CHAPTER 350 Carotid Endarterectomy
Treatment of carotid occlusive disease, in particular by carotid endarterectomy (CEA), is unique among neurosurgical procedures in that there is extensive evidence from large, well-conducted, multi-institutional, randomized controlled trials to guide physicians in their decision making. Specifically, the North American Symptomatic Carotid Endarterectomy Trial (NASCET)1 and the European Carotid Surgery Trial (ECST)2 for symptomatic carotid occlusive disease and the Asymptomatic Carotid Atherosclerosis Study (ACAS)3 and the Asymptomatic Carotid Surgery Trial (ACST)4 for asymptomatic disease provide very explicit guidelines for the advisability of CEA by taking into account a number of patient factors. Nonetheless, there can still be a fair amount of controversy and at times uncertainty when treating patients with cervical internal carotid artery (ICA) narrowing. This chapter reviews the indications, technical aspects of the procedure, postoperative care and potential complications, and follow-up for CEA.
History
C. Miller Fisher, a neurologist at Harvard, was the first to accurately report the clinical syndrome associated with cervical ICA occlusion in 1951.5 He reported the autopsy findings in 4 patients and suggested that the cause of occlusion appeared to be primary atherosclerosis. Previously, a variety of causes had been suggested for ICA thrombosis, including syphilis, hypertension, history of head injury (remote or recent), retrograde thrombosis from a cerebral aneurysm, and retrograde thrombosis from intracerebral arteries.6 That same year, Johnson and Walker from Johns Hopkins reported the angiographic findings from 6 of their patients who suffered cervical ICA thrombosis and reviewed the cases of an additional 101 patients in the world literature in whom thrombosis was diagnosed by angiography.6 They emphasized the value of angiography in establishing the diagnosis and agreed with Moniz and colleagues that spontaneous ICA thrombosis is probably much more common than originally thought inasmuch as many of these patients had been clinically suspected of having a brain tumor, aneurysm, or “some vague cerebrovascular accident” before angiography revealed the occluded ICA.6,7 During that decade, Millikan, Siekert, and Whisnant at the Mayo Clinic further defined the syndrome of transient ischemic attack (TIA) as a harbinger of carotid artery stenosis.8
Who actually performed the first CEA is hotly debated. Several surgeons pioneered work in this area shortly after Miller’s publications in the early 1950s. Ed Laws reported that a neurosurgeon, William Spence, performed the first successful CEA in 1951 but that his report was rejected for publication in the Journal of Neurosurgery.9 Numerous prominent surgeons did report successful surgical treatment of carotid occlusive disease with a variety of techniques around that time, including carotid-carotid anastomosis,10 primary resection of the diseased segment and reanastomosis,11 arterial grafting,12 interposition vein grafting,13 and endarterectomy.14–17 The concept of surgical revascularization of the cervical segment of the ICA for atherosclerotic narrowing or occlusion firmly took hold in the second half of the 20th century.
According to data from the National Hospital Discharge Survey, the number of CEAs performed in the United States dramatically increased from 15,000 in 1971 to 107,000 in 1985.18 Based on regression estimates, it was predicted that 127,000 CEAs would be performed in the United States in 1986. However, several important publications in the mid-1980s raised concern about higher than previously reported rates of morbidity and mortality and questioned the indications for CEA, particularly in asymptomatic patients, which probably quelled the otherwise almost exponential rise in the number of procedures performed.19–25 In fact, only 83,000 CEAs were performed in 1986.18
Interestingly, prerelease of the results from NASCET and ACAS in 1991 and 1994, respectively, again resulted in a surge in the number of CEAs performed in the United States.26 Data from acute care hospitals in seven states were reviewed between 1989 and 1996. For 2 years before 1991 the rate of CEA per 100,000 persons did not change. However, the CEA rate increased 3.4% each month for the 6 months after release of the NASCET clinical alert in February 1991. Even more notable, after publication of the ACAS clinical alert in 1994, the CEA rate increased approximately 7% each month for 7 months with a total increase of 42% per 100,000 persons.26 Other studies have also shown increased rates of CEA since release of the randomized controlled trials.27,28 By 1995, about 132,000 CEAs were performed in the United States. With the introduction of carotid artery angioplasty and stenting, the number of CEAs is probably decreasing. According to data from the most recently available Hospital Discharge Survey on the number of CEA procedures performed on patients aged 75 to 84 years (the age group with the highest rate), the rate of CEA in 1990 was 19.8 per 10,000 population, peaked at 32.8 per 10,000 in the year 2000, and was 29.5 per 10,000 in 1996.29
Preoperative Evaluation
Symptomatic Patients
The differential diagnosis and detailed work-up of every patient with possible symptoms of cerebral ischemia are beyond the scope of this chapter. Flemming and colleagues provided a detailed review of the evaluation and management of TIA and minor cerebral infarction.30 A patient with a history and findings on physical examination consistent with TIA or minor stroke of the anterior circulation should undergo routine laboratory evaluation (complete blood count, prothrombin time, activated partial thromboplastin time, electrolytes, fasting glucose, creatinine, erythrocyte sedimentation rate), electrocardiography, chest radiography, and computed tomography (CT) of the head without contrast enhancement. If carotid artery disease is still in the differential diagnosis, screening ultrasonography of the carotid arteries should be undertaken. If ultrasound demonstrates high-grade stenosis, a confirmatory examination is performed, typically gadolinium-enhanced magnetic resonance angiography (MRA).31 If there appears to be a discrepancy between the ultrasound and MRA findings, formal cerebral angiography may be performed.32 There continues to be much controversy regarding what imaging is necessary to establish the diagnosis of carotid stenosis. Ultrasound, MRA, and CT angiography have all been extensively compared with conventional digital subtraction angiography. All modalities have high specificity and sensitivity for detecting carotid stenosis, and MRA in particular may have some added value in detecting other characteristics of atherosclerotic plaque, such as lipid content and intraplaque hemorrhage, among other findings.33–40
Asymptomatic Patients
Although stroke is the leading cause of death and hospitalization in nearly all European countries and consistently the third major cause of death in the United States, widespread mass screening for carotid occlusive disease is not recommended.41,42 Whitty and associates argued that screening strategies for asymptomatic carotid stenosis are unlikely to be effective and might even be harmful in populations in which the prevalence is between 1% and 25%.43 The prevalence of moderate asymptomatic carotid stenosis (>50%) in a recent meta-regression analysis published in 2009 was 4.2%, and the prevalence of severe stenosis (>70%) was 1.7%.44 Age older than 70 years and male sex increased the chance of finding significant stenosis.44 The best screening strategy would involve evaluating patients at high risk for carotid stenosis (based on age, male sex, hypertension, tobacco abuse, peripheral vascular disease) who are surgical candidates and who would undergo surgery if recommended. Identifying this population and the true benefit to them and society as a whole remains highly controversial.45–51
Indications Based on Randomized Controlled Trials
Symptomatic Patients
As noted earlier, two major trials, NASCET and ECST, have clearly demonstrated the efficacy of CEA in selected patients with amaurosis fugax, hemispheric TIA, or nondisabling stroke within the previous 4 to 6 months.1,2 Several caveats of the trials are worth noting to guide patient selection.
