CHAPTER 351 Carotid Artery Angioplasty and Stenting
Ischemic stroke is caused by occlusion of an artery supplying the cerebral vasculature. Although most ischemic strokes result from release of an embolus from the heart, atherosclerotic disease in the carotid arteries is thought to be the cause in up to 30% of ischemic strokes. With approximately 795,000 new or recurrent (≈80% of which are ischemic) strokes occurring annually in the United States,1 carotid artery disease is a major cause of the disability and mortality associated with strokes. Landmark prospective randomized trials, including the North American Symptomatic Carotid Endarterectomy Trial (NASCET),2,3 Asymptomatic Carotid Atherosclerosis Study (ACAS),4 Asymptomatic Carotid Surgery Trial (ACST),5 and European Carotid Surgery Trial (ECST),6,7 established that carotid endarterectomy (CEA) was an effective means of future stroke prevention in at-risk populations with significant carotid disease. Similar to the evolution of endovascular strategies in the treatment of ischemic coronary artery disease resulting in a gradual shrinkage in the number of open coronary artery bypass grafting (CABG) procedures performed, there is an ongoing progressive shift toward endovascular strategies for revascularization of carotid artery disease. Ischemic stroke remains the major cause of adult disability and the third-leading cause of adult mortality, and carotid revascularization remains the principal surgical tool for the management of this disease.1 This is corroborated by an estimated 99,000 inpatient CEAs performed in the United States in 2006.1 In this chapter we briefly describe the historical development of endovascular strategies for the management of carotid artery disease, the current status of evidence supporting carotid angioplasty and stenting (CAS), current indications for and current techniques in performing CAS, perioperative management, complications and their management, and finally, new frontiers for the endovascular treatment of carotid disease.
Historical Perspective
Mathias and colleagues performed the first reported angioplasty of a carotid bifurcation in 1980.8 The indications initially were mostly nonatherosclerotic disease (radiation-induced or inflammatory stenosis); however, soon there was a push toward atherosclerotic disease, and that resulted in the discovery of an inordinate risk for iatrogenic dissection (5% to 8%) and distal embolic complications (8% to 10%).9,10 This initial finding resulted in the impetus for discovery of a distal embolic shower protection (DEP) device. The first attempts were simply distal occlusion of the internal carotid artery through a balloon parked in parallel to the more proximal stent delivery system, followed by aspiration of blood and debris after angioplasty. This was refined by the development of a wire-mounted balloon (PercuSurge GuardWire; Medtronic AVE/PercuSurge Inc., Sunnyvale, CA) that allowed a balloon to be passed through the lesion for angioplasty while there was distal flow arrest. However, the results from carotid angioplasty remained dismal, principally from the high rate of recurrent stenosis, as well as procedural complications. Parallel development of coronary and peripheral vascular stents eventually resulted in the trial of a stent at the carotid bifurcation with inherent jailing of the external carotid artery in 1990.11 This step single-handedly jettisoned CAS into a viable solution initially for patients considered very poor candidates for CEA.
The revolution that ensued has seen a wide variety of balloons for angioplasty, stents designed specifically for the carotid anatomy, an array of distal protection devices, and most recently, an entirely new way of distal protection achieved through flow reversal from the internal carotid artery into the arterial guide sheath, which is the conduit for deployment of devices across the carotid bifurcation. The major impetus for advancement of CAS came with publication of the results of the Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy (SAPPHIRE) trial,12 which effectively demonstrated that patients considered to be at high risk with CEA were less likely to have complications if treated by CAS. This trial resulted in Food and Drug Administration (FDA), Centers for Medicare & Medicaid Services (CMS), and Medicare approval of CAS as a viable option in such patients. This brings us to the current era, during which a large number of trials that have been completed and others that are ongoing continue to refine our understanding of the uses and restrictions of CAS. The future for CAS appears to be clear: it is here to stay.
Carotid Endarterectomy Trials
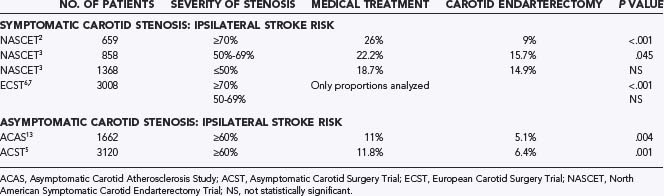
Indications for and outcomes of CEA have been extensively studied. Support for performance of CEA is generated from four well-designed multicenter, randomized clinical trials—NASCET,2,3 ECST,6,7 ACAS,13 and ACST.5 NASCET2,3 and ECST6,7 addressed the use of CEA for symptomatic patients with 70% to 99% carotid stenosis or selected patients with 50% to 69% stenosis (Table 351-1). These studies resulted in class IA indications for the use of CEA in symptomatic patients meeting the appropriate criteria.14 However, it is important to realize that the general population of patients with carotid stenosis has substantially different demographics than do those who met the strict eligibility criteria for these studies.15 For instance, NASCET excluded patients 80 years and older and those with intracranial carotid stenosis more severe than the surgically accessible lesion; liver, kidney, or lung failure; cardiac valve or rhythm disorder; previous ipsilateral CEA; uncontrolled hypertension or diabetes; recent myocardial infarction (MI); or major surgery.2 Such patients were considered to have excessive perioperative morbidity (i.e., high risk). Since publication of the NASCET results, patients considered for carotid revascularization are often divided into low- and high-risk groups, and this surgical risk stratification has been applied as an integral part of the study design in recent CAS trials.
The ACAS trial13 and the ACST5 addressed the use of CEA in asymptomatic patients. The degree of benefit from CEA for asymptomatic lesions is substantially less, and the indications for revascularization are still debated. ACAS and ACST demonstrated a 5.4% to 5.9% absolute reduction in risk over a 5-year period.5,13 Therefore, periprocedural risks are particularly relevant to the decision analysis for the treatment of asymptomatic patients, with a morbidity rate higher than 3% minimizing any benefit. Nonetheless, as a result of publication of the ACAS trial, nearly 75% of CEAs in the United States are performed on asymptomatic patients.16
In the aforementioned trials, carefully selected low-risk patients were treated by highly experienced surgeons at high-volume medical centers. The low complication rates seen in NASCET and ACAS are often not obtained in the general population. Studies have shown perioperative stroke and death rates to range from 0%10 to 11.1%17 for symptomatic patients and from 0%18 to 5.5%17 for asymptomatic patients. In fact, a study of Medicare mortality data from hospitals participating in NASCET and ACAS demonstrated a 1.4% perioperative mortality rate15 as compared with 0.6% reported in NASCET2 and 0.1% reported in ACAS.13 Perhaps equally concerning, CEA-related mortality rates have been demonstrated to be higher (2.5%) for low-volume hospitals,15 although other studies have argued that only small differences exist between mortality rates at high- and low-volume hospitals.19
Treatment decisions are also dependent on patient-specific factors. The presence of comorbid disease has a significant influence on outcome after CEA. Perioperative stroke and death rates for common comorbid conditions are 8.6% for congestive heart failure,20,21 7.5% for age older than 75 years,20,21 10.8% for postendarterectomy restenosis,22 13.9% for ipsilateral carotid siphon stenosis,20 10.7% to 17.9% for intraluminal thrombus,20,23 14.3% for contralateral carotid occlusion,24 and 16.4% to 26.2% for CEA combined with CABG.25,26 It is important to note that in the presence of such comorbidity, the natural history of carotid disease itself is grimmer. The investigators of the Asymptomatic Carotid Stenosis and Risk of Stroke (ACSRS) “natural history” study monitored 1115 patients with asymptomatic internal carotid artery stenosis treated with medical therapy alone and identified significant differences in patient subgroups with respect to risk for stroke and death.27 The highest risk group (82% to 99% stenosis according to NASCET criteria,2 history of contralateral transient ischemic attack [TIA], and serum creatinine level >0.085 mmol/L) had a 4.3% annual ipsilateral stroke rate as opposed to 0.7% in the lowest risk group.27,28
It should also be noted that since the aforementioned major randomized CEA trials began, best medical therapy has improved. In NASCET, the primary medical intervention was 1300 mg of aspirin on a daily basis.2 This dose of aspirin is no longer used because lower doses have been proved to be equally efficacious with fewer side effects.29–31 Other antiplatelet drugs, such as clopidogrel and ticlopidine, are also now available,32,33 and the combination of aspirin and dipyridamole was shown to be more efficacious than aspirin alone.34 Methods for blood pressure control were not specified in NASCET, whereas it is now known that blood pressure below 120 to 130/70 mm Hg is optimal for reduction of cardiovascular risk in patients with medical comorbid conditions14,35,36 and that for primary stroke prevention, a 10–mm Hg reduction in systolic blood pressure produces a 31% reduction in the relative risk for stroke.37 For secondary stroke prevention, angiotensin-converting enzyme (ACE) inhibitors35,38 and the combination of a thiazide diuretic with an ACE inhibitor38 have now been proved effective. Additionally, in the past decade, statins have assumed a prominent role in cerebrovascular and cardiovascular risk modification.39–43 In a study of patients receiving medical therapy for severe carotid artery disease, statin use was associated with significantly lower rates of stroke, MI, and death.44 It is likely that improvements in medical therapy for carotid atherosclerotic disease and related comorbidity should prompt periodic reevaluation and fine-tuning of the risk-benefit analysis for medical therapy versus surgical intervention.
With the great deal of complexity regarding risk assessment in this complex patient population, current standards are limited to minimizing overall surgical risk to maximize the probable benefit from surgery. Currently, the guidelines of the American Heart Association/American Stroke Assocation14 and the Canadian Neurosurgical Society45 have established an upper limit of 6% for perioperative risk in symptomatic patients14 and a 3% upper limit in asymptomatic patients, assuming a life expectancy exceeding 5 years.26
Publication of Carotid Angioplasty and Stenting Study Data
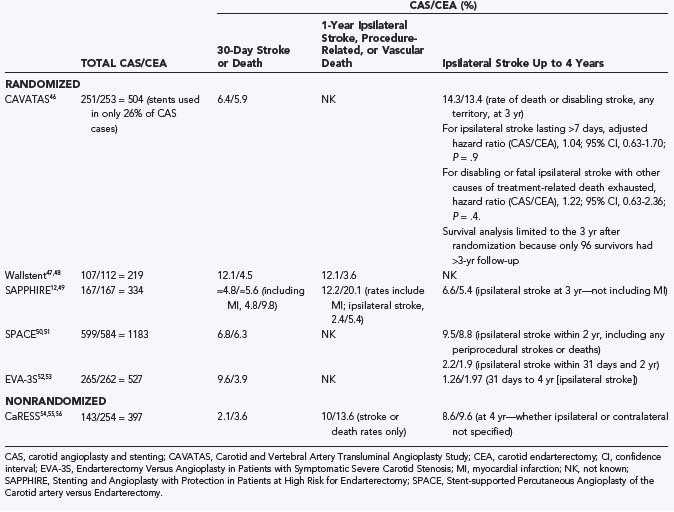
The results of trials comparing endovascular and surgical treatment of carotid stenosis are summarized in Table 351-2.46–56 The first randomized trial comparing these treatments, the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS), included 504 patients enrolled between 1992 and 1997 and was designed to compare balloon angioplasty alone with CEA.46 Stents, when they became available, were incorporated as well but accounted for only 26% of cases. Twenty-four centers in Europe, Australia, and Canada participated, and as in previous CEA trials, high-risk surgical patients were excluded from enrollment, including those with recent MI, poorly controlled hypertension or diabetes mellitus, renal disease, respiratory failure, inaccessible carotid stenosis, or severe cervical spondylosis. CAVATAS demonstrated no statistically significant difference between endovascular and surgical treatment in the rates of disabling stroke or death within 30 days (6.4% for CAS versus 5.9% for CEA) and no significant difference in 3-year ipsilateral stroke rates. It is interesting to note that only 26% of patients randomized to endovascular treatment actually received stents. This becomes even more pertinent when one considers the 3-year ipsilateral stroke rates. These early encouraging results generated a great deal of interest in CAS, and further studies were undertaken.
