Chapter 29 Cellular Effects of Detachment and Reattachment on the Neural Retina and the Retinal Pigment Epithelium
Introduction
In retinal detachment the separation of the neural retinal from the retinal pigment epithelium (RPE) initiates a complex series of cellular and molecular changes.1 Left untreated, retinal detachment results in permanent visual loss; however, early intervention may be associated with good visual outcomes, suggesting that some of these molecular changes may be arrested or reversed.2,3
Use and limitations of animal models in the study of retinal detachment
Human studies of cellular changes following acute retinal detachment are limited to isolated case reports as surgical management does not routinely involve removal of retinal tissue. More recently data have become available from patients undergoing macular translocation surgery in which the retina is detached as part of the procedure, allowing sampling of the retina as early as 1 hour following detachment.4 In patients with advanced stages of retinal detachment and PVR, surgical management may involve excising areas of scarred retinal tissue allowing histopathological analysis. However, the data from human studies is still limited by small numbers, the challenges of sampling and analyzing small retinal specimens, and an inability to study cellular recovery following reattachment.4–7
Ideally the characteristics of an experimental detachment should closely mimic those found in humans while allowing for precise control over the extent of separation between the two layers (detachment height), the location of the detachment, its surface area, and the onset of detachment (or reattachment). A number of methods have been used to simulate human retinal detachment in animal models. These range from creating large retinal tears to subretinal injections of fluid or viscous substances. Experiments where retinal detachment induction is standardized with a micropipette provide a very controlled environment for analysis; however they differ from the clinical pattern of events in which acute retinal tears of variable size are induced by vitreoretinal traction at the time of posterior vitreous detachment. It is possible that retinal tearing may act as a more potent stimulus for cellular disorganization, loss, and remodeling, leading more rapidly to the advanced pathology usually seen following longer periods of retinal detachment in animal models. Although experiments involving animal models may differ in methodology, species used, and outcome measures, they have yielded similar results to give a relatively detailed profile of the changes that occur after detachment. Retinal tissue removed from human postmortem specimens and from patients undergoing retinal detachment surgery has demonstrated changes similar to those seen in animal models.6–8
Cellular changes in response to retinal detachment
Acute retinal detachment
A rapid response to retinal detachment has been shown to occur within 15 minutes, including phosphorylation of fibroblast growth factor receptor (FGFR-1) and increased expression by RPE and Müller cells of extracellular signal-regulated kinase and activator protein transcription factor.9 This initiates a cascade of events that leads to a number of molecular and cellular changes within the retina and RPE.
RPE–photoreceptor interface
The earliest structural effects of retinal detachment are seen at the interface of photoreceptor outer segments and the RPE.10 The mature RPE is a polarized monolayer of neuroepithelial cells that rests on Bruch’s membrane, between the choriocapillaris and the neural retina.11 The relationship of the apical surface of the RPE to differentiated photoreceptors is anatomically complex. There are no actual cellular junctions between the two layers in the mature eye, but the two are adherent, with the degree of adhesion varying among species.12 With the onset of retinal detachment changes to this interface include alterations in the RPE apical surface, proliferation of RPE cells, migration of cells into the subretinal space, degeneration of photoreceptor outer segments, and changes in photoreceptor outer-segment renewal.1
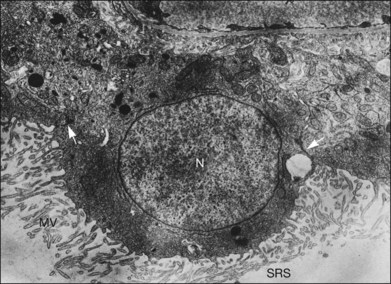
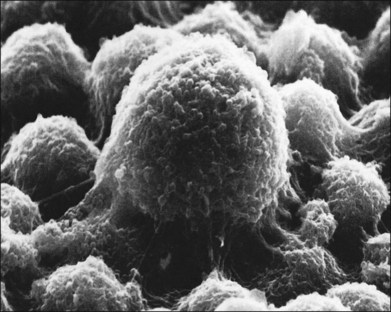
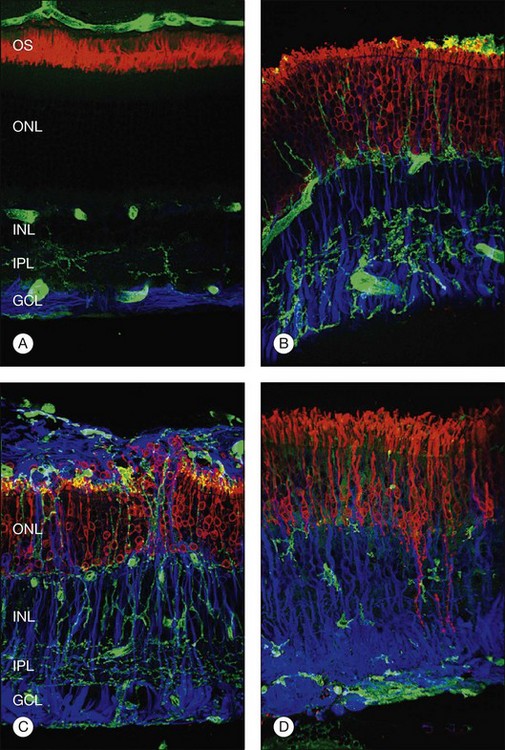
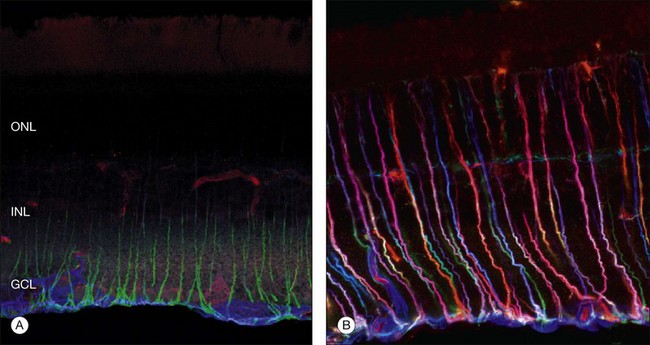
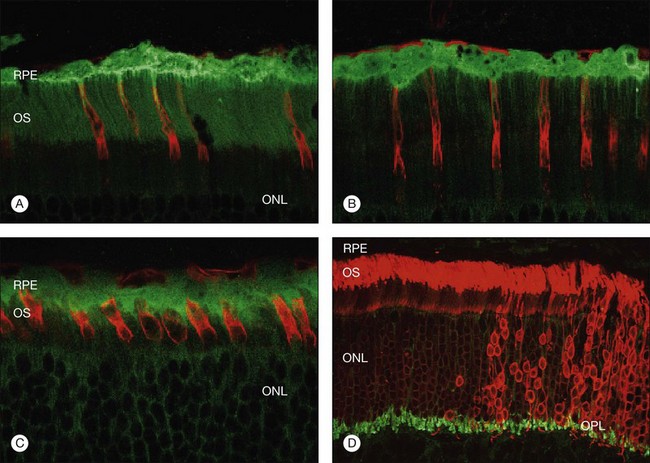
