Cell Wall–Deficient Bacteria
Mycoplasma and Ureaplasma
1. Describe the general characteristics of the Mycoplasmataceae, including microscopic and macroscopic appearance.
2. Identify key characteristic biochemical reactions for the differentiation of pathogenic Mycoplasma spp.
3. Explain the difficulties associated with isolation of these fastidious organisms, including nutritional requirements, immune response, cellular locations, and incubation requirements (length of time, temperature, and oxygenation requirements).
4. Compare the clinical presentation of M. pneumoniae to S. pneumoniae.
5. Describe the proper processing, collection, transport, and storage of specimens for the isolation of the organism discussed in this chapter.
6. State the site for colonization in the human host for M. genitalium, M. hominis, M. pneumoniae, and U. urealyticum.
7. Describe the clinical manifestations associated with each of the major species considered in this chapter.
8. Describe the complications of serologic diagnosis related to the variation in antibody formation in infection with M. pneumoniae.
9. Explain the current limitations and recommendations associated with susceptibility testing related to the Mycoplasmataceae.
10. Correlate signs and symptoms and evaluate laboratory data associated with the diagnosis of infections caused by the major pathogens discussed in this chapter.
General Characteristics
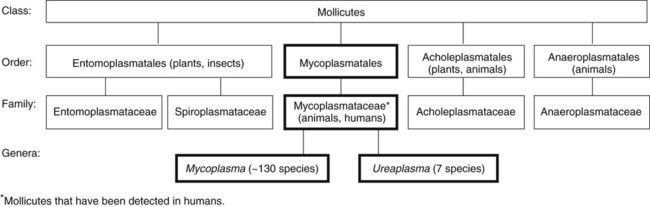
Organisms in this chapter belong to the class Mollicutes (Latin, meaning soft skin). This class comprises four orders, which, in turn, contain five families and eight genera (Figure 45-1). The mycoplasmas that colonize or infect humans belong to the family Mycoplasmataceae; this family comprises two genera, Mycoplasma and Ureaplasma. These organisms are highly fastidious, are slow growing, and most are facultative anaerobes that require nucleic acid precursor molecules, fatty acids, and sterols such as cholesterol for growth. These bacteria have a very small cell size (0.3 × 0.8 µm) and small genome. The Mollicutes appear most closely related to the gram-positive bacterial subgroup that includes bacilli, streptococci, and lactobacteria that diverged from the Streptococcus branch of gram-positive bacteria.
Epidemiology and Pathogenesis
Epidemiology
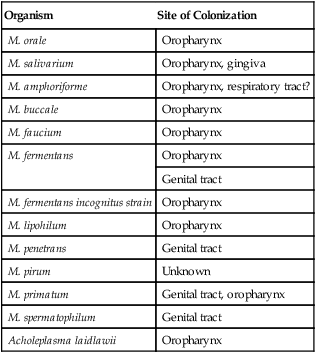
The mycoplasmas usually considered as commensals are listed in Table 45-1, along with their respective sites of colonization. These organisms may be transmitted by direct sexual contact, transplanted tissue from donor to recipient, or from mother to fetus during childbirth or in utero. M. pneumoniae may be transmitted by respiratory secretions. One species of Acholeplasma (these organisms are widely disseminated in animals), Acholeplasma laidlawii, has been isolated from the oral cavity of humans a limited number of times; however, the significance of these mycoplasmas and their colonization of humans remains uncertain.