The Wallstent trial,47,48 the first multicenter randomized trial designed from inception to evaluate CEA and CAS equivalence, enrolled a total of 219 symptomatic patients with 60% to 99% stenosis. Thirty-day stroke or death rates were 12.1% with CAS and 4.5% with CEA (P = .049). Additionally, 12.1% of CAS patients suffered ipsilateral stroke, procedure-related death, or vascular death at 1 year versus 3.6% of CEA patients (P = .022), and as a result, the trial was halted by the Data Safety and Monitoring Committee after an interim analysis demonstrated worse outcomes for the CAS group. Critical to interpreting these results is the fact that distal protection devices were not used in the Wallstent trial. A significant proportion of major CAS neurological complications are due to embolization of atheromatous material.57–60 Devices that capture embolic debris released during CAS have significantly improved procedural safety.57,60–64
One of the first trials to use embolic protection was Carotid Revascularization Using Endarterectomy or Stenting Systems (CaRESS),54,55 a multicenter, nonrandomized, prospective study comparing CAS with embolic protection (n = 143) and CEA (n = 254) in symptomatic (32%) and asymptomatic (68%) patients at low and high surgical risk. An important feature of CaRESS was that the treatment procedure was chosen by the treating physician and the patient, not randomized. Although this study design probably introduced selection bias, the CaRESS trial represents a generalized perspective on carotid revascularization and more closely represents its “real-world” application. Baseline group demographics were similar, except that patients with previous carotid interventions more often underwent CAS. No statistically significant difference between 30-day and 1-year death or stroke rates existed between CAS and CEA (2.1% versus 3.6% and 10.0% versus 13.6%, respectively), nor were significant differences found for restenosis, residual stenosis, repeat angiography, and need for carotid revascularization. Overall morbidity and mortality approached NASCET2,3 and ACAS13 standards and represent the lowest rates among the major CAS trials to date. The low stroke and death rates may be attributable to the ability of the treating physician to consider patient-specific factors and successfully assign each patient to the safest therapy.
CAS was well established as a treatment option for high-risk patients by SAPPHIRE,12 a randomized, multicenter trial designed to determine CAS noninferiority to CEA in high-risk patients. Eligible patients (n = 334) had symptomatic stenosis of at least 50% or asymptomatic stenosis of at least 80%. The 30-day MI, stroke, or death rate was 4.8% for CAS and 9.8% for CEA (P = .09). Much of this difference was secondary to MIs occurring in the CEA group, and although not reported in the SAPPHIRE publication, the 30-day rate of stroke and death was approximately 4.8% for CAS patients and around 5.6% for CEA patients. At 1 year, 12.2% of CAS patients had suffered stroke, MI, or death versus 20.1% of CEA patients (noninferiority analysis: P = .004; superiority analysis: intention to treat, P = .053; as treated, P = .048). MI and major ipsilateral stroke rates were significantly better after CAS than after CEA (2.5% versus 8.1%, P = .03; 0% versus 3.5%, P = .02; respectively).
Because SAPPHIRE had shown such clear noninferiority in high-risk patients, the Stent-supported Percutaneous Angioplasty of the Carotid artery versus Endarterectomy (SPACE) trial50 set out to establish noninferiority for CAS versus CEA in low-risk patients. In this multicenter trial, the safety and efficacy of CAS and CEA were compared in 1183 randomized patients with symptomatic carotid artery stenosis (≥70% by duplex ultrasonography, ≥50% by NASCET criteria,2 or ≥70% by ECST criteria6). The 30-day rates of ipsilateral stroke or death were 6.84% for CAS and 6.34% for CEA (P value not significant).59 It is important to note that embolic protection was not required and was used in just 27% of patients, although a subgroup analysis did not demonstrate a significant difference between patients with embolic protection and those without. Despite these encouraging results, “SPACE failed to prove the non-inferiority of carotid-artery stenting”50 statistically. This is because the trial was halted more than 700 patients shy of its goal enrollment of 1900 as a result of an interim analysis demonstrating that 2500 patients would be needed to reach significance given the results up to that point. The steering committee acknowledged a “lack of funds”50 to expand the trial to an enrollment of 2500 patients and therefore halted the trial. In essence, the study was underpowered to demonstrate noninferiority because of incorrect estimation of the anticipated effect sizes. Still, although its a priori goals were not realized, the 0.51% difference observed in perioperative stroke or death between CAS and CEA was not statistically significant and is well within the published differences between individuals, institutions, and variations of CEA.
The SPACE results, even though they were negative, were still quite encouraging to CAS proponents. Unfortunately, a second multicenter, randomized trial to assess the noninferiority of CAS versus CEA in patients with more than 60% stenosis, Endarterectomy Versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis (EVA-3S), was ended after interim analysis (n = 527) demonstrated the 30-day rate of any stroke or death to be significantly higher in the CAS group (9.6%) than in the CEA group (3.9%) (P = .01).52 Importantly, early in the trial the use of embolic protection was not required. Patients treated without embolic protection experienced a 25% 30-day rate of stroke or death (5 of 20 patients), which prompted changes in protocol by the EVA-3S safety committee. Additionally, EVA-3S compared groups of physicians with unequal experience. Surgeons performing CEA had performed at least 25 endarterectomies in the year before trial entry, yet endovascular physicians were certified after completing as few as 5 to 12 CAS procedures (5 CAS among at least 35 stent procedures on supra-aortic vessels or 12 CAS). Endovascular physicians were also allowed to enroll study patients while simultaneously undergoing training and certification. Subgroup analysis based on CAS physician experience demonstrated a 12.3% stroke and death rate for endovascular physicians tutored in CAS during the trial,52 7.1% for those tutored in CAS during their endovascular training, and 10.5% for physicians with experience in CAS. The resulting overall rate of stroke and death (9.6%) is substantially higher than that in other randomized trials. Therefore, it is hard to accept such an elevated complication rate as representative of the practice of CAS in general. It is more likely that EVA-3S emphasizes the importance of embolic protection, as well as rigorous training and credentialing for CAS physicians. The implied importance of embolic protection in EVA-3S has been further supported by numerous imaging studies examining the frequency of (mostly small, asymptomatic) ischemic lesions on postoperative magnetic resonance imaging (diffusion-weighted imaging [DWI]). These studies have demonstrated the following: a reduction in the frequency of lesions seen on DWI with distal embolic protection (49% versus 67%)62 and fewer lesions noted on DWI after CEA than after CAS (11.6% versus 42.6%, no significant clinical difference) with current embolic protection devices,65 as well as a low frequency of DWI-confirmed lesions with more recent embolic protection devices, such as the Parodi flow reversal system (NeuroProtection System, W. L. Gore & Associates, Flagstaff, AZ),66 at a rate not significantly different from that incurred by diagnostic cerebral angiography alone (18.2% versus 11.5%).67
Carotid registries are nonrandomized outcome records for symptomatic and asymptomatic high-risk CAS patients. These registries include Arbeitsgemeinschaft Leitende Kardiologische Krankenhausarzte (ALKK); ACCULINK for Revascularization of Carotids in High-Risk patients (ARCHeR); Boston Scientific EPI: A Carotid Stenting Trial for High-Risk Surgical Patients (BEACH); Carotid Artery Revascularization using the Boston Scientific FilterWire EX/EZ (CABERNET); Carotid Acculink/Accunet Post Approval Trial to Uncover Unanticipated or Rare Events (CAPTURE); Carotid Artery Stenting with Emboli protection Surveillance—Post Marketing Study (CASES-PMS); and Carotid Revascularization with ev3 Arterial Technology Evolution (CREATE) (Table 351-3).68–75 Although registries do not provide direct comparison data, they do help establish true adverse event rates in high-risk CAS patients and are a crucial component for improving our understanding of the risks associated with CAS. The collaborators of CABERNET found a 4.0% 30-day rate of death, stroke, and MI (N = 446 patients),71 whereas the investigators of ARCHeR (N = 581 patients) found a 30-day stroke or death rate of 6.9%, as well as a 1-year composite outcome (30-day rate of MI, stroke, or death plus the 1-year rate of ipsilateral stroke) of 9.6%.69 CREATE (N = 419 patients) demonstrated a 6.2% 30-day rate of MI, stroke, and death.75 The CAPTURE registry (N = 3500) determined that the post-CAS incidence of stroke, MI, and death was 6.3% for patients treated with the Acculink/AccuNet CAS system (Abbott Vascular, Santa Clara, CA), as well as a rate of major stroke or death of 2.9%.72,73 The BEACH investigators (N = 747 patients) found a 30-day MI, stroke, or death rate of 5.8%.70 These results were similar to those in the CASES-PMS registry (5.0%), which examined the use of distal protection by endovascular carotid surgeons who either had previous experience with the device (AngioGuard XP, Cordis Endovascular, Warren, NJ) or underwent formal training (N = 1493).74 Under these rigorous conditions, the 30-day major adverse event rate did not vary significantly between symptomatic and asymptomatic patients and among physicians with high and low volume or differing level of experience with the specific distal protection device. The German ALKK registry (N = 1888 patients), which included patients with standard risk, demonstrated an in-hospital death and stroke rate of 3.8%.68 Interestingly, when this risk was stratified by time, the investigators saw improvement from 6.3% in 1996 to 1.9% in 2004 (P = .021). Continued effort to maintain rigorous registries such as those just described is critical to our eventual understanding of appropriate patient selection and procedural risks.
| TRIAL | NO. OF PATIENTS | PERIOPERATIVE EVENT RATES (%) |
|---|---|---|
| ALKK68 | 1888 | 3.8 for in-hospital death and stroke |
| ARCHeR69 | 581 | 6.9 for stroke or death |
| BEACH70 | 747 | 5.8 for MI, stroke, or death |
| CABERNET71 | 446 | 4.0 for MI, stroke, and death |
| CAPTURE72,73 | 3500 | 6.3 for MI, stroke, and death |
| CASES-PMS74 | 1493 | 5.0 for MI, stroke, or death |
| CREATE75 | 419 | 6.2 for MI, stroke, or death |
ALKK, Arbeitsgemeinschaft Leitende Kardiologische Krankenhausarzte; ARCHeR, ACCULINK for Revascularization of Carotids in High-Risk patients; BEACH, Boston Scientific EPI: A Carotid Stenting Trial for High-Risk Surgical Patients; CABERNET, Carotid Artery Revascularization using the Boston Scientific FilterWire EX/EZ; CAPTURE, Carotid Acculink/Accunet Post Approval Trial to Uncover Unanticipated or Rare Events; CASES-PMS, Carotid Artery Stenting with Emboli protection Surveillance—Post Marketing Study; CREATE, Carotid Revascularization with ev3 Arterial Technology Evolution; MI, myocardial infarction.