North American Symptomatic Carotid Endarterectomy Trial
Between January 1988 and February 1991, NASCET enrolled 659 patients at 50 clinical centers in the United States and Canada with symptomatic carotid stenosis in whom symptoms occurred within 120 days before entry into the trial. To qualify as a trial center, each institution had to have performed at least 50 CEA procedures in the previous 2 years with a 30-day stroke and mortality rate of less than 6%. All patients included in the study had to be younger than 80 years and have ipsilateral ICA stenosis of 30% to 99% as assessed by angiography. Exclusion criteria are listed in Table 350-1. Patients were randomized to aspirin, 1300 mg/day, and other stroke reduction therapy as needed (e.g., antihypertensives, lipid-lowering therapy), and those assigned to surgery also underwent CEA. Three hundred thirty-one patients were randomized to medical therapy and 328 to surgery. There were no significant dissimilarities between the groups on careful analysis. The rate of occurrence of any perioperative stroke and death for the surgical group was 5.8%; for major stroke and death it was 2.1%.1
TABLE 350-1 Exclusion Criteria from the North American Symptomatic Carotid Endarterectomy Trial
|
Had a stroke on either hemisphere that would probably deprive the patient of useful function in the affected territory
|
The study was stopped early because of a clear and highly significant benefit in patients with high-grade (70% to 99%) stenosis. By 2 years after randomization, the risk for any ipsilateral stroke was 26% in the medical group and 9% in the surgical group (P < .001), a relative reduction in risk of 65%. The risk for ipsilateral major or fatal stroke was reduced from 13.1% in the medical group to 2.5% in the surgical group, and even the risk for any major stroke or death was reduced from 18.1% in the medical group to 8% in the surgical group. The treatment groups did not differ significantly in total mortality. The early disadvantage in the surgical group of the risk associated with the operation was negated by the 3-month follow-up, at which time the benefit of reduction in stroke and death overcame any perioperative risk.1
Additional analysis of the moderate stenosis group also revealed a statistical benefit in study patients who had 50% to 69% stenosis and underwent CEA in comparison to the medical group.52 The 5-year risk for any ipsilateral stroke was reduced from 22.2% in the medical cohort to 15.7% in the surgical group (P = .045).52
Further information acquired from analysis of the study revealed that patients 75 years and older benefited more from CEA than did younger patients.53 Baseline variables predictive of increased surgical risk for all patients undergoing CEA for symptomatic disease included hemispheric versus retinal TIA on initial evaluation, left-sided procedure, contralateral carotid occlusion, ipsilateral ischemic lesion on CT, and an irregular or ulcerated plaque.54
European Carotid Surgery Trial
A very similar trial to NASCET was undertaken at 97 centers in 12 European countries and the results reported in 1998.2 There were 1807 patients in the surgery arm and 1211 patients in the best medical management arm for analysis. To be eligible, patients had to have had symptoms within the previous 6 months of randomization. In this trial the overall perioperative (within 30 days of surgery) risk for nonfatal major stroke or death was 7.0%. The most striking finding in this trial was that for the combined outcome of surgical events, ipsilateral major ischemic strokes, and other major strokes, no overall effect was seen below about 70% to 80% stenosis.2
It is worth noting that one of the few controversial issues regarding these studies relates to how ICA stenosis was calculated. For NASCET, percent stenosis was determined by using the diameter at the point of the greatest narrowing as the numerator and the diameter of the artery beyond the bulb where the walls of the artery again become parallel as the denominator.1 ECST also used the lumen diameter at the most stenotic point as the numerator, but the estimated probable original diameter at the site of maximal stenosis was used as the denominator.2 This probably resulted in more observer variation in estimating the degree of stenosis, but it is generally thought that 80% stenosis in ECST would correlate to 70% stenosis in NASCET.2
Pooled analysis of the data from NASCET and ECST, as well as from the Veterans Affairs Trial 309,55 which was stopped early after the NASCET results became available, allows some additional conclusions.56 The degree of stenosis is very important in predicting the benefit from CEA. No benefit was seen with less than 50% stenosis of the luminal diameter. Benefit was noted in patients with 50% to 69% stenosis, but the number needed to treat to prevent one stroke over a 5-year period was 22. CEA was highly beneficial for symptomatic patients with stenosis greater than 70%, and the number needed to treat to prevent one stroke over a 5-year period was just 6.3. No benefit was observed when there was “near occlusion” of the symptomatic artery.56 Surgery within 2 weeks of the last symptom improved outcomes relative to later surgery (especially in women), and there was no increased operative risk when operating within 2 weeks of a nondisabling hemispheric stroke.57
Contralateral carotid stenosis in the face of an ipsilateral symptomatic high-grade stenosis deserves special mention. This subgroup of patients was analyzed in NASCET and the results may seem somewhat contradictory.58 Medically treated patients with an occluded contralateral ICA were twice as likely to have an ipsilateral stroke as patients who still had flow through the contralateral artery. However, the perioperative risk was higher in this subgroup, especially if the contralateral artery was occluded. Despite this risk, the overall evidence supports performing CEA on a recently symptomatic ICA with greater than 70% stenosis even if the contralateral artery is severely narrowed or occluded.58
Asymptomatic Patients
The indications for CEA in asymptomatic patients are much more controversial than those for symptomatic patients despite two very thorough randomized controlled trials.3,4 ACAS and ACST evaluated the efficacy of CEA in asymptomatic patients, and the results were published in 1995 and 2004, respectively.
