Chapter 29 Central Nervous System Dysfunction after Cardiopulmonary Bypass
Overt and subclinical perioperative cerebral injury remains an unresolved problem. Overall mortality for patients undergoing coronary artery bypass grafting (CABG) has decreased by 23% over the past decade despite a projected risk-adjusted mortality predicting a 33% increase in mortality, but the incidence of stroke has remained relatively unchanged.1
AGE-ASSOCIATED RISK OF CENTRAL NERVOUS SYSTEM INJURY
Current data show a persistent association between increased age and cerebral injury after cardiac surgery. In a review of 67,764 cardiac surgical patients, of whom there were 4743 octogenarians and who underwent cardiac surgery at 22 centers in the National Cardiovascular Network, Alexander and colleagues reported that the incidence of type I cerebral injury, defined as stroke, transient ischemic attack (TIA), or coma, was 10.2% in patients older than 80 years old versus 4.2% in patients younger than 80 years old.2 In addition to the age-related factor, reports from Europe and North America consistently describe previous cerebrovascular disease, diabetes mellitus, hypertension, peripheral vascular disease, aortic atherosclerosis, and intraoperative and postoperative complications as all being additional factors increasing the incidence of cerebral injury in cardiac surgical patients (Box 29-1).
The impact of age-associated cerebral injury in cardiac surgery is becoming more relevant owing to the progressive increase in the average age of the general population and, in particular, of the cardiac surgical population. As overall survival and quality of life after cardiac surgery continue to improve in elderly patients, advanced age alone is no longer considered a deterrent when evaluating a patient for cardiac surgery. The presence and extent of comorbidities should be considered as being of equal or greater importance than age itself as a risk factor for cerebral injury in cardiac surgical patients.3
CENTRAL NERVOUS SYSTEM INJURY
The incidence of stroke or type I injury after closed-chamber cardiac procedures is generally considered to be 1% to 4%, increasing to 8% to 9% in open-chamber (e.g., valvular surgery) or combined/complex procedures. The incidence of cognitive dysfunction (type II) is reported as ranging in incidence from 30% to 80% in the early postoperative period.4 To some extent there is a difference in the incidence of cerebral injury after cardiac surgery related to the type and complexity of the procedure, such as open chamber, combined valvular, and CABG.
Valvular versus Coronary Artery Bypass Graft Surgery
Increasing the complexity or undertaking open chamber-type procedures increases the risk of CNS injury. Ebert and coworkers prospectively studied 42 patients who underwent valve replacement surgery and 42 patients for CABG, with both groups matched post hoc for age, sex, and preoperative cognitive status.5 Patients were investigated preoperatively as well as 2 and 7 days postoperatively with a comprehensive neuropsychological and neuropsychiatric assessment. Valve replacement surgery patients exhibited more severe neuropsychological deficits and showed a slower recovery than patients who underwent CABG. In a study of 64,467 patients who underwent CABG alone and 3297 patients who underwent CABG in conjunction with aortic valve replacement (CABG/AVR) or CABG in conjunction with mitral valve repair or replacement (CABG/MVR), the incidence of type I cerebral injury in patients younger than 80 years of age was 4.2% for CABG, 9.1% for CABG/AVR, and 11.2% for CABG/MVR.2 It should be noted that the total CPB time was 96 minutes for CABG, 148 minutes for CABG/AVR, and 161 minutes for CABG/MVR.