Current Studies
The two major most recently completed randomized trials of CAS versus CEA are the Carotid Revascularization Endarterectomy versus Stent Trial (CREST) and the International Carotid Stenting Study (ICSS). CREST was a National Institutes of Health–funded, multicenter randomized trial that enrolled 2502 patients with greater than 50% symptomatic carotid stenosis or greater than 70% asymptomatic stenosis for randomization to CEA or CAS.76–80 Primary end points included death, stroke, or MI at 30 days and ipsilateral stroke within 60 days. CREST maintained a rigorous credentialing phase for CAS providers. CREST demonstrated no significant difference in the primary endpoint (30-day MI, stroke and death rate) between CAS (5.2%) as compared with CEA (4.5%) (p = 0.38). The major differences between the two treatments were that minor strokes (not major) were more common following CAS (3.2 vs. 1.7%, p = 0.01) whereas MI (1.1 vs. 2.3%, p = 0.03) and cranial never injury (0.3 vs. 4.7%, p < 0.0001) was more common after CEA. ICSS resulted from the favorable results of CAVATAS and is also known as CAVATAS-2. ICSS81 randomized 1713 patients; in this study, the primary endpoint, similar to CREST, were more common after CAS (8.5% vs. 5.2%, p < 0.006). However, similar to SPACE and EVA-3S, there were issues regarding credentialing being more rigorous for the group performing CEA, as compared to CAS.
ICSS resulted from the favorable findings of CAVATAS and is also known as CAVATAS-2.81 It is a multinational prospective trial randomizing symptomatic patients equally suited for CAS or CEA. Additionally, lessons learned from EVA-3S are being applied. Attendance at a CAS training course is required, as well as mandatory proctoring for centers with limited experience admitted to the trial on a probationary status. Furthermore, embolic protection is recommended whenever the endovascular physician believes that a protection device can be safely deployed.
An additional ongoing study is the Asymptomatic Carotid Stenosis, Stenting versus Endarterectomy Trial (ACT I), a randomized trial consisting of low-risk patients with asymptomatic 80% to 99% carotid stenosis at multiple centers across North America.82 The primary outcomes will be 30-day MI, stroke, and death rates and 5-year stroke-free survival. Another study, the Transatlantic Asymptomatic Carotid Interventional Trial (TACIT) will randomize standard- and high-risk patients with asymptomatic carotid stenosis into one of three treatment arms: optimal medical therapy only (antiplatelet, antilipidemic, antihypertensive, strict diabetes control, and smoking cessation), optimal medical therapy plus CEA, or optimal medical therapy plus CAS with embolic protection.83 Planned enrollment is 2400 patients with a primary end point of occurrence of stroke and death at 3 years. Secondary end points include rates of TIA and MI, economic cost, quality-of-life analysis, neurocognitive function, and carotid restenosis.
Most recently, preliminary results have been made available for the Parodi flow reversal system (Gore Neuroprotection System) in the Embolic Protection with Reverse Flow (EMPiRE) trial, which demonstrated a 30-day rate of TIA, stroke, MI, and death of 4.5%.84 Additionally, the results of the Evaluating the Use of the FiberNet Embolic Protection System (EPS) in Carotid Artery Stenting (EPIC) trial demonstrated a 30-day rate of 3% for TIA, stroke, MI, and death (N = 237 patients) when using the FiberNet distal embolic protection system (Lumen Biomedical, Plymouth MN).85 Continuing efforts and eventual completion of these trials or publication of the final results of these trials will improve our understanding of the relative indications for and contraindications to CAS and CEA.
Indications for Carotid Angioplasty and Stenting
The current indications for CAS are principally based on FDA and CMS approval for patients considered to be at high risk with CEA. As additional data become available from CREST, ICSS, ACT I, and other ongoing trials, further broadening of the indications for CAS is expected. However, at present, CAS should ideally be performed either in the setting of a patient with high surgical risk or after enrollment in an ongoing clinical trial. Guidelines for high surgical risk are principally derived from NASCET2 and other CEA studies, as are indications for CAS. Therefore, CAS is approved for patients who are at high risk with CEA and have greater than 70% symptomatic carotid stenosis.
High-risk features for CEA include medical comorbid conditions, most prominently concurrent coronary artery disease causing angina and requiring CABG, recent or ongoing MI, and congestive heart failure (Table 351-4). Other medical risk factors extrapolated from NASCET include uncontrolled hypertension or diabetes and lung, liver, or renal failure. Additionally, CEA is considered high risk in patients older than 80 years. Surgical high-risk features include previous ipsilateral CEA or other perilesional surgery, previous neck irradiation, tracheostomy, or contralateral laryngeal palsy, all of which are related to the higher risk posed by surgical dissection during CEA. Anatomic considerations include a carotid bifurcation (lesion) located above the C2 vertebral body or below the clavicle or a very short neck and relatively high bifurcation, as well as severe neck arthritis causing a marked reduction in neck mobility during positioning for CEA. Another category of risk factors pertains to the available cerebrovascular reserve that renders CEA high risk, such as contralateral carotid occlusion and tandem lesions beyond the accessible cervical carotid artery. Similarly, neurological instability is also a recognized high-risk feature for CEA, including crescendo TIAs, stroke in evolution, TIAs while receiving heparin, multiple strokes, recent stroke, and acute occlusion.
| Anatomic |
Procedural Technique
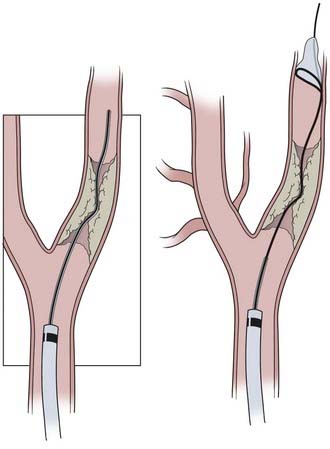
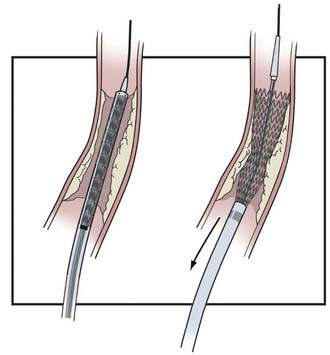
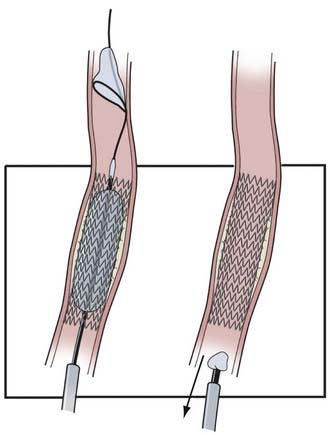
CAS continues to evolve as a result of developing technology, evidence-based medicine, and operator experience. Here we describe our current routine for most CAS procedures; major steps for the procedure are illustrated in Figures 351-1 to 351-5. We briefly describe specific technologic considerations to entertain when planning CAS.
The processes of angioplasty and stenting create intimal injury that promotes thrombosis.86 Therefore, patient preparation with adequate antiplatelet and anticoagulation therapy is essential. Patients receive a dual antiplatelet regimen consisting of aspirin (325 mg daily) and a thienopyridine derivative (i.e., clopidogrel, 75 mg daily, or ticlopidine, 250 mg twice daily) for at least 3 days before stent treatment. A loading dose of clopidogrel (300 to 600 mg) administered early on the day of the procedure is an alternative for patients who are already taking aspirin. An intravenous bolus dose of heparin (50 to 60 U/kg) is administered after catheterization of the common carotid artery. An activated coagulation time of 250 to 300 seconds is maintained throughout the procedure. The heparin infusion is usually discontinued at the conclusion of the procedure.
Device Selection
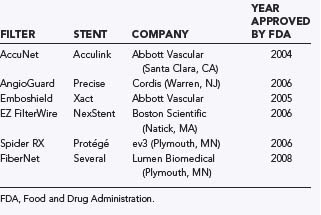
FDA-approved distal protection devices include the AngioGuard (Cordis), FilterWire EZ (Boston Scientific), AccuNet (Abbott), Emboshield (Abbott), Spider RX (ev3), and FiberNet (Lumen Biomedical) (Table 351-5). The AngioGuard has 100-µm pores and a crossing profile of 3.2 to 3.9 French. The FilterWire EZ has a crossing profile of 3.2 French with 80-µm pores. The AccuNet has 115-µm pores and a crossing profile of 3.5 to 3.7 French. The Emboshield has 140-µm pores and a 2.9 to 3.3 French crossing profile. Spider RX provides the additional option of being deployable across any 0.14-inch guidewire of the operator’s choice; however, its pore size varies, being smallest at the apex and larger at the periphery. All these devices are polyurethane membranes or nitinol mesh on a nitinol frame. The FiberNet has a very different design with radially arranged fibers that purportedly can filter particles as small as 40 µm without compromising flow. There are no clear data that support the use of one of these devices over another. We select the device on the basis of ease of use, crossing profile, and pore size.
