Chapter 129 Cavernous Hemangioma
Clinical findings
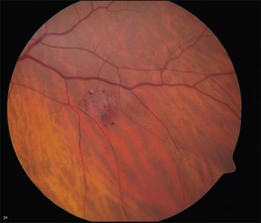
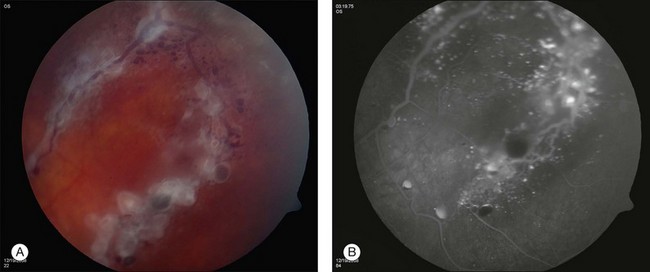
Retinal cavernous hemangiomas are composed of clusters of saccular aneurysms filled with dark blood. There is no intervening retinal tissue between the aneurysms. The aneurysms range in size from microaneurysms to a half-disc diameter.1 The typical tumor is isolated, one to two disc diameters in size, and resembles an intraretinal cluster of grapes (Fig. 129.1, see also Fig. 129.3).2,3 However, the clinical appearance can be quite variable; ranging from a single aneurysm to a wide distribution over the entire fundus or following the course of a major vein (see Fig. 129.5). Commonly, layering of the red blood cells within the aneurysms causes a plasma–erythrocytic separation that has been called a “pseudohypopyon.” Often a white or gray fibroglial membrane covers the surface of the tumor (see Fig. 129.5A). The adjacent retinal blood vessels appear unaffected by the tumor, with no evidence of feeder vessels. Although a mild lipid exudate has been reported adjacent to the hemangioma in one case,4 exudation from the tumor is extremely rare either at presentation or with long-term follow-up. The slow circulation through the aneurysms is responsible for the lack of exudation of lipid or fluid.
Retinal cavernous hemangiomas are symptomatic when they are located in or adjacent to the macula which has been reported in approximately 10% of the cases in the literature.1,5,6 In these cases, visual acuity can be decreased on the basis of both tumor location and amblyopia. Visual disturbance can also result from vitreous hemorrhage. Cavernous hemangioma can cause simultaneous subretinal, intraretinal, and preretinal hemorrhage (Fig. 129.2). Although vitreous hemorrhage has been reported in 10% of retinal cavernous hemangiomas, it is usually old, minimal, and unassociated with long-term loss of visual acuity.1 However, vitreous hemorrhage, if it occurs in a child, may cause amblyopia.7
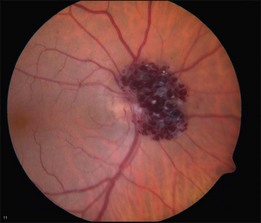
Cavernous hemangiomas have also been reported to be located on the optic nerve head.8–10 These tumors have a clinical appearance similar to the retinal lesions (Fig. 129.3). An enlarged blind spot may be demonstrated on the visual field, but visual acuity is normal. Gündüz et al.11 described a patient with a cavernous hemangioma in the superonasal quadrant of the fundus and unilateral decreased visual acuity, with associated red–green color defect. Cone response and 30 Hz flicker responses were nearly absent.
Differential diagnosis
The characteristic appearance of a retinal cavernous hemangioma is rarely confused with other conditions. Retinal telangiectasis can have a similar appearance, and several authors have suggested that older cases of cavernous hemangioma were mistakenly called Coats disease or Leber miliary aneurysms.3,8,12 The lack of intraretinal exudate distinguishes this tumor from Coats disease, but the differentiation from Leber can be more difficult. Gass points out that the retinal telangiectasis of Leber is a progressive condition affecting the integrity and structure of the “intrinsic” vasculature, whereas the cavernous hemangioma is a sessile tumor projecting from and partly isolated from the retinal blood vessels.8 On the other hand, Giuffre13 reported a case with a cavernous hemangioma and retinal telangiectasis closely located in the same eye, suggesting a relation between these two developmental conditions. Telangiectatic or microaneurysmal vascular dilation can also be seen as late sequelae in branch vein occlusions and diabetic retinopathy but should be easily differentiated from retinal cavernous hemangioma by the associated features.