The incidence of subtle neurologic dysfunction and cognitive abnormalities is similar in all adult patients undergoing surgical coronary artery revascularization. Increasing age has been repeatedly shown to be one of the major risk factors for stroke after CABG, likely related to the greater prevalence of severe aortic atherosclerosis in the elderly. This suggests that there may be different factors operative in the production of gross neurologic damage than in the genesis of cognitive dysfunction. Although calcific or atheromatous macroembolic debris from the ascending aorta or aortic arch appears to be a prime factor in the production of clinical stroke syndromes and it was formerly thought that microembolic elements, either gaseous or particulate, produced cognitive dysfunction, studies from beating-heart surgery in which CPB is avoided, despite a much lower incidence of embolic events, appear to have a relatively similar incidence of cognitive dysfunction to CABG using conventional CPB.6
Aortic Atherosclerosis
Atheroembolism from an atheromatous ascending aorta and aortic arch is recognized as a major risk factor in the patient undergoing cardiac surgery and is a widespread problem.7
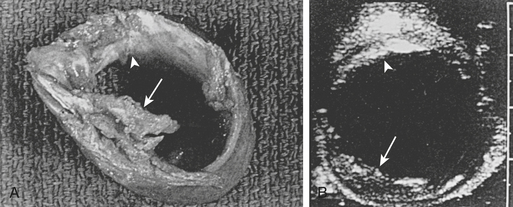
Atheroembolism in cardiac surgery has a broad spectrum of clinical presentations, including devastating injuries and death; yet its true incidence is probably underestimated. Thoracic aorta atheromatosis is associated with coronary artery disease and stroke in the general population. Yahia and associates prospectively studied patients with diagnoses of TIA or stroke using TEE for assessment of aortic atheromatosis.7 Thoracic aortic atheromas were present in 141 of 237 patients (59%); mild plaque (<2 mm) was present in 5%, moderate plaque (2 to 4 mm) in 21%, severe plaque (≥4 mm) in 33%, and complex plaque in 27%. Plaques were more frequently present in the descending aorta and the arch of the aorta than in the ascending aorta. Overall, atherosclerosis of the ascending aorta is present in 20% to 40% of cardiac surgical patients, with the percentage increasing with age, and it is an independent risk factor for type I cerebral injury.
Transesophageal Echocardiography versus Epiaortic Scanning
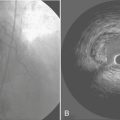
Djaiani and colleagues performed TEE and EAS to assess the severity of aortic atherosclerosis in the ascending aorta and the aortic arch.8 Patients were allocated to either low-risk or high-risk groups according to thickness of the intima of the aorta. Transcranial Doppler imaging was used to monitor the middle cerebral artery. Diffusion-weighted magnetic resonance imaging (MRI) was performed 3 to 7 days after surgery. The NEECHAM Confusion Scale was used for assessment and monitoring patient consciousness level. In the high-risk group (intimal thickness > 2 mm), confusion was present in six (16%) patients versus five (7%) patients in the low-risk group, and there was a threefold increase in median embolic count, 223.5 versus 70.0 (P = .0003). Diffusion-weighted MRI–detected brain lesions were only present in patients from the high-risk group, 61.5% versus 0% (P < .0001). There was significant correlation between the NEECHAM scores and embolic count in the high-risk group. Multiple studies have documented that most of the significant atherosclerotic lesions in the ascending aorta are missed by intraoperative palpation by the surgeon, and intraoperative echocardiographic studies of the aorta have been recommended (Fig. 29-1). However, the ability of TEE to reliably detect all ascending aorta and aortic arch lesions is limited.
The high acoustic reflectance attributable to the air-tissue interface resulting from overlying right main bronchus and trachea limits TEE assessment of the upper ascending aorta where cannulation is generally undertaken. Intraoperative EAS has emerged as a most helpful tool for the diagnosis of ascending aortic atherosclerosis and has revealed major insights into the nature and distribution of this disease. Konstadt and colleagues investigated 81 patients (57 male and 24 female; aged 32 to 88 years, mean age 64 years) scheduled for elective cardiac surgery.9 A comprehensive examination of the entire thoracic aorta in both the longitudinal and transverse planes was performed by biplane TEE. After pericardiotomy, a sterile-draped 7-MHz probe was used to scan the entire ascending aorta in both long- and short-axis views. In both echocardiographic examinations, the presence and location of protruding plaques and intimal thickening greater than 3 mm were recorded. Fourteen (17%) of the 81 patients had significant atherosclerotic disease of the ascending aorta as diagnosed by EAS echocardiography. The sensitivity of TEE was 100%, the specificity was 60%, the positive predictive value was 34%, and the negative predictive value was 100%. According to the authors, if the complete biplane TEE examination is negative for plaque, it is highly unlikely that there is significant plaque in the ascending aorta. If the TEE examination is positive for plaque, there is a 34% chance that there is significant disease of the ascending aorta, and EAS should be considered. TEE is a very sensitive but only mildly specific method of determining whether ascending aortic atherosclerosis is present.