Asymptomatic Carotid Atherosclerosis Study
ACAS was a very well-designed comparison of aspirin (325 mg/day) and stroke risk factor reduction strategies versus aspirin, CEA, and stroke risk factor reduction strategies in asymptomatic patients aged 40 to 79 years who had 60% to 99% luminal diameter stenosis of the cervical ICA as defined by angiography.3 Thirty-nine centers in the United States and Canada randomized patients between 1987 and 1993. Follow-up data were available for 1659 patients after more than 42,000 had been screened during the 6 years of the study. Exclusion criteria are detailed in Table 350-2 and were primarily designed to avoid enrolling patients who had an underlying condition that might complicate surgery or produce disability or death within 5 years of enrollment. After a mean follow-up of 2.7 years with 4657 patient-years of observation, the 5-year risk for ipsilateral stroke and any perioperative stroke or death was reduced from 11.0% in the medical group to 5.1% in the surgical patients (P = .004). This correlates to 19 CEAs necessary to prevent 1 stroke over a period of 5 years. The perioperative complication rate was very low at 2.3%, and the perioperative death rate was 0.1%.3
TABLE 350-2 Exclusion Criteria for the Asymptomatic Carotid Atherosclerosis Study
There are several additional caveats regarding the results of ACAS worth noting such that many neurologists caution against widespread application of the results to all patients with asymptomatic high-grade carotid stenosis.45,59 If the outcome was restricted to just disabling stroke or any stroke or death, the benefit of surgery was no longer statistically significant. It is worth emphasizing the very low morbidity in this study and in all the other randomized trials as well. If CEA is not performed by a skilled, experienced surgeon, even a small increase in the morbidity or mortality rate in comparison to the study results will negate the benefit of surgery. Interestingly, there are numerous natural history studies that document an increased risk for ipsilateral stroke as the degree of ICA stenosis increases.60–68 In fact, as noted earlier, this was confirmed in NASCET and ECST as well, but not seen in ACAS.1,2 Surprisingly, the benefit from surgery was higher in the 60% to 69% stenosis group than in the 80% to 89% group in ACAS.3 The reason for this is not entirely clear. Finally, although not designed to detect a difference between the sexes, a subgroup analysis showed no benefit for women and a higher perioperative complication rate than in men (3.6% versus 1.7%).3
Asymptomatic Carotid Surgery Trial
ACST was the largest study to evaluate the efficacy of CEA for asymptomatic carotid stenosis.4 Between 1993 and 2003, 3120 patients were randomized to CEA within 1 month to 1 year or to continued observation. Again, 60% or greater narrowing of the ipsilateral ICA was used as criteria for entry into the study, but for this study, the degree of stenosis was based on ultrasound evaluation rather than formal angiography. Mean follow-up for all patients was 3.4 years. The medical regimen was left to the discretion of the treating physicians, and approximately 4% of the observation group eventually crossed over to undergo CEA. Once again, the operative 30-day morbidity and mortality rate was very low (3.1%). The overall results were very similar to those of ACAS. The 5-year risk for any stroke was 6.4% in the surgery cohort and 11.8% in the observation group (P < .001). CEA was also beneficial in comparison to medical therapy for fatal or disabling stroke (P = .004) and fatal stroke alone (P = .006). The benefit was seen in both men and women in this study, thus suggesting that some of the additional morbidity in women seen in ACAS may have been due to increased morbidity from angiography rather than CEA itself. There was no benefit for patients 75 years or older. As in ACAS, the degree of stenosis was not significant in establishing benefit.4
Several other randomized trials for symptomatic55 and asymptomatic69–72 carotid stenosis were attempted both before and during the aforementioned four trials but were either stopped or found to be inconclusive for a variety of reasons, and because of space issues they are not discussed in this chapter.
In summary then, we recommend CEA for the following patients:
Of course, there are numerous additional patient factors that may influence a decision regarding CEA versus medical therapy for carotid artery disease. A multidisciplinary consensus statement from the American Heart Association was released in 1995 and again in 1998 in an attempt to provide guidelines for CEA.73,74 Patients in these publications were stratified according to symptomatology, degree of ICA stenosis, and perceived surgical risk, and this can serve as a starting reference when attempting to decide on surgery in difficult cases.73,74 Complicating this even further (and beyond the scope of this chapter) is the role of carotid angioplasty and stenting (CAS) and how the numerous trials already completed and currently under way to evaluate the safety and efficacy of CAS and to compare CAS and CEA might affect these recommendations in the future.75–79
Technique
The technique described in the following sections was developed primarily by Thor Sundt, Jr., M.D., and has changed very little over the past 30 years.80 The department of Neurologic Surgery at the Mayo Clinic has performed more than 3000 CEAs and has successfully trained generations of residents in this technique.
Positioning and Exposure
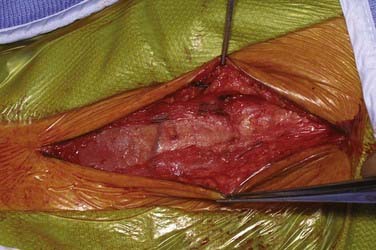
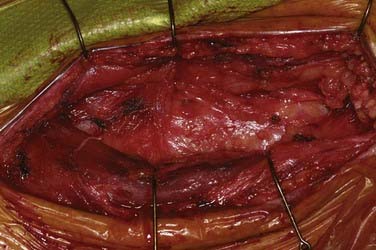
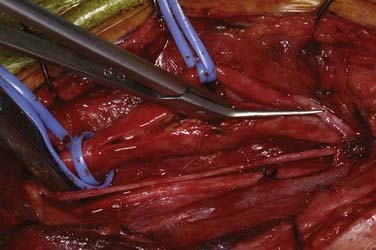
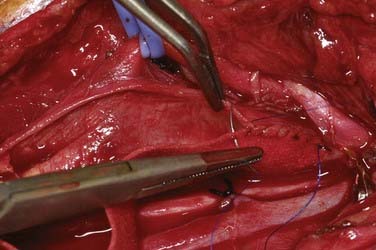
Patients undergo CEA under general anesthesia at our institution. Typically, this is done with a combination of an intravenous muscle relaxant and opioids and inhaled nitrous oxide and isoflurane. All patients are monitored with 16-channel EEG, and the inhaled anesthetic is titrated (usually slightly less than 1 minimum alveolar concentration) so that it does not interfere with the recorded EEG. Patients are positioned supine with their arms tucked at their sides and the head turned to the opposite side and resting on a soft headrest. For obese patients or large barrel-chested men, a rolled blanket placed under the shoulders can assist in the exposure. Some neck extension is very helpful, but in elderly patients, caution must be exercised to avoid overextension in those with possible underlying cervical spondylosis. A curvilinear incision is made parallel to and just posterior to the anterior border of the sternocleidomastoid (SCM) muscle. We have found that placing the incision directly on the anterior border results in a more conspicuous scar, particularly in thin patients. The incision begins about 1 cm below the tip of the mastoid, curves 1 cm below the angle of the mandible, and ends 1 cm above the sternoclavicular joint. A scalpel is used to incise the skin and platysma to expose the cervical fascia (Fig. 350-1). A large transverse cervical sensory nerve might be encountered in the superior portion of the wound and can be sacrificed if necessary. Care should be taken to not violate the parotid fascia, which can be adherent to the upper SCM. Dissection is performed with scissors in a spreading fashion deep to the anterior border of the SCM muscle to free it up from the underlying fascia (Fig. 350-2). The carotid system can then be easily and gently palpated. The cervical fascia is next opened sharply to first expose the common carotid artery. Careful spreading dissection is then continued in a caudal to cephalad direction to expose the carotid bifurcation, external carotid artery (ECA), and ICA. During this dissection, the common facial vein will usually be seen running transversely across the region of the carotid bifurcation (Fig. 350-3). This vein is doubly ligated and divided. The internal jugular vein (IJV) itself rarely needs to be exposed. The omohyoid muscle usually marks the inferior extent of the exposure, and the posterior belly of the digastric muscle marks the superior extent. The hypoglossal nerve (XII) is at risk during the dissection and should be identified and carefully protected. It almost always lies superficial to the ECA and ICA, just below the digastric muscle. The descendens hypoglossi can be seen arising from cranial nerve XII and can be sacrificed; however, we have found this rarely to be necessary. It can almost always be mobilized anterior or posterior to the arteries to provide the needed exposure. Although cutting it seldom has any clinical consequences, if it is going to be sacrificed, it is vital to make sure that it is indeed the descendens hypoglossi and not the vagus nerve. The vagus is almost always deep to the carotid system between the carotid and the IJV (Fig. 350-4). Any dissection deep to the carotid bifurcation should be done very gingerly to avoid injury to the superior laryngeal branch of the vagus nerve, which will result in significant dysphagia postoperatively. We typically use standard fish hooks (Deroyal, Powell, TN) to hold the wound open because they are less obtrusive than self-retaining retractors. Soft vessel loops (Cardinal Health, Chicago) are placed on the common carotid artery and ECA and usually the superior thyroid artery as well. The tape on the ECA should be placed more than a centimeter and preferably 2 cm from its origin from the common carotid artery to facilitate removal of plaque from the orifice of the ECA when the time comes. Great care should be taken when exposing the bifurcation and the proximal ICA, especially in a symptomatic patient, to not dislodge any emboli from the artery.
