Cardiovascular system
5.1
Gross cardiomegaly on chest x-ray
1. Ischaemic heart disease and other cardiomyopathies.
2. Pericardial effusion – globular or flask-shaped heart, crisp cardiac outline.
4. Multivalve disease – particularly regurgitation.
5. Congenital heart disease – notably Ebstein’s anomaly (congenital displacement of the septal and posterior leaflets of the tricuspid valve towards the apex of the RV producing atrialization of the RV and complex tricuspid regurgitation).
5.6
Cardiac calcification
Valves
1. Aortic valve calcification – bicuspid aortic valve, degenerative aortic sclerosis, previous rheumatic fever.
2. Mitral calcification – rheumatic fever, degenerative annular calcification.
3. Pulmonary calcification – pulmonary stenosis, rheumatic fever.
4. Tricuspid calcification – rare; rheumatic fever, endocarditis, ASD.
Gowda, R. M., Boxt, L. M. Calcifications of the heart. Radiol Clin North Am. 2004; 42:603–617.
Greenland, P., Bonow, R. O., Brundage, B. H., et al. ACCF/AHA 2007 Clinical expert consensus document on coronary artery calcium scoring by CT in global cardiovascular risk assessment and in evaluation of patients with chest pain. J Am Coll Cardiol. 2007; 49:378–402.
5.7
Myocardial diseases
Myocardial thinning
1. Generalized in LV dilatation due to ischaemic heart disease or DCM.
2. Focal LV thinning from previous infarction – may also be associated with focal calcification or focal fat deposition. Localized wall thinning can also be seen in focal LV non-compaction, where the normal compaction process of the LV trabecula arrests at an earlier than normal stage in the embryo, and thus there is focal prominent trabecula and thinned and often poorly contractile myocardium seen even in adult life; this is more commonly seen at the apex and in the distal lateral and anterior walls of the LV.
Fatty lesions
2. Lipomatous hypertrophy of the interatrial septum – normal variant associated with obesity and steroid use.
3. Fatty replacement of a myocardial infarct.
4. Fatty infiltration into the RV free wall – ARVD. This is associated with increased RV volume and regionally or globally reduced RV function. There may be evidence of RV scar tissue also on MRI. The LV is affected less frequently.
5.8
Pericardial diseases
Pericardial effusion
1. Transudate – from left ventricular failure, hypoalbuminaemia, renal failure.
2. Exudate – from collagen vascular diseases, infections, e.g. TB or viral, chronic renal failure.
3. Haemopericardium – from acute aortic dissection, trauma including surgery, acute myocardial infarction, tumour (primary, local tumour invasion, metastases, lymphoma).
Pericardial constriction
1. Can occur following pericarditis, trauma including surgery, radiotherapy – underlying cause is often idiopathic.
2. Cross-sectional imaging appearances include pericardial thickening and calcification, narrowed tubular ventricles, distortion and irregularity of the RV free wall, enlargement of the atria and the venae cavae, flattening and bowing of the interventricular septum, which is more marked during inspiration (seen on MRI).
5.9
Cardiac masses
1. Thrombus – ventricular after acute myocardial infarction and in aneurysms, left atrial in atrial fibrillation, particularly the left atrial appendage, right atrial usually associated with indwelling venous catheters with insufficient anticoagulation.
2. Benign tumours – the commonest are myxomas, which occur most frequently in the atria. There is often a pedicle attaching the mass to the region of the fossa ovalis. Other benign tumours include lipomas and fibromas. Usually discrete and well-defined.
3. Malignant tumours – primary malignant tumours are rare but tend to be sarcomas, particularly angiosarcomas and rhabdomyosarcomas. These are usually ill-defined and infiltrative with a pericardial effusion. Metastases are the commonest form of cardiac tumour (melanoma has a predilection).
5.10
Late gadolinium enhancement on cardiac MRI
1. Myocardial infarction – the distribution is subendocardial or full thickness and in a recognized coronary artery territory.
2. Cardiomyopathy – such as due to sarcoid, myocarditis, HCM, DCM or ARVD. The distribution is not that of infarction and is often mid-myocardial or subepicardial. The finding of myocardial scar tissue in this way tends to be associated with a worse prognosis.
3. Diffuse late enhancement – can be seen with extensive cardiac amyloid infiltration.
5.11
Malignant coronary artery anomalies in the adult
5.13
Causes of a perfusion defect on a cardiac spect scan
5.14
Acute aortic syndromes
Aortic dissection
1. Classified as type A if it involves the aorta proximal to the left subclavian artery and type B if it involves only the aorta distal to the left subclavian artery.
2. X-ray signs – include widening of the mediastinum, ill-defined mediastinal outline, left pleural effusion or pleural cap, displaced intimal calcification.
3. CT signs – include the dissection flap with flow in the true ± the false lumen, mediastinal haemorrhage, haemopericardium, haemothorax, mural haematoma. There may be no contrast opacification of the right coronary artery if it is involved with the dissection.
Aortic transection
1. Post-traumatic – particularly high-speed deceleration injuries.
2. Usually occurs at the isthmus in the proximal descending aorta – due to tethering by the ligamentum arteriosum.
3. X-ray signs – include widening and ill-definition of the mediastinal contour, left apical cap and left pleural effusion. The CXR may be normal.
4. CT signs – often subtle. The normal circular contour of the lumen of the aortic isthmus is lost and there may be a pseudoaneurysm. Mediastinal haemorrhage is usually present. May proceed to dissection. Extravasation of contrast is a poor prognostic sign.
Macura, K. J., Corl, F. M., Fishman, E. K., Bluemke, D. A. Pathogenesis in acute aortic syndromes: aortic dissection, intramural hematoma, and penetrating atherosclerotic aortic ulcer. AJR Am J Roentgenol. 2003; 181(2):309–316.
Vilacosta, I., San Roman, J. A. Acute aortic syndrome. Heart. 2001; 85:365–368.
5.15
Aortic arch anomalies
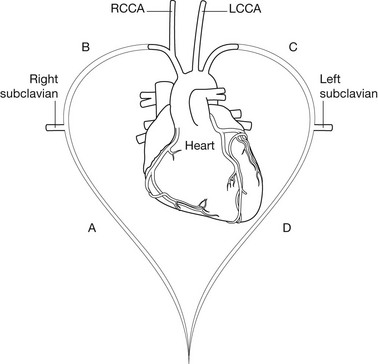
1. If segment A atrophies – produces the normal arrangement of the head and neck vessels from a left-sided aortic arch.
2. If segment B atrophies – there is a left-sided arch with an aberrant right subclavian artery.
3. If segment C atrophies – the aortic arch is right-sided with an aberrant left subclavian artery. This is associated with a low (~12%) incidence of congenital heart disease including Fallot’s tetralogy and coarctation.
4. If segment D atrophies – the aortic arch is right-sided with mirror-image branching of the head and neck vessels. This is associated with a high incidence (~98%) of congenital heart disease, particularly Fallot’s tetralogy.
5.18
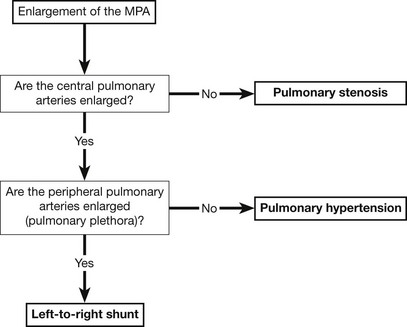
Pulmonary arterial enlargement
The figure shows a flowchart for the analysis of increased pulmonary arterial size on the CXR.
5.19
Pulmonary hypertension