Periprocedural Management
Hydration is essential before, during, and after the procedure. In patients who have concurrent renal dysfunction, hydration includes alkalinization of the urine with three ampules of sodium bicarbonate added to each liter of 5% dextrose in normal saline for prophylaxis against contrast-related nephropathy. In others, 5% dextrose in normal saline (0.9% NaCl) will suffice. Intravenous hydration should be maintained for up to 24 hours after the procedure. CAS at the carotid bulb often results in a sustained vagal bradycardia, which during the procedure may be minimized by the administration of anticholinergic agents such as glycopyrrolate. Postprocedure hypotension should be aggressively managed with vasoconstrictors such as Neo-Synephrine or intravenous infusion of dopamine. The goal is to keep the patient in a normotensive condition. Frequently, carotid artery disease is associated with coronary artery disease, and sustained hypotension is a harbinger of myocardial ischemia. Conversely, sustained hypertension after CAS revascularization may be associated with a cerebral hyperperfusion syndrome and, in some cases, with breakthrough hyperperfusion hemorrhage with disastrous consequences.87 Hence, normotension should be continuously maintained with a systolic blood pressure between 110 and 150 mm Hg. This requires postoperative observation in a continuously monitored setting. If a closure device has not been used, the arterial sheath should be removed when the activated coagulation time is less than 150 seconds. The patient is usually discharged the afternoon after the procedure if maintaining hemodynamic parameters without intravenous infusions and if neurologically unchanged after the procedure. Patients require surveillance imaging to evaluate vessel patency. Duplex ultrasonographic evaluation should be obtained before discharge; at 6 weeks, 3 months, 6 months, and 1 year; and then annually thereafter. The dual antiplatelet regimen of aspirin and clopidogrel (or ticlopidine) is maintained for 12 weeks after the procedure, after which patients remain on aspirin therapy.
Procedural Durability
The durability of carotid revascularization with CAS is a concern frequently expressed by the surgical community. In a retrospective study of patients undergoing stenting for de novo (119 arteries) and postendarterectomy (76 arteries) carotid stenosis, 80% or greater stenosis was detected by follow-up Doppler imaging in 5.2% of the vessels stented.88 Restenosis after endarterectomy was the major risk for in-stent restenosis. Significant (symptomatic or ≥80%) recurrent stenosis was detected by follow-up Doppler imaging in 6 (5%) of 112 patients in our CAS series.89 The 3-year follow-up of the SAPPHIRE trial revealed a 4% recurrent stenosis rate after CAS.49 This rate compares favorably with the 0% to 7.9% risk for restenosis reported after endarterectomy in large series.90 Furthermore, an increasing body of evidence is indicating a persistent benefit after CAS. It appears that the majority of the risk associated with the procedure occurs within the periprocedural period and up to 30 days afterward. Thereafter, the risk falls significantly and remains low for the duration of these studies. SAPPHIRE suggested a 6.6% ipsilateral stroke rate at 3 years as compared with 5.4% after CEA, including the periprocedural risk (see Table 351-2).49 The 2-year data from the SPACE trial suggest a 2.2% risk for ipsilateral stroke after CAS versus 1.9% after CEA (between postprocedure day 30 and 2 years).88 Similarly, EVA-3S had a 1.26% stroke rate after CAS as opposed to 1.97% after CEA (between postprocedure day 30 and 4 years).53
Complications and Their Management
Risk Stratification
Patient selection is the most important factor in minimizing complications associated with CAS.20,91 Categories of major risk factors for CAS include medical, neurological, anatomic, and genetic arteriopathy. Age is often listed as a risk factor, but it is the anatomic challenges and medical comorbid conditions associated with age that increase the risk for most patients.64,92,93 Medically, the major risk factor in patients with carotid stenosis is MI. A sudden decline in blood pressure and the onset of severe bradycardia present major risk for MI in patients with severe left main coronary artery disease or severe triple-vessel disease (or both). In this group, if CAS is necessary before coronary intervention, minimal or no dilation of the stent after deployment will generally avoid major hemodynamic swings, and the patient can undergo cardiac surgery, with a plan made for follow-up and retreatment if necessary. Neurological risk increases with recent large infarction, crescendo TIAs, and stroke in evolution. Large infarctions present a significant risk for hemorrhage.94,95 Traditionally, patients with large infarction are allowed to “heal” their stroke for 6 weeks before intervention. Patients with active TIAs or stroke in evolution need to be treated but are at higher risk for neurological injury.
Patients with high-risk anatomy may include those with calcified tortuous aortic arches, tortuous and severe iliofemoral disease, and proximal or distal tortuosity of the common carotid artery or internal carotid artery (Table 351-6). Patients considered at high risk for performance of CAS include those with long, irregular or concentrically calcified stenosis, pseudo-occlusion, the string sign, carotid artery kinking, and intraluminal thrombus. Not all high-risk patients can be avoided. For example, treatment should probably be delayed for 6 weeks in a patient who has a large completed infarction with territory still at risk. Conversely, treatment should be undertaken promptly in a patient in need of urgent CABG who has crescendo TIAs and an MI. Each case should be evaluated on an individual basis. For patients who are candidates for carotid intervention, it should be remembered that CEA remains a safe and effective operation if CAS is thought to be too risky. In fact, CEA and CAS are amazingly complementary procedures; in situations in which one procedure is high risk, the other is usually feasible with acceptable risk. It is important to remember that backing out of a CAS procedure is rarely a problem for the patient whereas persisting in the face of technical challenges may result in an avoidable stroke.
TABLE 351-6 High-Risk Features for Carotid Artery Angioplasty and Stenting
| ANATOMY | UNFAVORABLE CHARACTERISTIC |
|---|---|
| Iliac vessels and abdominal aorta |
Creative endovascular solutions can be found even for high-risk patients when treatment is deemed necessary. For patients with intraluminal thrombus and symptomatic carotid disease, the traditional treatment has been heparin and warfarin therapy with reevaluation in 6 weeks to 3 months. In four patients with multiple TIA episodes, we used a trapping technique with proximal and distal balloon occlusion with good success.96,97 Flow reversal systems may prove useful in this setting and in the context of kinks that make landing of a DEP device infeasible.98
Low-risk patients are those with either asymptomatic or single retinal or hemispheric TIAs and no previous cardiac history.21 Anatomically, type I aortic arches99 with both straight proximal and distal anatomy provide the easiest anatomic substrate for CAS. Many of these patients have not been treated in the carotid stenting pool that has been reserved for “high-risk” patients. Ongoing low-risk clinical trials such as ACT I will determine how these patients fare with CAS versus CEA.
Complications Associated with the Steps and Tools for Carotid Angioplasty and Stenting
Femoral Artery Access
The femoral artery approach is most commonly used for CAS. Because approximately 33% of patients have both significant carotid artery disease and severe symptomatic peripheral vascular disease,100,101 the operator should also be familiar with radial and brachial approaches. In addition, femoral artery complications are probably more common in this population inasmuch as many have femoral artery disease. Access site complications do not make the surface of primary or secondary end points in most of the major carotid stent trials; consequently, the incidence is not clearly known. Micropuncture kits, single wall puncture, and femoral angiography to evaluate access site anatomy and disease will mitigate many of these complications, such as dissection, occlusion related to the closure device, and groin or retroperitoneal hematoma.
Guide Catheter Placement
The external carotid artery and its branches are often used to support a guidewire during the exchange of a diagnostic catheter for a guide sheath or catheter. This maneuver is thought to be safer than exchanging devices within the common carotid artery because it prevents premature crossing of the lesion and showering of emboli. Additionally, it gives the purchase essential in the setting of a difficult aortic arch that might not be otherwise accessible. In our experience of more than 2000 stenting procedures, five external carotid artery branch artery ruptures occurred as a result of wire perforation in four cases and a misdeployed PercuSurge embolic protection balloon in an additional case.102 The four wire perforations were secondary to advancement of the stiff exchange wire through the facial artery (two patients) and the lingual branch and artery to the sternocleidomastoid (one patient each). These perforations resulted in hematomas that responded to manual pressure in one patient, required embolization with N-butyl cyanoacrylate (glue) or coils in two patients, and required emergency tracheostomy for airway control secondary to massive tongue swelling in one patient.
An equally rare event is external carotid artery embolism to the carotid ophthalmic collaterals, which may lead to retinal embolism and blindness. Anatomically, embolization can occur through middle meningeal and superficial temporal collaterals to the ophthalmic artery or via direct internal carotid artery reconstitution associated with embolic phenomena or retrograde embolization through the external carotid artery to the ophthalmic and retinal branches. Wilentz and colleagues studied 188 consecutive patients undergoing CAS with the Theron-type DEP system (in which debris is flushed into the external carotid artery) or the PercuSurge system with careful funduscopy, fluorescein angiography, and visual field testing.103 Overall, 6 of 118 patients had retinal emboli, which were symptomatic in 2 patients (1.7%). Looking at the two different systems, 13.2% (5 of 38 patients) treated with the Theron system had emboli versus 1.25% in the PercuSurge group. In fact, in the setting of internal carotid artery occlusion, amaurosis fugax from the external carotid artery collateral through the ophthalmic artery retrograde to the retinal artery can occur and responds well to carotid revascularization.103 The predominance of the literature regarding external carotid artery revascularization is from the CEA literature.104–106
Distal Embolic Protection
The technique of DEP has lowered morbidity and mortality rates associated with CAS. Additionally, DEP devices provide excellent platforms on which to perform CAS. Nonetheless, each step of CAS, from crossing the stenosis to retrieval of the DEP device, has potential for complication. Transcranial Doppler (TCD) data have documented hits indicative of embolism at all stages of CAS; however, most TCD-detected emboli are clinically silent. On the basis of TCD data in which protected stenting with the PercuSurge device was compared with unprotected stenting, the highest risk maneuvers for emboli in unprotected stenting, in order from lowest to highest, were predilation angioplasty, stenting, and postdilation angioplasty.53 In the PercuSurge-protected stenting group, guide sheath placement, guidewire manipulation, and deflation of the device were high embolic periods. DEP significantly lowered the risk of hits documented by TCD imaging. Stenting performed in conjunction with filter devices for DEP (FilterWire EX or AccuNet) in a series of 10 patients was monitored with a multifrequency TCD imaging system.107 The system used was capable of distinguishing between gaseous and solid emboli. During deployment of the DEP device, more than 8000 microemboli were detected, with more than 40% being solid. During the stenting procedure, more than 7000 microemboli were detected, again with more than 40% being solid. In none of the patients did clinical sequelae develop. Microemboli occur even in the gentlest hands, but with good patient selection and technique, the occurrence of symptomatic embolic phenomena can be kept low.
The indication for proximal versus distal protection has not been determined. Logically, intraluminal thrombus, soft plaque, and a poor distal landing zone for DEP would be indications for proximal protection. The results of the European Imaging in Carotid Angioplasty and Risk of Stroke (ICAROS) study showed that gray-scale median (GSM) scores of 25 or less (representing echogenic plaque) are associated with higher embolic potential.108 The ICAROS investigators created a prospective registry of 418 CAS cases from 11 centers and recorded GSM scores preprocedurally. Eleven of 155 (7.1%) patients with GSM scores of 25 or less had strokes versus 4 of 263 (1.5%) patients with GSM scores higher than 25 (P = .005). Taking this one step further, the ICAROS investigators validated the use of DEP in patients with GSM scores higher than 25 (P = .01), but not in those with GSM scores of 25 or lower. For these patients, stenting with proximal embolic protection devices or CEA may prove safer.