Within a few hours of retinal detachment, the long and elaborate sheet-like and villous processes that normally ensheath the outer segments are lost and replaced by a “fringe” of short microvilli (Fig. 29.1).13 At the same time, the overall surface morphology of the RPE cells changes into a rounded contour, as cytoplasm protrudes past the normal limits of the apical surface into the subretinal space, and the nucleus becomes displaced to a more apical position10,14 (Fig. 29.2).
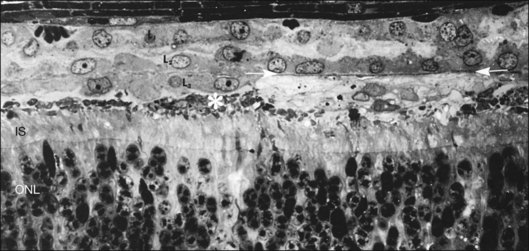
In the feline model, experiments using 3H-thymidine have shown that within 72 hours of retinal detachment the RPE has begun to proliferate and may be observed as areas of hyperplasia within the RPE monolayer.10 This proliferative response transforms the RPE’s uniform monolayer into a heterogeneous morphology in which strands of cells extend from the original monolayer into the subretinal space or result in the formation of multiple layers of cells whose polarity does not necessarily match that of the original monolayer (Fig. 29.3). This effect is limited to the region of detachment; in attached regions the RPE remains mitotically quiet, suggesting that attachment of the RPE to the neural retina acts to keep the RPE mitotically inactive and its apical surface highly differentiated.15–17 The proliferative response of the RPE cells also appears to be self-limiting with only low levels of proliferation observed after long detachment intervals (e.g., 12–14 months) in owl, monkey, and cat retinas10,16 (Box 29.1 and Fig. 29.4).
Box 29.1
Clinical correlates
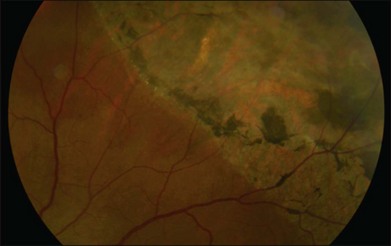
Retinal pigment epithelial (RPE) proliferation can be seen as subretinal pigment deposition in chronic retinal detachments. It is likely that the demarcation lines noted in human retinal detachments represent zones of proliferated RPE occurring at transitions between detached and attached regions of the eye (Fig. 29.4).
The subretinal space is usually free of cells; however within 24 hours of retinal detachment a number of cell types (polymorphonuclear neutrophils, monocytes, and macrophages) migrate into this space from the choroidal and retinal capillaries.10,18 Free RPE cells are also seen in the subretinal space within 72 hours of retinal detachment and frequently contain outer-segment fragments, indicating that they may play a role in phagocytosis of cellular debris.10,18
Photoreceptors
Within 12 hours of experimental retinal detachment, photoreceptor outer segments show evidence of structural damage. Initially, the distal end of the outer segment becomes vacuolated or distorted and by 24–72 hours all rod and cone outer segments are significantly shorter and distorted with disoriented discs.19 The degeneration of outer segments may proceed until those in the zone of detachment appear only as empty sacs of membrane attached to the connecting cilium.10
Outer-segment debris is shed into the subretinal space where it is phagocytosed by RPE cells and macrophages which have migrated into the area.10,18
Although retinal detachment interrupts the process of disc production and shedding, outer-segment specific proteins continue to be produced but localize to abnormal cellular locations. Opsin, normally concentrated in the outer segment, begins to accumulate in the plasma membrane vitread to the outer segment within a day following experimental retinal detachment (Fig. 29.5).19 Peripherin/rds, another outer-segment protein specific to the disc rims, is also redistributed and begins to appear in cytoplasmic vesicles.20 Cone outer-segment proteins appear to be more susceptible to damage, with redistributed cone opsins persisting for just 1 week following retinal detachment, after which their expression is downregulated.21
During the first day of a detachment the inner segments appear essentially normal, but between the first and third days they begin to show signs of degeneration: most commonly swelling, disruption, and loss of mitochondria (and loss of anticytochrome oxidase labeling) in the ellipsoid region,3,22 an overall disruption of the organized rough endoplasmic reticulum and Golgi apparatus in the myoid region, and, within a few days, an overall size reduction of the inner segment. It is interesting to note that the connecting cilium, which is essential for production of the outer segment, is retained even in severely affected inner segments in long-term detachments. This is crucial as its loss would prevent regeneration of outer segments following reattachment. Similarly, the loss of mitochondria also has the potential to affect the photoreceptors’ ability to regenerate significantly, because the metabolic rate in these cells is among the highest of any in the body.