TABLE 45-1
Mycoplasmas That Are Considered Normal Flora of the Oropharynx or Genital Tract
| Organism | Site of Colonization |
| M. orale | Oropharynx |
| M. salivarium | Oropharynx, gingiva |
| M. amphoriforme | Oropharynx, respiratory tract? |
| M. buccale | Oropharynx |
| M. faucium | Oropharynx |
| M. fermentans | Oropharynx |
| Genital tract | |
| M. fermentans incognitus strain | Oropharynx |
| M. lipohilum | Oropharynx |
| M. penetrans | Genital tract |
| M. pirum | Unknown |
| M. primatum | Genital tract, oropharynx |
| M. spermatophilum | Genital tract |
| Acholeplasma laidlawii | Oropharynx |
M. pneumoniae is a cause of community-acquired atypical pneumonia, often referred to as walking pneumonia (see Chapter 69); infections caused by this agent are distributed worldwide, with an estimated 2 million cases per year in the United States. M. pneumoniae infection may also result in bronchitis or pharyngitis. M. pneumoniae may be transmitted person-to-person by respiratory secretions as previously stated or indirectly by inanimate objects contaminated with respiratory secretions (fomites). Infections can occur singly or as outbreaks in closed populations such as families and military recruit camps. Pneumonia caused by M. pneumoniae may present as asymptomatic to mild disease, with early nonspecific symptoms including malaise, fever, headache, sore throat, earache, and nonproductive cough. This differs significantly from the classic symptoms associated with pneumonia as a result of infection with Streptococcus pneumoniae (see Chapters 15 and 69). M. pneumoniae strongly attaches to the mucosal cells and may reside intracellularly within host cells, resulting in a chronic persistent infection that may last for months to years. The infections do not follow seasonal patterns as seen with influenzae and other respiratory pathogens. Besides respiratory infection, M. pneumoniae can cause extrapulmonary manifestations such as pericarditis, hemolytic anemia, arthritis, nephritis, Bell’s palsy, and meningoencephalitis resulting in various additional forms of paralysis.
Spectrum of Disease
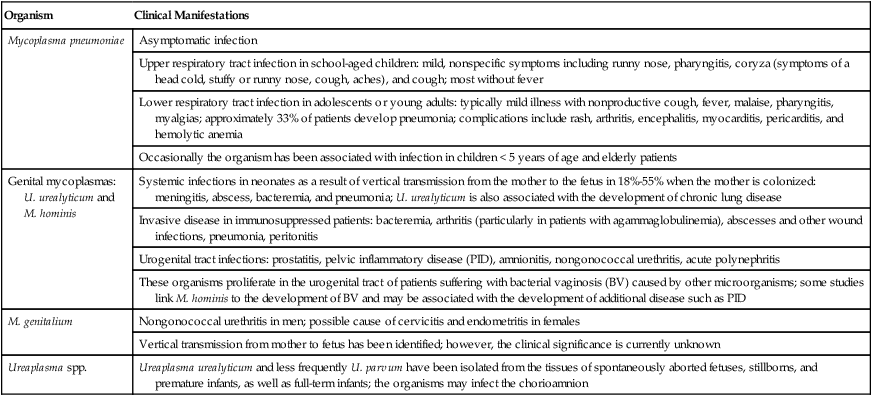
The clinical manifestations of infections caused by M. pneumoniae and the pathogenic genital mycoplasmas, U. urealyticum, U. parvum, M. hominis, and M. genitalium are summarized in Table 45-2.
TABLE 45-2
| Organism | Clinical Manifestations |
| Mycoplasma pneumoniae | Asymptomatic infection |
| Upper respiratory tract infection in school-aged children: mild, nonspecific symptoms including runny nose, pharyngitis, coryza (symptoms of a head cold, stuffy or runny nose, cough, aches), and cough; most without fever | |
| Lower respiratory tract infection in adolescents or young adults: typically mild illness with nonproductive cough, fever, malaise, pharyngitis, myalgias; approximately 33% of patients develop pneumonia; complications include rash, arthritis, encephalitis, myocarditis, pericarditis, and hemolytic anemia | |
| Occasionally the organism has been associated with infection in children < 5 years of age and elderly patients | |
| Genital mycoplasmas: U. urealyticum and M. hominis | Systemic infections in neonates as a result of vertical transmission from the mother to the fetus in 18%-55% when the mother is colonized: meningitis, abscess, bacteremia, and pneumonia; U. urealyticum is also associated with the development of chronic lung disease |
| Invasive disease in immunosuppressed patients: bacteremia, arthritis (particularly in patients with agammaglobulinemia), abscesses and other wound infections, pneumonia, peritonitis | |
| Urogenital tract infections: prostatitis, pelvic inflammatory disease (PID), amnionitis, nongonococcal urethritis, acute polynephritis | |
| These organisms proliferate in the urogenital tract of patients suffering with bacterial vaginosis (BV) caused by other microorganisms; some studies link M. hominis to the development of BV and may be associated with the development of additional disease such as PID | |
| M. genitalium | Nongonococcal urethritis in men; possible cause of cervicitis and endometritis in females |
| Vertical transmission from mother to fetus has been identified; however, the clinical significance is currently unknown | |
| Ureaplasma spp. | Ureaplasma urealyticum and less frequently U. parvum have been isolated from the tissues of spontaneously aborted fetuses, stillborns, and premature infants, as well as full-term infants; the organisms may infect the chorioamnion |
Data from Versalovic J: Manual of clinical microbiology, ed 10, Washington, DC, 2011, ASM Press.
