were reported for the next 15 years. Other investigators used indirect estimates of CBF (e.g., A-Vdo2, TCD CBF velocities, or thermodilution techniques) to estimate CBF.191
pH Management and Cerebral Blood Flow
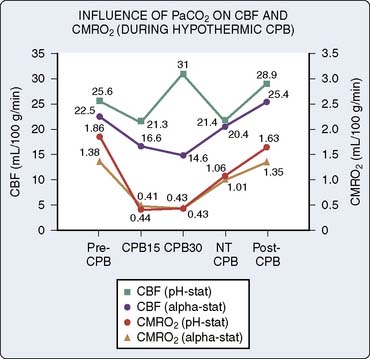
Relatively little new information regarding the cerebral circulation in human beings during CPB appeared until 1983, when Henriksen et al192 reported evidence of cerebral hyperemia occurring during CPB. This report was followed in 1984 by a seminal paper from Govier et al,165 who not only incited controversy with their observations of ischemic threshold levels of CBF during CPB, in direct contrast with the hyperperfusion reported by Henriksen, but also made preliminary observations on many of the other critical variables thought to influence CBF during CPB.
Without the measurement of concomitant cerebral metabolism, these apparently discordant observations of CBF could not be reconciled. Murkin et al66 subsequently reported their observations of both CBF and CMRO2 during hypothermic CPB in humans, using a xenon-133 clearance technique for measurement of CBF, similar to techniques used by Kubota, Govier et al, and Henriksen et al, but with the addition of a jugular bulb catheter for sampling effluent cerebral venous blood for measurement of cerebral metabolic activity. It was hypothesized that differences in pH management accounted for the divergent values previously reported for CBF during hypothermic CPB. Accordingly, patients were managed with either alpha-stat or pH-stat pH management during hypothermic CPB. A similar and pronounced reduction in CMRO2 was observed in both groups during hypothermia (Figure 36-10), and in the alpha-stat group, global cerebral flow/metabolism coupling was preserved in comparison with the group managed with pH-stat (Figure 36-11). Decreases in CBF and CMRO2, significantly lower than similar measures before and after CPB, were still evident after rewarming during normothermic nonpulsatile CPB. These low values for CBF and CMRO2 were restored to control levels shortly after separation from CPB. Alpha-stat management preserved autoregulation and the relation between CBF and metabolism.66
Temperature and Coronary Artery Bypass Grafting
How much does cerebral hyperthermia superimposed on a brain suffused with focal ischemic lesions contribute to postoperative CNS dysfunction? It is known that the vulnerability of the normothermic brain to focal ischemic insult demonstrates a surprising variability in the presence of small gradations in temperature. Busto et al194 demonstrated that at 33° C, expression of cerebral ischemia was virtually eliminated compared with controls maintained at 36° C. In fact, a large measure of the apparent cerebroprotective efficacy of the glutamate-receptor antagonist MK-801 in global ischemia was demonstrated by Buchan and Pulsinelli195 to be mediated by just such a small secondary decrease in brain temperature. Conversely, small increases in brain temperature, such as to 39° C increments as may occur during CABG (Figure 36-12), have been shown to profoundly enhance the susceptibility of the brain to focal ischemic insult and result in ischemic lesions of much greater extent in comparison with controls at 37° C.196
Normothermic Cardiopulmonary Bypass
The demonstration of apparently improved myocardial performance and shorter CPB and operating room times after normothermic CPB has prompted several outcome studies to assess the efficacy of this therapy, with particular focus now centered on CNS outcomes. Hypothermia reduces cerebral metabolic rate; thus, mild hypothermia might protect the brain by preferentially suppressing energy utilization to maintain cellular integrity.197 In support of this are results from a subset of 138 patients randomized to normothermic or hypothermic CPB, in whom a detailed prospective neurologic examination and a series of cognitive tests were performed before surgery, at postoperative days 1 to 3 and 7 to 10, and again at 1 month after surgery.198 Seven of 68 patients in the normothermic group were found to have a central neurologic deficit compared with none of the patients in the hypothermic group, a significantly greater incidence. In a separate study of 96 patients undergoing CABG and randomized to CPB at either 28° C, 32° C, or 37° C, patients managed at 37° C had a significantly greater incidence of deterioration on cognitive test scores than did those managed at either 28° C or 32° C. No additional benefit in terms of cognitive function was conferred by cooling to 28° C versus 32° C.199 Nathan et al200 reported on CABG patients operated under hypothermic (32° C) CPB and then randomly assigned to rewarming to 37° C (control) or 34° C (hypothermic), with no further intraoperative warming. Neurocognitive testing was performed 1 week and 3 months after surgery. Eleven tests were combined into three cognitive domains: memory, attention, and psychomotor speed and dexterity. The incidence of cognitive deficits 1 week after surgery was 62% in the control group and 48% in the hypothermic group (relative risk, 0.77; P = 0.048). In the hypothermic group, the magnitude of deterioration in attention and in speed and dexterity was reduced by 55.6% (P = 0.038) and 41.3% (P = 0.042), respectively. At 3 months, the hypothermic group still performed better on one test of speed and dexterity.201
Other studies have demonstrated apparently different results, however. Engelman et al201 randomized a series of 291 patients undergoing coronary revascularization to either hypothermic or tepid/normothermic perfusion. Twelve intraoperative ischemic strokes occurred; six of these were in the group receiving hypothermic perfusion, and six were in the group receiving the tepid/normothermic perfusion. Measuring the infarct volume documented that three of the strokes in each group resulted in minor or small infarcts, and that three in each group were significant, major strokes. The volume of infarction, whether including all six patients in each group or only those with major strokes, was no different between the hypothermic and the tepid/normothermic groups. The authors observed no relation between the size of a cerebral ischemic infarct and the perfusate temperature during coronary revascularization.201 Similarly, Dworschak et al202 found no difference in brain isoenzyme S-100β release pattern or in clinical neurologic complications between two groups of CABG patients randomly assigned to normothermic or hypothermic (32° C) CPB. In a large, prospective study, Grigore et al118 randomly assigned 300 patients undergoing elective CABG to tepid/normothermic (35.5° C to 36.5° C) or hypothermic (28° C to 30° C) CPB and used a battery of neurocognitive tests evaluating four distinct cognitive domains administered before surgery and at 6 weeks after surgery. Again, there were no differences in neurologic or neurocognitive outcomes between normothermic and hypothermic groups in multivariable models. In a separate study, Grimm et al203 actually found subclinical impairment of cognitive brain function to be more pronounced in CABG patients undergoing mildly hypothermic CPB compared with normothermic CPB. Accordingly, a protective effect of hypothermia on neurologic outcome in CABG has not been verified in most clinical trials, possibly because of too fast and/or excessive rewarming of patients or, more likely, because the impact of only transient cooling during CPB does not extend into the postoperative interval when cerebral hyperthermia has also been demonstrated and correlated with impaired cognitive performance at 6 weeks after surgery.21,204–208
Cerebral Hyperthermia
Cerebral hyperthermia during the rewarming phase of CPB can exacerbate a preexisting injury before rewarming and may be detrimental in itself. Hyperthermia can have a strong impact on cerebral oxygen transfer and neurologic outcome. Glutamate levels can increase during cerebral hyperthermia, leading to eventual cell death. Rapid rewarming decreases jugular venous hemoglobin saturation, creating a mismatch between cerebral oxygen consumption and delivery.209,210 Okano et al211 assessed the effects of normothermia and mild hypothermia (32° C) during CPB on jugular oxygen saturation (Sjvo2) in 20 patients scheduled for elective CABG. The Sjvo2 in the normothermic group was decreased significantly at 20 and 40 minutes after the onset of CPB compared with pre-CPB, whereas there was no change in Sjvo2 in the mild hypothermic group during the study. The authors concluded that cerebral oxygenation, as assessed by Sjvo2, was increased during mild hypothermic CPB compared with normothermic CPB. Kawahara et al210 examined the effect of rewarming rates on Sjvo2 in 100 patients scheduled for elective CABG and randomly divided into two groups: a control group and a slow rewarming group. Cerebral desaturation (defined as an Sjvo2 value < 50%) during rewarming was more frequent in the control group than in the slow group. Cerebral desaturation time was defined as duration when Sjvo2 was less than 50% and the ratio of the cerebral desaturation time to the total CPB time in the control group differed significantly from those in the slow group (control group: 17 ± 11 minutes, 12% ± 4%; slow group: 10 ± 8 minutes, 7% ± 4%, respectively; P < 0.05).210 Consistent with this, in a study of the impact of rate of rewarming on cognitive outcomes in 165 CABG patients randomized to two differing rewarming strategies, Grigore et al118 demonstrated a significant association between change in cognitive function and rate of rewarming.
Grocott et al208 recorded hourly postoperative temperatures in 300 patients undergoing CABG on CPB and determined the degree of postoperative hyperthermia using the maximum temperature within the first 24 hours, as well as by calculating the area under the curve for temperatures greater than 37° C. Patients underwent a battery of cognitive testing both before surgery and 6 weeks after surgery. The maximum temperature within the first 24 hours after CABG ranged from 37.2° C to 39.3° C, and these investigators demonstrated that the maximum postoperative temperature was independently associated with cognitive dysfunction at 6 weeks.176 Accordingly, slower rewarming rate with lower peak temperatures during CPB may be an important factor in the prevention of neurocognitive decline after hypothermic CPB, and interventions to avoid postoperative hyperthermia may be warranted to improve cerebral outcome after cardiac surgery.
Cerebrovascular Disease
Relatively few studies have examined the cerebrovascular responses to CPB in patients with known cerebrovascular disease. Because of the vasodilatory effects of increased carbon dioxide in patients with cerebrovascular disease, pH-stat management could theoretically induce redistribution of regional CBF from marginally perfused to well-perfused regions (i.e., an intracerebral steal). Gravlee et al212 investigated patients with cerebrovascular disease undergoing CABG and assessed the CBF responses to varying pH management, between alpha-stat and pH-stat. They confirmed the responsiveness of the cerebral vasculature to changes in Paco2 during hypothermic CPB but did not demonstrate evidence of intracerebral steal at greater Paco2 levels in any of these patients. In all patients, however, arterial perfusion pressure was greater than 65 mm Hg during CBF measurements, which may have offset any tendency for regional CBF inhomogeneities.
Using TCD monitoring of CBF velocity, 18 patients with severe carotid stenosis and 37 with no or mild stenosis were monitored during CPB.213 Although not specified, it appears as though pH-stat management was used, because flow velocities correlated with Paco2 and arterial pressure. There were no significant differences detectable in flow velocity between patients with or without significant carotid stenosis. In a case report of a single patient with bilateral carotid stenoses, alpha-stat pH management was used and arterial pressure was varied from 35 to 85 mm Hg, whereas CBF was measured during hypothermic CPB.214 The CBF values obtained from contralateral hemispheres were essentially equal and remained so throughout the range of different perfusion pressures used. These studies suggest that the cerebrovascular responses to CPB in patients with cerebrovascular disease do not differ significantly from those of normal patients with respect to gross measures of cerebrovascular responsiveness. This suggests that other factors, including associated aortic atherosclerosis, may be a more important factor.
Hogue et al7 examined demographic and perioperative data prospectively collected from 2972 patients undergoing cardiac surgery. Carotid artery ultrasound examination was performed before surgery for patients aged 65 years or older or when there was a history of TIAs or prior stroke. Epiaortic ultrasound was performed at the time of surgery in all patients to assess for atherosclerosis of the ascending aorta. Strokes occurred after surgery in 30 women and 18 men (P < 0.0001). A history of a stroke was the strongest predictor of new stroke for both women and men. A prior cerebrovascular event was a more important predictor of stroke for men than women.7
Delirium
In Bucerius et al’s study5 assessing CNS outcomes from 16,184 patients undergoing cardiac operations, the overall prevalence rate of postoperative delirium was 8.4%. Stepwise logistic regression revealed history of cerebrovascular disease, peripheral vascular disease, atrial fibrillation, diabetes mellitus, left ventricular ejection fraction of 30% or less, preoperative cardiogenic shock, urgent operation, intraoperative hemofiltration, operation time of 3 hours or more, and a high perioperative transfusion requirement as being independent predictors of delirium. In a prospective follow-up study of 112 cardiac surgical patients, the incidence rate of postoperative delirium was 21% and was associated with significantly increased mortality and readmission to hospital, as well as significantly greater incidences of cognitive and sleep disturbances.215
Carotid Endarterectomy
In the current cardiac surgical population, 17% to 22% of patients have a moderate carotid artery stenosis of 50% or more, and 6% to 12% have a severe stenosis of 80% or more.21 The risk for postoperative stroke is 10% in patients with moderate and 11% to 19% in patients with severe stenosis, whereas it remains 2% or less in patients with a stenosis of less than 50%. Although in patients presenting for cardiac surgery severe bilateral carotid artery disease is rare, the risk for perioperative stroke is as high as 20%.21,216–218 It is not clear that carotid endarterectomy decreases this rate, however, because in a meta-analysis, pooled data for stroke or death did not support carotid endarterectomy for risk reduction from asymptomatic carotid stenosis during CABG (relative risk, 0.9; P = 0.5).219 In a review, it was estimated that only about 40% of perioperative strokes (at most) could be directly attributable to ipsilateral carotid artery disease.220 Accordingly, in a patient with asymptomatic carotid stenosis, combined surgery should not be undertaken unless the surgical team is very experienced in combined carotid endarterectomy/CABG procedures. Concomitant carotid endarterectomy is unlikely to decrease a patient’s stroke risk. Rather, carotid stenosis should be regarded as indicating a high likelihood of aortic and/or concomitant intracerebral disease, and there is increasing evidence that use of EAS with appropriate modification of surgical approach and, potentially, applied neuromonitoring can be of particular benefit in this high-risk group.