The outer nuclear layer contains the cell bodies of the photoreceptor cells. These cell bodies extend a process toward the outer plexiform layer, where they form synapses with second-order neurons. Rods and cones have characteristic synaptic terminals called spherules and pedicles respectively.23 The outer plexiform layer also contains the processes of second-order neurons, the cell bodies of which lie in the inner nuclear layer. These processes synapse with each other and with the photoreceptors. The photoreceptor cell bodies and synaptic terminals show a rapid response to detachment with extensive vacuolization, degeneration of mitochondria, and disorganization of the microtubules and actin filaments. Cell death via the apoptotic pathway peaks at day 3 following retinal detachment but continues at low levels for as long as the retina is detached, a process that appears to be mediated via caspases 3, 7, and 9.24,25 Recent studies have shown that, when caspase pathways are blocked, receptor interacting protein (RIP) kinases promote necrosis and overcome apoptosis inhibition. Therefore, targeting of both caspase and RIP kinase pathways is required for effective photoreceptor protection26 (Box 29.2).
Box 29.2
Clinical correlates
More recently, photoreceptor apoptosis has also been demonstrated in human retinal specimens, with a peak at day 2 following retinal detachment.27
Following cell death some photoreceptors are extruded into the subretinal space where they are phagocytosed by macrophages while others appear to undergo degeneration and phagocytosis within the outer nuclear layer.28
Not all photoreceptors degenerate at the same rate; areas of extensive degeneration coexist with areas of relatively intact photoreceptors.19 It does appear that rod cell bodies appear to degenerate quicker than cones following retinal detachment.21 In a region in which nearly all of the rod cell bodies show signs of degeneration and even cell death, neighboring cone cell bodies may look relatively intact. Consistent with this observation, the rod spherules appear to be particularly susceptible to the effects of detachment. These synaptic terminals are normally filled with synaptic vesicles and contain one or two large presynaptic ribbons. When the retina has been detached for 3 days, many of these terminals appear depleted of vesicles, except for a few that remain as a halo around a greatly truncated ribbon.29 Many terminals appear as if they have “retracted” into the cell body, and some synaptic structures generally associated with the outer plexiform layer now occur within the outer nuclear layer (Fig. 29.6).29,30 As with the cone and rod photoreceptor cell bodies, the cone synaptic terminals seem to survive the early effects of detachment better than the rod terminals do. Although their shape can change fairly dramatically, they do not appear to retract and by electron microscopy they remain filled with synaptic vesicles.30,31
Second-order neurons and nonneuronal cell types
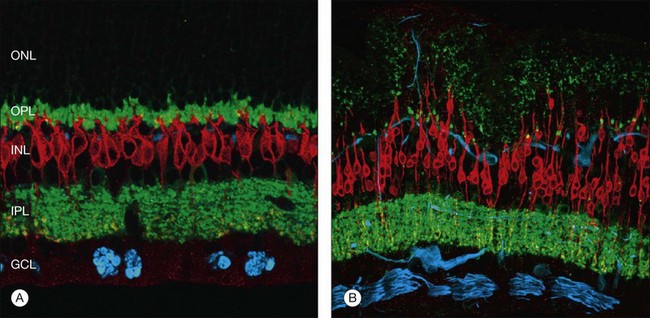
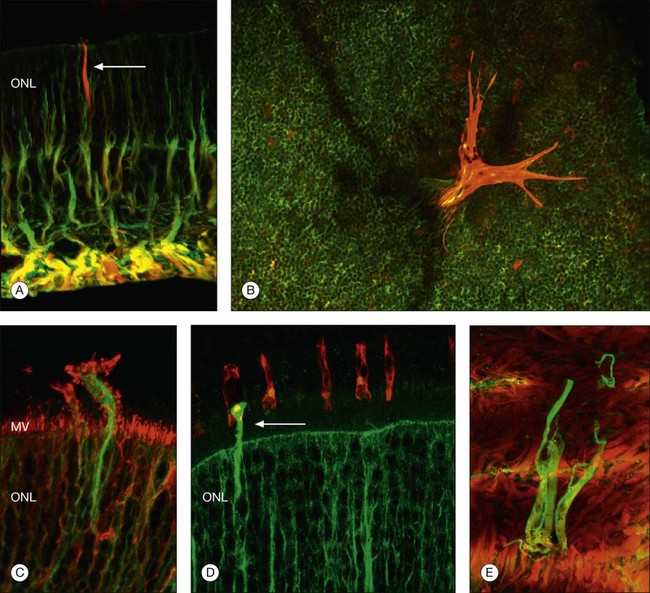
At the same time as rod spherules are retracting, processes from the rod bipolar cells and horizontal cells (labeled with antibodies to protein kinase C, and neurofilament protein, respectively) begin to grow beyond the normal layer of photoreceptor synaptic terminals and into the outer nuclear layer and even beyond, sometimes extending into the subretinal space (Fig. 29.7).30
Just as changes in the synaptic terminals are accompanied by the growth of processes from the rod bipolar cells and horizontal cells into the outer nuclear layer,29,30 ganglion cells also become reactive and begin to re-express growth-associated protein (GAP)-43, a protein expressed early in cell body development for the formation of synaptic connections between ganglion cell axons and the brain.32 Horizontal and ganglion cells show dramatic and extensive remodeling, growing processes that appear to be attracted to Müller cells.33 By contrast, rod bipolar cell dendrite growth is less aggressive, being targeted towards the retracted terminals of the rod photoreceptors.