Laboratory Diagnosis
Specimen Collection, Transport, and Processing
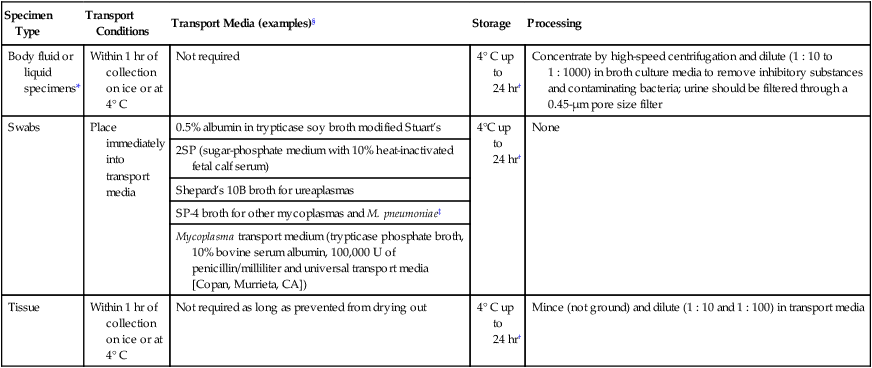
Because mycoplasmas have no cell wall, they are highly susceptible to drying; therefore, transport media are necessary, particularly when specimens are collected on swabs. Liquid specimens such as body fluids do not require transport media if inoculated to appropriate media within 1 hour of collection. Tissues should be kept moist; if a delay in processing is anticipated, they should also be placed in transport media. Specific media for the isolation of Mycoplasma spp. include those containing 10% heat-inactivated calf serum containing 0.2 M sucrose in a 0.02 M phosphate buffer, pH 7.2, such as SP4, Shepard’s 10B broth or 2 SP. Additional commercial media available for cultivation of these organisms include Stuart’s medium, trypticase soy broth supplemented with 0.5% bovine serum albumin, Mycotrans (Irvine Scientific, Irvine, California), and A3B broth (Remel, Inc.). Excessive delays in processing can result in decreased viability and recovery of organisms from clinical specimens. If the storage time is expected to exceed 24 hours prior to cultivation, the samples should be placed in transport media and frozen at −80°C. Frozen samples should be thawed in a hot water bath at 37°C. Transport and storage conditions of various types of specimens are summarized in Table 45-3.
TABLE 45-3
Transport and Storage Conditions for Mycoplasma pneumoniae, Ureaplasma urealyticum, and M. hominis
| Specimen Type | Transport Conditions | Transport Media (examples)§ | Storage | Processing |
| Body fluid or liquid specimens* | Within 1 hr of collection on ice or at 4° C | Not required | 4° C up to 24 hr† | Concentrate by high-speed centrifugation and dilute (1 : 10 to 1 : 1000) in broth culture media to remove inhibitory substances and contaminating bacteria; urine should be filtered through a 0.45-µm pore size filter |
| Swabs | Place immediately into transport media | 0.5% albumin in trypticase soy broth modified Stuart’s | 4°C up to 24 hr† | None |
| 2SP (sugar-phosphate medium with 10% heat-inactivated fetal calf serum) | ||||
| Shepard’s 10B broth for ureaplasmas | ||||
| SP-4 broth for other mycoplasmas and M. pneumoniae‡ | ||||
| Mycoplasma transport medium (trypticase phosphate broth, 10% bovine serum albumin, 100,000 U of penicillin/milliliter and universal transport media [Copan, Murrieta, CA]) | ||||
| Tissue | Within 1 hr of collection on ice or at 4° C | Not required as long as prevented from drying out | 4° C up to 24 hr† | Mince (not ground) and dilute (1 : 10 and 1 : 100) in transport media |
†Can be stored indefinitely at -80° C if diluted in transport media following centrifugation.