Exposure for a High Bifurcation or Plaque
Occasionally, the surgeon will encounter a patient who has a high carotid bifurcation (above the C3 level) or a plaque that extends high up the ICA. This may require further cephalad exposure of the carotid system. Additional steps in the dissection can easily allow safe exposure of the very distal cervical ICA.81 The incision can be extended cephalad to the mastoid tip, curved forward and inferior in the postauricular sulcus while skirting the earlobe, and then ascend in the pretragal skin crease. Care needs to be taken again to not incise the fascia over the parotid gland with the skin incision. The posterior border of the parotid gland can then be grasped with forceps and elevated off the upper SCM. If this is continued, the facial nerve can be located just deep to the temporoparotid fascia. With the surgeon’s finger on the mastoid tip and directed anteriorly, its tip overlies the main trunk of the nerve. We fortunately rarely find it necessary to actually expose the facial nerve, even with very high bifurcations. Elevating the parotid further exposes the posterior belly of the digastric muscle and the hypoglossal nerve just deep and inferior to it. The occipital artery often needs to be ligated and divided. The posterior belly of the digastric muscle is then divided between two 2-0 silk sutures and retracted out of the way. These sutures can be tied back together to reapproximate the muscle at the end of the operation, but this is not really necessary. The stylohyoid muscle, which lies parallel and just superior to the digastric muscle, can then be identified and also divided. The deeper stylomandibular ligament then needs to be resected, and the distal ICA lies just below this ligament.81 Some authors advocate mobilization or resection of a portion of the mandible to aid in the exposure, but we have not found this to be especially helpful or necessary.82,83
Endarterectomy
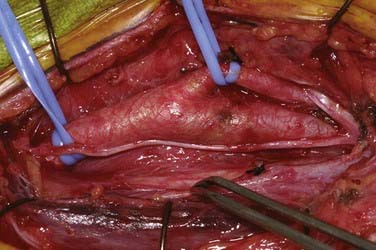
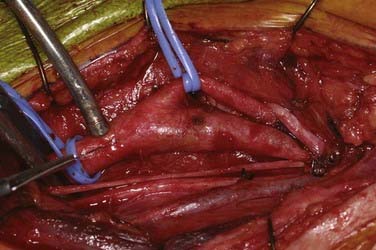
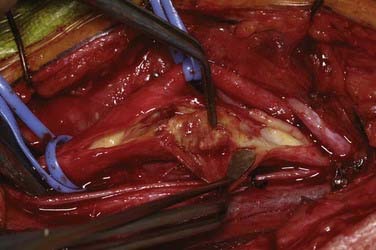
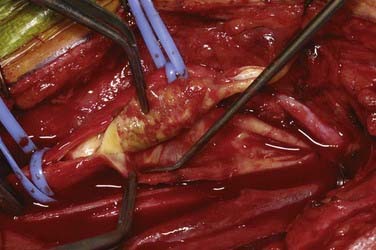
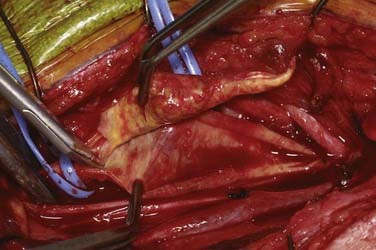
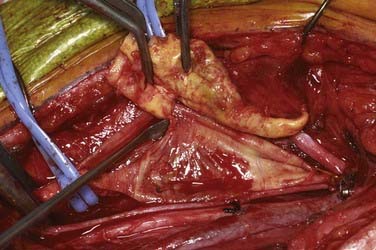
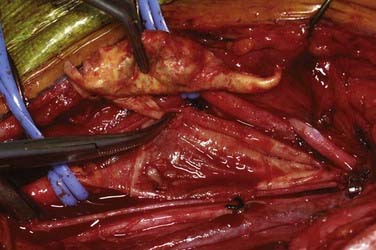
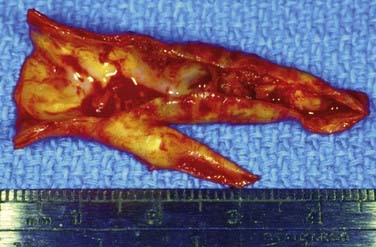
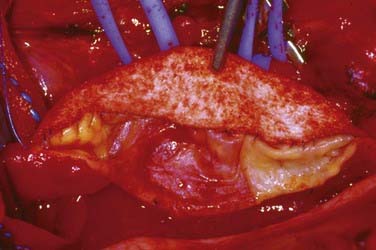
An arteriotomy is made in the mid common carotid artery with a No. 11 knife blade and extended up the ICA with Pott’s scissors (Fig. 350-5). The tips of Pott’s scissors will guide the surgeon through the lumen of even the most tightly stenosed artery. The arteriotomy should be extended distally in the ICA until normal intima is obvious (Fig. 350-6). A common initial mistake is to attempt to make a smaller arteriotomy than necessary. The temporary aneurysm clip on the distal ICA is momentarily opened to ensure that backflow is present. The endarterectomy is then begun at the carotid bifurcation, where the plaque is almost always the thickest. The plaque separates from the media of the native artery fairly readily with mild dissection with a small spatula (Fig. 350-7). Dissection is initially carried distally up the ICA. Almost always, the plaque breaks distally from the normal intima with just gentle dissection. Occasionally, if the intima is very thick, a distal intimal break point becomes obvious at the spot where the intima becomes very adherent, and the plaque no longer wants to dissect off the artery. In this case, curved microscissors can be used to trim the plaque distally and create the distal break point. The dissection is then carried proximal into the common carotid artery (Fig. 350-8). The proximal intimal break point is always made with Pott’s scissors once a relatively uniform section of intima is reached (Fig. 350-9). This will create a small “ledge” where the remaining proximal intima meets the endarterectomy site, but this is rarely a concern because it is in the direction of flow. The plaque is then followed up the ECA by circumferentially dissecting it free from the lumen of this vessel (Fig. 350-10). The vascular tape on the ECA can be loosened and the ECA somewhat everted to allow more distal dissection. Once the plaque starts to thin out, it is grasped and removed (Fig. 350-11), thus removing the entire plaque as one specimen (Fig. 350-12).