Within 24 hours of retinal detachment nonneuronal cell types, for example, astrocytes, Müller cells, pericytes, capillary endothelial cells, and microglia, also display signs of proliferation.16,17 By 2 days, some labeled Müller cell nuclei are translocated from their normal positions on the vitreal border of the inner nuclear layer into the outer plexiform and outer nuclear layers (Fig. 29.8).34 This response peaks 3–4 days after detachment and declines slowly to very low levels several weeks later. Microglia, a form of macrophage in the central nervous system that usually lie dormant in normal retina, are activated and begin to divide and migrate to the outer retina (Fig. 29.5). Microglial proliferation is observed to differing degrees in different species, and is thought to play a role in photoreceptor cell death, possibly by modifying the production of neuroprotective trophic factors by Müller cells.28
Changes in the Müller cell are seen within 1 day of retinal detachment, including changes in protein expression and early growth of Müller cell processes.35 Within 3 days Müller cell bodies have migrated to the outer nuclear and outer plexiform layers (Fig. 29.9) and processes begin to extend into the subretinal space through localized disruptions in the outer limiting membrane. Müller cell processes within the feline retina express both vimentin and glial fibrillary acidic protein (GFAP); however segments of the processes extending through the outer limiting membrane into the subretinal space preferentially express vimentin (Fig. 29.10).35 These processes appear to penetrate the outer limiting membrane preferentially adjacent to cone photoreceptors, often growing for long distances on the photoreceptor border.35 Interestingly, it is those Müller cells stimulated to divide on day 3 that ultimately contribute to the formation of subretinal scars.34
Chronic retinal detachment and proliferative vitreoretinopathy
Although animal models remain the main source of data regarding more long-term neuronal remodeling following retinal detachment, human studies of retinectomy specimens removed at the time of surgery also exist.6 These studies show close correlation with results from feline and primate models.
Photoreceptors
The degree of photoreceptor cell death and its timing are species-dependent. In the feline model, there is a significant decrease in the number of photoreceptor cells by 1 month after detachment and this number continues to decline until the outer nuclear layer loses about 80% of its cell population by 90 days after detachment.19 In regions severely affected by photoreceptor degeneration, the outer nuclear layer can be reduced in thickness to one or two cell layers. In humans, a histopathological study by Wilson and Green of retinal detachment in postmortem eyes also showed atrophy of the photoreceptor layer in 26.5% of retinas examined7 (Box 29.3).
Box 29.3
Clinical correlates
Cell death in the photoreceptor cell layer is likely to be a significant factor in visual recovery after reattachment, particularly in detachments of more than a few days’ duration. Finding a strategy for preserving photoreceptors may lead to an improvement of the visual outcome after reattachment surgery. A number of factors have been successful at reducing cell death in animal models, including brain-derived neurotrophic factor (BDNF), ciliary neurotrophic factor (CNTF), and basic fibroblast growth factors (FGF-1, FGF-2), the free radical scavenger edaravone, the antibiotic minocycline, and the Fas receptor inhibitor MET12.36–42 Increasing the concentration of environmental oxygen breathed has also yielded positive results.22,43
The loss of cells from the photoreceptor layer occurs by cell death and the extrusion of photoreceptor cell bodies past the outer limiting membrane into the subretinal space. Cell death among photoreceptor cells has been shown by the TUNEL technique to occur by apoptosis, but may also involve photoreceptor necrosis,24,26,44,45 a finding also documented in human studies.8,46,47 The mechanism by which cells are extruded into the subretinal space is not understood, but these cells have clearly lost their differentiated phenotype and appear as rounded cells with very little cytoplasm. With increasing chronicity of retinal detachment, disorganized lamellar debris rather than a discrete packet of discs is found in the subretinal space, providing additional evidence that discs are not shed and phagocytosed in the normal manner.