‡SP-4 broth: sucrose phosphate buffer, 20% horse serum, Mycoplasma base, and neutral red.
§Not a complete list. A variety of commercial media is available.
Direct Detection Methods
Molecular Diagnostics
Several amplification methods, such as polymerase chain reaction (PCR), have been developed for the detection of the clinically relevant Mycoplasma and Ureaplasma species. Various targets including 16srRNA sequences, insertion sequences, and organism specific genes have been used in the development of these assays. As a result of the fast turnaround time, specificity, and lack of need to cultivate fastidious organisms, PCR amplification for the diagnosis of these organisms is particularly attractive. When considering the use of molecular amplification methods for the detection of infectious diseases, it is important to note that although an organism is detectable, the patient’s signs and symptoms must be correlated with the identified agent. It is possible to detect an organism by one method and not another—in other words, a patient may be PCR positive but culture negative or serologically negative for a Mycoplasma based on the patient’s response to infection and current disease manifestation. Chapter 8 provides a more detailed description of the advantages, limitations, and methods used in the development of amplification assays. Multiplexed real-time PCR assays that detect M. pneumoniae as well as other atypical respiratory tract pathogens such as Chlamydophila pneumoniae and Legionella pneumoniae have been developed.
Cultivation
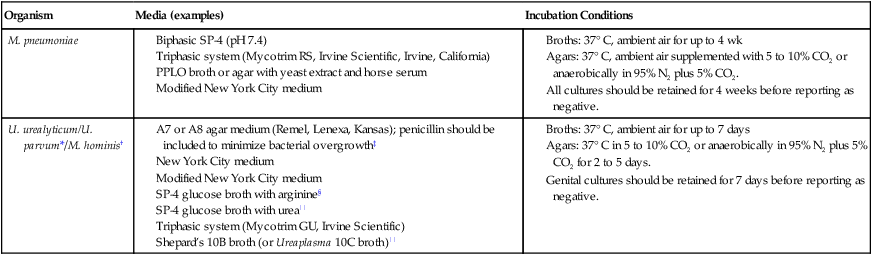
In general, the medium for mycoplasma isolation contains a beef or soybean protein with serum, fresh yeast extract, and other factors. As a result of the slow growth of these organisms, the medium must be selective to prevent overgrowth of faster-growing organisms that may be present in a clinical sample. Culture media and incubation conditions for these organisms are summarized in Table 45-4. Culture methods for M. pneumoniae, U. urealyticum, and M. hominis are provided on the Evolve site in Procedures 45-1, 45-2, and 45-3, respectively. The quality control of the growth media with a fastidious isolate is of great importance.
TABLE 45-4
Cultivation of Mycoplasma pneumoniae, Ureaplasma spp., and M. hominis
| Organism | Media (examples) | Incubation Conditions |
| M. pneumoniae |
*Utilizes urea and requires acidic medium.
†Converts arginine to ornithine and grows over a broad pH range.
||For U. urealyticum isolation.
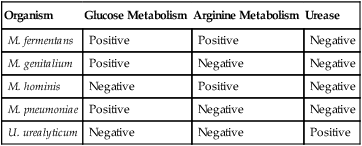
For the most part, the different metabolic activity of the mycoplasmas for different substrates is used to detect their growth. Glucose (dextrose) is incorporated into media selective for M. pneumoniae, because this mycoplasma ferments glucose to lactic acid; the resulting pH change is then detected by a color change in a dye indicator. Similarly, urea or arginine can be incorporated into media to detect U. urealyticum and M. hominis, respectively (Table 45-5). If a color change—that is, a pH change—is detected, a 0.1- to 0.2-mL aliquot is immediately subcultured to fresh broth and agar media.