Ancillary studies
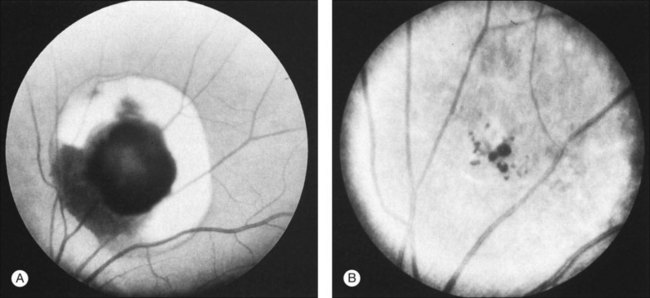
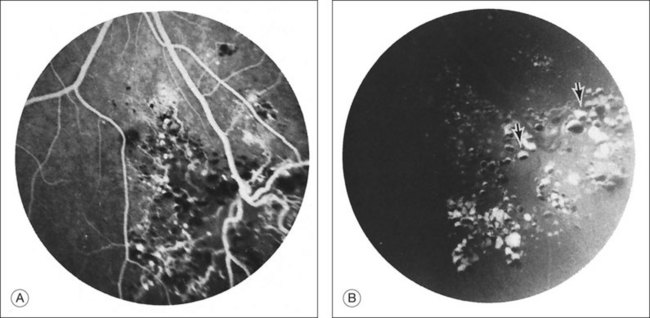
Fluorescein angiography may show autofluorescence of the gray-white epiretinal membrane overlying the tumor. The aneurysms will fill slowly and often incompletely up to 30 minutes after dye injection. Dye will rarely extravasate from the tumor in the late phase of the angiogram. In addition, the plasma–erythrocytic separation often seen on clinical examination is dramatically demonstrated in the later phases of the angiogram. The fluorescein collects in the superior portion of the aneurysm, with the gravitated blood cells blocking its accumulation in the inferior area (Figs 129.4 and 129.5B).
Although performing echography is rarely necessary, the ultrasonographic features have been described by Shields3 in cases in which diagnosis was rendered difficult by vitreous hemorrhage. A-scan shows a high initial spike and high internal reflectivity. B-scan shows an irregular surface, large internal acoustic density, and absence of choroidal excavation. Many cavernous hemangiomas are too small to exhibit a characteristic echographic pattern.
Natural history
Growth of a retinal cavernous hemangioma is exceedingly rare. Klein et al.4 reported the only case of enlargement, and this developed after photocoagulation. Messmer et al.1 noted enlargement of the fibroglial component, which may represent progressive thrombosis of some of the aneurysms. In a later report, Kushner et al.14 monitored two patients over 5–10 years and reported definitive growth.
Visual loss can occur from growth of an associated epiretinal membrane. In addition, macular pucker has been reported separate from the membrane overlying the hemangioma; the membrane and the tumor in this case were probably unrelated.1 Vitreoretinal or vitreopapillary traction may occur occasionally.15
Vitreous hemorrhage is the most common cause of progressive visual disturbance in patients with cavernous hemangioma. Although usually mild due to slow blood flow through the hemangioma, isolated cases of severe hemorrhage have been reported.16–18 Impairment of visual acuity from vitreous hemorrhage is a very uncommon event.