In arteries in which a kink in the common carotid artery or internal carotid artery has been moved cranially with significant movement of the filter DEP device and in some particularly sensitive arteries, carotid vasospasm can occur. Generally, this is not a clinically or an angiographically significant problem, but stroke has been described in conjunction with sudden severe spasm just after deployment of an AngioGuard embolic protection device.109 Spasm developed in three patients in one of the initial series in which the Emboshield was used, which was flow limiting in two patients.110 In non–flow-limiting spasm, the procedure should be completed and the filter recaptured. Usually, the spasm clears after the passage of a few minutes. Contrast material remaining within the arterial wall should cause concern for dissection. In flow-limiting situations or if significant time has passed and the spasm persists, intra-arterial nitroglycerin (100 to 200 µg) or verapamil (5 to 10 mg) is usually effective. Because these pharmacologic injections can certainly aggravate any ongoing hypotension, intravenous pressors should be at hand to maintain normotension. Distal dissection at the level of the filter can occur, more commonly so with the PercuSurge device than with filter devices. In patients with small, asymptomatic, and non–flow-limiting dissections, clinical observation is recommended. Stenting is warranted if the dissection is symptomatic or flow limiting. Spasm also occurs when a kink in the carotid artery is moved cranially by the DEP device and guide catheter. This can be ignored because it will resolve with retrieval of the device and often resolves after stenting and postdilation angioplasty.
Immediate complications associated with stenting are unusual. With dual antiplatelet therapy for 12 weeks and with arteries larger than 3 mm, acute and subacute thrombosis is uncommon. Subacute thrombosis has occurred twice at the University at Buffalo neurosurgery practice when cardiac surgeons stopped the patients’ antiplatelet medications within the first 2 weeks before CABG. Aspirin use was discontinued before urologic surgery in another patient 3 months after stenting of a carotid dissection with stents extending from the proximal segment of the internal carotid artery to the petrous segment of the internal carotid artery, and the stents became occluded; some degree of abnormal endothelialization was probably present in this patient, and a hypercoagulable state may have been implicated. A dual antiplatelet regimen for 12 weeks after the procedure and aspirin use for life appear to be essential. Drawing on the cardiology literature, early stent thrombosis is probably due to a dissection unrecognized at treatment or an undersized or expanded stent; late thrombosis is probably due to stent-artery mismatch, hypersensitivity, abnormal endothelialization, or poor compliance with antiplatelet medications.111
If arterial occlusion occurs acutely during the procedure, the diagnosis includes severe spasm, dissection, thrombosis from plaque or platelet aggregation, and a filter that is filled with embolic material. As long as there is no dissection, the spasm will resolve. Nitrates or calcium channel blockers can be given, as mentioned earlier. Treatment is required for an occlusive dissection. A microcatheter and microwire need to be brought past the flap into the true lumen to accomplish this. If the clot has not embolized to the intracranial circulation and the patient is asymptomatic, consideration may be given to administering heparin to the patient overnight, checking collateralization with angiography, and performing stenting under proximal flow arrest. In an acutely symptomatic patient, stenting after the administration of a lytic agent and glycoprotein IIb/IIIa inhibitor may be necessary. If the filter is filled with embolic debris, a utility catheter can be used to perform a suction thrombectomy and the filter carefully captured and brought through the stent so that the captured debris is not disturbed. Successful outcomes have been reported in a small series of patients undergoing operative rescue after acute or subacute stent thrombosis.112 However, if the experience with emergency CEA for treatment of acute carotid occlusion is a guide, the associated morbidity and mortality rate is greater than 20%.113 The hope is that with CAS, where we have the ability to visualize and access the entire cerebral vascular system, and with newly evolving techniques for acute stroke treatment, we will have better salvage procedures and success with endovascular approaches.
Intracranial Complications
The intracranial complications of carotid stenting can be grouped into large-vessel occlusion, shower of emboli, and hemorrhage. All three sources should be entertained in a patient with an acute or delayed neurological change not explained at the cervical level. In the acute setting, cerebral angiography should be performed to look for vessel cutoff or slow flow and emptying. If a clear large-vessel cutoff can be seen, an immediate attempt should be undertaken to recanalize the occluded vessel. A microcatheter (0.014-inch lumen or larger) should be brought through the lesion to confirm patency of the distal vessel and the length of the occlusion. In cases of acute stroke at the University at Buffalo, we have been using the microcatheter for the Merci device (Concentric Medical, Mountain View, CA) as the initial catheter to obviate the necessity for additional exchange maneuvers. One or two passes with the Merci retriever should be attempted. The other approved device in this setting is the Penumbra catheter system (Penumbra, Inc., Alameda, CA), which allows aspiration of distal debris and clot. If these devices are not available, thrombolytics can be used initially. There is some evidence that glycoprotein IIb/IIIa inhibitors give additive benefit.114 Other devices that can be used to open occlusions of large intracranial vessels (M1 segment of the middle cerebral artery, A1 segment of the anterior cerebral artery, P1 segment of the posterior cerebral artery, basilar artery, carotid artery) include balloon angioplasty, snares, large microcatheters, and stents.115
In settings other than clear large-vessel cutoff, if the DEP device has already been deployed, the procedure should be completed and a cranial CT scan obtained. Intracranial hemorrhage can be manifested as slow flow, and before expansion of the hematoma and development of a significant mass effect, it may appear as a shower of emboli. If an angiogram documents slow flow and the CT scan is negative for hemorrhage, glycoprotein IIb/IIIa antiplatelet agents are administered as a bolus dose followed by a 23-hour infusion. The patient’s blood pressure should be controlled tightly, with a systolic blood pressure of less than 160 mm Hg. If hemorrhage is identified, the systemic heparin anticoagulation should be reversed with protamine, blood pressure tightly controlled, and a repeat CT scan obtained in 6 to 12 hours. Life-threatening hematomas in neurologically salvageable patients can be evacuated. In the setting of hematoma expansion but no operative indication, activated factor VII can be given to stop progression of the hemorrhage. Most intracranial hematomas expand within the first 12 hours.116 Strong consideration should be given to stopping the patient’s dual antiplatelet therapy. The source of reperfusion hemorrhage remains debatable; some advocate a hyperperfusion origin, whereas others have suggested hemorrhagic conversion of a shower of emboli. In different cases, both sources are probably possible. However, once the hemorrhage has occurred, the treatment is identical.
Occasionally, a patient will have an intracranial aneurysm ipsilateral to a critically severe carotid stenosis. In a review of NASCET data, 1 in 90 patients with aneurysms known before the performance of CEA experienced aneurysm rupture (at 6 days after CEA).117 On the basis of the findings of the International Study of Unruptured Intracranial Aneurysms (ISUIA),118 the following approach seems rational: for patients with unruptured, asymptomatic aneurysms smaller than 6 mm, CAS can be performed before any treatment of the aneurysm, whereas for patients with unruptured, symptomatic aneurysms 6 mm or larger, the aneurysm should probably be treated before CAS is performed.
Systemic Complications
The rate of MI in the SAPPHIRE trial was 2.5% at 1 year, which was statistically lower than the risk for MI during CEA.12 One of the major benefits of CAS in the major studies completed is the lower incidence of intraprocedural and postprocedural MI. However, MI still occurs in the CAS population. Standard measures, including nitrate infusion, beta blockade, and heparin administration, should be initiated if MI is diagnosed by cardiac enzyme studies and electrocardiography. A cardiologist should be consulted early because patients with Q-wave infarction seen on the electrocardiogram will usually require acute revascularization.
The exact incidence of contrast-associated nephropathy is not clear. In one study of patients with creatinine levels lower than 1.5 mg/dL, only 8% had a 0.5-mg/dL increase in their level, and none had rises greater than 1.0 mg/dL with angiography.119 Other data suggest that contrast-induced nephropathy is the third most common cause of renal failure in hospitalized patients.120 Certainly, the state of a patient’s renal function before angiography is the greatest determinant of post-treatment renal failure. A recent exhaustive review of contrast-related nephropathy120 gave a few practical guidelines for renal prophylaxis in patients with preexisting elevated creatinine levels. Hydration with normal saline for 2 to 12 hours at a level of 1 mL/kg per hour before administration of contrast material is recommended. Low doses of low-osmolar contrast agents such as iodixanol should be given. Doses greater than 5 mL/kg of body weight divided by the serum creatinine level are associated with higher risk.
New Frontiers
Nonatherosclerotic Carotid Artery Disease
Dissection
Carotid artery dissections can occur spontaneously or after trauma, including seemingly minor events or more significant forces, such as chiropractic manipulation or motor vehicle accidents with fracture-dislocations. The dissection flap can be associated with a significant decrease in flow or thrombus and occlusion, or it can lead to subtle stasis of contrast material with essentially normal hemodynamics. Traditionally, carotid dissection has been treated with heparin and warfarin therapy for two reasons: the majority of patients in large retrospective series have done well, and until the maturation of techniques for carotid stenting over the past 10 years, little else was available.121 However, what should one do about patients with a history of repetitive ischemic attacks or a high National Institutes of Health Stroke Scale score on initial evaluation? In this subset of dissection, primary stenting can be performed safely.122
Acute aortic dissection with great-vessel involvement carries high mortality with medical and surgical management.123 There are case reports of successful stenting of the great vessels to the level of the arch.124
Fibromuscular Dysplasia
FMD is a nonatherosclerotic, noninflammatory arteriopathy of small and medium-sized arteries that often affects the renal and carotid arteries.125 Angiographically, it is diagnosed by a distinct beading of the artery with alternating areas of dilation and stenosis. Most often, this finding is of little clinical significance. However, secondary dissections and symptomatic stenosis requiring treatment can develop; frequently, they track high in the cervical segment of the carotid artery where filters are not easily used, but flow reversal might be possible. Soft, trackable self-expanding stents such as the Xpert (Abbott; off-label for this indication) can produce excellent results, and balloon angioplasty alone can often be effective without stenting for pure FMD. Patients with Ehlers-Danlos syndrome, especially type IV, as the underlying condition are a high-risk group for treatment-related morbidity and long-term failure, and the decision to treat such patients should be carefully weighed against the risk for further vessel dissection.