TABLE 45-5
Basic Biochemical Differentiation of the Major Mycoplasma spp. and Ureaplasma urealyticum
| Organism | Glucose Metabolism | Arginine Metabolism | Urease |
| M. fermentans | Positive | Positive | Negative |
| M. genitalium | Positive | Negative | Negative |
| M. hominis | Negative | Positive | Negative |
| M. pneumoniae | Positive | Negative | Negative |
| U. urealyticum | Negative | Negative | Positive |
Approach to Identification
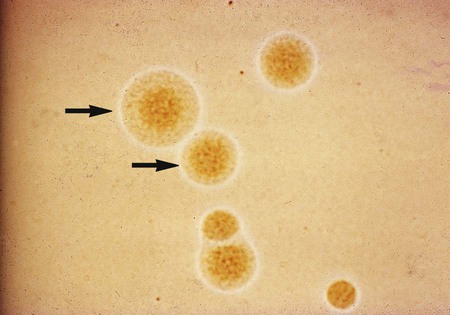
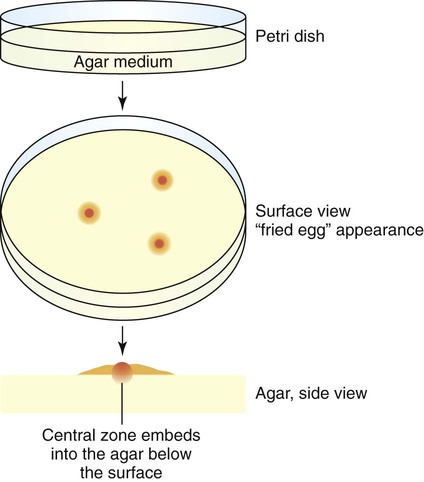
On agar, M. pneumoniae will appear as spherical, grainy, yellowish forms that are embedded in the agar, with a thin outer layer similar to those shown in Figure 45-2. The agar surface is examined under 20 to 60× magnification using a stereomicroscope daily for Ureaplasma spp., at 24 to 72 hours for M. hominis, and every 3 to 5 days for M. pneumoniae and other slow-growing species. Because only M. pneumoniae and one serovar of U. urealyticum hemadsorb, M. pneumoniae is definitively identified by overlaying suspicious colonies with 0.5% guinea pig erythrocytes in phosphate-buffered saline instead of water. After 20 to 30 minutes at room temperature, colonies are observed for adherence of red blood cells.
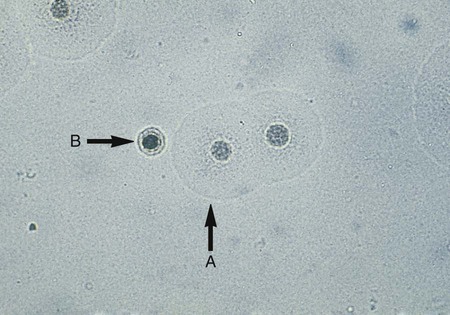
Cultures for the genital mycoplasmas are handled in a similar fashion, including culture examination and the requirement for subculturing. Colonies may be definitively identified on A8 agar as U. urealyticum by urease production in the presence of a calcium chloride indicator. U. urealyticum colonies (15 to 60 µm in diameter) will appear as dark brownish clumps. Colonies that are typical in appearance for U. urealyticum are shown in Figure 45-3. M. hominis are large (about 20 to 300 µm in diameter) and are urease negative (see Figure 45-3), with a characteristic “fried egg” appearance (Figure 45-4). On conventional blood agar, strains of M. hominis, but not of U. urealyticum, produce nonhemolytic, pinpoint colonies that do not stain with Gram stain. These colonies can be stained with the Dienes or acridine orange stains. Numerous transport and growth media systems for the detection, quantitation, identification, and antimicrobial susceptibility testing of the genital mycoplasmas are commercially available in the United States and Europe.