Second-order neurons and nonneuronal cell types
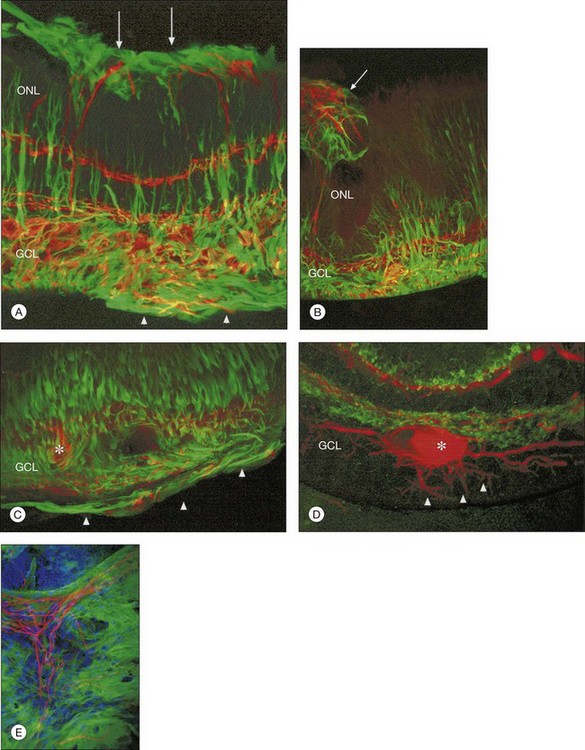
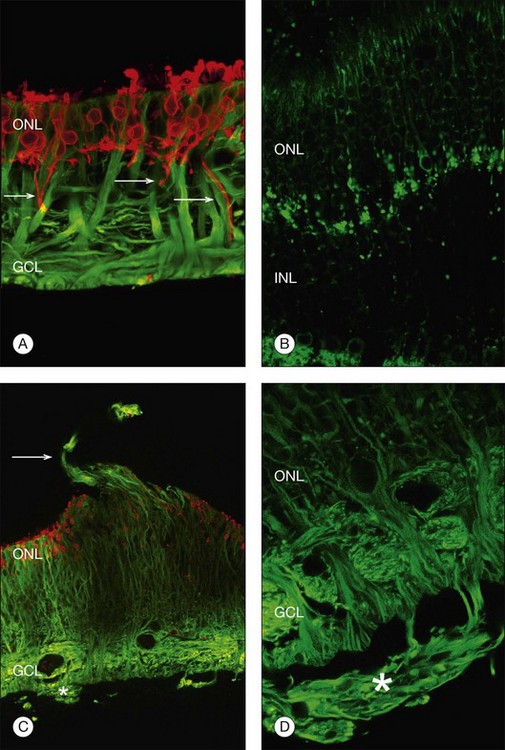
Beyond 3 days of detachment, Müller cell processes often extend into the subretinal space through localized disruptions in the outer limiting membrane. These processes become more commonplace and elaborate as detachment time lengthens. Immunocytochemical labeling and confocal imaging studies demonstrate the unique nature of Müller cell processes extending into the subretinal space. In felines, Müller cell processes within the retina that preferentially express vimentin in the outer portion of the cell grow into the subretinal space.35 The vimentin-expressing processes that grow beyond the outer limiting membrane and into the subretinal space assume the appearance of filopodia, while retaining their preferential expression of vimentin over GFAP. Microvilli normally extend from the apical surface of the Müller cells, just beyond the outer limiting membrane. These processes are richly decorated with the protein CD-44. As the Müller cell filopodia intrude into the subretinal space, their surface remains decorated with “microspikes” of CD-44. The presence of CD-44 on the apical microvilli and subretinal outgrowths may provide for sites of molecular interaction between the growing processes and components of the subretinal space, perhaps providing some clue to their preference for growing adjacent to cone photoreceptors.48 These Müller cell processes can grow for long distances on the photoreceptor border, often forming a multilayered “glial scar” within the subretinal space (Fig. 29.5C and Box 29.4).
In a study of 16 retinectomy specimens taken from patients with PVR, Sethi et al. demonstrated that the response to prolonged retinal detachment with PVR in humans was similar to that observed in chronic detachment animal models.6 In humans, as in animal models, photoreceptors were degenerate and intracellular redistribution of opsin proteins to the plasma membrane was observed. In cones, labeling with anti-M/L cone opsin showed degenerate outer segments and faint staining of swollen inner segments and, in severe PVR, staining of cone opsins was absent. Rod synaptic terminals showed remodeling with extension of rod bipolar cell dendrites and horizontal cell processes into the outer nuclear layer. There was also upregulation of neurofilament and GAP-43 expression in large ganglion cells with neurite sprouting. All retinectomy specimens showed a marked upregulation of Müller cell and astrocyte expression of GFAP and vimentin, with areas of increased glial tissue replacing degenerated retinal neurons (Fig. 29.11).6 In some sections Müller cells, together with microglia, breached the inner limiting membrane and extended on to the retinal surface where they formed one of the components of an epiretinal membrane (Fig. 29.5D). Müller cell processes also formed confluent subretinal membranes (Fig. 29.11). Ongoing photoreceptor apoptosis has also been demonstrated in human PVR.47
Retinal reattachment
Clinical evidence shows that retinal reattachment is associated with good visual outcomes, particularly if the macula is rapidly reattached, and that visual recovery may continue for some time following surgery.49,50 This suggests that some of the changes described above may be reversible or have little effect on visual function, when measured by Snellen acuities and patient reported outcomes.51 A number of factors are associated with poor visual outcomes, including involvement of the macula, the duration and extent of retinal detachment, and the development of PVR.2 Development of PVR is associated with a significant deterioration in visual outcomes and this is likely to reflect more than just the length of detachment as good outcomes can still be achieved in patients who undergo multiple operations for retinal detachment with no clinical PVR.49
Feline models have shown that rapid retinal reattachment is associated with a good but not complete restoration of neural circuitry.3,19 This has also been observed clinically in human case series, for example in patients undergoing translocation surgery: despite only minor changes in neuronal remodeling being observed prior to reattachment, some patients still developed PVR postoperatively.4 This suggests that the retina had been primed or activated by retinal detachment and that this process remained active despite subsequent rapid reattachment.4 This is consistent with the observation that within 15 minutes of retinal detachment there are changes in growth factor expression that may lead to irreversible changes in cellular structure.9 For example, in rabbits and cats a single intravitreal injection of 1 µg of basic fibroblast growth factor (bFGF) leads to FGFR internalization, Müller cell proliferation, an increased expression of GFAP and vimentin, and the growth of Müller cell processes on to the vitreal surface of the retina.52,53 It is possible therefore that, in some patients, the cellular events initiated by this rapid response to retinal detachment may persist despite reattachment, resulting in the development of PVR following retinal reattachment or translocation surgery.