Shunt Placement
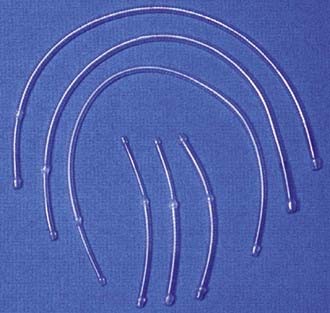
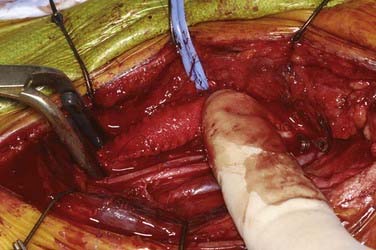
We place a shunt only if there is a change in the monitored 16-channel EEG ipsilateral to the occluded ICA. This is a very reliable indicator of ischemia (attenuation of faster frequencies of >4 Hz by >50% along with an increase in the amplitude of delta activity by 50%), and when it does occur, we do not hesitate to place a shunt.84 Almost always there is time from occlusion to the point when the first change in the EEG occurs to allow proceeding with the arteriotomy and initial dissection of the plaque from the ICA before the shunt has to be inserted. The exception to this strategy is a patient in whom immediate and profound change in the EEG is seen on occluding flow through the ICA. In this case, we perform the arteriotomy and place the shunt right away even before any dissection of the plaque is performed. Placement of a shunt can be somewhat daunting (and bloody) to an inexperienced surgeon and requires good teamwork between the surgeon and assistant. We prefer to use the internal Sundt shunt (Integra, Cincinnati, OH) (Fig. 350-13). A medium-sized shunt (proximal diameter of 3 × 5 mm) works well for most arteries. The proximal end of the shunt is placed first in the common carotid artery. This may require moving the Fogarty clamp more proximal on the artery, which is easily accomplished by holding the artery closed with the thumb and forefinger and simply removing the clamp, sliding it more proximally, and reapplying it to the vessel. The shunt should be flushed with heparinized saline before insertion. After the proximal, larger end is placed in the common carotid artery, the vascular tape around the common carotid is secured around the shunt to hold it in place. A second vascular tape may also be used to ensure that it stays in place. The Fogarty catheter is temporarily opened to flush the shunt with blood and remove any air bubbles. The distal end of the shunt is held with forceps in the surgeon’s dominant hand and the artery is steadied with forceps held in the nondominant hand. The assistant removes the aneurysm clip on the ICA as the surgeon slides the shunt into the distal ICA. The Fogarty clamp is then removed to restore flow up the ICA. Rarely, there may be backbleeding around the shunt from the ICA. Vascular tape placed around the ICA and held secure with a small vascular clip usually controls the backbleeding nicely. If there is a large amount of bleeding from the ICA around the shunt or the EEG does not improve after flow is restored, one should be concerned about the possibility of a distal embolus or occlusion. The arteries need to be reoccluded as the shunt is removed, and backflow from the ICA needs to be checked. If there is no backflow, a Fogarty balloon catheter may need to be passed distally to see whether a clot is occluding the distal cervical ICA.
Patch Closure of the Arteriotomy
When a shunt is not required, backflow from the ICA is checked once again, the entire endarterectomy bed is irrigated copiously with heparinized saline, and the endarterectomy bed is inspected once more. We prefer to close the arteriotomy with a knitted Dacron patch (Hemashield Patch Co., Boston Scientific, Wayne, NJ). The distal end of the patch is trimmed to a “V” to match the arteriotomy (Fig. 350-14). It is sewn in with double-armed 5-0 Prolene suture (Ethicon–Johnson & Johnson, Somerville, NJ). The stitch is placed through the apex of the patch and then through the apex of the arteriotomy and tied. A running stitch is then used to close whatever wall of the arteriotomy is most easily done with a forehand stitch first. The initial six or seven stitches have to be placed very precisely to assist in tacking the patch to the native intima. The needle is passed first through the patch and then through the artery in two steps to ensure that placement is precisely where the surgeon wants it. Each suture should be 1 mm deep and 1 mm distal to the previous stitch. Once the endarterectomy site is reached, the patch can be sutured to the artery wall in one throw while taking care to keep the depth and distance between stitches uniform (Fig. 350-15). Closure of the arteriotomy continues until the proximal intima of the common carotid artery is encountered. The proximal end of the patch is then trimmed, and once again it is best to resume placing the sutures in two steps to ensure that the full thickness of the common carotid is engaged with the stitch. Closure continues around the proximal apex of the arteriotomy onto the opposite side. The suture line can then be inspected from inside the artery to make sure that it is uniform (Fig. 350-16). The second arm of the suture is next begun from the apex down the opposite side of the arteriotomy in similar fashion to the first. This can be moderately challenging because it needs to be done in a backhanded fashion to continue to sew from the patch to the artery, and the stiffness of the patch can sometimes make adequate visualization difficult. Patience and a steady hand, however, will guarantee a good, durable closure.
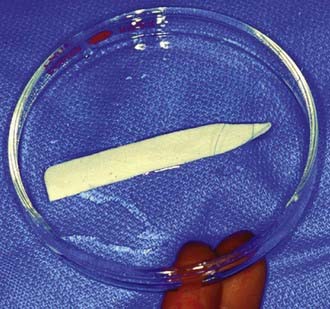
FIGURE 350-14 The knitted Dacron patch trimmed for placement with the apical 5-0 Prolene suture in place.
Restoration of Flow
Once the closure has proceeded to within one or two stitches of being complete, systolic blood pressure is lowered to 130 to 150 mm Hg. The ICA is once again backbled to purge the artery of any air (Fig. 350-17). This continues for 8 to 10 seconds and the ICA is reoccluded. The Fogarty clamp on the common carotid artery is then temporarily opened in an attempt to flush any remaining debris and air from the artery (Fig. 350-18). The Fogarty clamp is closed once again and the final stitches are placed. As the two ends of the Prolene suture are tied, flow is restored up the common carotid artery, the tape on the ECA and superior thyroid artery is relaxed to allow flow to first go up the ECA and its branches, and then the aneurysm clip is removed from the ICA to restore flow to the cerebral circulation (Fig. 350-19). Our typical occlusion times range from 30 to 60 minutes. In our experience the EEG is an extremely reliable indicator of ischemia, and if the patient can tolerate 15 minutes of occlusion, hours of occlusion can probably be tolerated and the operation should not be rushed.

FIGURE 350-17 Before tying the last suture, the internal carotid artery is backbled to purge any air or debris from the artery.
It is very common for significant bleeding to arise from several places along the suture line. Almost always, with just gentle pressure from the surgeon’s fingertip and irrigation with warm saline, these areas will spontaneously stop bleeding (Fig. 350-20). Occasionally, a small piece of Gelfoam may help in hemostasis. In rare cases there will be a large enough gap between the stitches that an additional single 6-0 Prolene suture will be required to obtain hemostasis. We seldom reocclude the common carotid artery when we place this stitch. We try to avoid having to place any additional sutures where there is native intima for fear of causing a dissection, but on occasion this may be necessary.