Treatment
Because cavernous hemangiomas rarely increase in size or cause lipid exudation or severe vitreous hemorrhage, these tumors generally do not require treatment. Photocoagulation or cryotherapy has been advocated if vitreous hemorrhage occurs. However, Gass8 has reported one case of vitreous hemorrhage following xenon photocoagulation, and Klein et al.4 described a patient in whom the hemangioma enlarged greatly after xenon treatment. The value of laser treatment or cryopexy remains unproven and periodic observation is standard care. However, for rare cases of nonclearing vitreous hemorrhage, vitrectomy has proved to be effective. Haller and Knox18 reported a case of dense, nonclearing vitreous hemorrhage due to persistent vitreous traction to a cavernous hemangioma of the optic disc. During vitrectomy, the vitreopapillary traction was relieved and the preretinal portion of the hamartoma was excised. Minimal bleeding occurred and was easily controlled with intraocular diathermy. Vision improved from light perception to 20/40.
Pathology
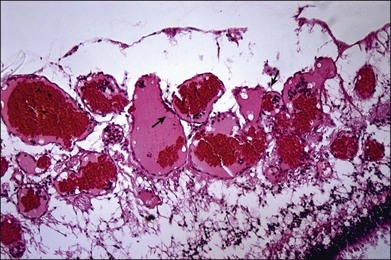
Histopathologic study of retinal cavernous hemangioma demonstrates a tumor arising from the inner half of the retina (Fig. 129.6).19 The tumor comprises of multiple endothelial-lined, thin-walled aneurysms. These aneurysms are separated by thin, fibrous septa. There is no evidence of intraretinal or subretinal exudate.
The cavernous hemangioma is a localized vascular hamartoma arising from the capillaries and is partly isolated from the normal vascular tree as seen on fluorescein angiography.20 Electron microscopy performed on an eye enucleated for leukocoria in a 6-month-old infant showed the aneurysms to be lined by nonfenestrated, flat endothelial cells with terminal bars on the luminal side and a thin basement membrane.21 Pericytes were also present. The vessels were surrounded by processes of fibrous astrocytes invested by a basal lamina. These features of normal retinal vessels in association with the slow flow through the vascular abnormality probably account for the lack of exudates and hemorrhage.20
Systemic and familial involvement
Reports of several individuals and pedigrees with retinal cavernous hemangiomas, as well as cutaneous and central nervous system angiomatous lesions led to the concept that this entity is part of a neuro-oculocutaneous syndrome. Weskamp and Cotlier22 first reported such a patient with occipital cutaneous angiomas, intracranial calcifications, and seizures who turned out to have a cavernous hemangioma in the pre-Rolandic area of the cerebral cortex diagnosed at the time of craniotomy. Subsequently, several familial cases have been seen, and it is now established that the incidence is higher in Hispanic Americans than non Hispanic Americans.6
A variety of cutaneous lesions has been reported in association with this tumor.23–26 Most commonly, patients have cutaneous capillary malformations on the back of the neck. However, Schwartz et al.25 reported a patient with cutaneous angiomas on the abdomen, and Mildner discussed a 14-year-old with a cavernous hemangioma of the chest.24 In addition, Gautier-Smith et al.23 have reported an association with angioma serpiginosum, a progressive condition with widespread dilation of subpapillary venous plexuses. These cutaneous lesions are associated with motor and sensory loss and have peripheral nerve involvement.23–26
Angiomas of the central nervous system are the more consistent association with retinal cavernous hemangiomas. In fact, cerebro cavernous malformations (CCMs) are far more common than retinal cavernomas. The overall incidence of cerebro cavernous hemangioma is between 0.1% and 0.5% in the general population. Only 5% of these patients have an associated retinal hemangioma. Among those with CCMs, the proportion of familial cases has been estimated to be as high as 50% in Hispanic American patients and 10–40% in other populations. In addition to the patient reported by Weskamp and Cotlier,22 Gass8 has reported one patient with a retinal cavernous hemangioma and seizures whose father had cavernous hemangiomas of the midbrain, pons, and cerebellum. He reported another patient with a retinal cavernous hemangioma, a cutaneous angioma, and