NEUROPSYCHOLOGICAL DYSFUNCTION
Compared with stroke, cognitive dysfunction is a considerably more frequent sequela of cardiac surgery and has been demonstrated in up to 80% of patients early postoperatively.10 The pathogenesis of cognitive dysfunction after cardiac surgery is still uncertain. Variables that have been postulated to explain the development of postoperative neurocognitive decline include advanced age, concomitant cerebrovascular disease and severity of cardiovascular disease, and intraoperative factors such as embolization, cerebral hypoperfusion or hypoxia, activation of inflammatory processes, aortic cross-clamp or CPB time, and possibly low mean arterial pressure in patients with impaired cerebrovascular autoregulation. In many instances, subtle signs of neuropsychological dysfunction are detectable only with sophisticated cognitive testing strategies. The fact that many of these abnormalities are difficult to properly assess on routine clinical work and their apparently transient nature have led to a tendency to minimize their clinical relevance. The mid- and long-term impact of cognitive dysfunction on quality of life after cardiac surgery has been addressed by different studies.
MECHANISMS OF BRAIN INJURY
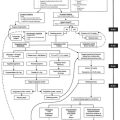
Determining which factor or, more likely, which combination of factors is responsible for postoperative neurologic or behavioral dysfunction in patients undergoing cardiac surgery using CPB is problematic (Box 29-2). Based on postmortem studies, as well as correlative analysis of intraoperative events with neurologic outcomes, two primary mechanisms appear to be responsible for brain injury in otherwise uncomplicated cardiac operations: cerebral hypoperfusion and cerebral emboli.
Intraoperative cerebral embolization of particulate and microgaseous elements has been demonstrated to have a significant role in the genesis of cerebral events in postoperative cardiac surgical patients. Increasing attention is also being paid to the role of perioperative hypoperfusion, particularly in patients with intracranial and extracranial atherosclerosis, and to the effect of inflammatory processes triggered during exposure to surgery and CPB (Box 29-3).11
Watershed Infarctions
These areas are also highly susceptible to ischemia due to end-artery embolization, and it is also recognized that although severe hypotension is the most common cause, showers of microemboli may lodge preferentially in these areas and cause infarcts in the underlying brain.12 As such, although they are commonly due to profoundly hypotensive episodes, watershed lesions are not pathognomonic of a hypotensive episode and may be the result of cerebral emboli. Embolization and hypoperfusion acting together play a synergistic role and either cause or magnify the brain damage of cardiac surgical patients.
Cerebral Perfusion Pressure
The interaction of emboli, perfusion pressure, and the particular conditions of the regional cerebral circulation (e.g., preexisting cerebral intravascular lesions) will determine the final expression of brain damage in the cardiac surgical patient. Combinations of hemodynamic events from apparently normal CPB procedures are related to the development of postoperative neurologic complications and affect the impact of common clinical risk factors. A multivariate statistical procedure (cluster analysis) was applied to a dataset of automatically recorded perfusions from 1395 patients who underwent CABG.13 The following five parameters emerged for cluster analysis: MAP, dispersion of MAP, dispersion of systemic vascular resistance, dispersion of arterial pulse pressure, and the maximum value of mixed venous saturation. Patients who underwent CPB procedures with large fluctuations in hemodynamic parameters particularly showed an increased risk for the development of postoperative neurologic complications.