Long-Term Results
As previously emphasized, the randomized trials for both symptomatic and asymptomatic carotid stenosis provide excellent benchmarks for the morbidity and mortality associated with CEA. The risk for any perioperative stroke or death in NASCET and ECST was 5.8% and 7.0%, respectively.1,2 For asymptomatic patients in ACAS and ACST, it was even lower (2.4% and 3.1%, respectively).3,4
Ecker and coworkers prospectively reviewed our experience with 1000 consecutive CEAs performed on 975 patients between 1988 and 2000 with the exact technique as described in this chapter.85 The mean follow-up was 7.1 years (range, 2.0 to 11 years). All patients had greater than 70% stenosis of the luminal diameter. There were 680 men and 320 women with a mean age of 69 years. The stenosis was symptomatic preoperatively in 59% of the patients. All the arteries were patched for closure. The combined 30-day stroke and death rate was 1.9%. The mortality rate alone was 0.9%. Three patients suffered cardiac death, 4 died of intracerebral hyperperfusion hemorrhage, and 2 suffered a fatal postoperative stroke. Any patient in whom postoperative stroke was a concern was evaluated by a neurologist. There were 10 (1%) documented postoperative strokes. Seven of the 10 patients recovered to functional independence, 2 strokes were fatal, and 1 was disabling.
It is our practice to examine all patients with carotid ultrasound at a 3-month follow-up visit and then yearly thereafter. Ten patients (1%) in this series of 1000 experienced restenosis of greater than 70% of the operated artery. The restenosis was confirmed by angiography or MRA in all cases. Eight of the 10 were symptomatic recurrences. Five were reoperated and 3 were treated with carotid angioplasty and stenting.85 Multiple other large case series published since the release of NASCET and ACAS that included from 517 to 2723 CEA procedures each have revealed 30-day stroke and mortality rates between 1% and 4% and restenosis rates varying from 0.7% to 5.8%, depending on the definition used for restenosis.86–93
Barnett HJ, Taylor DW, Eliasziw M, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1998;339:1415-1425.
Biller J, Feinberg WM, Castaldo JE, et al. Guidelines for carotid endarterectomy: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 1998;29:554-562.
Brott T, Thalinger K. The practice of carotid endarterectomy in a large metropolitan area. Stroke. 1984;15:950-955.
Dodick DW, Meissner I, Meyer FB, et al. Evaluation and management of asymptomatic carotid artery stenosis. Mayo Clin Proc. 2004;79:937-944.
Ecker RD, Pichelmann MA, Meissner I, et al. Durability of carotid endarterectomy. Stroke. 2003;34:2941-2944.
European Carotid Surgery Trial. Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet. 1998;351:1379-1387.
Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. Endarterectomy for asymptomatic carotid artery stenosis. JAMA. 1995;273:1421-1428.
Ferguson GG, Eliasziw M, Barr HW, et al. The North American Symptomatic Carotid Endarterectomy trial. Surgical results in 1415 patients. Stroke. 1999;30:1751-1758.
Flemming KD, Brown RDJr, Petty GW, et al. Evaluation and management of transient ischemic attack and minor cerebral infarction. Mayo Clin Proc. 2004;79:1071-1086.
Gasecki A, Eliasziw M, Ferguson GG, et al. Long-term prognosis and effect of endarterectomy in patients with symptomatic severe carotid stenosis and contralateral carotid stenosis or occlusion: results from NASCET. J Neurosurg. 1995;83:778-782.
Gross CP, Steiner CA, Bass EB, et al. Relation between prepublication release of clinical trial results and the practice of carotid endarterectomy. JAMA. 2000;284:2886-2893.
Halliday A, Mansfield A, Marro J, et al. MRC Asymptomatic Carotid Surgery Trial (ACST) Collaborative Group. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomized controlled trial. Lancet. 2004;363:1491-1502.
Moore WS, Barnett HJM, Beebe HG, et al. Guidelines for carotid endarterectomy. A multidisciplinary consensus statement from the Ad Hoc Committee, American Heart Association. Stroke. 1995;26:188-201.
North American Symptomatic Carotid Endarterectomy Trial (NASCET) Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325:445-453.
Pearson BW, Sundt TMJr. Exposure of the distal internal carotid artery. In: Sundt TMJr, editor. Occlusive Cerebrovascular Disease. Diagnosis and Surgical Management. Philadelphia: Saunders; 1987:298-302.
Rothwell PM, Eliasziw M, Gutnikov SA, et al. Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery. Lancet. 2004;363:915-924.
Rothwell PM, Eliasziw M, Gutnikov SA, et al. Analysis of pooled data from the randomized controlled trials of endarterectomy for symptomatic carotid stenosis. Lancet. 2003;361:107-116.
Sundt TMJr. Techniques of carotid endarterectomy. In: Sundt TMJr, editor. Occlusive Cerebrovascular Disease. Diagnosis and Surgical Management. Philadelphia: Saunders; 1987:191-225.
1 North American Symptomatic Carotid Endarterectomy Trial (NASCET) Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325:445-453.
2 European Carotid Surgery Trial. Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet. 1998;351:1379-1387.
3 Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. Endarterectomy for asymptomatic carotid artery stenosis. JAMA. 1995;273:1421-1428.
4 Halliday A, Mansfield A, Marro J, et al. MRC Asymptomatic Carotid Surgery Trial (ACST) Collaborative Group. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomized controlled trial. Lancet. 2004;363:1491-1502.
5 Fisher M. Occlusion of the internal carotid artery. Arch Neurol Psychiatry. 1951;65:346-377.
6 Johnson HC, Walker AE. The angiographic diagnosis of spontaneous thrombosis of the internal and common carotid arteries. J Neurosurg. 1951;8:631-659.
7 Moniz E, Lima A, De Lacerda R. Hemiplegies par thrombose de la carotide interne. Presse Med. 1937;45:977-980.
8 Millikan CH, Siekert RG, Whisnant JP. The clinical pattern in certain types of occlusive cerebrovascular disease. Circulation. 1960;22:1002-1010.
9 Laws ERJr. Comment to: Zuccarello M, Yeh H-S, Tew JM Jr. Morbidity and mortality of carotid endarterectomy under local anesthesia: a retrospective study. Neurosurgery. 1988;23:450.
10 Carrea R, Molins M, Murphy G. Surgical treatment of spontaneous thrombosis of the internal carotid artery in the neck. Carotid-carotideal anastomosis. Report of a case. Acta Neurol Latinoam. 1955;1:71-78.
11 Eastcott HH, Pickering GW, Rob CG. Reconstruction of internal carotid artery in a patient with intermittent attacks of hemiplegia. Lancet. 1954;2:994-996.
12 Denman FR, Ehni G, Duty WS. Insidious thrombotic occlusion of cervical carotid arteries, treated by arterial graft, a case report. Surgery. 1955;38:569-577.
13 Lin PM, Javid H, Doyle EJ. Partial internal carotid artery occlusion treated by primary resection and vein graft. Report of case. J Neurosurg. 1956;13:650-655.
14 Cooley DA, Al-Naaman YD, Carton CA. Surgical treatment of arteriosclerotic occlusion of common carotid artery. J Neurosurg. 1958;13:500-514.
15 DeBakey ME, Crawford ES, Cooley DA, et al. Surgical considerations of occlusive diseases of innominate, carotid, subclavian, and vertebral arteries. Ann Surg. 1959;149:690-710.
16 Strully KJ, Hurwitt ES, Blankenberg HW. Thrombo-endarterectomy for thrombosis of the internal carotid in the neck. J Neurosurg. 1953;10:474-482.