seizures who had a child who died after excision of the cavernous hemangioma of the third and lateral ventricles.20 Schwartz et al.25 reported a patient with a retinal cavernous hemangioma, cutaneous angiomas, and a cerebrovascular lesion consistent with cavernous hemangioma on computed tomography (CT) scan and magnetic resonance imaging (MRI). Subsequently, Pancurak et al.5 reported a family with two members having CT scans consistent with cavernous hemangioma of the brain. Bell et al.27 reported a case of bilateral cavernous hemangioma associated with a cavernous hemangioma in the left parietal lobe. Most recent is the report by Sarraf et al.28 of a family of 12 affected members spanning three generations. The proband had multiple CCMs and a choroidal hemangioma; her son had a retinal cavernous hemangioma; her daughter had CCMs; a sister manifested cutaneous hemangiomas, CCMs and a choroidal hemangioma, and her father had multiple cutaneous hemangiomas. This is the first association with choroidal hemangioma.28
CCMs occur predominantly in the cerebrum and pons, rarely in the spinal cord and about 60% are symptomatic (Fig. 129.7). They can present with focal or generalized seizures (45%), cerebral hemorrhages (41%), focal neurological deficits or headaches.30 Some 40% of patients with cavernous hemangioma of the brain have calcifications apparent on skull radiographs,31 but CT is currently the best diagnostic procedure to demonstrate calcium. Magnetic resonance imaging (MRI) is most sensitive of all imaging modalities. Gradient echo sequences can identify three times more lesions than turbo spin echo on MRI and is recommended. Four specific MRI patterns have been identified based on the hemangioma characteristics, presence of old or fresh blood and calcium.5,32
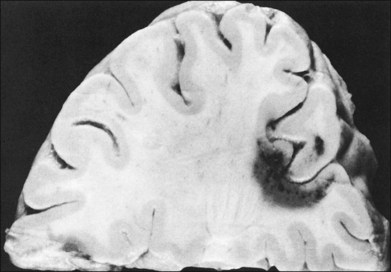
(Reproduced with permission from Rubenstein LJ. Tumors of the central nervous system. Washington, DC: Armed Forces Institute of Pathology; 1972.29)
Patients have also been reported with extraocular muscle paresis. Although this may be related to a cavernous hemangioma of the midbrain, Yen and Wu33 have reported a patient with agenesis of the internal carotid artery associated with retinal cavernous hemangioma and bilateral oculomotor palsies.
Genetics
Several pedigrees have been reported with cavernous hemangioma of the retina in which various family members had retinal, cutaneous, or neurologic lesions. The most extensive pedigree, reported by Goldberg et al.,15 spanned four generations. One daughter had a retinal cavernous hemangioma, another daughter had seizures and cutaneous lesions, six relatives had seizures, and 14 other relatives had cutaneous vascular lesions. In a family reported by Pancurak et al.,5 the proband had a retinal cavernous hemangioma; his sister had cavernous hemangiomas of both retina and brain; his mother had a cavernous hemangioma of the brain, and a niece had a retinal cavernous hemangioma.
Three separate genes have been identified in association with familial CCMs: CCM1/KRIT1, CCM2/MGC4607, and CCM3/PDCD10.30,34–37 CCM1 is located at chromosome locus 7q11-q22 and was the first one identified with the familial form of CCMs. Linkage studies have shown that a CCM1 mutation is involved in 40–53% of familial CCMs and nearly half of these patients have neurological manifestations before 25 years of age.38,39 The product of CCM1, named Krev interaction trapped 1 or KRIT1, is an ankyrin repeat-containing protein that interacts with RAP-1A (Krev-1), which is a member of the RAS family of GTPases.38,39 CCM2 is located at 7p15–13; mutations in this gene are involved in up to 25–40% of familial CCMs. Patients with CCM2-associated disease have a lower number of gradient-echo sequence lesions than those with CCM1 or CCM3 disease, and the number of lesions increase less rapidly with age than in patients with CCM1 disease. CCM3, localized at 3q25.2-q27, encodes programmed cell death protein 10 (PDCD10) and is the most recently discovered gene involved in familial CCMs.40 CCM3 mutation carriers are less common (10%) than CCM1 or CCM2 carriers, but they have near 100% penetrance and these patients are more likely to present with hemorrhage and have symptoms before 15 years of age.