Animal models have given us a clearer understanding of cellular recovery following retinal reattachment. In the feline model, retinal reattachment within 1–3 days is very effective at reversing the cellular changes induced by retinal detachment.3,19 Retinal recovery relies on re-establishing the cell-to-cell contact of RPE cells and photoreceptors. This involves redifferentiation of the RPE apical surface, re-ensheathment of the regenerating outer segments (which differs for rods and cones), and probably synthesis of interphotoreceptor matrix components. Finally, the photoreceptors and RPE must also re-establish a functional relationship. For example, a normal balance between disc addition and disc shedding must be restored if the outer segments are going to attain normal length. Clinical evidence indicates that this process may occur over months or even years.50 In addition, the transport of ions and molecules between the retina and RPE, which is affected to an unknown degree when the two cell layers are separated from each other, must also be restored. For example, retinoids (chemically distinct forms of vitamin A), coupled with their binding proteins, must be transported back and forth between the neural retina and the RPE as part of the visual cycle.54
In the feline model, retinal reattachment following a 3-day detachment results in recovery of the outer segments to approximately 70% of their length at 28 days, arrested photoreceptor apoptosis, and reduction in cellular proliferation.55 Regenerating outer segments may appear shortened and misaligned with respect to each other and stacking of the disc membranes is often abnormal. In monkey retinas detached for 1 week, rod and cone outer segments regain approximately 30% of their normal mean length within 7 days of reattachment, 60% of their length after 30 days, and 100% by 150 days.56 In the first 30-day interval, the mean disc membrane assembly rate in rods is approximately one-third slower than the normal rate. Disc shedding, on the other hand, appears to engage after the first reattachment week. Neuronal recovery may also be determined by the duration of retinal detachment prior to reattachment. In cat retinas detached for periods longer than 7 days, many outer segments remained shorter than normal several months after reattachment,57 implying that defects in the assembly or shedding phases (or both) of the renewal process may persist well beyond 30 days of reattachment in retinas detached for longer durations. Long-term reattachment experiments are expensive because of the cost of maintaining experimental animals, but they may be highly informative given our lack of understanding of long-term recovery and indications that visual recovery may continue for a very long time after reattachment in humans.
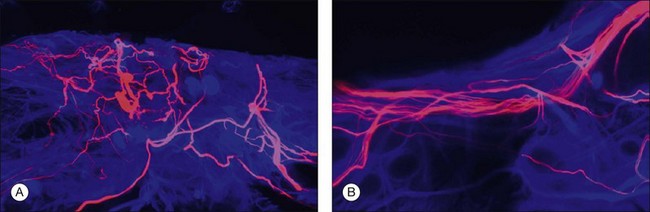
Retinal reattachment may also stimulate the formation of scar tissue. The development of PVR following retinal reattachment surgery remains the major cause of surgical failure. In the feline model, reattachment induces growth into the vitreous of Müller cell processes, which form epiretinal membranes.19,33 These have a different structure and a different intermediate filament composition compared to those that grow into the subretinal space.35 The initial outgrowths into the vitreous occur as thin “wispy” extensions of the endfoot region and have an intermediate filament population that is dominated by GFAP instead of vimentin. These Müller cell processes then act as a substrate for ganglion cell growth and neurite extension.33,58,59 In humans and animal models rod axons are also stimulated to grow by retinal reattachment, and this may be seen as neurite extensions into the inner retina and beyond to the vitreal surface within epiretinal membranes (Fig. 29.12).6,19,58
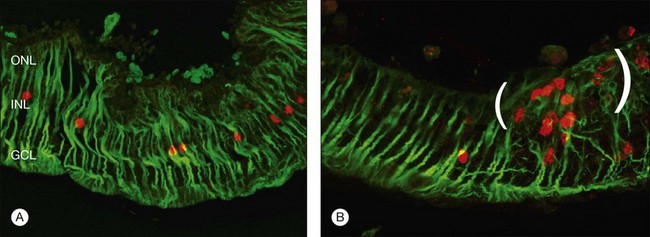
Restoration of retinal anatomy following detachment is not uniform. The morphological appearance has been described as a “patchwork,” with areas of variability in outer-segment length and levels of protein expression within areas of the same retina (Fig. 29.13).19,55 There is also variability between different retinas.19,55 Although this may reflect the variations in the extent (detachment height) and duration of detachment seen clinically in humans, this patchwork appearance is still observed in controlled detachment experiments. In the feline model, in areas where the apposition of the retina with the apical surface of the RPE is restored, photoreceptors are quick to regenerate. In some areas where RPE cells may have proliferated with rounded apical surfaces and/or reversal of cellular polarity, they bear little resemblance to normal RPE. Here photoreceptor outer-segment regeneration following retinal reattachment is far more likely to be abnormal.60 In areas where photoreceptor recovery is poor, microglia remain activated, suggesting that they may play a role in modulating recovery.28 Similarly, in areas where Müller cell hypertrophy has led to the formation of a subretinal scar, photoreceptors only show limited signs of recovery with no outer-segment regeneration.
At present it seems reasonable to conclude that a return towards completely normal retinal morphology occurs gradually over a timespan of months or years, even after brief episodes of detachment.50,57 Incomplete morphologic recovery may, however, be sufficient to subserve near-normal vision, although functional correlates in humans are lacking.
1 Fisher SK, Lewis GP, Linberg KA, et al. Cellular remodelling in mammalian retina: results from studies of experimental retinal detachment. Prog Retin Eye Res. 2005;24:395–431.
2 Burton TC. Recovery of visual acuity after retinal detachment involving the macula. Trans Am Ophthalmol Soc. 1982;80:475–497.
3 Lewis GP, Charteris DG, Sethi CS, et al. The ability of rapid retinal reattachment to stop or reverse the cellular and molecular events initiated by detachment. Invest Ophthalmol Vis Sci. 2002;43:2412–2420.
4 Wickham L, Lewis GP, Charteris DG, et al. Histological analysis of retinas sampled during translocation surgery: a comparison with normal and transplantation retinas. Br J Ophthalmol. 2009;93:969–973.