Diabetes Mellitus and Hyperglycemia
The presence of diabetes is recognized as a factor related to increased morbidity and mortality in cardiac surgical patients.221–225 The incidence of diabetes mellitus increases with age, and its presence is known to accelerate the damage caused by atherosclerosis; thus, an increasingly greater percentage of patients coming for CABG have concomitant diabetes, currently estimated as a comorbidity in approximately 30% to 40% of CABG patients. Bucerius et al226 found diabetes to be associated with increased incidence of stroke and delirium, and prolonged ICU and hospital stay. Mortasawi et al227 and Nussmeier26 describe diabetes mellitus as associated with increased incidences of stroke and mortality. In a large study, McKhann et al19 prospectively collected data on 2711 CABG patients and identified diabetes mellitus as an independent risk factor for both strok and encephalopathy. Part of the risk may involve cerebral hypoperfusion because cerebral oxygen desaturation during CPB has been documented in diabetic patients, with patients with insulin-dependent diabetes demonstrating the lowest values as measured via jugular oximetry and the poorest response to increases in MAP.228
Studies identify normoglycemia as a desirable perioperative goal in cardiac surgical patients regardless of whether they are diabetic.223,225 There is both experimental and clinical evidence that hyperglycemia is associated with exacerbation of neurologic injury.229 Approaches to maintain serum glucose values less than 150 mg/dL have shown favorable results. Furnary et al221 reported on 3554 patients who underwent CABG from 1987 through 2001, demonstrating that the observed mortality in the group managed with tighter glucose values was lower and concluded that continuous intravenous insulin infusion added a protective effect against death. Carvalho et al224 used an aggressive approach to maintain serum glucose values between 80 and 110 mg/dL and reported that this is a safely attainable goal. One study positively correlated average blood glucose on the first postoperative day with a variety of adverse outcomes (stroke, myocardial infarction, septic complication, or death). For each 1-mmol/L increase greater than 6.1 mmol/L (1 mmol = 18 mg/dL), risk increased by 17%.230 The ideal value of serum glucose in cardiac surgical patients remains unknown, but the evidence available suggests that maintenance of euglycemia is related to a better prognosis.
In accordance with these data, recent Society of Thoracic Surgeons guidelines have been published outlining recommendations for glucose control in diabetic and nondiabetic patients undergoing cardiac surgery with an overall recommendation that in both diabetic and nondiabetic patients, blood glucose levels should be maintained at less than or equal to 180 mg/dL with intravenous insulin as required.231 However, because of concerns regarding potential adverse effects associated with hypoglycemia, including both increased risk for mortality associated with even a single episode of severe hypoglycemia as seen in medical/surgical intensive care patients,232 and because in a randomized, prospective study of 400 cardiac surgical patients managed either with tight glucose control (intravenous insulin to maintain intraoperative glucose between 80 and 100 mg/dL) or conventional management (glucose level < 200 mg/dL) a significantly greater incidence of stroke was found in the treatment group,233 avoidance of hypoglycemia should be paramount. Accordingly, an important caveat recommending preservation of lower limit of glucose level greater than 100 mg/dL should be appended to the guidelines.234 Overall, it would appear that maintenance of perioperative serum glucose between 100 and 180 mg/dL in both diabetic and nondiabetic patients is desirable.
Hemodynamic Instability
Hemodynamic complications, either before, during, or after surgery, have been found to increase cerebral injury in cardiac surgical patients. In Bucerius et al’s5 report, ejection fraction less than 30%, urgent operations, and preoperative cardiogenic shock were related to increased postoperative delirium. Ridderstolpe et al27 found that hypotension and postoperative arrhythmias were related to cerebral complications, whereas Stanley et al132 reported that postoperative atrial fibrillation was related to increased cognitive decline. Ganushchak et al172 reported in a retrospective analysis of 1395 patients that the frequency of neurologic complications was 3.9% in the group of patients who experienced large fluctuations in hemodynamic parameters while on CPB, whereas in the group of patients with more stable values on CPB, the incidence rate of neurologic complications was 0.3%. These studies indicate an increased susceptibility of the brain in cardiac surgical patients to apparently “benign” hemodynamic alterations that either produce or enhance cerebral injury, probably through hypoperfusion of the brain tissue, particularly because it has been estimated that more than 50% of CABG patients have coexisting cerebrovascular disease.137,138
Cerebroprotective strategies
Cardiopulmonary Bypass Equipment
Early studies demonstrated increased microemboli in patients undergoing CPB using bubble oxygenators, with a reduction in cerebral embolization with the use of membrane oxygenators and arterial line filtration124,127,235–237 (Box 36-4). Using intraoperative fluorescein retinal angiography, Blauth et al124 reported retinal microembolizations occurring during CPB with much greater frequency in patients in whom bubble versus membrane oxygenators were used. Using TCD for detection of cerebral emboli, Padayachee et al236 demonstrated continuous generation of cerebral emboli in all patients managed using bubble oxygenators and none in patients in whom a membrane oxygenator was used. They also demonstrated the efficacy of arterial line filtration to significantly decrease cerebral embolic load.237 It is apparent that emboli may be generated continuously during CPB and that equipment modification (e.g., arterial line microfiltration and preferential usage of membrane oxygenators) can decrease the generation of such emboli.238 Membrane oxygenators are currently recommended for CPB.131 (See Chapters 28 and 29.)
BOX 36-4 Clinical Strategies that may Decrease Neurologic Complications in Cardiac Surgery
It is equally evident that equipment modifications, although decreasing the embolic load, cannot completely eliminate it.94,236 Georgiadis et al239 used Doppler ultrasound and evaluated the percentage of MES reduction caused by the arterial filter and the proportion of MES actually reaching the brain by comparing the MES counts detected before the arterial filter, after the arterial filter, and in both middle cerebral arteries. Eleven patients underwent surgery using normothermic CPB, alpha-stat, a membrane oxygenator, and a 40-μm arterial filter. Evaluation of MES was only performed during extracorporeal circulation, was initiated after cannulation and clamping of the ascending aorta, and was terminated shortly before the aortic clamp was removed. The arterial filter resulted in a 58.9% reduction of MES, with only 4.4% (2624/59,132) of the MES detected after the arterial filter. The proportion of MES detected in the middle cerebral artery corresponded to the total cerebral perfusion under CPB, estimated as 5% to 10% of the total perfusion volume.239
Schoenburg et al240 used a dynamic bubble trap, incorporated in the arterial line after a 40-μm filter, to reduce the number of gaseous microemboli in 50 patients undergoing CABG. In 26 patients, a dynamic bubble trap was placed between the arterial filter and the aortic cannula (group 1), and in 24 patients, a placebo dynamic bubble trap was used (group 2) with TCD continuously measured on both sides during bypass, which was separated into four periods: phase 1, start of bypass until aortic clamping; phase 2, aortic clamping until rewarming; phase 3, rewarming until clamp removal; and phase 4, clamp removal until end of bypass. The bubble elimination rate during bypass was 77% in group 1 and 28% in group 2. The number of high-intensity signals was lower in group 1 during phase 1 (5.8 ± 7.3 vs. 16 ± 15.4; P < 0.05 vs. group 2) and phase 2 (6.9 ± 7.3 vs. 24.2 ± 27.3; P < 0.05 vs. group 2) but not during phases 3 and 4.240 The authors found that the dynamic bubble trap can remove gaseous microemboli. Unfortunately, no psychometric studies were performed on either group of patients, but future research in this area might yield positive influence on neurologic outcome by decreasing gas microemboli. Other investigators have demonstrated that air within the venous line of the CPB circuit, resulting from air entrainment at the venous cannulation site, injection of drugs into the venous line, or use of cardiotomy suction can pass through the oxygenator and appear as microemboli within the arterial line, even in the presence of a venous line defoamer and with use of a membrane oxygenator.239,240 Georgiadis et al239 used tubing systems that included an arterial line 40-μm filter and demonstrated that the arterial filter resulted in a 58.9% reduction of microemboli signals with only 4.4% of the MESs detected after the arterial filter being actually detected in the middle cerebral artery.
Modification of the inflammatory response to the CPB using modified surface CPB circuits and leukocyte-depleting filters has been explored. Hamada et al134 examined the combined use of heparin coating of the CPB circuit and a leukocyte-depleting arterial line filter in 30 patients allocated randomly to equal groups with a conventional circuit and arterial line filter, a heparin-coated circuit with a conventional filter, or a heparin-coated circuit with a leukocyte-depleting arterial line filter. Plasma interleukin-6 and -8 concentrations in the heparin bonded with leukocyte-depleting filter group were lower than in the conventional circuit group. Although a decrease in inflammatory mediator release has been observed by using leukocyte-depleting filters, the impact of these types of filters on neurologic outcome is less clear. In a meta-analysis of 28 relevant clinical studies, Whitaker et al243 concluded that conventional arterial line filtration had a definite effect in reducing neuropsychological deficit post-CPB. The results of studies using the leucocyte-depleting filter were less clear-cut.
In an attempt to decrease emboli originating from the surgical field, cell savers have been used for processing cardiotomy suction blood before returning it to the CPB circuit. Jewell et al244 reported on 20 patients prospectively randomized to either cell saver or cardiotomy suction and demonstrated that compared with cardiotomy suction, cell saver removed significantly more fat from shed blood, such that the percentage reduction in fat weight achieved by cell saver or cardiotomy suction was 87% compared with 45%. de Vries et al245 published a study on patients randomly assigned to have a fat removal filter for the cardiotomy suction. The fat filter removed 40% fat, leukocytes, and platelets from cardiotomy suction blood during cardiac surgery compared with the control group without the filter.
In addition, various intraoperative manipulations, particularly instrumentation of the atherosclerotic aorta, are independent risks for the generation of cerebral emboli and likely produce particulate or macrogaseous emboli, rather than oxygenator-generated microgaseous and microaggregate emboli.80,84,246,247 Avoidance of manipulation of a diseased aorta seems to decrease embolization and cerebral injury.91,97,248 An alternative approach, emboli reduction by capture using an intra-aortic filter inserted through a side chamber of a modified aortic cannula, has also been assessed (Figure 36-13).
In a nonrandomized study, Schmitz et al133 examined the impact of intra-aortic filtration during CPB.134,249 Three hundred four cardiac surgical patients had intra-aortic filtration using a 150-μm net deployed through the aortic cannula, whereas a further 278 patients formed the control group. Patients in the filter group experienced a lower incidence of adverse neurologic outcomes than patients in the control group (4.3% vs. 11.9%), with significantly fewer TIAs (0% vs. 1.4%), delirium (3.0% vs. 6.5%), and memory deficit (1.3% vs. 6.2%). There were also fewer strokes in the filter group compared with the control group (0.7% vs. 2.2%). Although the sample size was relatively small and the patients were not randomly assigned, the study findings suggest a protective effect of intra-aortic filters on CNS injury.133 In a large, multi-institutional, prospective, randomized trial of 1289 patients in whom intra-aortic filtration or conventional CPB cannula was used, emboli were identified in 598 (96.8%) of 618 filters successfully deployed, and their post hoc analysis indicated a reduction in postoperative renal complications.249 In other studies, intra-aortic emboli trapping devices have been used with varied results.97,239 In a small study by Eifert et al94 in 24 patients, the use of the intra-aortic filter device did not show any difference in neurologic, neuroradiographic, or neuropsychological outcomes, yet the intra-aortic filter was effective in capturing particulate material. Similar efficacy in capturing intraoperative material using intraaortic filters during CPB was reported by Reichenspurner et al.250 It does appear as though intra-aortic filters can be safely deployed, and that they do capture particulate emboli, the predominant origin of which is atheromatous.