Acute Carotid Occlusion
Competence of the circle of Willis renders an acute carotid occlusion asymptomatic in many patients. There are other patients who have a minor TIA or an episode of amaurosis fugax and are discovered to have an acute occlusion, but hemodynamic physiologic assessment with CT scanning, magnetic resonance perfusion imaging, or TCD renders revascularization more risky than the expected benign course of these occlusions. There are still others who have an acute stroke with a major intracranial branch occlusion or symptoms that are confirmed to be related to an inadequacy of intracranial collateral circulation, such as watershed region infarcts with a large preserved at-risk ischemic penumbra. It is these patients who can be helped most by revascularization. Patients seen in delayed fashion can be considered for enrollment in the ongoing Carotid Occlusion Surgery Study (COSS), which is designed to evaluate the very small cohort that has ischemic penumbra and survives long enough to be evaluated for enrollment. Several case series have evaluated the feasibility of endovascular revascularization in the acute setting for at-risk patients with ongoing ischemia.126 We have used CAS in this setting frequently. The principal strategy here is arrest of proximal flow when performing revascularization of the occluded carotid artery so that further distal embolism is prevented. We have used balloon guides such as the Concentric 7 to 9 French guides (Concentric Medical) in the distal common or proximal internal carotid artery and performed CAS under flow arrest, followed by angiographic assessment of the distal intracranial vasculature. If there is a distal embolus, it is retrieved separately with a standard stroke intervention, such as with the Merci or Penumbra systems, intra-arterial thrombolysis, mechanical disruption of the clot with a microwire and microcatheter, and even intracranial stenting. The Gore Neuroprotection proximal flow reversal system may be very useful in these circumstances.
Intraluminal Thrombus
Intraluminal thrombus in the internal carotid artery is an ominous lesion that carries a high risk for stroke. In NASCET, the 30-day risk for stroke in medically treated patients with intraluminal thrombus was triple that of medically treated patients without thrombus.2,23 The risk for stroke in surgically treated patients with thrombus was double that of those without thrombus. In patients with intraluminal thrombus, the 1-year risk for stroke in surgically and medically treated patients was 16% and 25.3%, respectively.127 We recently presented our data for nine patients with nondisabling stroke in whom CAS was performed with proximal and distal flow arrest in both the external carotid artery and internal carotid artery and achieved a 30-day stroke and morbidity rate of 0%.128 We placed a PercuSurge balloon outside the guide sheath in the external carotid artery and then inserted a balloon guide into the common carotid artery, followed by flow reversal, which was used to cross the intraluminal thrombus with yet another PercuSurge balloon placed in the distal cervical internal carotid artery. Therefore, under complete flow arrest, we placed a stent across the thrombus. We selected a Wallstent in these cases because of the small stent tines and low porosity, thereby reducing the chance of a cheese-grating effect on the thrombus. Once the stent is deployed, intravascular ultrasound (IVUS) is used to confirm the absence of any residual intraluminal debris. The external carotid artery and common carotid artery balloons are then deflated, as after CEA, to allow debris to be washed into the external carotid artery, followed by release of the internal carotid artery balloon, which results in restoration of intracranial flow. The preliminary results as reported by us appear very promising.128
Intravascular Ultrasound
We now routinely use IVUS during CAS procedures.129,130 The endoluminal visualization provided by IVUS has allowed us to detect extruded plaque debris on many occasions when it had been missed on conventional angiography. This has resulted in additional maneuvers that enable trapping of the debris by placement of yet another stent or advancing the guide catheter to perform suction while the DEP device is still deployed, thus enabling aspiration of extruded debris. We prefer to perform IVUS after deployment of the DEP device but before stenting and angioplasty to evaluate plaque morphology, which has clearly been associated with embolization risk. In many cases, IVUS has resulted in an alteration of strategies with respect to the choice of the stent deployed and the performance and vigor of poststenting angioplasty. Furthermore, after completion of CAS, we perform IVUS once more to ensure that no residual cheese-grated plaque debris is evident through the stent tines before withdrawal of the DEP device. In our estimation, IVUS is a valuable tool for further reduction of the embolization risk associated with CAS. As one may gather from the aforementioned CAS registry data, procedure-related strokes occur with relative infrequency (1% to 6%); therefore, prevention of one stroke can have a significant effect on overall CAS-related outcomes for each practitioner.
Amarenco P, Bogousslavsky J, Callahan A3rd, et al. High-dose atorvastatin after stroke or transient ischemic attack. The Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. N Engl J Med. 2006;355;:549-559.
Angelini A, Reimers B, Della Barbera M, et al. Cerebral protection during carotid artery stenting: collection and histopathologic analysis of embolized debris. Stroke. 2002;33:456-461.
Barnett HJ, Taylor DW, Eliasziw M, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1998;339:1415-1425.
Bates ER, Babb JD, Casey DEJr, et al. ACCF/SCAI/SVMB/SIR/ASITN 2007 clinical expert consensus document on carotid stenting: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents (ACCF/SCAI/SVMB/SIR/ASITN Clinical Expert Consensus Document Committee on Carotid Stenting). J Am Coll Cardiol. 2007;49:126-170.
Biasi GM, Froio A, Diethrich EB, et al. Carotid plaque echolucency increases the risk of stroke in carotid stenting: the Imaging in Carotid Angioplasty and Risk of Stroke (ICAROS) study. Circulation. 2004;110:756-762.
CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet. 1996;348:1329-1339.
Eckstein HH, Ringleb P, Allenberg JR, et al. Results of the Stent-Protected Angioplasty versus Carotid Endarterectomy (SPACE) study to treat symptomatic stenoses at 2 years: a multinational, prospective, randomised trial. Lancet Neurol. 2008;7:893-902.
European Carotid Surgery Trialists’ Collaborative Group. Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet. 1998;351:1379-1387.
Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. Endarterectomy for asymptomatic carotid artery stenosis. JAMA. 1995;273:1421-1428.
Goldstein LB, Samsa GP, Matchar DB, et al. Multicenter review of preoperative risk factors for endarterectomy for asymptomatic carotid artery stenosis. Stroke. 1998;29:750-753.
Gray WA, Hopkins LN, Yadav S, et al. Protected carotid stenting in high-surgical-risk patients: the ARCHeR results. J Vasc Surg. 2006;44:258-268.
Gurm HS, Yadav JS, Fayad P, et al. Long-term results of carotid stenting versus endarterectomy in high-risk patients. N Engl J Med. 2008;358:1572-1579.
Hobson RWII, Howard VJ, Roubin GS, et al. Carotid artery stenting is associated with increased complications in octogenarians: 30-day stroke and death rates in the CREST lead-in phase. J Vasc Surg. 2004;40:1106-1111.
Kadkhodayan Y, Jeck DT, Moran CJ, et al. Angioplasty and stenting in carotid dissection with or without associated pseudoaneurysm. AJNR Am J Neuroradiol. 2005;26:2328-2335.
Lawes CM, Bennett DA, Feigin VL, et al. Blood pressure and stroke: an overview of published reviews. Stroke. 2004;35:776-785.
Levy EI, Ecker RD, Horowitz MB, et al. Stent-assisted intracranial recanalization for acute stroke: early results. Neurosurgery. 2006;58:458-463.
Levy EI, Hanel RA, Lau T, et al. Frequency and management of recurrent stenosis after carotid artery stent implantation. J Neurosurg. 2005;102:29-37.
Mas JL, Trinquart L, Leys D, et al. Endarterectomy Versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis (EVA-3S) trial: results up to 4 years from a randomised, multicentre trial. Lancet Neurol. 2008;7:885-892.
Moore WS, Barnett HJ, Beebe HG, et al. Guidelines for carotid endarterectomy. A multidisciplinary consensus statement from the Ad Hoc Committee, American Heart Association. Stroke. 1995;26:188-201.
Ringleb PA, Allenberg J, Bruckmann H, et al. 30 day results from the SPACE trial of stent-protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non-inferiority trial. Lancet. 2006;368:1239-1247.
Sacco RL, Adams R, Albers G, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Stroke. 2006;37:577-617.
Villalobos HJ, Harrigan MR, Lau T, Wehman JC, et al. Advancements in carotid stenting leading to reductions in perioperative morbidity among patients 80 years and older. Neurosurgery. 2006;58:233-240.
Yadav JS, Wholey MH, Kuntz RE, et al. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med. 2004;351:1493-1501.
1 Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21-181.
2 North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325:445-453.
3 Barnett HJ, Taylor DW, Eliasziw M, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1998;339:1415-1425.
4 Asymptomatic Carotid Atherosclerosis Study Group. Study design for randomized prospective trial of carotid endarterectomy for asymptomatic atherosclerosis. Stroke. 1989;20:844-849.
5 Halliday A, Mansfield A, Marro J, et al. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial. Lancet. 2004;363:1491-1502.
6 European Carotid Surgery Trialists’ Collaborative Group. MRC European Carotid Surgery Trial: interim results for symptomatic patients with severe (70-99%) or with mild (0-29%) carotid stenosis. Lancet. 1991;337:1235-1243.
7 European Carotid Surgery Trialists’ Collaborative Group. Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet. 1998;351:1379-1387.
8 Mathias K, Gospos C, Thron A, et al. Percutaneous transluminal treatment of supraaortic artery obstruction. Ann Radiol (Paris). 1980;23:281-282.
9 Diethrich EB, Ndiaye M, Reid DB. Stenting in the carotid artery: initial experience in 110 patients. J Endovasc Surg. 1996;3:42-62.
10 Jordan WDJr, Voellinger DC, Fisher WS, et al. A comparison of carotid angioplasty with stenting versus endarterectomy with regional anesthesia. J Vasc Surg. 1998;28:397-403.
11 Theron J, Courtheoux P, Alachkar F, et al. Intravascular technics of cerebral revascularization. J Mal Vasc. 1990;15:245-256.
12 Yadav JS, Wholey MH, Kuntz RE, et al. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med. 2004;351:1493-1501.
13 Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. Endarterectomy for asymptomatic carotid artery stenosis. JAMA. 1995;273:1421-1428.
14 Sacco RL, Adams R, Albers G, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Stroke. 2006;37:577-617.
15 Wennberg DE, Lucas FL, Birkmeyer JD, et al. Variation in carotid endarterectomy mortality in the Medicare population: trial hospitals, volume, and patient characteristics. JAMA. 1998;279:1278-1281.
16 Halm EA, Chassin MR, Tuhrim S, et al. Revisiting the appropriateness of carotid endarterectomy. Stroke. 2003;34:1464-1471.
17 Hartmann A, Hupp T, Koch HC, et al. Prospective study on the complication rate of carotid surgery. Cerebrovasc Dis. 1999;9:152-156.
18 Naylor AR, Hayes PD, Allroggen H, et al. Reducing the risk of carotid surgery: a 7-year audit of the role of monitoring and quality control assessment. J Vasc Surg. 2000;32:750-759.
19 Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128-1137.
20 Goldstein LB, McCrory DC, Landsman PB, et al. Multicenter review of preoperative risk factors for carotid endarterectomy in patients with ipsilateral symptoms. Stroke. 1994;25:1116-1121.