5 Wickham L, Sethi CS, Lewis GP, et al. Glial and neural response in short-term human retinal detachment. Arch Ophthalmol. 2006;124:1779–1782.
6 Sethi CS, Lewis GP, Fisher SK. Glial remodelling and neuronal plasticity in human retinal detachment with proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci. 2005;46:329–342.
7 Wilson DJ, Green WR. Histopathological study of the effect of retinal detachment surgery on 49 eyes obtained post mortem. Am J Ophthalmol. 1987;103:167–179.
8 Chang CJ, Lai WW, Edward DP, et al. Apoptotic photoreceptor cell death after traumatic retinal detachment in humans. Arch Ophthalmol. 1995;113:880–886.
9 Geller SF, Lewis GP, Fisher SK. FGFR1 signaling and AP-1 expression after retinal detachment: Reactive Müller and RPE cells. Invest Ophthalmol Vis Sci. 2001;42:1363–1369.
10 Anderson DH, Stern WH, Fisher SK, et al. Retinal detachment in the cat: The pigment epithelial–photoreceptor interface. Invest Ophthalmol Vis Sci. 1983;24:906–926.
11 Campochiaro PA, Jerdan JA, Glaser BM. The extracellular matrix of human retinal pigment epithelial cells in vivo and its synthesis in vitro. Invest Ophthalmol Vis Sci. 1986;27:1615–1621.
12 Steinberg RH, Wood I. The relationship of the retinal pigment epithelium to photoreceptor outer segments in the human retina. In: Zimm KM, Marmor ME. The retinal pigment epithelium. Cambridge, MA: Harvard University Press, 1979.
13 Fisher SK, Anderson DH. Cellular responses of the RPE to retinal detachment and reattachment. In: Marmor MF, Wolfensberger TJ. The retinal pigment epithelium. Oxford: Oxford University Press, 1999.
14 Immel J, Negi A, Marmor MF. Acute changes in RPE apical morphology after retinal detachment in rabbit. A SEM study. Invest Ophthalmol Vis Sci. 1986;27:1770–1776.
15 Anderson DH, Stern WH, Fisher SK, et al. The onset of pigment epithelial proliferation after retinal detachment. Invest Ophthalmol Vis Sci. 1981;21:10–16.
16 Fisher SK, Erickson PA, Lewis GP, et al. Intraretinal proliferation induced by retinal detachment. Invest Ophthalmol Vis Sci. 1991;32:1739–1748.
17 Geller SF, Lewis GP, Anderson DH, et al. Use of MB1 antibody for detecting proliferating cells in the retina. Invest Ophthalmol Vis Sci. 1995;36:737–744.
18 Johnson NF, Foulds WS. Observations on the retinal pigment epithelium and retinal macrophages in experimental retinal detachment. Br J Ophthalmol. 1977;61:564–572.
19 Lewis GP, Sethi CS, Linberg KA, et al. Experimental retinal reattachment – a new perspective. Mol Neurobiol. 2003;28:159–175.
20 Fariss RN, Molday RS, Fisher SK, et al. Evidence from normal and degenerating photoreceptors that two outer segment integral membrane proteins have separate transport pathways. J Comp Neurol. 1997;387:148–156.
21 Rex TS, Fariss RN, Lewis GP, et al. A survey of molecular expression by photoreceptors after experimental retinal detachment. Invest Ophthalmol Vis Sci. 2002;43:1234–1247.
22 Mervin K, Valter K, Maslim J, et al. Limiting photoreceptor death and deconstruction during experimental retinal detachment: the value of oxygen supplementation. Am J Ophthalmol. 1999;128:155–164.
23 Rodieck RW. The vertebrate retina. San Francisco: W H Freeman; 1973.
24 Cook B, Lewis GP, Fisher SK, et al. Apoptotic photoreceptor degeneration in experimental retinal detachment. Invest Ophthalmol Vis Sci. 1995;36:990–996.
25 Zacks DN, Hanninen V, Pantcheva M, et al. Caspase activation in an experimental model of retinal detachment. Invest Ophthalmol Vis Sci. 2003;44:1262–1267.
26 Murakami Y, Miller JW, Vavvas DG. RIP kinase-mediated necrosis as an alternative mechanism of photoreceptor death. Oncotarget. 2011;2:497–509.
27 Arroyo JG, Yang L, Bula D, et al. Photoreceptor apoptosis in human retinal detachment. Am J Ophthalmol. 2005;139:605–610.
28 Lewis GP, Sethi CS, Carter KM, et al. Microglial cell activation following retinal detachment: a comparison between species. Mol Vis. 2005;11:491–500.
29 Lewis GP, Linberg KA, Fisher SK. Neurite outgrowth from bipolar and horizontal cells after experimental retinal detachment. Invest Ophthalmol Vis Sci. 1998;39:424–434.
30 Linberg KA, Lewis GP, Fisher SK. Retraction and remodeling of rod spherules are early events following experimental retinal detachment: an ultrastructural study using serial sections. Mol Vis. 2009;15:10–25.
31 Erickson PA, Fisher SK, Anderson DH, et al. Retinal detachment in the cat: the outer nuclear and outer plexiform layers. Invest Ophthalmol Vis Sci. 1983;24:927–942.
32 Coblentz FE, Radeke MJ, Lewis GP, et al. Evidence that ganglion cells react to retinal detachment. Exp Eye Res. 2003;76:333–342.
33 Fisher SK, Lewis GP. Mueller cell and neuronal remodelling in retinal detachment and reattachment and their potential consequences for visual recovery: a review and reconsideration of recent data. Vision Res. 2003;43:887–897.