Cerebral Emboli and Outcome
Gaseous emboli are not innocuous. It has been demonstrated that the effects of air emboli on the cerebral vasculature not only are due to bubble entrapment with direct blockage of cerebral vessels but also represent the effects that such bubbles have on vascular endothelial cells.14 Ultrastructural examinations of pial vessels exposed to cerebral air emboli demonstrated severe injury to endothelial plasmalemma, leading to loss of cellular integrity and endothelial cell swelling. Such endothelial damage produces disruptions of vasoreactivity. Air embolism also produces changes in blood elements, leading to formation of a proteinaceous capsule around the bubbles, marked dilation of pial vessels, platelet sequestration, and damage to endothelial cells. Air-induced mechanical trauma to the endothelium causes basement membrane disruption, thrombin production, release of P-selectin from intracellular vesicles, synthesis of platelet-activating factor, and a reperfusion-like injury with perturbations in inflammation and thrombotic processes. These phenomena likely impair nitric oxide production, giving rise to alterations in cerebral microvascular regulation.
CEREBROPROTECTIVE STRATEGIES
Cardiopulmonary Bypass Equipment
Early studies demonstrated increased microemboli in patients undergoing CPB using bubble oxygenators, with a reduction in cerebral embolization with the use of membrane oxygenators and arterial line filtration15 (Boxes 29-4 and 29-5). It is apparent that emboli may be generated continuously during CPB and that equipment modification (e.g., arterial line microfiltration and preferential usage of membrane oxygenators) can decrease the generation of such emboli. Membrane oxygenators are currently recommended for CPB. It is equally evident that such equipment modifications, while decreasing the embolic load, cannot completely eliminate it.
BOX 29-5 Strategies to Decrease Impact of CPB on Neurologic Complications
In an attempt to decrease emboli originating from the surgical field, cell savers have been used for processing cardiotomy suction blood before returning it to the CPB circuit. Jewell and coworkers16 reported on 20 patients prospectively randomized to either cell saver or cardiotomy suction and demonstrated that compared with cardiotomy suction, cell saver removed significantly more fat from shed blood, such that the percentage reduction in fat weight achieved by cell saver or cardiotomy suction was 87% compared with 45%.
Applied Neuromonitoring
Intraoperative neurophysiologic monitoring may be of benefit to decrease CNS injury.17 Brain oximetry studies using noninvasive near-infrared spectrophotometry (NIRS) have shown promising results.
Intraoperative transcranial Doppler imaging has been demonstrated to detect embolic events in real time and allows modification of perfusion and surgical techniques. It has been shown that the numbers of emboli generated by perfusionist interventions (e.g., drug injection, blood return), as well as episodes of entrainment of air from the surgical field, are rapidly identified and corrected by transcranial Doppler detection of intraoperative emboli. Even during beating-heart procedures, compromised cerebral perfusion can occur relatively frequently, and, if unrecognized, may account for the relative lack of difference in CNS outcomes between CABG and off-pump CABG.18 Combined EEG and cerebral oximetry identified episodes of cerebral ischemia in 15% of a series of 550 beating-heart patients; all were treated successfully by a combination of pharmacologically improved cardiac output, increased perfusion pressure, and cardiac repositioning.
Pharmacologic Cerebral Protection
In general, pharmacologic protection from cerebral ischemia remains an elusive goal. While results suggest that there is as yet no pharmacologic “magic bullet” that can be used to reduce neurologic injury in patients undergoing cardiac surgery, there is a combination of technical and pharmacologic measures currently available that might positively affect the outcome of these patients. In patients identified as being at risk for perioperative cerebral injury, preventive measures as outlined in Box 29-6 should be instituted with organ-targeted management to guide the whole intraoperative and postoperative period. As the age and incidence of comorbid disease in the cardiac surgical population continue to rise, the importance of these issues becomes ever more acute because primary prevention continues to be the only effective measure to decrease cerebral injury in cardiac surgical patients.