In a recent study of 150 CABG patients randomized to either intra-aortic filtration, dynamic bubble trap or conventional management, no difference was found in the overall incidence of postoperative MRI-detected small ischemic brain lesions (17/143) between groups, whereas dynamic bubble trap was associated with significantly fewer TCD-detected cerebral emboli and improved cognitive performance at 3 months after surgery in comparison with control and intra-aortic filtration groups.251
Applied Neuromonitoring
Intraoperative neurophysiologic monitoring may be of benefit to decrease CNS injury.252 Intraoperative TCD has been demonstrated to detect embolic events in real time and allows modification of perfusion and surgical techniques. It has been shown that the numbers of emboli generated by perfusionist interventions (e.g., drug injection, blood return), as well as episodes of entrainment of air from the surgical field, are rapidly identified and corrected by TCD detection of intraoperative emboli.253 (See Chapter16.)
Brain oximetry studies using noninvasive near-infrared spectrophotometry (NIRS) have shown promising results.254–260 In Goldman’s259 large, nonrandomized cohort study, NIRS was used to monitor cerebral oxygen saturation in 1034 cardiac surgical patients and was compared with outcomes in 1245 patients who underwent cardiac surgery immediately before cerebral oximetry was incorporated. The study group had significantly more patients in New York Heart Association (NYHA) Classes III and IV than the control group, but the study group overall had fewer permanent strokes (10 [0.97%] vs. 25 [2.5%]; P < 0.044) and the proportion of patients requiring prolonged ventilation was significantly smaller in the study group, as was the length of hospital stay.259 Murkin et al260 reported a prospective, blinded, randomized study of NIRS cerebral oximetry in 200 cardiac surgical patients and demonstrated significantly fewer adverse clinical outcomes in NIRS versus control groups (P = 0.027).
Even during beating-heart procedures, compromised cerebral perfusion can occur relatively frequently, and if unrecognized, may account for the relative lack of difference in CNS outcomes between CABG and OPCAB surgery.261 Combined EEG and cerebral oximetry identified episodes of cerebral ischemia in 15% of a series of 550 beating-heart patients; all were treated successfully by a combination of pharmacologically improved cardiac output, increased perfusion pressure, and cardiac repositioning.261
In a study utilizing cerebral oximetry in 265 patients undergoing primary CAB surgery and randomized to active monitoring and a series of interventions designed to improve rSo2 or to a control group in which blinded monitoring was used, a significant association was found between prolonged cerebral desaturation and early cognitive decline, as well as a threefold increased risk for prolonged hospital stay.54 However, cerebral desaturation rates were similar between groups and ascribed to poor compliance with the treatment protocol, resulting in no difference in the incidence of cognitive dysfunction between groups. In a study of 103 patients undergoing valvular heart surgery in whom blinded cerebral NIRS monitoring was used, cerebral oxygen desaturation was again found to be associated with significantly longer duration of postoperative hospitalization.262 In a prospective, randomized, blinded study in 200 patients undergoing coronary artery grafting, Murkin et al260 demonstrated that active treatment of declining cerebral rSo2 values prevented prolonged cerebral desaturations and was associated with a shorter ICU length of stay and a significantly reduced incidence of major organ morbidity or mortality in comparison with a similar control group. In this study, the intervention protocol undertaken to return rSo2 to baseline resulted in a rapid improvement in rSo2 in 84% of cases and did not add undue risk to the patient, including no increase in allogeneic blood transfusions.260,263 There were also numerically fewer clinical cerebrovascular accidents in the monitored patients directionally consistent with previous studies.259 As such, a physiologically derived treatment algorithm for management of perioperative cerebral oxygen desaturation has been proposed and is shown in Figure 36-14.264 An important confounder in evaluating the role of cerebral NIRS devices is the efficacy of treatment for cerebral desaturation.
Figure 36-14 Algorithm for the use of brain oximetry.
(Reprinted from Denault A, Deschamps A, Murkin JM: A proposed algorithm for the intraoperative use of cerebral near-infrared spectroscopy.Semin Cardiothorac Vasc Anesth 11:274–281, 2007.)
Neuromonitoring during Deep Hypothermic Circulatory Arrest
Moderate (25° C to 30° C) and deep (< 25° C) hypothermia remain a mainstay for cerebral and systemic protection during complex aortic arch repair because surgical access may require interruption of systemic perfusion for relatively protracted periods. As there is relatively little ability to monitor cerebral well-bring during such times because EEG becomes progressively attenuated at less than 25° C, cerebral NIRS has been advocated as a means of monitoring and detecting onset of cerebral ischaemia during deep HCA.265,266 Although some groups monitor jugular venous oxygen saturation (Sjo2) using retrograde cannulation of the internal jugular vein as an index of cerebral metabolic suppression during cooling, correlation has not been demonstrated between Sjo2 and cerebral NIRS during deep HCA,267 likely indicative of the fact that NIRS is a highly regional measure of cerebral cortical oxygen tissue saturation, whereas Sjo2 is a measure of cerebral mixed venous oxygen saturation, and thus reflective of global changes in venous oxygenation, and as such, potentially less sensitive to regional perfusion inhomogeneities.
In addition to deep HCA, some centers use RCP via the superior vena cava or, increasingly, selective anterograde cerebral perfusion (SACP) via the innominate or subclavian artery. There have been a variety of case reports of the ability of cerebral NIRS to detect onset of cerebral ischemia during aortic arch surgeries, and there is growing interest in the role of cerebral NIRS as a measure of adequacy of perfusion in this setting.268–272 There is increasing recognition that RCP does not provide sufficient nutritive flow to sustain cerebral integrity for an extended interval,273 as has been reflected in lower rSo2 values seen during NIRS monitoring in RCP versus SACP.271–273
In a review of the role of NIRS monitoring during SACP, a study was undertaken in 46 consecutive patients in whom SACP was established by separate concomitant perfusion of the innominate and the left carotid arteries or by perfusion of the right subclavian artery (with or without left carotid artery perfusion), and during which bilateral regional cerebral tissue oxygen saturation index was monitored by INVOS 4100 NIRS (Somanetics Corporation, Troy, MI) and that used stroke as the primary clinical end point, together with indices of diagnostic performance of the NIRS device.274 In this series, six patients died in the hospital and six patients (13%) in whom regional cerebral tissue oxygen saturation values were significantly lower during SACP experienced a perioperative stroke. Regional cerebral tissue oxygen saturation decreasing to between 76% and 86% of baseline during SACP had a sensitivity of up to 83% and a specificity of up to 94% in identifying individuals with stroke. It was concluded that using NIRS monitoring of regional cerebral tissue oxygen saturation during SACP allows detection of clinically important cerebral desaturations and can help predict perioperative neurologic sequelae, supporting its use as a noninvasive trend monitor of cerebral oxygenation.274
In adult patients, cerebral malperfusion can occur either as a consequence of ascending aortic dissection with occlusion of carotid lumen,275 kinking or obstruction of perfusion cannula during selective cerebral perfusion for circulatory arrest procedures, or due to migration of aortic endoclamp cannula during minimal-access cardiac surgery with potential compromise of cerebral perfusion.276,277 There are increasing reports that bilateral rSo2 monitoring can detect contralateral desaturation during unilateral selective cerebral perfusion. This can result from an incomplete circle of Willis, which in some series has a prevalence rate of up to 50% and has been estimated to be a factor in cerebral malperfusion in approximately 15% of patients.278,279 In a more recent case report, cerebral rSo2 monitoring was used during selective cerebral perfusion in the absence of systemic CPB during repair of traumatic aortic arch rupture and detected both episodes of cerebral malperfusion and, most critically, acute thrombosis of carotid artery graft leading to thrombectomy and restoration of flow.280
There have also been a number of case reports of aortic arch surgery in which cerebral oximetry has been shown to detect cerebral hypoperfusion from a variety of factors including diminished Blalock–Taussig shunt flow after pediatric cardiac surgery.281
Pharmacologic Cerebral Protection
In general, pharmacologic protection from cerebral ischemia remains an elusive goal. Although there had been one clinical study in which a significant reduction in persistent neurologic defects after open-chamber cardiac surgery was reported after administration of high-dose thiopental, this was not confirmed in closed-chamber CABG procedures.282,283 Other data have suggested that if there is any such thiopental-derived cerebroprotective effect, the mechanism may be caused by a metabolically driven decrease in CBF, with a concomitant reduction in the delivery of emboli into the brain, rather than occurring primarily as a result of a decrease in CMRO2.284 However, a three-center study in 225 patients undergoing mitral or aortic valve surgery and randomized to high-dose propofol with induction of burst-suppression on EEG or a sufentanil control group was unable to detect any significant differences in neurologic or neuropsychological outcomes between groups.285 These authors concluded that neither cerebral metabolic suppression nor reduction in CBF reliably provides neuroprotection in open-chamber cardiac surgery. A large trial of perioperative nimodipine in valve replacement surgery had to be terminated prematurely after enrollment of only 150 of 400 patients because of a significant increase in major bleeding and an increased mortality in the 6-month follow-up period.286
There are some interesting associations between certain drug therapies having anti-inflammatory properties and lowered incidences of stroke and adverse CNS events. In a retrospective review of 2575 CABG patients, Amory et al287 reported that patients who received perioperative β-blockers had a significantly lower incidence of severe neurologic outcomes versus those who did not receive these drugs, demonstrating a 1.9% incidence rate of stroke and coma versus 4.3%.
However, concerns regarding perioperative β-blocker therapy has been raised by the results of the PeriOperative ISchemic Evaluation (POISE) trial in which 8351 patients with, or at risk for, atherosclerotic disease who were undergoing noncardiac surgery were randomized to receive extended-release metoprolol (n = 4174) or placebo (n = 4177).288 Although significantly fewer patients in the metoprolol group than in the placebo group had a myocardial infarction, there were more deaths in the metoprolol group than in the placebo group (3.1% vs. 2.3%), and more patients in the metoprolol group than in the placebo group had a stroke (1.0% vs. 0.5%).285 The implications of this study for cardiac surgical patients remains unclear, but there does not appear to be any increased risk for perioperative stroke in noncardiac surgery patients chronically maintained on β-blocker therapy.289
The serine protease inhibitor aprotinin has been shown to positively impact coagulation and inflammatory alterations triggered by CPB and has also been associated with decreased incidences of stroke and major CNS injury in cardiac surgical patients.135,136 Aprotinin has also been demonstrated to have anti-inflammatory and antithrombotic effects.290 Frumento et al8 retrospectively analyzed stroke outcomes in 1524 patients undergoing cardiac surgery and identified a subset of 149 patients older than 70, with history of hypertension, diabetes mellitus, stroke or TIA, and presence of aortic atheroma who were deemed to be at similarly high risk for stroke. The authors reported that in this high-risk subset of patients, intraoperative administration of full-dose aprotinin, but not half-dose, was associated with a lower incidence of stroke. However, because the clinical usage of aprotinin has been suspended indefinitely because of several reports of increased mortality and adverse events associated with aprotinin therapy in cardiac surgical patients,291–293 the future of this drug remains controversial.294,295
In a prospective study of 5065 patients undergoing CABG conducted at 70 centers in 17 countries, the relation between early aspirin use and fatal and nonfatal outcomes was investigated.296 Among patients who received aspirin within 48 hours after revascularization, subsequent mortality rate was 1.3% compared with 4% among those who did not receive aspirin during this period. Aspirin therapy was associated with a 48% reduction in the incidence of myocardial infarction (2.8% vs. 5.4%; P < 0.001), a 50% reduction in the incidence of stroke (1.3% vs. 2.6%; P = 0.01), a 74% reduction in the incidence of renal failure (0.9% vs. 3.4%; P < 0.001), and a 62% reduction in the incidence of bowel infarction (0.3% vs. 0.8%; P = 0.01). Taken together, these studies seem to indicate that therapies associated with decreased inflammatory and sympathetic responses appear to be associated with decreased incidence of stroke and adverse CNS events.