21 Goldstein LB, Samsa GP, Matchar DB, et al. Multicenter review of preoperative risk factors for endarterectomy for asymptomatic carotid artery stenosis. Stroke. 1998;29:750-753.
22 Meyer FB, Piepgras DG, Fode NC. Surgical treatment of recurrent carotid artery stenosis. J Neurosurg. 1994;80:781-787.
23 Villarreal J, Silva J, Eliasziw M, et al. North American Symptomatic Carotid Endarterectomy Trial. Prognosis of patients with intraluminal thrombus in the internal carotid artery [abstract 18]. Stroke. 1998;29:276.
24 Gasecki AP, Eliasziw M, Ferguson GG, et al. Long-term prognosis and effect of endarterectomy in patients with symptomatic severe carotid stenosis and contralateral carotid stenosis or occlusion: results from NASCET. North American Symptomatic Carotid Endarterectomy Trial (NASCET) Group. J Neurosurg. 1995;83:778-782.
25 Kresowik TF, Bratzler D, Karp HR, et al. Multistate utilization, processes, and outcomes of carotid endarterectomy. J Vasc Surg. 2001;33:227-235.
26 Moore WS, Barnett HJ, Beebe HG, et al. Guidelines for carotid endarterectomy. A multidisciplinary consensus statement from the Ad Hoc Committee, American Heart Association. Stroke. 1995;26:188-201.
27 Nicolaides AN, Kakkos SK, Griffin M, et al. Severity of asymptomatic carotid stenosis and risk of ipsilateral hemispheric ischaemic events: results from the ACSRS study. Eur J Vasc Endovasc Surg. 2005;30:275-284.
28 Nicolaides AN, Kakkos S, Griffin M, et al. Severity of asymptomatic carotid stenosis and risk of ipsilateral hemispheric ischaemic events: results from the ACSRS study. Eur J Vasc Endovasc Surg. 2006;31:336.
29 Dutch TIA Trial Study Group. A comparison of two doses of aspirin (30 mg vs. 283 mg a day) in patients after a transient ischemic attack or minor ischemic stroke. N Engl J Med. 1991;325:1261-1266.
30 Antiplatelet Trialists’ Collaboration. Collaborative overview of randomised trials of antiplatelet therapy—I: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. BMJ. 1994;308:81-106.
31 Farrell B, Godwin J, Richards S, et al. The United Kingdom transient ischaemic attack (UK-TIA) aspirin trial: final results. J Neurol Neurosurg Psychiatry. 1991;54:1044-1054.
32 CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet. 1996;348:1329-1339.
33 Gent M, Blakely JA, Easton JD, et al. The Canadian American Ticlopidine Study (CATS) in thromboembolic stroke. Lancet. 1989;1:1215-1220.
34 Diener HC, Cunha L, Forbes C, et al. European Stroke Prevention Study. 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci. 1996;143:1-13.
35 Progressive Collaborative Group. Randomised trial of a perindopril-based blood-pressure–lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet. 2001;358:1033-1041.
36 Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560-2572.
37 Lawes CM, Bennett DA, Feigin VL, et al. Blood pressure and stroke: an overview of published reviews. Stroke. 2004;35:776-785.
38 Yusuf S, Sleight P, Pogue J, et al. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342;:145-153.
39 Adult Treatment Panel III. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. JAMA. 2001;285:2486-2497.
40 Amarenco P, Bogousslavsky J, Callahan A3rd, et al. High-dose atorvastatin after stroke or transient ischemic attack. The Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. N Engl J Med. 2006;355;:549-559.
41 Amarenco P, Labreuche J, Lavallee P, et al. Statins in stroke prevention and carotid atherosclerosis: systematic review and up-to-date meta-analysis. Stroke. 2004;35:2902-2909.
42 Collins R, Armitage J, Parish S, et al. Effects of cholesterol-lowering with simvastatin on stroke and other major vascular events in 20536 people with cerebrovascular disease or other high-risk conditions. Heart Protection Study Collaborative Group. Lancet. 2004;363;:757-767.
43 Plehn JF, Davis BR, Sacks FM, et al. Reduction of stroke incidence after myocardial infarction with pravastatin: the Cholesterol and Recurrent Events (CARE) study. Care Investigators. Circulation. 1999;99;:216-223.
44 Ravipati G, Aronow WS, Ahn C, et al. Incidence of new stroke or new myocardial infarction or death in patients with severe carotid arterial disease treated with and without statins. Am J Cardiol. 2006;98:1170-1171.
45 Findlay JM, Tucker WS, Ferguson GG, et al. Guidelines for the use of carotid endarterectomy: current recommendations from the Canadian Neurosurgical Society. CMAJ. 1997;157:653-659.
46 CAVATAS Investigators. Endovascular versus surgical treatment in patients with carotid stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): a randomised trial. Lancet. 2001;357:1729-1737.
47 Alberts MJ. Results of a multicenter prospective randomized trial of carotid artery stenting vs. carotid endarterectomy [abstract 53]. for the Publications Committee of WALLSTENT. Stroke. 2001;32;:325.
48 Alberts MJ, McCann R, Smith TP, et al. A randomized trial of carotid stenting vs. endarterectomy in patients with symptomatic carotid stenosis: study design. for the Schneider Wallstent Endoprosthesis Clinical Investigators. J Neurovasc Dis. 1997;2;:228-234.
49 Gurm HS, Yadav JS, Fayad P, et al. Long-term results of carotid stenting versus endarterectomy in high-risk patients. N Engl J Med. 2008;358:1572-1579.
50 Ringleb PA, Allenberg J, Bruckmann H, et al. 30 day results from the SPACE trial of stent-protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non-inferiority trial. Lancet. 2006;368:1239-1247.
51 Eckstein HH, Ringleb P, Allenberg JR, et al. Results of the Stent-Protected Angioplasty versus Carotid Endarterectomy (SPACE) study to treat symptomatic stenoses at 2 years: a multinational, prospective, randomised trial. Lancet Neurol. 2008;7:893-902.
52 Mas JL, Chatellier G, Beyssen B, et al. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med. 2006;355:1660-1671.
53 Mas JL, Trinquart L, Leys D, et al. Endarterectomy Versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis (EVA-3S) trial: results up to 4 years from a randomised, multicentre trial. Lancet Neurol. 2008;7:885-892.
54 CaRESS Steering Committee. Carotid revascularization using endarterectomy or stenting systems (CARESS): phase I clinical trial. J Endovasc Ther. 2003;10:1021-1030.
55 CaRESS Steering Committee. Carotid Revascularization Using Endarterectomy or Stenting Systems (CaRESS) phase I clinical trial: 1-year results. J Vasc Surg. 2005;42:213-219.
56 Zarins C. CaRESS: Carotid stenting equivalent to carotid endarterectomy. Presented at the 20th Transcatheter Cardiovascular Therapeutics Annual Scientific Symposium, Washington, DC, Oct 14, 2008.
57 Angelini A, Reimers B, Della Barbera M, et al. Cerebral protection during carotid artery stenting: collection and histopathologic analysis of embolized debris. Stroke. 2002;33:456-461.
58 Castellan L, Causin F, Danieli D, et al. Carotid stenting with filter protection. Correlation of ACT values with angiographic and histopathologic findings. J Neuroradiol. 2003;30:103-108.
59 Jaeger HJ, Mathias KD, Hauth E, et al. Cerebral ischemia detected with diffusion-weighted MR imaging after stent implantation in the carotid artery. AJNR Am J Neuroradiol. 2002;23:200-207.
60 Kastrup A, Groschel K, Krapf H, et al. Early outcome of carotid angioplasty and stenting with and without cerebral protection devices: a systematic review of the literature. Stroke. 2003;34:813-819.
61 Al-Mubarak N, Roubin GS, Vitek JJ, et al. Microembolization during carotid stenting with the distal-balloon antiemboli system. Int Angiol. 2002;21:344-348.
62 Kastrup A, Nagele T, Groschel K, et al. Incidence of new brain lesions after carotid stenting with and without cerebral protection. Stroke. 2006;37:2312-2316.
63 Theron JG, Payelle GG, Coskun O, et al. Carotid artery stenosis: treatment with protected balloon angioplasty and stent placement. Radiology. 1996;201:627-636.
64 Villalobos HJ, Harrigan MR, Lau T, et al. Advancements in carotid stenting leading to reductions in perioperative morbidity among patients 80 years and older. Neurosurgery. 2006;58:233-240.
65 Lacroix V, Hammer F, Astarci P, et al. Ischemic cerebral lesions after carotid surgery and carotid stenting. Eur J Vasc Endovasc Surg. 2007;33:430-435.
66 Adami CA, Scuro A, Spinamano L, et al. Use of the Parodi anti-embolism system in carotid stenting: Italian trial results. J Endovasc Ther. 2002;9:147-154.
67 Asakura F, Kawaguchi K, Sakaida H, et al. Diffusion-weighted MR imaging in carotid angioplasty and stenting with protection by the reversed carotid arterial flow. AJNR Am J Neuroradiol. 2006;27:753-758.
68 Zahn R, Roth E, Ischinger T, et al. Carotid artery stenting in clinical practice results from the Carotid Artery Stenting (CAS)-registry of the Arbeitsgemeinschaft Leitende Kardiologische Krankenhausarzte (ALKK). Z Kardiol. 2005;94:163-172.
69 Gray WA, Hopkins LN, Yadav S, et al. Protected carotid stenting in high-surgical-risk patients: the ARCHeR results. J Vasc Surg. 2006;44:258-268.
70 White CJ, Iyer SS, Hopkins LN, et al. Carotid stenting with distal protection in high surgical risk patients: The BEACH trial 30 day results. Catheter Cardiovasc Interv. 2006;67:503-512.
71 Hopkins LN, Myla S, Grube E, et al. Carotid artery revascularization in high surgical risk patients with the NexStent and the Filterwire EX/EZ: 1-year results in the CABERNET trial. Catheter Cardiovasc Interv. 2008;71:950-960.
72 Fairman R, Gray WA, Scicli AP, et al. The CAPTURE registry: analysis of strokes resulting from carotid artery stenting in the post approval setting: timing, location, severity, and type. Ann Surg. 2007;246:551-558.
73 Gray WA, Yadav JS, Verta P, et al. The CAPTURE registry: results of carotid stenting with embolic protection in the post approval setting. Catheter Cardiovasc Interv. 2007;69:341-348.
74 Katzen BT, Criado FJ, Ramee SR, et al. Carotid artery stenting with emboli protection surveillance study: thirty-day results of the CASES-PMS study. Catheter Cardiovasc Interv. 2007;70:316-323.
75 Safian RD, Bresnahan JF, Jaff MR, et al. Protected carotid stenting in high-risk patients with severe carotid artery stenosis. J Am Coll Cardiol. 2006;47:2384-2389.