1 Messmer E, Laqua H, Wessing A, et al. Nine cases of cavernous hemangioma of the retina. Am J Ophthalmol. 1983;95:383–390.
2 Colvard DM, Robertson DM, Trautmann JC. Cavernous hemangioma of the retina. Arch Ophthalmol. 1978;96:2042–2044.
3 Shields J. Diagnosis and management of intraocular tumors. St Louis: Mosby; 1987.
4 Klein M, Goldberg MF, Cotlier E. Cavernous hemangioma of the retina: report of four cases. Ann Ophthalmol. 1975;7:1213–1221.
5 Pancurak J, Goldberg MF, Frenkel M, et al. Cavernous hemangioma of the retina. Genetic and central nervous system involvement. Retina. 1985;5:215–220.
6 Naftchi S, la Cour M. A case of central visual loss in a child due to macular cavernous haemangioma of the retina. Acta Ophthalmol Scand. 2002;80:550–552.
7 Yamaguchi K, Tamai M. Cavernous hemangioma of the retina in a pediatric patient. Ophthalmologica. 1988;197:127–129.
8 Gass JD. Cavernous hemangioma of the retina. A neuro-oculo-cutaneous syndrome. Am J Ophthalmol. 1971;71:799–814.
9 Nicholson DH. Tumors of the optic disc. Trans Sect Ophthalmol Am Acad Ophthalmol Otolaryngol. 1977;83:751–754.
10 Drummond JW, Hall DL, Steen WH, Jr., et al. Cavernous hemangioma of the optic disc. Ann Ophthalmol. 1980;12:1017–1018.
11 Gündüz K, Ozbayrak N, Okka M, et al. Cavernous hemangioma with cone dysfunction. Ophthalmologica. 1996;210:367–371.
12 Lewis RA, Cohen MH, Wise GN. Cavernous haemangioma of the retina and optic disc. A report of three cases and a review of the literature. Br J Ophthalmol. 1975;59:422–434.
13 Giuffre G. Cavernous hemangioma of the retina and retinal telangiectasis. Distinct or related vascular malformations? Retina. 1985;5:221–224.
14 Kushner MS, Jampol LM, Haller JA. Cavernous hemangioma of the optic nerve. Retina. 1994;14:359–361.
15 Goldberg RE, Pheasant TR, Shields JA. Cavernous hemangioma of the retina. A four-generation pedigree with neurocutaneous manifestations and an example of bilateral retinal involvement. Arch Ophthalmol. 1979;97:2321–2324.
16 Witmer R, Verrey F, Speiser P. Retinal angiomatosis. Atypical cases of retinal angiomatosis and telangiectases. Bibl Ophthalmol. 1968;76:113–123.
17 Gíslason I, Stenkula S, Alm, et al. Cavernous haemangioma of the retina. Acta Ophthalmol (Copenh). 1979;57:709–717.
18 Haller JA, Knox DL. Vitrectomy for persistent vitreous hemorrhage from a cavernous hemangioma of the optic disk. Am J Ophthalmol. 1993;116:106–107.
19 Agarwal A. Gass’ atlas of macular diseases. Philadelphia: Elsevier; 2011.
20 Gass J. Differential diagnosis of intraocular tumors: a stereoscopic presentation. St Louis: Mosby; 1974.
21 Messmer E, Font RL, Laqua H, et al. Cavernous hemangioma of the retina. Immunohistochemical and ultrastructural observations. Arch Ophthalmol. 1984;102:413–418.