Several preliminary studies had suggested that intraoperative administration of lidocaine infusion during cardiac surgery was associated with a decreased incidence of postoperative cognitive dysfunction.297,298 However, in larger, randomized, prospective trials, this was not demonstrated.299 In view of these divergent results, whether lidocaine infusion will have any further role as a cerebroprotectant remains uncertain.300 An increasingly promising line of investigation for cerebral protection is the role of perioperative statin therapy, with evidence accruing that increased statin dosages and combination with other antiinflammatory agents such as angiotensin converting enzyme inhibitors can significantly decrease inflammatory markers and associated stroke risk.301,302
Although these results suggest that there is as yet no pharmacologic “magic bullet” that can be used to reduce neurologic injury in patients undergoing cardiac surgery, a combination of technical and pharmacologic measures is currently available that might positively affect the CNS outcomes of these patients.160 In patients identified as being at risk for perioperative cerebral injury, preventive measures as outlined in Box 36-4 should be instituted with organ-targeted management to guide the whole intraoperative and postoperative period. The Best Practice Cardiopulmonary Bypass Group summarized these measures as the avoidance of embolic injury by means of intraoperative EAS before aortic instrumentation, the employment of arterial line filters, modified-surface and reduced-area CPB circuits and membrane oxygenators, minimization of transfusion of unprocessed cardiotomy suction blood, the use of alpha-stat pH management during moderate hypothermic CPB, monitoring cerebral venous outflow pressure via proximal jugular venous pressure, avoidance of hypotension, the avoidance of cerebral hyperthermia during rewarming, maintenance of euglycemia, and the use of “tepid” rather than normothermic perfusion during CPB. As the age and incidence of comorbid disease in the cardiac surgical population continue to increase, the importance of these issues becomes ever more acute. In summary, primary prevention continues to be the only effective measure to decrease cerebral injury in cardiac surgical patients.
1 Ferguson T.B.Jr, Hammill B.G., Peterson E.D., et al. A decade of change—risk profiles and outcomes for isolated coronary artery bypass grafting procedures, 1990-1999: A report from the STS National Database Committee and the Duke Clinical Research Institute. Society of Thoracic Surgeons. Ann Thorac Surg. 2002;73:480.
2 Gardner T.J., Horneffer P.J., Manolio T.A., et al. Stroke following coronary artery bypass grafting: A ten-year study. Ann Thorac Surg. 1985;40:574.
3 Tuman K.J., McCarthy R.J., Najafi H., et al. Differential effects of advanced age on neurologic and cardiac risks of coronary artery operations. J Thorac Cardiovasc Surg. 1992;104:1510.
4 Bucerius J., Gummert J.F., Borger M.A., et al. Stroke after cardiac surgery: A risk factor analysis of 16,184 consecutive adult patients. Ann Thorac Surg. 2003;75:472.
5 Bucerius J., Gummert J.F., Borger M.A., et al. Predictors of delirium after cardiac surgery: Effect of beating-heart (off-pump) surgery. J Thorac Cardiovasc Surg. 2004;127:57.
6 Ricotta J.J., Char D.J., Cuadra S.A., et al. Modeling stroke risk after coronary artery bypass and combined coronary artery bypass and carotid endarterectomy. Stroke. 2003;34:1212.
7 Hogue C.W.Jr, De Wet C.J., Schechtman K.B., et al. The importance of prior stroke for the adjusted risk of neurologic injury after cardiac surgery for women and men. Anesthesiology. 2003;98:823.
8 Frumento R.J., O’Malley C.M., Bennett-Guerrero E. Stroke after cardiac surgery: A retrospective analysis of the effect of aprotinin dosing regimens. Ann Thorac Surg. 2003;75:479.
9 Ridderstolpe L., Ahlgren E., Gill H., et al. Risk factor analysis of early and delayed cerebral complications after cardiac surgery. J Cardiothorac Vasc Anesth. 2002;16:278.
10 D’Alfonso A., Mariani M.A., Amerini A., et al. Off-pump coronary surgery improves in-hospital and early outcomes in octogenarians. Ital Heart J. 2004;5:197.
11 Gerrah R., Izhar U., Elami A., et al. Cardiac surgery in octogenarians—a better prognosis in coronary artery disease. Isr Med Assoc J. 2003;510:713.
12 Alexander K.P., Anstrom K.J., Muhlbaier L.H., et al. Outcomes of cardiac surgery in patients ≥80 years: Results from the National Cardiovascular Network. J Am Coll Cardiol. 2000;135:731.
13 Shahian D.M., O’Brien S.M., Filardo G., et al. Society of Thoracic Surgeons Quality Measurement Task Force. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: Part 1—coronary artery bypass grafting surgery. Ann Thorac Surg. 2009;88(Suppl 1):S2-S22.
14 Roach G.W., Kanchuger M., Mangano C.M., et al. Adverse cerebral outcomes after coronary bypass surgery. Multicenter Study of Perioperative Ischemia Research Group and the Ischemia Research and Education Foundation Investigators. N Engl J Med. 1996;525:1857.
15 Blacker D.J., Flemming K.D., Link M.J., et al. The preoperative cerebrovascular consultation: Common cerebrovascular questions before general or cardiac surgery. Mayo Clin Proc. 2004;79:223.
16 Naylor A.R., Mehta Z., Rothwell P.M., et al. Carotid artery disease and stroke during coronary artery bypass: A critical review of the literature. Eur J Vasc Endovasc Surg. 2002;23:283.
17 Hewitt T.D., Santa Maria P.L., Alvarez J.M. Cardiac surgery in Australian octogenarians: 1996-2001. Aust N Z J Surg. 2003;73:749.
18 Eagle K.A., Guyton R.A., Davidoff R., et al. ACC/AHA guidelines for coronary artery bypass graft surgery: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines Committee to Revise the 1991 Guidelines for Coronary Artery Bypass Graft Surgery. American College of Cardiology/American Heart Association. J Am Coll Cardiol. 1999;34:1262.
19 McKhann G.M., Grega M.A., Borowicz L.M.Jr, et al. Encephalopathy and stroke after coronary artery bypass grafting: Incidence, consequences, and prediction. Arch Neurol. 2002;59:1422.
20 Wolman R.L., Nussmeier N.A., Aggarwal A., et al. Cerebral injury after cardiac surgery: Identification of a group at extraordinary risk. Multicenter Study of Perioperative Ischemia Research Group (McSPI) and the Ischemia Research Education Foundation (IREF) Investigators. Stroke. 1999;30:514.
21 Ahonen J., Salmenpera M. Brain injury after adult cardiac surgery. Acta Anaesthesiol Scand. 2004;48:4.
22 Arrowsmith J.E., Grocott H.P., Reves J.G., et al. Central nervous system complications of cardiac surgery. Br J Anaesth. 2000;84:378.
23 Baker R.A., Andrew M.J., Knight J.L. Evaluation of neurologic assessment and outcomes in cardiac surgical patients. Semin Thorac Cardiovasc Surg. 2001;13:149.
24 van Dijk D., Keizer A.M., Diephuis J.C., et al. Neurocognitive dysfunction after coronary artery bypass surgery: A systematic review. J Thorac Cardiovasc Surg. 2000;120:632.
25 Hogue C.W.Jr, Barzilai B., Pieper K.S., et al. Sex differences in neurological outcomes and mortality after cardiac surgery: A Society of Thoracic Surgery National Database report. Circulation. 2001;12:133.
26 Nussmeier N.A. A review of risk factors for adverse neurologic outcome after cardiac surgery. J Extra Corpor Technol. 2002;34:4.
27 Ridderstolpe L., Ahlgren E., Gill H., et al. Risk factor analysis of early and delayed cerebral complications after cardiac surgery. J Cardiothorac Vasc Anesth. 2002;16:278.
28 Newman M.F., Grocott H.P., Mathew J.P., et al. Report of the substudy assessing the impact of neurocognitive function on quality of life 5 years after cardiac surgery. Stroke. 2001;1:2874.
29 Newman M.F., Wolman R., Kanchuger M., et al. Multicenter preoperative stroke risk index for patients undergoing coronary artery bypass graft surgery. Multicenter Study of Perioperative Ischemia (McSPI) Research Group. Circulation. 1996;1949(Suppl II):II-174.
30 Maruff P., Silbert B., Evered L. Cognitive decline following cardiac surgery. Br J Anaesth. 2001;87:518.
31 Sotaniemi K.A. Cerebral outcome after extracorporeal circulation. Comparison between prospective and retrospective evaluations. Arch Neurol. 1983;40:75.
32 Murkin J.M., Newman S.P., Stump D.A., et al. Statement of consensus on assessment of neurobehavioral outcomes after cardiac surgery. Ann Thorac Surg. 1995;59:1289.
33 Ebert A.D., Walzer T.A., Huth C., et al. Early neurobehavioral disorders after cardiac surgery: A comparative analysis of coronary artery bypass graft surgery and valve replacement. J Cardiothorac Vasc Anesth. 2001;15:15.
34 Murkin J.M. Neurologic dysfunction after CAB or valvular surgery: Is the medium the miscreant? Anesth Analg. 1993;76:213.
35 Kalpokas M.V., Nixon I.K., Kluger R., et al. Carbon dioxide field flooding versus mechanical de-airing during open-heart surgery: A prospective randomized controlled trial. Perfusion. 2003;18:291-294.
36 Svenarud P., Persson M., van der Linden J. Effect of CO2 insufflation on the number and behavior of air microemboli in open-heart surgery: A randomized clinical trial. Circulation. 2004;109:1127-1132.
37 Martens S., Neumann K., Sodemann C., et al. Carbon dioxide field flooding reduces neurologic impairment after open heart surgery. Ann Thorac Surg. 2008;85:543-547.
38 Giordano S., Biancari F. Does the use of carbon dioxide field flooding during heart valve surgery prevent postoperative cerebrovascular complications? Interact Cardiovasc Thorac Surg. 2009;9:323-326.
39 Al-Rashidi F., Blomquist S., Höglund P., et al. A new de-airing technique that reduces systemic microemboli during open surgery: A prospective controlled study. J Thorac Cardiovasc Surg. 2009;138:157-162.
40 Ho P.M., Arciniegas D.B., Grigsby J., et al. Predictors of cognitive decline following coronary artery bypass graft surgery. Ann Thorac Surg. 2004;77:597.
41 Arrowsmith J.E., Grocott H.P., Newman M.F. Neurologic risk assessment, monitoring and outcome in cardiac surgery. J Cardiothorac Vasc Anesth. 1999;13:736.
42 Fearn S.J., Pole R., Wesnes K., et al. Cerebral injury during cardiopulmonary bypass: Emboli impair memory. J Thorac Cardiovasc Surg. 2001;121:1150.
43 Almassi G.H., Sommers T., Moritz T.E., et al. Stroke in cardiac surgical patients: Determinants and outcome. Ann Thorac Surg. 1999;68:391.
44 Omar Y., Balacumaraswami L., Pigott D.W., et al. Solid and gaseous cerebral microembolization during off-pump, on-pump, and open cardiac surgery procedures. J Thorac Cardiovasc Surg. 2004;127:1759.
45 Watters M.P., Cohen A.M., Monk C.R., et al. Reduced cerebral embolic signals in beating heart coronary surgery detected by transcranial Doppler ultrasound. Br J Anaesth. 2000;84:629.
46 van D.D., Jansen E.W., Hijman R., et al. Cognitive outcome after off-pump and on-pump coronary artery bypass graft surgery: A randomized trial. JAMA. 2002;287:1405.
47 Selnes O.A., Gottesman R.F. Neuropsychological outcomes after coronary artery bypass grafting. J Int Neuropsychol Soc. 2010;16:221-226.
48 Selnes O.A., Grega M.A., Bailey M.M., et al. Do management strategies for coronary artery disease influence 6-year cognitive outcomes? Ann Thorac Surg. 2009;88:445-454.
49 Shroyer A.L., Grover F.L., Hattler B., et al. Veterans Affairs Randomized On/Off Bypass (ROOBY) Study Group: On-pump versus off-pump coronary-artery bypass surgery. N Engl J Med. 2009;361:1827-1837.
50 Sweet J.J., Finnin E., Wolfe P.L., et al. Absence of cognitive decline one year after coronary bypass surgery: Comparison to nonsurgical and healthy controls. Ann Thorac Surg. 2008;85:1571-1578.
51 Tully P.J., Baker R.A., Knight J.L., et al. Neuropsychological function 5 years after cardiac surgery and the effect of psychological distress. Arch Clin Neuropsychol. 2009;24:741-751.
52 Newman M.F., Kirchner J.L., Phillips-Bute B., et al. Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N Engl J Med. 2001;8344:395.
53 Barber P.A., Hach S., Tippett L.J., et al. Cerebral ischemic lesions on diffusion-weighted imaging are associated with neurocognitive decline after cardiac surgery. Stroke. 2008;39:1427-1433.
54 Slater J.P., Guarino T., Stack J., et al. Cerebral oxygen desaturation predicts cognitive decline and longer hospital stay after cardiac surgery. Ann Thorac Surg. 2009;87:36-44.
55 Reich D.L., Uysal S., Ergin M.A., et al. Retrograde cerebral perfusion during thoracic aortic surgery and late neuropsychological dysfunction. Eur J Cardiothorac Surg. 2001;19:594-600.