17 Lyons C, Galbraith G. Surgical treatment of atherosclerotic occlusion of the internal carotid artery. Ann Surg. 1957;145:487-496.
18 Pokras R, Dyken ML. Dramatic changes in the performance of endarterectomy for diseases of the extracranial arteries of the head. Stroke. 1988;19:1289-1290.
19 Dyken ML, Pokras R. The performance of endarterectomy for disease of the extracranial arteries of the head. Stroke. 1984;15:948-950.
20 Brott T, Thalinger K. The practice of carotid endarterectomy in a large metropolitan area. Stroke. 1984;15:950-955.
21 Slavish LG, Nicholas GG, Gee W. Review of a community hospital experience with carotid endarterectomy. Stroke. 1984;15:956-959.
22 Muuronen A. Outcome of surgical treatment of 110 patients with transient ischemic attacks. Stroke. 1984;15:959-964.
23 Barnett HJM, Plum F, Walton JN. Carotid endarterectomy—an expression of concern [editorial]. Stroke. 1984;15:941-1943.
24 Dyken ML. Carotid endarterectomy studies: a glimmering of science [editorial]. Stroke. 1986;17:355-358.
25 Modi JR, Finch WT, Sumner DS. Update of carotid endarterectomy in two community hospitals. Springfield revisited [abstract]. Stroke. 1983;14:128.
26 Gross CP, Steiner CA, Bass EB, et al. Relation between prepublication release of clinical trial results and the practice of carotid endarterectomy. JAMA. 2000;284:2886-2893.
27 Maxwell JG, Rutledge R, Covington DL, et al. A statewide, hospital-based analysis of frequency and outcomes in carotid endarterectomy. Am J Surg. 1997;174:655-661.
28 Morasch MD, Parker MA, Feinglass J, et al. Carotid endarterectomy: characterization of recent increases in procedure rates. J Vasc Surg. 2000;31:901-909.
29 Discharges with at least one procedure in nonfederal short-stay hospitals by sex, age, and selected procedures: United States selected years 1990-2006. Table 105. Available at http://www.cdc.gov/nchs/data/hus/hus08.pdf#105
30 Flemming KD, Brown RDJr, Petty GW, et al. Evaluation and management of transient ischemic attack and minor cerebral infarction. Mayo Clin Proc. 2004;79:1071-1086.
31 Huston J3rd, Fain SB, Riederer SJ, et al. Carotid arteries: maximizing arterial to venous contrast in fluoroscopically triggered contrast-enhanced MR angiography with elliptic centric view ordering. Radiology. 1999;211:265-273.
32 U-King-Im JM, Hollingworth W, Trivedi RA, et al. Cost-effectiveness of diagnostic strategies prior to carotid endarterectomy. Ann Neurol. 2005;58:506-515.
33 Vanninen R, Manninen H, Koivisto K, et al. Carotid stenosis by digital subtraction angiography: reproducibility of the European Carotid Surgery Trial and the North American Symptomatic Carotid Endarterectomy Trial measurement methods and visual interpretation. AJNR Am J Neuroradiol. 1994;15:1635-1641.
34 Toole JF, Castaldo JE. Accurate measurement of carotid stenosis. Chaos in methodology. J Neuorimaging. 1994;4:222-230.
35 Neale ML, Chambers JL, Kelly AT, et al. Reappraisal of duplex criteria to assess significant carotid stenosis with special reference to reports from the North American Symptomatic Carotid Endarterectomy Trial and the European Carotid Surgery Trial. J Vasc Surg. 1994;20:642-649.
36 Erickson BJ, Cole BA, Huston J. Semiautomated quantitation of carotid artery stenosis in gadolinium-bolus magnetic resonance angiography. J Digit Imaging. 2002;15:69-77.
37 Scaroni R, Cardaioli G, Pelliccioli GP, et al. Spiral computed tomography angiography (SCTA) and color coded duplex ultrasound (CCDUS): two complementary diagnostic techniques for assessment of extracranial cerebral artery stenosis. Clin Exp Hypertens. 2002;24:659-668.
38 Honish C, Sadanand V, Fladeland D, et al. The reliability of ultrasound measurements of carotid stenosis compared to MRA and DSA. Can J Neurol Sci. 2005;32:465-471.
39 Hacklander T, Wegner H, Hoppe S, et al. Agreement of multislice CT angiography and MR angiography in assessing the degree of carotid artery stenosis in consideration of different methods of postprocessing. J Comput Assist Tomogr. 2006;30:433-442.
40 Yuan C, Kerwin WS, Yarnykh VL. MRI of atherosclerosis in clinical trials. NMR Biomed. 2006;19:636-654.
41 Primatesta P, Allender S, Ciccarelli P, et al. Cardiovascular surveys: manual of operations. Eur J Cardiovasc Prev Rehabil. 2007;14:S43-S61.
42 Thom T, Haase N, Rosamond W, et al. Heart disease and stroke statistics—2006 update: a report from the American Heart Associate Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:e85-e151.
43 Whitty CJ, Sudlow CL, Warlow CP. Investigating individual subjects and screening populations for asymptomatic carotid stenosis can be harmful. J Neurol Neuorsurg Psychiatry. 1998;64:619-623.
44 Prevalence of asymptomatic carotid artery stenosis according to age and sex. Stroke. 2009;40:1105-1113.
45 Dodick DW, Meissner I, Meyer FB, et al. Evaluation and management of asymptomatic carotid artery stenosis. Mayo Clin Proc. 2004;79:937-944.
46 Clase CM, Cina CS. Medical management versus investigate-and-operate strategy in asymptomatic carotid stenosis: a decision analysis. J Vasc Surg. 2002;36:541-548.
47 Roederer GO, Langlois YE, Jager KA, et al. The natural history of carotid arterial disease in asymptomatic patients with cervical bruits. Stroke. 1984;15:605-613.
48 Grotta JC, Yatsu FM, Pettigrew LC, et al. Prediction of carotid stenosis progression by lipid and hematologic measurements. Neurology. 1989;39:1325-1331.
49 Fowl RJ, Marsch JG, Love M, et al. Prevalence of hemodynamically significant stenosis of the carotid artery in an asymptomatic veteran population. Surg Gynecol Obstet. 1991;172:13-16.
50 Zhu CZ, Norris JW. Role of carotid stenosis in ischemic stroke. Stroke. 1990;21:1131-1134.
51 AbuRahma AF, Robinson PA. Prospective clinicopathophysiologic follow-up study of asymptomatic neck bruit. Am Surg. 1990;56:108-113.
52 Barnett HJ, Taylor DW, Eliasziw M, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1998;339:1415-1425.
53 Alamowitch S, Eliasziw M, Algra A, et al. Risk, causes, and prevention of ischaemic stroke in elderly patients with symptomatic internal carotid artery stenosis. Lancet. 2001;357:1154-1160.
54 Ferguson GG, Eliasziw M, Barr HW, et al. The North American Symptomatic Carotid Endarterectomy trial. Surgical results in 1415 patients. Stroke. 1999;30:1751-1758.
55 Mayberg MR, Wilson SE, Yatsu F, et al. Carotid endarterectomy and prevention of cerebral ischemia in symptomatic carotid stenosis. Veterans Affairs Cooperative Studies Program 309 Trialist Group. JAMA. 1991;266:3289-3294.