22 Weskamp C, Cotlier I. Angioma del cerebro y de la retina con malformaciones capilares de la piel. Arch Oftalmol B Aires. 1940;15:1–10.
23 Gautier-Smith PC, Sanders MD, Sanderson KV. Ocular and nervous system involvement in angioma serpiginosum. Br J Ophthalmol. 1971;55:433–443.
24 Mildner I. Cavernous hemangioma of the retina as a symptom of phakomatosis. Ber Zusammenkunft Dtsch Ophthalmol Ges. 1972;71:610–613.
25 Schwartz AC, Weaver RG, Jr., Bloomfield R, et al. Cavernous hemangioma of the retina, cutaneous angiomas, and intracranial vascular lesion by computed tomography and nuclear magnetic resonance imaging. Am J Ophthalmol. 1984;98:483–487.
26 Backhouse O, O’Neill D. Cavernous haemangioma of retina and skin. Eye (Lond). 1998;12:1027–1028.
27 Bell D, Yang HK, O’Brien C. A case of bilateral cavernous hemangioma associated with intracerebral hemangioma. Arch Ophthalmol. 1997;115:818–819.
28 Sarraf D, Payne AM, Kitchen ND, et al. Familial cavernous hemangioma: An expanding ocular spectrum. Arch Ophthalmol. 2000;118:969–973.
29 Rubenstein L. Tumors of the central nervous system. Washington, DC: Armed Forces Institute of Pathology; 1972.
30 Labauge P, Denier C, Bergametti F, et al. Genetics of cavernous angiomas. Lancet Neurol. 2007;6:237–244.
31 Giombini S, Morello G. Cavernous angiomas of the brain. Account of fourteen personal cases and review of the literature. Acta Neurochir (Wien). 1978;40:61–82.
32 Vaquero J, Leunda G, Martínez R, et al. Cavernomas of the brain. Neurosurgery. 1983;12:208–210.
33 Yen MY, Wu CC. Cavernous hemangioma of the retina and agenesis of internal carotid artery with bilateral oculomotor palsies. J Clin Neuroophthalmol. 1985;5:258–262.
34 Ji BH, Qin W, Sun T, et al. A novel deletion mutation in CCM1 gene (krit1) is detected in a Chinese family with cerebral cavernous malformations. Yi Chuan Xue Bao. 2006;33:105–110.
35 Campione E, Terrinoni A, Orlandi A, et al. Cerebral cavernomas in a family with multiple cutaneous and uterine leiomyomas associated with a new mutation in the fumarate hydratase gene. J Invest Dermatol. 2007;127:2271–2273.
36 Stahl S, Gaetzner S, Voss K, et al. Novel CCM1, CCM2, and CCM3 mutations in patients with cerebral cavernous malformations: in-frame deletion in CCM2 prevents formation of a CCM1/CCM2/CCM3 protein complex. Hum Mutat. 2008;29:709–717.
37 Lee YW, Lee ST, Cha JG, et al. A novel KRIT1 gene mutation in a patient with cerebral and multiple spinal cavernous malformations. Ann Clin Lab Sci. 2010;40:290–294.
38 Denier C, Gasc JM, Chapon F, et al. Krit1/cerebral cavernous malformation 1 mRNA is preferentially expressed in neurons and epithelial cells in embryo and adult. Mech Dev. 2002;117:363–367.
39 Denier C, Goutagny S, Labauge P, et al. Société Française de Neurochirurgie. Mutations within the MGC4607 gene cause cerebral cavernous malformations. Am J Hum Genet. 2004;74:326–337.
40 Chen L, Tanriover G, Yano H, et al. Apoptotic functions of PDCD10/CCM3, the gene mutated in cerebral cavernous malformation 3. Stroke. 2009;40:1474–1481.