56 Okita Y., Minatoya K., Tagusari O., et al. Prospective comparative study of brain protection in total aortic arch replacement: Deep hypothermic circulatory arrest with retrograde cerebral perfusion or selective antegrade cerebral perfusion. Ann Thorac Surg. 2001;72:72-79.
57 Svensson L.G., Nadolny E.M., Penney D.L., et al. Prospective randomized neurocognitive and S-100 study of hypothermic circulatory arrest, retrograde brain perfusion, and antegrade brain perfusion for aortic arch operations. Ann Thorac Surg. 2001;71:1905-1912.
58 Boeckxstaens C.J., Flameng W.J. Retrograde cerebral perfusion does not perfuse the brain in nonhuman primates. Ann Thorac Surg. 1995;60:319-327.
59 Ehrlich M.P., Hagl C., McCullough J.N., et al. Retrograde cerebral perfusion provides negligible flow through brain capillaries in the pig. J Thorac Cardiovasc Surg. 2001;122:331-338.
60 Juvonen T., Weisz D.J., Wolfe D., et al. Can retrograde perfusion mitigate cerebral injury after particulate embolization? A study in a chronic porcine model. J Thorac Cardiovasc Surg. 1998;115:1142-1159.
61 du Plessis A.J., Jonas R.A., Wypij D., et al. Perioperative effects of alpha-stat versus pH-stat strategies for deep hypothermic cardiopulmonary bypass in infants. J Thorac Cardiovasc Surg. 1997;114:991-1000.
62 Murkin J.M., Martzke J.S., Buchan A.M., et al. A randomized study of the influence of perfusion technique and pH management strategy in 316 patients undergoing coronary artery bypass surgery. II. Neurologic and cognitive outcomes. J Thorac Cardiovasc Surg. 1995;110:349-362.
63 Patel R.L., Turtle M.R., Chambers D.J., et al. Alpha-stat acid-base regulation during cardiopulmonary bypass improves neuropsychologic outcome in patients undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. 1996;111:1267-1279.
64 Stephan H., Weyland A., Kazmaier S., et al. Acid-base management during hypothermic cardiopulmonary bypass does not affect cerebral metabolism but does affect blood flow and neurological outcome. Br J Anaesth. 1992;69:51-57.
65 Cook D.J., Plochl W., Orszulak T.A. Effect of temperature and PaCO2 on cerebral embolization during cardiopulmonary bypass in swine. Ann Thorac Surg. 2000;69:415-420.
66 Murkin J.M., Farrar J.K., Tweed W.A., et al. Cerebral autoregulation and flow/metabolism coupling during cardiopulmonary bypass: The influence of PaCO2. Anesth Analg. 1987;66:825-832.
67 Hagl C., Ergin M.A., Galla J.D., et al. Neurologic outcome after ascending aorta-aortic arch operations: Effect of brain protection technique in high-risk patients. J Thorac Cardiovasc Surg. 2001;121:1107-1121.
68 Apostolakis E., Koletsis E.N., Dedeilias P., et al. Antegrade versus retrograde cerebral perfusion in relation to postoperative complications following aortic arch surgery for acute aortic dissection type A. J Card Surg. 2008;23:480-487.
69 Halkos M.E., Kerendi F., Myung R., et al. Selective antegrade cerebral perfusion via right axillary artery cannulation reduces morbidity and mortality after proximal aortic surgery. J Thorac Cardiovasc Surg. 2009;138:1081-1089.
70 Harrington D.K., Bonser M., Moss A., et al. Neuropsychometric outcome following aortic arch surgery: A prospective randomized trial of retrograde cerebral perfusion. J Thorac Cardiovasc Surg. 2003;126:638-644.
71 Abdul, Aziz K.A., Meduoye A. Is pH-stat or alpha-stat the best technique to follow in patients undergoing deep hypothermic circulatory arrest? Interact Cardiovasc Thorac Surg. 2010;10:271-282.
72 Sundt T.M.3rd, Orszulak T.A., Cook D.J., et al. Improving results of open arch replacement. Ann Thorac Surg. 2008;86:787-796.
73 Jaffer F.A., O’Donnell C.J., Larson M.G., et al. Age and sex distribution of subclinical aortic atherosclerosis: A magnetic resonance imaging examination of the Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2002;122:849.
74 Macleod M.R., Amarenco P., Davis S.M., et al. Atheroma of the aortic arch: An important and poorly recognised factor in the aetiology of stroke. Lancet Neurol. 2004;3:408.
75 Macleod M.R., Donnan G.A. Atheroma of the aortic arch: The missing link in the secondary prevention of stroke? Expert Rev Cardiovasc Ther. 2003;1:487.
76 Li A.E., Kamel I., Rando F., et al. Using MRI to assess aortic wall thickness in the multiethnic study of atherosclerosis: Distribution by race, sex, and age. AJR Am J Roentgenol. 2004;182:593.
77 Yahia A.M., Kirmani J.F., Xavier A.R., et al. Characteristics and predictors of aortic plaques in patients with transient ischemic attacks and strokes. J Neuroimaging. 2004;14:16.
78 Watanabe K., Hiroki T., Koga N. Relation of thoracic aorta calcification on computed tomography and coronary risk factors to obstructive coronary artery disease on angiography. Angiology. 2003;54:433.
79 Agmon Y., Khandheria B.K., Meissner I., et al. Relation of coronary artery disease and cerebrovascular disease with atherosclerosis of the thoracic aorta in the general population. Am J Cardiol. 2002;189:262.
80 Blauth C.I., Cosgrove D.M., Webb B.W., et al. Atheroembolism from the ascending aorta. An emerging problem in cardiac surgery. J Thorac Cardiovasc Surg. 1992;103:1104.
81 Doty J.R., Wilentz R.E., Salazar J.D., et al. Atheroembolism in cardiac surgery. Ann Thorac Surg. 2003;75:1221.
82 Goodwin A.T., Goddard M., Taylor G.J., et al. Clinical versus actual outcome in cardiac surgery: A postmortem study. Eur J Cardiothorac Surg. 2000;17:747.
83 Mackensen G.B., Swaminathan M., Ti L.K., et al. Preliminary report on the interaction of apolipoprotein E polymorphism with aortic atherosclerosis and acute nephropathy after CABG. Ann Thorac Surg. 2004;78:520.
84 Davila-Roman V.G., Barzilai B., Wareing T.H., et al. Atherosclerosis of the ascending aorta. Prevalence and role as an independent predictor of cerebrovascular events in cardiac patients. Stroke. 1994;25:2010-2016.
85 Leacche M., Carrier M., Bouchard D., et al. Improving neurologic outcome in off-pump surgery: The “no touch” technique. Heart Surg Forum. 2003;6:169.
86 Mackensen G.B., Ti L.K., Phillips-Bute B.G., et al. Cerebral embolization during cardiac surgery: Impact of aortic atheroma burden. Br J Anaesth. 2003;91:656.
87 Sharony R., Bizekis C.S., Kanchuger M., et al. Off-pump coronary artery bypass grafting reduces mortality and stroke in patients with atheromatous aortas: A case control study. Circulation. 2003;9(Suppl I):II-115.
88 Wareing T.H., Davila-Roman V.G., Daily B.B., et al. Strategy for the reduction of stroke incidence in cardiac surgical patients. Ann Thorac Surg. 1993;55:1400.
89 Sekoranja L., Vuille C., Bianchi-Demicheli F., et al. Thoracic aortic plaques, transoesophageal echocardiography and coronary artery disease. Swiss Med Wkly. 2004;134:75-78.
90 Djaiani G., Fedorko L., Borger M., et al. Mild to moderate atheromatous disease of the thoracic aorta and new ischemic brain lesions after conventional coronary artery bypass graft surgery. Stroke. 2004;35:e356-e358.
91 Royse A.G., Royse C.F., Ajani A.E., et al. Reduced neuropsychological dysfunction using epiaortic echocardiography and the exclusive Y graft. Ann Thorac Surg. 2000;69:1431.
92 St Amand M.A., Murkin J.M., Menkis A.H., et al. Aortic atherosclerotic plaque identified by epiaortic scanning predicts cerebral embolic load in cardiac surgery. Can J Anaesth. 1997;44:A7.
93 Konstadt S.N., Reich D.L., Kahn R., et al. Transesophageal echocardiography can be used to screen for ascending aortic atherosclerosis. Anesth Analg. 1995;81:225.
94 Eifert S., Reichenspurner H., Pfefferkorn T., et al. Neurological and neuropsychological examination and outcome after use of an intra-aortic filter device during cardiac surgery. Perfusion. 2003;18(Suppl 1):55.
95 Bonatti J. Ascending aortic atherosclerosis—a complex and challenging problem for the cardiac surgeon. Heart Surg Forum. 1999;2:125.
96 Gaspar M., Laufer G., Bonatti J., et al. Epiaortic ultrasound and intraoperative transesophageal echocardiography for the thoracic aorta atherosclerosis assessment in patient undergoing CABG. Surgical technique modification to avoid cerebral stroke. Chirurgia (Bucur). 2002;97:529.
97 Konstadt S.N., Reich D.L., Quintana C., et al. The ascending aorta: How much does transesophageal echocardiography see? Anesth Analg. 1994;78:240.
98 Wareing T.H., Davila-Roman V.G., Barzilai B., et al. Management of the severely atherosclerotic ascending aorta during cardiac operations. A strategy for detection and treatment. J Thorac Cardiovasc Surg. 1992;103:453.
99 Ohteki H., Itoh T., Natsuaki M., et al. Intraoperative ultrasonic imaging of the ascending aorta in ischemic heart disease. Ann Thorac Surg. 1990;50:539.
100 Murkin J.M. Attenuation of neurologic injury during cardiac surgery. Ann Thorac Surg. 2001;72:S1838.
101 Takami Y., Tajima K., Terazawa S., et al. Safer aortic crossclamping during short-term moderate hypothermic circulatory arrest for cardiac surgery in patients with a bad ascending aorta. J Thorac Cardiovasc Surg. 2009;137:875-880.
102 Royse A.G., Royse C.F. Epiaortic ultrasound assessment of the aorta in cardiac surgery. Best Pract Res Clin Anaesthesiol. 2009;23:335-341.
103 Bergman P., Hadjinikolaou L., Dellgren G., et al. A policy to reduce stroke in patients with extensive atherosclerosis of the ascending aorta undergoing coronary surgery. Interact Cardiovasc Thorac Surg. 2004;3:28-32.
104 Calafiore A.M., Di M.M., Teodori G., et al. Impact of aortic manipulation on incidence of cerebrovascular accidents after surgical myocardial revascularization. Ann Thorac Surg. 2002;73:1387.
105 Knipp S.C., Matatko N., Wilhelm H., et al. Evaluation of brain injury after coronary artery bypass grafting. A prospective study using neuropsychological assessment and diffusion-weighted magnetic resonance imaging. Eur J Cardiothorac Surg. 2004;25:791.
106 Murkin J.M. Long-term neurological and neuropsychological outcome 3 years after coronary artery bypass surgery. Anesth Analg. 1996;82:S328.
107 Taggart D.P., Westaby S. Neurological and cognitive disorders after coronary artery bypass grafting. Curr Opin Cardiol. 2001;16:271.
108 Bar-Yosef S., Anders M., Mackensen G.B., et al. Aortic atheroma burden and cognitive dysfunction after coronary artery bypass graft surgery. Ann Thorac Surg. 2005;78:1556.
109 Ahlgren E., Lundqvist A., Nordlund A., et al. Neurocognitive impairment and driving performance after coronary artery bypass surgery. Eur J Cardiothorac Surg. 2003;23:334.
110 Di Carlo C.A., Perna A.M., Pantoni L., et al. Clinically relevant cognitive impairment after cardiac surgery: A 6-month follow-up study. J Neurol Sci. 2001;188:85-93.
111 Fox H.M., Rizzo N.D., Gifford S. Psychological observations of patients undergoing mitral surgery: A study of stress. Psychosom Med. 1954;16:186.
112 Tufo H.M., Ostfeld A.M., Shekelle R. Central nervous system dysfunction following open-heart surgery. JAMA. 1970;212:1333.
113 Kornfeld D.S., Heller S.S., Frank K.A., et al. Delirium after coronary artery bypass surgery. J Thorac Cardiovasc Surg. 1978;76:93.
114 Millar K., Asbury A.J., Murray G.D. Pre-existing cognitive impairment as a factor influencing outcome after cardiac surgery. Br J Anaesth. 2001;86:63.
115 Rankin K.P., Kochamba G.S., Boone K.B., et al. Presurgical cognitive deficits in patients receiving coronary artery bypass graft surgery. J Int Neuropsychol Soc. 2003;9:913.