SUMMARY
1. Ferguson T.B.Jr., Hammill B.G., Peterson E.D., et al. A decade of change: Risk profiles and outcomes for isolated coronary artery bypass grafting procedures, 1990-1999. A report from the STS National Database Committee and the Duke Clinical Research Institute. Society of Thoracic Surgeons. Ann Thorac Surg. 2002;73:480.
2. Alexander K.P., Anstrom K.J., Muhlbaier L.H., et al. Outcomes of cardiac surgery in patients ≥ 80 years: Results from the National Cardiovascular Network. J Am Coll Cardiol. 2000;135:731.
3. Blacker D.J., Flemming K.D., Link M.J., Brown R.D.Jr. The preoperative cerebrovascular consultation: Common cerebrovascular questions before general or cardiac surgery. Mayo Clin Proc. 2004;79:223.
4. Ahonen J., Salmenpera M. Brain injury after adult cardiac surgery. Acta Anaesthesiol Scand. 2004;48:4.
5. Murkin J.M., Newman S.P., Stump D.A., Blumenthal J.A. Statement of consensus on assessment of neurobehavioral outcomes after cardiac surgery. Ann Thorac Surg. 1995;59:1289.
6. Omar Y., Balacumaraswami L., Pigott D.W., et al. Solid and gaseous cerebral microembolization during off-pump, on-pump, and open cardiac surgery procedures. J Thorac Cardiovasc Surg. 2004;127:1759.
7. Yahia A.M., Kirmani J.F., Xavier A.R., et al. Characteristics and predictors of aortic plaques in patients with transient ischemic attacks and strokes. J Neuroimaging. 2004;14:16.
8. Djaiani G., Fedorko L., Borger M., et al. Mild to moderate atheromatous disease of the thoracic aorta and new ischemic brain lesions after conventional coronary artery bypass graft surgery. Stroke. 2004;35:e356.
9. Konstadt S.N., Reich D.L., Kahn R., Viggiani R.F. Transesophageal echocardiography can be used to screen for ascending aortic atherosclerosis. Anesth Analg. 1995;81:225.
10. Knipp S.C., Matatko N., Wilhelm H., et al. Evaluation of brain injury after coronary artery bypass grafting: A prospective study using neuropsychological assessment and diffusion-weighted magnetic resonance imaging. Eur J Cardiothorac Surg. 2004;25:791.
11. Murkin J.M. Inflammatory responses and CNS injury: Implications, prophylaxis, and treatment. Heart Surg Forum. 2003;6:193.
12. Boyajian R.A., Otis S.M. Embolic stroke syndrome underlies encephalopathy and coma following cardiac surgery. Arch Neurol. 2003;60:291.
13. Ganushchak Y.M., Fransen E.J., Visser C., et al. Neurological complications after coronary artery bypass grafting related to the performance of cardiopulmonary bypass. Chest. 2004;125:2196.
14. Eckmann D.M., Armstead S.C., Mardini F. Surfactants reduce platelet-bubble and platelet-platelet binding induced by in vitro air embolism. Anesthesiology. 2005;103:1204.
15. Haworth W.S. The development of the modern oxygenator. Ann Thorac Surg. 2003;76:S2216.
16. Jewell A.E., Akowuah E.F., Suvarna S.K., et al. A prospective randomised comparison of cardiotomy suction and cell saver for recycling shed blood during cardiac surgery. Eur J Cardiothorac Surg. 2003;23:633.
17. Murkin J.M. Perioperative multimodality neuromonitoring: An overview. Semin Cardiothorac Vasc Anesth. 2004;8:167.
18. van Dkijk D., Moons K.G., Keizer A.M., et al. Association between early and three month cognitive outcome after off-pump and on-pump coronary bypass surgery. Heart. 2004;90:431.