Cardiovascular Clinical Assessment
Physical assessment of the patient with cardiovascular disease is a skill that must not be overlooked in the middle of all the technology of the critical care setting.1 Data collected from a thorough, thoughtful history taking and examination contribute to both the nursing and the medical decisions about therapeutic interventions.
History
Patient history is important because it provides data that contribute to diagnosis of cardiovascular disease and treatment plan. For a patient in acute distress, the history taking is shortened to just a few questions about the patient’s chief complaint, precipitating events, and current medications (Box 13-1). For a patient who is not in obvious distress, the history focuses on the following four areas:
1. Review of the patient’s present illness
2. Overview of the patient’s general cardiovascular status, including previous cardiac diagnostic studies, interventional procedures, cardiac surgeries, and current medications (i.e., cardiac, noncardiac, and over-the-counter medications)
3. Review of the patient’s general health status, including family history of coronary artery disease (CAD), hypertension, diabetes, peripheral arterial disease, or stroke
4. Survey of the patient’s lifestyle, including risk factors for CAD
• If any evidence of CAD or risk of heart disease exists, assume that the chest pain is caused by myocardial ischemia until proven otherwise.
• Questions to elicit the nature of the chest pain cover five basic areas: 1) quality, 2) location, 3) duration of pain, 4) factors that provoke the pain, and 5) factors that relieve the pain. Questions that may help elicit this information are listed in Table 13-1.
TABLE 13-1
CLARIFYING CHEST PAIN SYMPTOMS BY ASKING SPECIFIC QUESTIONS
| DETERMINE | TYPICAL QUESTION |
| Location, radiation | Where is it? |
| Does it move or stay in one place? | |
| Quality | What is it like? |
| Quantity | How severe is it? How frequent? |
| How long does it last? | |
| Chronology | When did it begin? |
| How has it progressed? | |
| What are you doing when it occurs? | |
| What do you do to get rid of it? | |
| Associated findings | Do you feel any other symptoms at the same time? |
| Treatment sought and effect | Have you seen a physician in the past for this same problem? |
| What was the treatment? | |
| Personal | What do you think this is from? |
| Perception | Why do you think it happened now? |
• Little correlation may exist between the severity of chest discomfort and the gravity of its cause. This is a result of the subjective nature of pain and the unique presentation of ischemic disease in women, older patients, and individuals with diabetes.
• Subjective descriptors vary greatly among individuals. Not all patients use the word “pain”; some may describe their problem as “pressure,” heaviness,” discomfort,” or “indigestion.”
• A correlation is not always present between the location of chest discomfort and its source because of referred pain. For example, in patients with gastroesophageal reflux disease (GERD), esophageal spasm can cause visceral substernal chest pain that radiates to the left arm and jaw and may be described by patients as “heartburn.”2
• Other nonpainful symptoms that may signal cardiac dysfunction are dyspnea, palpitations, cough, fatigue, edema, ischemic leg pain, nocturia, syncope, and cyanosis.
In a meta-analysis of the evaluation of stable, intermittent chest pain, a patient’s description of chest pain was found to be the most important predictor of underlying coronary disease.3 In the evaluation of acute chest pain, the 12-lead electrocardiogram (ECG) was the most useful bedside predictor for a diagnosis of ST-elevation myocardial infarction (STEMI).3
Physical Examination
Inspection
Face
Nail Beds and Cyanosis
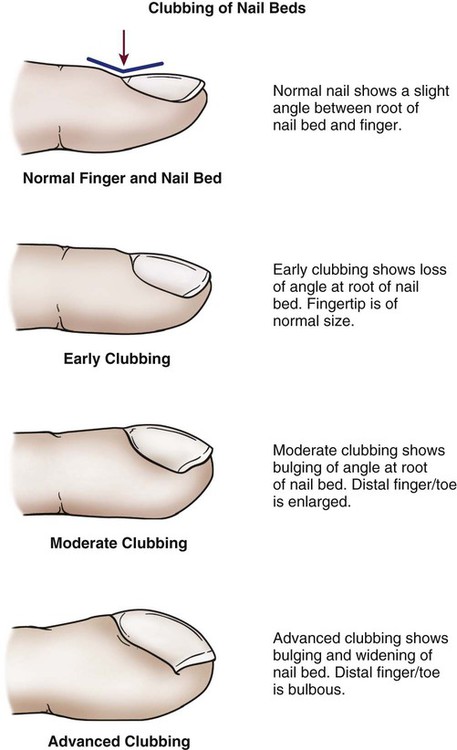
The nail beds are inspected for signs of discoloration or cyanosis.4 Clubbing in the nail bed is a sign associated with long-standing central cyanotic heart disease or pulmonary disease with hypoxemia.4,5 “Clubbing” of the nail refers to a nail that has lost the normal angle between the finger and the nail root; the nail becomes wide and convex. The terminal phalanx of the finger also becomes bulbous and swollen.6 Clubbing is rare and is a sign of severe central cyanosis (Fig. 13-1). Peripheral cyanosis, a bluish discoloration of the nail bed, is seen more commonly. Peripheral cyanosis results from a reduction in the quantity of oxygen in the peripheral extremities from arterial disease or decreased cardiac output (CO). Clubbing never occurs as a result of peripheral cyanosis.
Lower Extremities
Legs are inspected for signs of peripheral arterial or venous vascular disease. The visible signs of peripheral arterial vascular disease include pale, shiny legs with sparse hair growth. Recent guidelines indicate that many individuals, especially women, can have peripheral atherosclerosis without obvious signs or symptoms.7 Venous disease causes edema of the limb, with deep red rubor, brown discoloration, and, frequently, leg ulceration. A comparison of typical assessment findings in arterial and venous diseases is presented in Table 13-2.