116 Muramoto O., Kuru Y., Sugishita M., et al. Pure memory loss with hippocampal lesions: A pneumoencephalographic study. Arch Neurol. 1979;36:54.
117 Grant I., Neuropsychological findings in hypoxemic chronic obstructive pulmonary disease, Proceedings of the 10th Annual Meeting of the International Neuropsychology Society, Pittsburgh PA; 1982;
118 Grigore A.M., Grocott H.P., Mathew J.P., et al. The rewarming rate and increased peak temperature alter neurocognitive outcome after cardiac surgery. Anesth Analg. 2002;94:4.
119 Harrington D.K., Bonser M., Moss A., et al. Neuropsychometric outcome following aortic arch surgery: A prospective randomized trial of retrograde cerebral perfusion. J Thorac Cardiovasc Surg. 2003;126:638.
120 Rasmussen L.S., Sperling B., Abildstrom H.H., et al. Neuron loss after coronary artery bypass detected by SPECT estimation of benzodiazepine receptors. Ann Thorac Surg. 2002;74:1576.
121 Wang D., Wu X., Li J., et al. The effect of lidocaine on early postoperative cognitive dysfunction after coronary artery bypass surgery. Anesth Analg. 2002;95:1134.
122 Basile A.M., Fusi C., Conti A.A., et al. S-100 protein and neuron-specific enolase as markers of subclinical cerebral damage after cardiac surgery: Preliminary observation of a 6-month follow-up study. Eur Neurol. 2001;45:151.
123 Shaw P.J., Bates D., Cartlidge N.E., et al. Neurologic and neuropsychological morbidity following major surgery: Comparison of coronary artery bypass and peripheral vascular surgery. Stroke. 1987;18:700.
124 Blauth C.I., Smith P.L., Arnold J.V., et al. Influence of oxygenator type on the prevalence and extent of microembolic retinal ischemia during cardiopulmonary bypass. Assessment by digital image analysis. J Thorac Cardiovasc Surg. 1990;99:61.
125 Harrison M.J., Pugsley W., Newman S., et al. Detection of middle cerebral emboli during coronary artery bypass surgery using transcranial Doppler sonography. Stroke. 1990;2110:1512.
126 Moody D.M., Bell M.A., Challa V.R., et al. Brain microemboli during cardiac surgery or aortography. Ann Neurol. 1990;28:477.
127 Blauth C., Arnold J., Kohner E.M., et al. Retinal microembolism during cardiopulmonary bypass demonstrated by fluorescein angiography. Lancet. 1986;2:837.
128 bu-Omar Y., Balacumaraswami L., Pigott D.W., et al. Solid and gaseous cerebral microembolization during off-pump, on-pump, and open cardiac surgery procedures. J Thorac Cardiovasc Surg. 2004;127:1759.
129 Motallebzadeh R., Kanagasabay R., Bland M., et al. S100 protein and its relation to cerebral microemboli in on-pump and off-pump coronary artery bypass surgery. Eur J Cardiothorac Surg. 2004;25:409.
130 Wimmer-Greinecker G. Reduction of neurologic complications by intra-aortic filtration in patients undergoing combined intracardiac and CABG procedures. Eur J Cardiothorac Surg. 2003;23:159.
131 Hindman B.J. Emboli, inflammation, and CNS impairment: An overview. Heart Surg Forum. 2002;5:249.
132 Stanley T.O., Mackensen G.B., Grocott H.P., et al. The impact of postoperative atrial fibrillation on neurocognitive outcome after coronary artery bypass graft surgery. Anesth Analg. 2002;94:290.
133 Schmitz C., Weinreich S., White J., et al. Can particulate extraction from the ascending aorta reduce neurologic injury in cardiac surgery? J Thorac Cardiovasc Surg. 2003;126:1829.
134 Hamada Y., Kawachi K., Nakata T., et al. Antiinflammatory effect of heparin-coated circuits with leukocyte-depleting filters in coronary bypass surgery. Artif Organs. 2001;25:1004.
135 Greilich P.E., Brouse C.F., Whitten C.W., et al. Antifibrinolytic therapy during cardiopulmonary bypass reduces proinflammatory cytokine levels: A randomized, double-blind, placebo-controlled study of epsilon-aminocaproic acid and aprotinin. J Thorac Cardiovasc Surg. 2003;126:1498.
136 Murkin J.M. Inflammatory responses and CNS injury: Implications, prophylaxis, and treatment. Heart Surg Forum. 2003;6:193.
137 Uehara T., Tabuchi M., Kozawa S., et al. MR angiographic evaluation of carotid and intracranial arteries in Japanese patients scheduled for coronary artery bypass grafting. Cerebrovasc Dis. 2001;11:341.
138 Yoon B.W., Bae H.J., Kang D.W., et al. Intracranial cerebral artery disease as a risk factor for central nervous system complications of coronary artery bypass graft surgery. Stroke. 2001;32:94.
139 Abildstrom H., Rasmussen L.S., Rentowl P., et al. Cognitive dysfunction 1-2 years after noncardiac surgery in the elderly. ISPOCD group. International Study of Post-Operative Cognitive Dysfunction. Acta Anaesthesiol Scand. 2000;44:1246.
140 Linstedt U., Meyer O., Kropp P., et al. Serum concentration of S-100 protein in assessment of cognitive dysfunction after general anesthesia in different types of surgery. Acta Anaesthesiol Scand. 2002;46:384.
141 Connolly E.S.Jr, Winfree C.J., Rampersad A., et al. Serum S100B protein levels are correlated with subclinical neurocognitive declines after carotid endarterectomy. Neurosurgery. 2001;49:1076.
142 Edmonds C.R., Barbut D., Hager D., et al. Intraoperative cerebral arterial embolization during total hip arthroplasty. Anesthesiology. 2000;93:315.
143 Colonna D.M., Kilgus D., Brown W., et al. Acute brain fat embolization occurring after total hip arthroplasty in the absence of a patent foramen ovale. Anesthesiology. 2002;96:1027.
144 Kinoshita H., Iranami H., Fujii K., et al. The use of bone cement induces an increase in serum astroglial S-100B protein in patients undergoing total knee arthroplasty. Anesth Analg. 2003;97:1657.
145 Serruys P.W., Morice M.C., Kappetein A.P., SYNTAX Investigators. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360:961-972.
146 Aguilar M.J., Gerbode F., Hill J.D. Neuropathologic complications of cardiac surgery. J Thorac Cardiovasc Surg. 1971;61:676.
147 Aberg T., Kihlgren M. Cerebral protection during open-heart surgery. Thorax. 1977;32:525.
148 Emmrich P., Hahn J., Ogunlade V., et al. [Neuropathological findings after cardiac surgery—retrospective study over 6 years]. Z Kardiol. 2003;92:925.
149 Torvik A. The pathogenesis of watershed infarcts in the brain. Stroke. 1984;15:221.
150 Salazar J.D., Wityk R.J., Grega M.A., et al. Stroke after cardiac surgery: Short- and long-term outcomes. Ann Thorac Surg. 2001;72:1195.
151 Koga M., Shimokawa S., Moriyama Y., et al. Watershed infarction after combined coronary and axillobifemoral bypass surgery. Jpn J Thorac Cardiovasc Surg. 2000;48:258.
152 Malone M. Brain damage after cardiopulmonary bypass: Correlations between neurophysiological and neuropathological findings. J Neurol Neurosurg Psychiatry. 1981;44:924.
153 Wityk R.J., Goldsborough M.A., Hillis A., et al. Diffusion- and perfusion-weighted brain magnetic resonance imaging in patients with neurologic complications after cardiac surgery. Arch Neurol. 2001;58:571.
154 Torvik A., Skullerud K. Watershed infarcts in the brain caused by microemboli. Clin Neuropathol. 1982;1:99.
155 Boyajian R.A., Otis S.M. Embolic stroke syndrome underlies encephalopathy and coma following cardiac surgery. Arch Neurol. 2003;60:291.
156 Boyajian R.A., Otis S.M., Tyner J.J., et al. Study design validation for consolidating global with focal neurological events in cardiac surgery stroke risk factor analyses. Eur J Neurol. 2003;10:71.
157 Browne S.M., Halligan P.W., Wade D.T., et al. Postoperative hypoxia is a contributory factor to cognitive impairment after cardiac surgery. J Thorac Cardiovasc Surg. 2003;126:1061.
158 Koga M., Shimokawa S., Moriyama Y., et al. Watershed infarction after combined coronary and axillobifemoral bypass surgery. Jpn J Thorac Cardiovasc Surg. 2000;48:258.
159 Caplan L.R., Hennerici M. Impaired clearance of emboli (washout) is an important link between hypoperfusion, embolism, and ischemic stroke. Arch Neurol. 1998;5511:1475.
160 Shann K.G., Likosky D.S., Murkin J.M., et al. An evidenced-based review of the practice of cardiopulmonary bypass in adults. J Thorac Cardiovasc Surg. 2006;132:283-290.
161 Bolsin S.N. Detection of neurological damage during cardiopulmonary bypass. Anaesthesia. 1986;41:61.
162 Levy W.J. Electroencephalographic evidence of cerebral ischemia during acute extracorporeal hypoperfusion. J Cardiothorac Anesth. 1987;1:300.
163 Russ W., Kling D., Sauerwein G., et al. Spectral analysis of the EEG during hypothermic cardiopulmonary bypass. Acta Anaesthesiol Scand. 1987;31:111.
164 Edmonds H.L.Jr, Griffiths L.K., Slater A.D., et al. Quantitative electroencephalographic monitoring during myocardial revascularization predicts postoperative disorientation and improves outcome. J Thorac Cardiovasc Surg. 1992;103:555.
165 Govier A.V., Reves J.G., McKay R.D., et al. Factors and their influence on regional cerebral blood flow during nonpulsatile cardiopulmonary bypass. Ann Thorac Surg. 1984;38:592.
166 Prough D.S., Stump D.A., Roy R.C., et al. Response of cerebral blood flow to changes in carbon dioxide tension during hypothermic cardiopulmonary bypass. Anesthesiology. 1986;64:576.
167 Gold J.P., Charlson M.E., Williams-Russo P., et al. Improvement of outcomes after coronary artery bypass. A randomized trial comparing intraoperative high versus low mean arterial pressure. J Thorac Cardiovasc Surg. 1995;110:1302.
168 Lundar T., Froysaker T., Lindegaard K.F., et al. Some observations on cerebral perfusion during cardiopulmonary bypass. Ann Thorac Surg. 1985;39:318.
169 Avraamides E.J. The effect of surgical dislocation of the heart on cerebral blood flow in the presence of a single, two-stage venous cannula during cardiopulmonary bypass. Can J Anaesth. 1996;43:A36.
170 Russell R.W., Bharucha N. The recognition and prevention of border zone cerebral ischaemia during cardiac surgery. Q J Med. 1978;47187:303.
171 Newman M.F., Kramer D., Croughwell N.D., et al. Differential age effects of mean arterial pressure and rewarming on cognitive dysfunction after cardiac surgery. Anesth Analg. 1995;81:236.
172 Ganushchak Y.M., Fransen E.J., Visser C., et al. Neurological complications after coronary artery bypass grafting related to the performance of cardiopulmonary bypass. Chest. 2004;125:2196.
173 Ura M., Sakata R., Nakayama Y., et al. Ultrasonographic demonstration of manipulation-related aortic injuries after cardiac surgery. J Am Coll Cardiol. 2000;35:1303.
174 Sylivris S., Levi C., Matalanis G., et al. Pattern and significance of cerebral microemboli during coronary artery bypass grafting. Ann Thorac Surg. 1998;66:1674.
175 Eckmann D.M., Armstead S.C., Mardini F. Surfactants reduce platelet-bubble and platelet-platelet binding induced by in vitro air embolism. Anesthesiology. 2005;103:1204.
176 Persson L.I., Johansson B.B., Hansson H.A. Ultrastructural studies on blood-brain barrier dysfunction after cerebral air embolism in the rat. Acta Neuropathol (Berl). 1978;1344:53.
177 Philp R.B., Inwood M.J., Warren B.A. Interactions between gas bubbles and components of the blood: Implications in decompression sickness. Aerosp Med. 1972;43:946.
178 Warren B.A., Philp R.B., Inwood M.J. The ultrastructural morphology of air embolism: Platelet adhesion to the interface and endothelial damage. Br J Exp Pathol. 1973;54:163.
179 Helps S.C., Parsons D.W., Reilly P.L., et al. The effect of gas emboli on rabbit cerebral blood flow. Stroke. 1990;21:94.
180 Haller C., Sercombe R., Verrecchia C., et al. Effect of the muscarinic agonist carbachol on pial arteries in vivo after endothelial damage by air embolism. J Cereb Blood Flow Metab. 1987;7:605.