56 Rothwell PM, Eliasziw M, Gutnikov SA, et al. Analysis of pooled data from the randomized controlled trials of endarterectomy for symptomatic carotid stenosis. Lancet. 2003;361:107-116.
57 Rothwell PM, Eliasziw M, Gutnikov SA, et al. Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery. Lancet. 2004;363:915-924.
58 Gasecki A, Eliasziw M, Ferguson GG, et al. Long-term prognosis and effect of endarterectomy in patients with symptomatic severe carotid stenosis and contralateral carotid stenosis or occlusion: results from NASCET. J Neurosurg. 1995;83:778-782.
59 Barnett HJ. The inappropriate use of carotid endarterectomy. CMAJ. 2004;171:473-474.
60 Hennerici M, Hulsbomer HB, Hefter H, et al. Natural history of asymptomatic extracranial arterial disease: results of a long-term prospective study. Brain. 1987;110:777-791.
61 Chambers BR, Norris JW. Outcome in patients with asymptomatic neck bruits. N Engl J Med. 1986;315:860-865.
62 Lewis RF, Abrahamowicz M, Cote R, et al. Predictive power of duplex ultrasonography in asymptomatic carotid disease. Ann Intern Med. 1997;127:13-20.
63 Autret A, Pourcelot L, Saudeau D, et al. Stroke risk in patients with carotid stenosis. Lancet. 1987;1:888-890.
64 Roederer GO, Langlois YE, Jager KA, et al. The natural history of carotid arterial disease in asymptomatic patients with cervical bruits. Stroke. 1984;15:605-613.
65 Norris JW, Zhu CZ. Stroke risk and critical carotid stenosis. J Neurol Neurosurg Psychiatry. 1990;53:235-237.
66 Shanik GD, Moore DJ, Leahy A, et al. Asymptomatic carotid stenosis: a benign lesion? Eur J Vasc Surg. 1992;6:10-15.
67 Bock RW, Gray-Weale AC, Mock PA, et al. The natural history of asymptomatic carotid artery disease. J Vasc Surg. 1993;17:160-169.
68 Javid H, Ostermiller WEJr, Hengesh JW, et al. Natural history of carotid bifurcation atheroma. Surgery. 1970;67:80-86.
69 CASANOVA Study Group. Carotid surgery versus medical therapy in asymptomatic carotid stnosis. Stroke. 1991;22:1229-1235.
70 Mayo Asymptomatic Carotid Endarterectomy Study Group. Results of a randomized controlled trial of carotid endarterectomy for asymptomatic carotid stenosis. Mayo Clin Proc. 1992;67:513-518.
71 Hobson RWII, Weiss DG, Fields WS, et al. Veterans Affairs Cooperative Study Group. Efficacy of carotid endarterectomy for asymptomatic carotid stenosis. N Engl J Med. 1993;328:221-227.
72 Clagett GP, Youkey JR, Brigham RA, et al. Asymptomatic cervical bruit and abnormal ocular pneumoplethysmography: a prospective study comparing two approaches to management. Surgery. 1984;96:823-830.
73 Moore WS, Barnett HJM, Beebe HG, et al. Guidelines for carotid endarterectomy. A multidisciplinary consensus statement from the Ad Hoc Committee, American Heart Association. Stroke. 1995;26:188-201.
74 Biller J, Feinberg WM, Castaldo JE, et al. Guidelines for carotid endarterectomy: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 1998;29:554-562.
75 Endovascular versus surgical treatment in patients with carotid stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): a randomized trial. Lancet. 2001;357:1729-1737.
76 Alberts MJ. Results of a multicenter prospective randomized trial of carotid artery stenting vs. carotid endarterectomy [abstract 53]. Stroke. 2001;32:325.
77 Yadav JS. Carotid stenting in high-risk patients: design and rationale of the SAPPHIRE trial. Cleve Clin J Med. 2004;71(Suppl 1):S45-S46.
78 CARESS Steering Committee. Carotid revascularization using endarterectomy or stenting systems (CARESS): phase I clinical trial. J Endovasc Ther. 2003;10:1021-1030.
79 Howard VJ, Voeks JH, Lutsep HL, et al. Does sex matter? Thirty-day stroke and death rates after carotid artery stenting in women versus men: results from the Carotid Revascularization Endarterectomy versus Stenting Trial (CREST) lead-in phase. Stroke. 2009;40:1140-1147.
80 Sundt TMJr. Techniques of carotid endarterectomy. In: Sundt TMJr, editor. Occlusive Cerebrovascular Disease. Diagnosis and Surgical Management. Philadelphia: Saunders; 1987:191-225.
81 Pearson BW, Sundt TMJr. Exposure of the distal internal carotid artery. In: Sundt TMJr, editor. Occlusive Cerebrovascular Disease. Diagnosis and Surgical Management. Philadelphia: Saunders; 1987:298-302.
82 Fortes FS, da Silva ES, Sennes LU. Mandibular subluxation for distal cervical exposure of the internal carotid artery. Laryngoscope. 2007;117:890-893.
83 Beretta F, Hemida SA, Andaluz N, et al. Exposure of the cervical internal carotid artery: surgical steps to the cranial base and morphometric study. Neurosurgery. 59(1 suppl 1), 2006.
84 Sharbrough FW, Messick JMJr, Sundt TMJr. Correlation of continuous electroencephalograms with cerebral blood flow measurements during carotid endarterectomy. Stroke. 1973;4:674-683.
85 Ecker RD, Pichelmann MA, Meissner I, et al. Durability of carotid endarterectomy. Stroke. 2003;34:2941-2944.
86 Archie JPJr. A fifteen-year experience with carotid endarterectomy after a formal operative protocol requiring highly frequent patch angioplasty. J Vasc Surg. 2000;31:724-735.
87 Biasi GM, Sternjakob S, Mingassini PM, et al. Nine-year experience of bovine pericardium patch angioplasty during carotid endarterectomy. J Vasc Surg. 2002;36:271-277.
88 Cao P, Giordano G, De Rango P, et al. Eversion versus conventional carotid endarterectomy: late results of a prospective multicenter randomized trial. J Vasc Surg. 2000;31:19-30.
89 Hertzer NR, Beven EG, O’Hara PJ, et al. A prospective study of vein patch angioplasty during carotid endarterectomy: three-year results for 801 patients and 917 operations. Ann Surg. 1987;206:628-635.
90 Lawhorne TWJr, Brooks HB, Cunningham JM. Five hundred consecutive carotid endarterectomies: emphasis on vein patch closure. Cardiovasc Surg. 1997;5:141-144.
91 Scavee V, Viejo D, Buche M, et al. Six hundred consecutive carotid endarterectomies with temporary shunt and vein patch angioplasty: early and long-term results. Cardiovasc Surg. 2001;9:463-468.
92 Shah DM, Darling RCIII, Chang BB, et al. Carotid endarterectomy by eversion technique: its safety and durability. Ann Surg. 1998;228:471-478.
93 Trisal V, Paulson T, Hans SS, et al. Carotid artery restenosis: an ongoing disease process. Am Surg. 2002;68:275-280.