34 Lewis GP, Chapin EA, Luna G, et al. The fate of Müller’s glia following experimental retinal detachment: nuclear migration, cell division, and subretinal glial scar formation. Mol vis. 2010;16:1361–1372.
35 Lewis GP, Fisher SK. Upregulation of glial fibrillary acidic protein in response to retinal injury: its potential role in glial remodelling and a comparison of vimentin expression. Int Rev Cytol. 2003;230:263–290.
36 Lewis GP, Linberg KA, Geller SF, et al. Effects of the neurotrophin brain-derived neurotrophic factor in an experimental model of retinal detachment. Invest Ophthalmol Vis Sci. 1999;40:1530–1544.
37 Faktorovich EG, Steinberg RH, Yasumura D, et al. Basic fibroblast growth factor and local injury protect photoreceptors from light damage in the rat. J Neurosci. 1992;12:3554–3567.
38 LaVail MM, Unoki K, Yasumura D, et al. Multiple growth factors, cytokines, and neurotrophins rescue photoreceptors from the damaging effects of constant light. Proc Natl Acad Sci U S A. 1992;89:11249–11253.
39 LaVail MM, Yasumura D, Matthes MT, et al. Protection of mouse photoreceptors by survival factors in retinal degenerations. Invest Ophthalmol Vis Sci. 1998;39:592–602.
40 Roh MI, Murakami Y, Thanos A, et al. Edaravone, an ROS scavenger, ameliorates photoreceptor cell death after experimental retinal detachment. Invest Ophthalmol Vis Sci. 2011;52:3825–3831.
41 Yang L, Kim JH, Kovacs KD, et al. Minocycline inhibition of photoreceptor degeneration. Arch Ophthalmol. 2009;127:1475–1480.
42 Besirli CG, Chinskey ND, Zheng QD, et al. Inhibition of retinal detachment-induced apoptosis in photoreceptors by a small peptide inhibitor of the Fas receptor. Invest Ophthalmol Vis Sci. 2010;51:2177–2184.
43 Lewis GP, Talaga KC, Linberg KA, et al. The efficacy of delayed oxygen therapy in the treatment of experimental retinal detachment. Am J Ophthalmol. 2004;137:1085–1095.
44 Sakai T, Lewis GP, Linberg KA, et al. The ability of hyperoxia to limit the effects of experimental detachment in cone-dominated retina. Invest Ophthalmol Vis Sci. 2001;42:3264–3273.
45 Linberg KA, Sakai T, Lewis GP, et al. Experimental retinal detachment in the cone-dominant ground squirrel retina: morphology and basic immunocytochemistry. Vis Neurosci. 2002;19:603–619.
46 Xu GZ, Li WW, Tso MO. Apoptosis in human retinal degenerations. Trans Am Ophthalmol Soc. 1996;94:411–430.
47 Charteris DG, Downie J, Aylward GW, et al. Intraretinal and periretinal pathology in anterior proliferative vitreoretinopathy. Graefe Arch Clin Exp Ophthalmol. 2007;245:93–100.
48 Lewis GP, Fisher SK. Müller cell outgrowth after retinal detachment: association with cone photoreceptors. Invest Ophthalmol Vis Sci. 2000;41:1542–1545.
49 Wickham L, Ho-Yen GO, Bunce C, et al. Surgical failure following primary retinal detachment surgery by vitrectomy: risk factors and functional outcomes. Br J Ophthalmol. 2011;95:1234–1238.
50 Kusaka S, Toshino A, Ohashi Y, et al. Long-term visual recovery after scleral buckling for macular-off retinal detachments. Jpn J Ophthalmol. 1998;42:218–222.
51 Sullivan P, Luff AJ, Julious SA, et al. Patient satisfaction following vitreoretinal surgery. Eye. 1993;7:433–435.
52 Lewis GP, Erickson PA, Guerin CJ, et al. Basic fibroblast growth factor: a potential regulator of proliferation and intermediate filament expression in the retina. J Neurosci. 1992;12:3968–3978.
53 Lewis GP, Fisher SK, Anderson DH. Fate of biotinylated basic fibroblast growth factor in the retina following intravitreal injection. Exp Eye Res. 1996;62:309–324.
54 Bunt-Milam AH, Saari JC. Immunocytochemical localization of two retinoid-binding proteins in vertebrate retina. J Cell Biol. 1983;97:703–712.
55 Lewis GP, Charteris DG, Sethi CS, et al. Animal models of retinal detachment and reattachment: identifying cellular events that may affect visual recovery. Eye. 2002;16:375–387.
56 Guerin CJ, Lewis GP, Fisher SK, et al. Recovery of photoreceptor outer segment length and analysis of membrane assembly rates in regenerating primate photoreceptor outer segments. Invest Ophthalmol Vis Sci. 1993;34:175–183.
57 Anderson DH, Guerin CJ, Erickson PA, et al. Morphological recovery in the reattached retina. Invest Ophthalmol Vis Sci. 1986;27:168–183.
58 Lewis GP, Betts KE, Sethi CS, et al. Identification of ganglion cell neurites in human subretinal and epiretinal membranes. Br J Ophthalmol. 2007;91:1234–1238.
59 Lesnik Oberstein SY, Lewis GP, Chapin EA, et al. Ganglion cell neurites in human idiopathic epiretinal membranes. Br J Ophthalmol. 2008;92:981–985.
60 Guerin CJ, Anderson DH, Fariss RN, et al. Retinal reattachment of the primate macula: photoreceptor recovery after short-term retinal detachment. Invest Ophthalmol Vis Sci. 1989;30:1708–1725.