76 Brott TG, Hobson RW2nd, Howard G, et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med. 2010;363:11-23.
77 Howard VJ, Brott TG, Qureshi AI, et al. Gender and periprocedural stroke and death following carotid artery stenting: results from the CREST lead-in phase [abstract P5]. for the CREST Investigators. Stroke. 2004;35;:253.
78 Roubin GS, Brott TG, Hopkins LN. Developing embolic protection for carotid stenting in the Carotid Revascularization Endarterectomy vs Stenting Trial (CREST) [abstract 3124]. for the CREST Investigators. Circulation. 2003;suppl 4;108:IV-687.
79 Hobson RWII, Howard VJ, Roubin GS, et al. Carotid artery stenting is associated with increased complications in octogenarians: 30-day stroke and death rates in the CREST lead-in phase. J Vasc Surg. 2004;40:1106-1111.
80 Howard G, Hobson RWII, Brott TG. Does the stroke risk of stenting increase at older ages? Thirty-day stroke death rates in the CREST lead-in phase [abstract 2116]. for the CREST Investigators. Circulation. 2003;suppl 4;8:V-461.
81 Ederle J, Dobson J, Featherstone RL, et al. Carotid artery stenting compared with endarterectomy in patients with symptomatic carotid stenosis (International Carotid Stenting Study): an interim analysis of a randomized controlled trial. Lancet. 2010;375:985-997.
82 . Carotid stenting vs. surgery of severe carotid artery disease and stroke prevention in asymptomatic patients (ACT I). Available at http://www.clinicaltrials.gov/ct/show/NCT00106938?order=1 Accessed February 9, 2009
83 Katzen B. The Transatlantic Asymptomatic Carotid Intervention Trial. Endovasc Today. 2005;September:49-50.
84 Hopkins LN. Results of the Gore EMPIRE Clinical Study. Presented at the Transcatheter Cardiovascular Therapeutics Meeting, Washington DC, Oct 17, 2008.
85 Myla SV. Evaluating the use of the FiberNet embolic protection system in carotid artery stenting. Presented at the Transcatheter Cardiovascular Therapeutics Meeting, Washington DC, Oct 17, 2008.
86 Lam JY, Chesebro JH, Steele PM, et al. Deep arterial injury during experimental angioplasty: relation to a positive indium-111–labeled platelet scintigram, quantitative platelet deposition and mural thrombosis. J Am Coll Cardiol. 1986;8:1380-1386.
87 Kang HS, Han MH, Kwon OK, et al. Intracranial hemorrhage after carotid angioplasty: a pooled analysis. J Endovasc Ther. 2007;14:77-85.
88 Setacci C, Pula G, Baldi I, et al. Determinants of in-stent restenosis after carotid angioplasty: a case-control study. J Endovasc Ther. 2003;10:1031-1038.
89 Levy EI, Hanel RA, Lau T, et al. Frequency and management of recurrent stenosis after carotid artery stent implantation. J Neurosurg. 2005;102:29-37.
90 Ecker RD, Pichelmann MA, Meissner I, et al. Durability of carotid endarterectomy. Stroke. 2003;34:2941-2944.
91 Ouriel K, Hertzer NR, Beven EG, et al. Preprocedural risk stratification: identifying an appropriate population for carotid stenting. J Vasc Surg. 2001;33:728-732.
92 Ballotta E, Renon L, Da Giau G, et al. Octogenarians with contralateral carotid artery occlusion: a cohort at higher risk for carotid endarterectomy? J Vasc Surg. 2004;39:1003-1008.
93 Reed AB, Gaccione P, Belkin M, et al. Preoperative risk factors for carotid endarterectomy: defining the patient at high risk. J Vasc Surg. 2003;37:1191-1199.
94 Meyer FB, editor. Sundt’s Occlusive Cerebrovascular Disease, 2nd ed, Philadelphia: WB Saunders, 2004.
95 Pritz MB. Timing of carotid endarterectomy after stroke. Stroke. 1997;28:2563-2567.
96 Ecker RD, Tummala RP, Levy EI, et al. “Internal cross-clamping” for symptomatic internal carotid artery thrombus. Report of two cases. J Neurosurg. 2007;107:1223-1227.
97 Tummala RP, Jahromi BS, Yamamoto J, et al. Carotid artery stenting under flow arrest for the management of intraluminal thrombus: technical case report. Neurosurgery. 2008;63:ONSE87-88.
98 Parodi JC, Ferreira LM, Sicard G, et al. Cerebral protection during carotid stenting using flow reversal. J Vasc Surg. 2005;41:416-422.
99 Bates ER, Babb JD, Casey DEJr, et al. ACCF/SCAI/SVMB/SIR/ASITN 2007 clinical expert consensus document on carotid stenting: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents (ACCF/SCAI/SVMB/SIR/ASITN Clinical Expert Consensus Document Committee on Carotid Stenting). J Am Coll Cardiol. 2007;49:126-170.
100 de Virgilio C, Toosie K, Arnell T, et al. Asymptomatic carotid artery stenosis screening in patients with lower extremity atherosclerosis: a prospective study. Ann Vasc Surg. 1997;11:374-377.
101 House AK, Bell R, House J, et al. Asymptomatic carotid artery stenosis associated with peripheral vascular disease: a prospective study. Cardiovasc Surg. 1999;7:44-49.
102 Ecker RD, Guidot CA, Hanel RA, et al. Perforation of external carotid artery branch arteries during endoluminal carotid revascularization procedures: consequences and management. J Invasive Cardiol. 2005;17:292-295.
103 Wilentz JR, Chati Z, Krafft V, et al. Retinal embolization during carotid angioplasty and stenting: mechanisms and role of cerebral protection systems. Catheter Cardiovasc Interv. 2002;56:320-327.
104 Walker PJ, May J, Harris JP, et al. External carotid endarterectomy for amaurosis fugax in the presence of internal carotid artery occlusion. Aust N Z J Surg. 1994;64:48-52.
105 Ong TJ, Harper CA, O’Day J. Restoration of ocular circulation and some visual function following external carotid endarterectomy. Clin Experimental Ophthalmol. 2000;28:329-331.
106 Rush DS, Holloway WO, Fogartie JEJr, et al. The safety, efficacy, and durability of external carotid endarterectomy. J Vasc Surg. 1992;16:407-413.
107 Chen CI, Iguchi Y, Garami Z, et al. Analysis of emboli during carotid stenting with distal protection device. Cerebrovasc Dis. 2006;21:223-228.
108 Biasi GM, Froio A, Diethrich EB, et al. Carotid plaque echolucency increases the risk of stroke in carotid stenting: the Imaging in Carotid Angioplasty and Risk of Stroke (ICAROS) study. Circulation. 2004;110:756-762.
109 Cardaioli P, Giordan M, Panfili M, et al. Complication with an embolic protection device during carotid angioplasty. Catheter Cardiovasc Interv. 2004;62:234-236.
110 Macdonald S, Venables GS, Cleveland TJ, et al. Protected carotid stenting: safety and efficacy of the MedNova NeuroShield filter. J Vasc Surg. 2002;35:966-972.
111 Serruys PW, Kutryk MJ, Ong AT. Coronary-artery stents. N Engl J Med. 2006;354:483-495.
112 Setacci C, de Donato G, Setacci F, et al. Surgical management of acute carotid thrombosis after carotid stenting: a report of three cases. J Vasc Surg. 2005;42:993-996.
113 Meyer FB, Sundt TMJr, Piepgras DG, et al. Emergency carotid endarterectomy for patients with acute carotid occlusion and profound neurological deficits. Ann Surg. 1986;203:82-89.
114 Deshmukh VR, Fiorella DJ, Albuquerque FC, et al. Intra-arterial thrombolysis for acute ischemic stroke: preliminary experience with platelet glycoprotein IIb/IIIa inhibitors as adjunctive therapy. Neurosurgery. 2005;56:46-55.
115 Levy EI, Ecker RD, Horowitz MB, et al. Stent-assisted intracranial recanalization for acute stroke: early results. Neurosurgery. 2006;58:458-463.
116 Kazui S, Naritomi H, Yamamoto H, et al. Enlargement of spontaneous intracerebral hemorrhage. Incidence and time course. Stroke. 1996;27:1783-1787.
117 Kappelle LJ, Eliasziw M, Fox AJ, et al. Small, unruptured intracranial aneurysms and management of symptomatic carotid artery stenosis. North American Symptomatic Carotid Endarterectomy Trial Group. Neurology. 2000;55:307-309.
118 Wiebers DO, Whisnant JP, Huston J3rd, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362:103-110.
119 Tepel M, van der Giet M, Schwarzfeld C, et al. Prevention of radiographic-contrast-agent–induced reductions in renal function by acetylcysteine. N Engl J Med. 2000;343:180-184.
120 Barrett BJ, Parfrey PS. Clinical practice. Preventing nephropathy induced by contrast medium. N Engl J Med. 2006;354:379-386.
121 Schievink WI, Mokri B, O’Fallon WM. Recurrent spontaneous cervical-artery dissection. N Engl J Med. 1994;330:393-397.
122 Kadkhodayan Y, Jeck DT, Moran CJ, et al. Angioplasty and stenting in carotid dissection with or without associated pseudoaneurysm. AJNR Am J Neuroradiol. 2005;26:2328-2335.
123 Fann JI, Sarris GE, Miller DC, et al. Surgical management of acute aortic dissection complicated by stroke. Circulation. 1989;80:I257-263.
124 Pavkov I, Horner S, Klein GE, et al. Percutaneous transluminal angioplasty with stenting in extended supra-aortic artery dissection. Croat Med J. 2004;45:217-219.
125 Slovut DP, Olin JW. Fibromuscular dysplasia. N Engl J Med. 2004;350:1862-1871.
126 Terada T, Yamaga H, Tsumoto T, et al. Use of an embolic protection system during endovascular recanalization of a totally occluded cervical internal carotid artery at the chronic stage. Case report. J Neurosurg. 2005;102:558-564.
127 Barnett HJ, Meldrum HE, Eliasziw M. The appropriate use of carotid endarterectomy. CMAJ. 2002;166:1169-1179.
128 Jahromi BS, Tummala RP, Yamamoto J, et al. Early carotid stenting for symptomatic stenosis and intraluminal thrombus presenting with stroke. Neurology. 2008;71:1831-1833.
129 Wehman JC, Holmes DRJr, Ecker RD, et al. Intravascular ultrasound identification of intraluminal embolic plaque material during carotid angioplasty with stenting. Catheter Cardiovasc Interv. 2006;68:853-857.
130 Wilson EP, White RA, Kopchok GE. Utility of intravascular ultrasound in carotid stenting. J Endovasc Surg. 1996;3:63-68.