181 Mangano C.M. Scuds, scads, and other air-borne hazards. Anesthesiology. 1997;87:476.
182 Mitchell S., Gorman D. The pathophysiology of cerebral arterial gas embolism. J Extra Corpor Technol. 2002;34:18.
183 Moody D.M., Brown W.R., Challa V.R., et al. Brain microemboli associated with cardiopulmonary bypass: A histologic and magnetic resonance imaging study. Ann Thorac Surg. 1995;59:1304.
184 Challa V.R., Lovell M.A., Moody D.M., et al. Laser microprobe mass spectrometric study of aluminum and silicon in brain emboli related to cardiac surgery. J Neuropathol Exp Neurol. 1998;57:140.
185 Kincaid E.H., Jones T.J., Stump D.A., et al. Processing scavenged blood with a cell saver reduces cerebral lipid microembolization. Ann Thorac Surg. 2000;70:1296.
186 Djaiani G., Fedorko L., Borger M.A., et al. Continuous-flow cell saver reduces cognitive decline in elderly patients after coronary bypass surgery. Circulation. 2007;116:1888-1895.
187 Rubens F.D., Boodhwani M., Mesana T., et al. Cardiotomy Investigators: The cardiotomy trial: A randomized, double-blind study to assess the effect of processing of shed blood during cardiopulmonary bypass on transfusion and neurocognitive function. Circulation. 2007;116(Suppl 11):I89-I97.
188 Koch C.G., Li L., Duncan A.I., et al. Morbidity and mortality risk associated with red blood cell and blood-component transfusion in isolated coronary artery bypass grafting. Crit Care Med. 2006;34:1608-1616.
189 Wollman H., Stephen G.W., Clement A.J., et al. Cerebral blood flow in man during extracorporeal circulation. J Thorac Cardiovasc Surg. 1966;52:558.
190 Kubota Y. Clinical study of the cerebral hemodynamics during extracorporeal circulation. Nagoya J Med Sci. 1968;31:117.
191 Branthwaite M.A. Cerebral blood flow and metabolism during open-heart surgery. Thorax. 1974;29:633.
192 Henriksen L., Hjelms E., Lindeburgh T. Brain hyperperfusion during cardiac operations. Cerebral blood flow measured in man by intra-arterial injection of xenon 133: Evidence suggestive of intraoperative microembolism. J Thorac Cardiovasc Surg. 1983;86:202.
193 Taylor K.M. Brain damage during cardiopulmonary bypass. Ann Thorac Surg. 1998;654(Suppl):S20.
194 Busto R., Dietrich W.D., Globus M.Y., et al. The importance of brain temperature in cerebral ischemic injury. Stroke. 1989;20:1113.
195 Buchan A., Pulsinelli W. Hypothermia but not the N-methyl-D-aspartate antagonist, MK801, attenuates neuronal damage in gerbils subjected to transient global ischemia. J Neurosci. 1990;10:311.
196 Chopp M., Knight R., Tidwell C.D., et al. The metabolic effects of mild hypothermia on global cerebral ischemia and recirculation in the cat: Comparison to normothermia and hyperthermia. J Cereb Blood Flow Metab. 1989;9:141.
197 Nemoto E.M., Klementavicius R., Melick J.A., et al. Suppression of cerebral metabolic rate for oxygen CMRO(2) by mild hypothermia compared with thiopental. J Neurosurg Anesthesiol. 1996;8:52.
198 Mora C.T., Henson M.B., Weintraub W.S., et al. The effect of temperature management during cardiopulmonary bypass on neurologic and neuropsychological outcomes in patients undergoing coronary revascularization. J Thorac Cardiovasc Surg. 1996;112:514.
199 Murkin J.M. Hypothermic cardiopulmonary bypass—time for a more temperate approach. Can J Anaesth. 1995;42:663.
200 Nathan H.J., Wells G.A., Munson J.L., et al. Neuroprotective effect of mild hypothermia in patients undergoing coronary artery surgery with cardiopulmonary bypass: A randomized trial. Circulation. 2001;18(Suppl I):I-85.
201 Engelman R.M., Pleet A.B., Hicks R., et al. Is there a relationship between systemic perfusion temperature during coronary artery bypass grafting and extent of intraoperative ischemic central nervous system injury? J Thorac Cardiovasc Surg. 2000;119:230.
202 Dworschak M., Lassnigg A., Tenze G., et al. Perfusion temperature during cardiopulmonary bypass does not affect serum S-100beta release. Thorac Cardiovasc Surg. 2004;52:29.
203 Grimm M., Czerny M., Baumer H., et al. Normothermic cardiopulmonary bypass is beneficial for cognitive brain function after coronary artery bypass grafting—a prospective randomized trial. Eur J Cardiothorac Surg. 2000;18:270.
204 Arrowsmith J.E., Dunning J.L. Normothermic cardiopulmonary bypass is beneficial for cognitive brain function after coronary artery bypass grafting—a prospective randomized trial. Eur J Cardiothorac Surg. 2001;19:732.
205 Engelman R., Pleet A.B. Cardiopulmonary bypass temperature and extension of intraoperative brain damage: Controversies persist. J Thorac Cardiovasc Surg. 2000;120:1014.
206 Gaudino M., Possati G. Cardiopulmonary bypass temperature and extension of intraoperative brain damage: Controversies persist. J Thorac Cardiovasc Surg. 2000;120:1013.
207 Murkin J.M. Pathophysiological basis of CNS injury in cardiac surgical patients: Detection and prevention. Perfusion. 2006;21:203-208.
208 Grocott H.P., Mackensen G.B., Grigore A.M., et al. Postoperative hyperthermia is associated with cognitive dysfunction after coronary artery bypass graft surgery. Stroke. 2002;33:537.
209 Scheffer T., Sanders D.B. The neurologic sequelae of cardiopulmonary bypass-induced cerebral hyperthermia and cerebroprotective strategies. J Extra Corpor Technol. 2003;35:317.
210 Kawahara F., Kadoi Y., Saito S., et al. Slow rewarming improves jugular venous oxygen saturation during rewarming. Acta Anaesthesiol Scand. 2003;47:419.
211 Okano N., Owada R., Fujita N., et al. Cerebral oxygenation is better during mild hypothermic than normothermic cardiopulmonary bypass. Can J Anaesth. 2000;47:131.
212 Gravlee G.P., Roy R.C., Stump D.A., et al. Regional cerebrovascular reactivity to carbon dioxide during cardiopulmonary bypass in patients with cerebrovascular disease. J Thorac Cardiovasc Surg. 1990;99:1022.
213 von Reutern G.M., Hetzel A., Birnbaum D., et al. Transcranial Doppler ultrasonography during cardiopulmonary bypass in patients with severe carotid stenosis or occlusion. Stroke. 1988;19:674.
214 Brusino F.G., Reves J.G., Prough D.S., et al. Cerebral blood flow during cardiopulmonary bypass in a patient with occlusive cerebrovascular disease. J Cardiothorac Anesth. 1989;3:87.
215 Koster S., Hensens A.G., van der Palen J. The long-term cognitive and functional outcomes of postoperative delirium after cardiac surgery. Ann Thorac Surg. 2009;87:1469-1474.
216 Berens E.S., Kouchoukos N.T., Murphy S.F., et al. Preoperative carotid artery screening in elderly patients undergoing cardiac surgery. J Vasc Surg. 1992;15:313.
217 Schwartz L.B., Bridgman A.H., Kieffer R.W., et al. Asymptomatic carotid artery stenosis and stroke in patients undergoing cardiopulmonary bypass. J Vasc Surg. 1995;21:146.
218 Salasidis G.C., Latter D.A., Steinmetz O.K., et al. Carotid artery duplex scanning in preoperative assessment for coronary artery revascularization: The association between peripheral vascular disease, carotid artery stenosis, and stroke. J Vasc Surg. 1995;21:154.
219 Palerme L.P., Hill A.B., Obrand D., et al. Is Canadian cardiac surgeons’ management of asymptomatic carotid artery stenosis at coronary artery bypass supported by the literature? A survey and a critical appraisal of the literature. Can J Surg. 2000;43:93.
220 Naylor A.R. A critical review of the role of carotid disease and the outcomes of staged and synchronous carotid surgery. Semin Cardiothorac Vasc Anesth. 2004;8:37.
221 Furnary A.P., Gao G., Grunkemeier G.L., et al. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2003;125:1007.
222 Latham R., Lancaster A.D., Covington J.F., et al. The association of diabetes and glucose control with surgical-site infections among cardiothoracic surgery patients. Infect Control Hosp Epidemiol. 2001;22:607.
223 Coursin D.B., Prielipp R.C. The new anesthesia diet plan: Keeping perioperative carbs in check. Anesth Analg. 2004;99:316.
224 Carvalho G., Moore A., Qizilbash B., et al. Maintenance of normoglycemia during cardiac surgery. Anesth Analg. 2004;99:319.
225 Jessen M.E. Glucose control during cardiac surgery: How sweet it is. J Thorac Cardiovasc Surg. 2003;125:985.
226 Bucerius J., Gummert J.F., Walther T., et al. Impact of diabetes mellitus on cardiac surgery outcome. Thorac Cardiovasc Surg. 2003;51:11.
227 Mortasawi A., Arnrich B., Rosendahl U., et al. Is age an independent determinant of mortality in cardiac surgery as suggested by the EuroSCORE? BMC Surg. 2002;72:8.
228 Kadoi Y., Saito S., Yoshikawa D., et al. Increasing mean arterial blood pressure has no effect on jugular venous oxygen saturation in insulin-dependent patients during tepid cardiopulmonary bypass. Anesth Analg. 2002;95:266.
229 Murkin J.M. Pro: Tight intraoperative glucose control improves outcome in cardiovascular surgery. J Cardiothorac Vasc Anesth. 2000;14:475.
230 McAlister F.A., Man J., Bistritz L., et al. Diabetes and coronary artery bypass surgery: An examination of perioperative glycemic control and outcomes. Diabetes Care. 2003;26:1518.
231 Lazar H.L., McDonnell M., Chipkin S.R., et al. Society of Thoracic Surgeons Blood Glucose Guideline Task Force: The Society of Thoracic Surgeons practice guideline series: Blood glucose management during adult cardiac surgery. Ann Thorac Surg. 2009;87:663-669.
232 Krinsley J.S., Grover A. Severe hypoglycemia in critically ill patients: Risk factors and outcomes. Crit Care Med. 2007;35:2262-2267.
233 Gandhi G.Y., Nuttall G.A., Abel M.D., et al. Intensive intraoperative insulin therapy versus conventional glucose management during cardiac surgery: A randomized trial. Ann Intern Med. 2007;146:233-243.
234 Sheehy A.M., Coursin D.B., Keegan M.T. Risks of tight glycemic control during adult cardiac surgery. Ann Thorac Surg. 2009;88:1384-1385.
235 Blauth C.I., Arnold J.V., Schulenberg W.E., et al. Cerebral microembolism during cardiopulmonary bypass. Retinal microvascular studies in vivo with fluorescein angiography. J Thorac Cardiovasc Surg. 1988;95:668.
236 Padayachee T.S., Parsons S., Theobold R., et al. The detection of microemboli in the middle cerebral artery during cardiopulmonary bypass: A transcranial Doppler ultrasound investigation using membrane and bubble oxygenators. Ann Thorac Surg. 1987;44:298.
237 Padayachee T.S., Parsons S., Theobold R., et al. The effect of arterial filtration on reduction of gaseous microemboli in the middle cerebral artery during cardiopulmonary bypass. Ann Thorac Surg. 1988;45:647.
238 Williams I.M., Stephens J.F., Richardson E.P.Jr, et al. Brain and retinal microemboli during cardiac surgery. Ann Neurol. 1991;30:736.
239 Georgiadis D., Hempel A., Baumgartner R.W., et al. Doppler microembolic signals during cardiac surgery: Comparison between arterial line and middle cerebral artery. J Thorac Cardiovasc Surg. 2003;126:1638.
240 Schoenburg M., Kraus B., Muehling A., et al. The dynamic air bubble trap reduces cerebral microembolism during cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2003;126:1455.
241 Edmunds L.H.Jr. Advances in the heart-lung machine after John and Mary Gibbon. Ann Thorac Surg. 2003;76:S2220.
242 Haworth W.S. The development of the modern oxygenator. Ann Thorac Surg. 2003;76:S2216.
243 Whitaker D.C., Stygall J.A., Newman S.P., et al. The use of leukocyte-depleting and conventional arterial line filters in cardiac surgery: A systematic review of clinical studies. Perfusion. 2001;16:433.
244 Jewell A.E., Akowuah E.F., Suvarna S.K., et al. A prospective randomised comparison of cardiotomy suction and cell saver for recycling shed blood during cardiac surgery. Eur J Cardiothorac Surg. 2003;23:633.
245 de Vries A.J., Gu Y.J., Douglas Y.L., et al. Clinical evaluation of a new fat removal filter during cardiac surgery. Eur J Cardiothorac Surg. 2004;25:261.
246 Marschall K., Kanchuger M., Kessler K., et al. Superiority of transesophageal echocardiography in detecting aortic arch atheromatous disease: Identification of patients at increased risk of stroke during cardiac surgery. J Cardiothorac Vasc Anesth. 1994;8:5.
247 Hosoda Y., Watanabe M., Hirooka Y., et al. Significance of atherosclerotic changes of the ascending aorta during coronary bypass surgery with intraoperative detection by echography. J Cardiovasc Surg (Torino). 1991;32:301.
248 Van Boven W.J., Berry G. Intra-aortic filtration captures particulate debris in OPCAB cases using anastomotic devices. Heart Surg Forum. 2002;5(Suppl 4):S461.
249 Banbury M.K., Kouchoukos N.T., Allen K.B., et al. Emboli capture using the Embol-X intraaortic filter in cardiac surgery: A multicentered randomized trial of 1,289 patients. Ann Thorac Surg. 2003;76:508.
250 Reichenspurner H., Navia J.A., Berry G., et al. Particulate emboli capture by an intra-aortic filter device during cardiac surgery. J Thorac Cardiovasc Surg. 2000;119:233.
251 Gerriets T., Schwarz N., Sammer G., et al. Protecting the brain from gaseous and solid micro-emboli during coronary artery bypass grafting: A randomized controlled trial. Eur Heart J. 2010;31:360-368.
252 Murkin J.M. Perioperative multimodality neuromonitoring: An overview. Semin Cardiothorac Vasc Anesth. 2004;8:167.
253 Borger M.A., Djaiani G. Reduction of cerebral emboli during cardiac surgery: Influence of surgeon and perfusionist feedback. Heart Surg Forum. 2003;6:204.
254 Bar-Yosef S., Sanders E.G., Grocott H.P. Asymmetric cerebral near-infrared oximetric measurements during cardiac surgery. J Cardiothorac Vasc Anesth. 2003;17:773.
255 Daubeney P.E., Smith D.C., Pilkington S.N., et al. Cerebral oxygenation during paediatric cardiac surgery: Identification of vulnerable periods using near-infrared spectroscopy. Eur J Cardiothorac Surg. 1998;13:370.
256 Austin E.H.III, Edmonds H.L.Jr, Auden S.M., et al. Benefit of neurophysiologic monitoring for pediatric cardiac surgery. J Thorac Cardiovasc Surg. 1997;114:707.
257 Chen C.S., Leu B.K., Liu K. Detection of cerebral desaturation during cardiopulmonary bypass by cerebral oximetry. Acta Anaesthesiol Sin. 1996;34:173.
258 Murkin J.M., Arango M. Near-infrared spectroscopy as an index of brain and tissue oxygenation. Br J Anaesth. 2009;103(Suppl 1):i3-i13.
259 Goldman S. Optimizing intraoperative cerebral oxygen delivery using noninvasive cerebral oximetry decreases the incidence of stroke for cardiac surgical patients. Heart Surg Forum. 2004;7:E376-E381.
260 Murkin J.M., Adams S.J., Novick R.J., et al. Monitoring brain oxygen saturation during coronary bypass surgery: A randomized, prospective study. Anesth Analg. 2007;104:51-58.
261 Novitzky D., Boswell B.B. Total myocardial revascularization without cardiopulmonary bypass utilizing computer-processed monitoring to assess cerebral perfusion. Heart Surg Forum. 2000;3:198.
262 Hong S.W., Shim J.K., Choi Y.S., et al. Prediction of cognitive dysfunction and patients’ outcome following valvular heart surgery and the role of cerebral oximetry. Eur J Cardiothorac Surg. 2008;33:560-565.
263 Murkin J.M., Bainbridge D., Novick R. In response. Do the data really support the conclusion [Letter]? Anesth Analg. 2007;105:536-538.
264 Denault A., Deschamps A., Murkin J.M. A proposed algorithm for the intraoperative use of cerebral near-infrared spectroscopy. Semin Cardiothorac Vasc Anesth. 2007;11:274-281.
265 Kurth C.D., Steven J.M., Nicolson S.C. Cerebral oxygenation during pediatric cardiac surgery using deep hypothermic circulatory arrest. Anesthesiology. 1995;82:74-82.
266 Kurth C.D., Steven J.M., Nicolson S.C., et al. Kinetics of cerebral deoxygenation during deep hypothermic circulatory arrest in neonates. Anesthesiology. 1992;77:656-661.
267 Leyvi G., Bello R., Wasnick J.D., et al. Assessment of cerebral oxygen balance during deep hypothermic circulatory arrest by continuous jugular bulb venous saturation and near-infrared spectroscopy. J Cardiothorac Vasc Anesth. 2006;20:826-833.
268 Ogino H., Ueda Y., Sugita T., et al. Monitoring of regional cerebral oxygenation by near-infrared spectroscopy during continuous retrograde cerebral perfusion for aortic arch surgery. Eur J Cardiothorac Surg. 1998;14:415-418.
269 Orihashi K., Sueda T., Okada K., et al. Near-infrared spectroscopy for monitoring cerebral ischemia during selective cerebral perfusion. Eur J Cardiothorac Surg. 2004;26:907-911.
270 Hofer A., Haizinger B., Geiselseder G., et al. Monitoring of selective antegrade cerebral perfusion using near infrared spectroscopy in neonatal aortic arch surgery. Eur J Anaesthesiol. 2005;22:293-298.
271 Higami T., Kozawa S., Asada T., et al. A comparison of changes of cerebrovascular oxygen saturation in retrograde and selective cerebral perfusion during aortic arch surgery. Nippon Kyobu Geka Gakkai Zasshi. 1995;43:1919-1923.
272 Matalanis G., Hata M., Buxton B.F. A retrospective comparative study of deep hypothermic circulatory arrest, retrograde, and antegrade cerebral perfusion in aortic arch surgery. Ann Thorac Cardiovasc Surg. 2003;9:174-179.
273 Higami T., Kozawa S., Asada T., et al. Retrograde cerebral perfusion versus selective cerebral perfusion as evaluated by cerebral oxygen saturation during aortic arch reconstruction. Ann Thorac Surg. 1999;67:1091-1096.
274 Olsson C., Thelin S. Regional cerebral saturation monitoring with near-infrared spectroscopy during selective antegrade cerebral perfusion: Diagnostic performance and relationship to postoperative stroke. J Thorac Cardiovasc Surg. 2006;131:371-379.
275 Janelle G.M., Mnookin S., Gravenstein N., et al. Unilateral cerebral oxygen desaturations during emergent repair of DeBakey type 1 aortic dissection: Potential aversion of a major catastrophe. Anesthesiology. 2002;96:1263-1265.
276 Sakaguchi G., Komiya T., Tamura N., et al. Cerebral malperfusion in acute type A dissection: Direct innominate artery cannulation. J Thorac Cardiovasc Surg. 2005;129:1190-1191.
277 Schneider F., Falk V., Walther T., et al. Control of endoaortic clamp position during port-access mitral valve operations using transcranial Doppler echography. Ann Thorac Surg. 1998;65:1481.
278 Hoksbergen A.W., Legemate D.A., Csiba L., et al. Absent collateral function of the circle of Willis as risk factor for ischemic stroke. Cerebrovasc Dis. 2003;16:191-198.
279 Merkkola P., Tulla H., Ronkainen A., et al. Incomplete circle of Willis and right axillary artery perfusion. Ann Thorac Surg. 2006;82:74-79.
280 Santo K.C., Barrios A., Dandekar U., et al. Near-infrared spectroscopy: An important monitoring tool during hybrid aortic arch replacement. Anesth Analg. 2008;107:793-796.
281 Rossi M., Tirotta C.F., Lagueruela R.G., et al. Diminished Blalock-Taussig shunt flow detected by cerebral oximetry. Paediatr Anaesth. 2007;17:72-74.
282 Nussmeier N.A., Arlund C., Slogoff S. Neuropsychiatric complications after cardiopulmonary bypass: Cerebral protection by a barbiturate. Anesthesiology. 1986;64:165.
283 Zaidan J.R., Klochany A., Martin W.M., et al. Effect of thiopental on neurologic outcome following coronary artery bypass grafting. Anesthesiology. 1991;74:406.
284 Woodcock T.E., Murkin J.M., Farrar J.K., et al. Pharmacologic EEG suppression during cardiopulmonary bypass: Cerebral hemodynamic and metabolic effects of thiopental or isoflurane during hypothermia and normothermia. Anesthesiology. 1987;67:218.
285 Roach G.W., Newman M.F., Murkin J.M., et al. Ineffectiveness of burst suppression therapy in mitigating perioperative cerebrovascular dysfunction. Multicenter Study of Perioperative Ischemia (McSPI) Research Group. Anesthesiology. 1999;90:1255-1264.
286 Legault C., Furberg C.D., Wagenknecht L.E., et al. Nimodipine neuroprotection in cardiac valve replacement: Report of an early terminated trial. Stroke. 1996;27:593.
287 Amory D.W., Grigore A., Amory J.K., et al. Neuroprotection is associated with beta-adrenergic receptor antagonists during cardiac surgery: Evidence from 2,575 patients. J Cardiothorac Vasc Anesth. 2002;16:270.
288 POISE Study Group, Devereaux P.J., Yang H. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): A randomised controlled trial. Lancet. 2008;371:1839-1847.
289 van Lier F., Schouten O., van Domburg R.T., et al. Effect of chronic beta-blocker use on stroke after noncardiac surgery. Am J Cardiol. 2009;104:429-433.
290 Landis R.C., Asimakopoulos G., Poullis M., et al. The antithrombotic and antiinflammatory mechanisms of action of aprotinin. Ann Thorac Surg. 2001;72:2169.
291 Mangano D.T., Tudor I.C., Dietzel C., Multicenter Study of Perioperative Ischemia Research Group. Ischemia Research and Education Foundation: The risk associated with aprotinin in cardiac surgery. N Engl J Med. 2006;354:353-365.
292 Fergusson D.A., Hébert P.C., Mazer C.D., BART Investigators. A comparison of aprotinin and lysine analogues in high-risk cardiac surgery. N Engl J Med. 2008;358:2319-2331.
293 Mangano D.T., Miao Y., Vuylsteke A., Investigators of The Multicenter Study of Perioperative Ischemia Research Group. Ischemia Research and Education Foundation: Mortality associated with aprotinin during 5 years following coronary artery bypass graft surgery. JAMA. 2007;297:471-479.
294 Later A.F., Maas J.J., Engbers F.H., et al. Tranexamic acid and aprotinin in low- and intermediate-risk cardiac surgery: A non-sponsored, double-blind, randomised, placebo-controlled trial. Eur J Cardiothorac Surg. 2009;36:322-329.
295 Immer F.F., Jent P., Englberger L., et al. Aprotinin in cardiac surgery: A different point of view. Heart Surg Forum. 2008;11:E9-E12.
296 Mangano D.T. Aspirin and mortality from coronary bypass surgery. N Engl J Med. 2002;2434:1309.
297 Wang D., Wu X., Li J., et al. The effect of lidocaine on early postoperative cognitive dysfunction after coronary artery bypass surgery. Anesth Analg. 2002;95:1134-1141.
298 Mitchell S.J., Merry A.F., Frampton C., et al. Cerebral protection by lidocaine during cardiac operations: A follow-up study. Ann Thorac Surg. 2009;87:820-825.
299 Mathew J.P., Mackensen G.B., Phillips-Bute B., et al. Neurologic Outcome Research Group (NORG) of the Duke Heart Center: Randomized, double-blinded, placebo controlled study of neuroprotection with lidocaine in cardiac surgery. Stroke. 2009;40:880-887.
300 Mitchell S.J., Merry A.F. Lignocaine: Neuro-protective or wishful thinking? J Extra Corpor Technol. 2009;41:37-42.
301 Aboyans V., Labrousse L., Lacroix P., et al. Productive factors of stroke in patients undergoing coronary bypass grafting: Statins are protective,. Eur J Cardiothorac Surg. 2006;30:300-304.
302 Radaelli A., Loardi C., Cazzaniga M., et al. Inflammatory activation during coronary artery surgery and its dose-dependent modulation by statin/ACE-inhibitor combination,. Arterioslcer Thromb Vasc Biol. 2007;27(12):2750-2755.