TABLE 13-2
INSPECTION AND PALPATION OF EXTREMITIES: COMPARISON OF ARTERIAL AND VENOUS DISEASE
| CHARACTERISTIC | ARTERIAL DISEASE | VENOUS DISEASE |
| Hair loss | Present | Absent |
| Skin texture | Thin, shiny, dry | Flaking, stasis, dermatitis, mottled |
| Ulceration | Located at pressure points; painful, pale, dry with little drainage; well-demarcated with eschar or dried; surrounded by fibrous tissue; granulation tissue scant and pale | Usually at the ankle; painless, pink, moist with large amount of drainage; irregular, dry, and scaly; surrounded by dermatitis; granulation tissue healthy |
| Skin color | Elevational pallor, dependent rubor | Brown patches, rubor, mottled cyanotic color when dependent |
| Nails | Thick, brittle | Normal |
| Varicose veins | Absent | Present |
| Temperature | Cool | Warm |
| Capillary refill | Greater than 3 seconds | Less than 3 seconds |
| Edema | None or mild, usually unilateral | Usually present foot to calf, unilateral or bilateral |
| Pulses | Weak or absent (0 to 1+) | Normal, strong, and symmetric |
Modified from Krenzer ME. Peripheral vascular assessment: finding your way through arteries and veins. AACN Clin Issues. 1995;6(4):631.
Jugular Veins
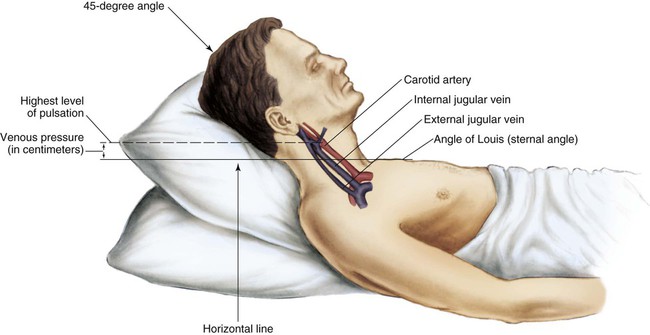
The jugular veins of the neck are inspected for a noninvasive estimate of intravascular volume and pressure. The internal jugular veins are observed for jugular vein distention (JVD) (Fig. 13-2; Box 13-2). JVD is caused by an elevation in central venous pressure (CVP). This occurs with fluid volume overload, right ventricular dysfunction, pericardial effusion, or any condition that elevates right atrial pressure.9,10 The right internal jugular vein can be palpated for measurement of CVP in centimeters of water (Fig. 13-3; Box 13-3). Bedside ultrasound is also used to evaluate JVD and estimate CVP.11
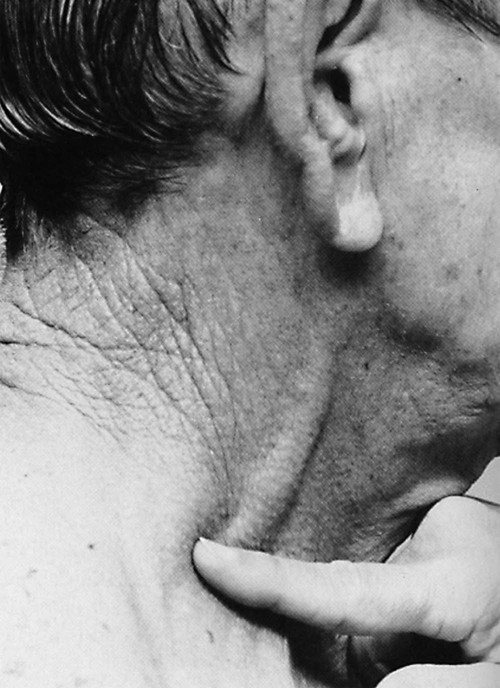
Applying light finger pressure over the sternocleidomastoid muscle, parallel to the clavicle, helps identify the external jugular vein by occluding flow and distending it. The finger pressure is released, and the patient is observed for true distention. If the patient’s trunk is elevated to 30 degrees or more, JVD should not be present.
Abdominojugular Reflux
The abdominojugular reflux sign can assist with the diagnosis of right ventricular failure. This noninvasive test is used in conjunction with measurement of JVD. The procedure for assessing abdominojugular reflux is described in Box 13-4. A positive abdominojugular reflux sign is an increase in the jugular venous pressure (CVP equivalent) of 4 cm or more sustained for at least 15 seconds.12
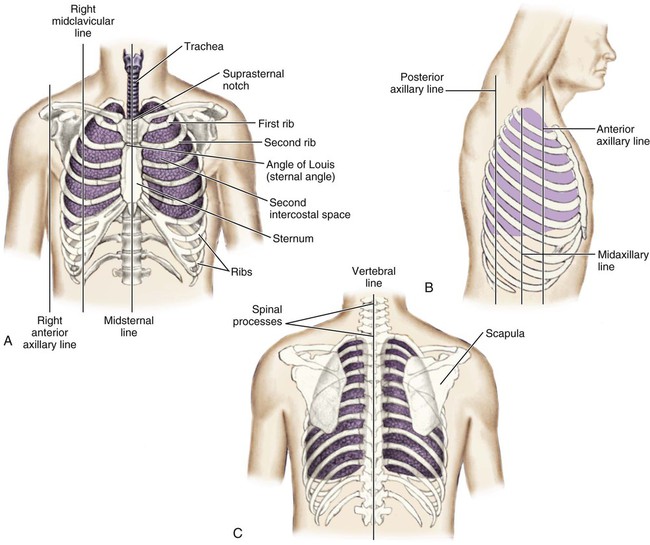
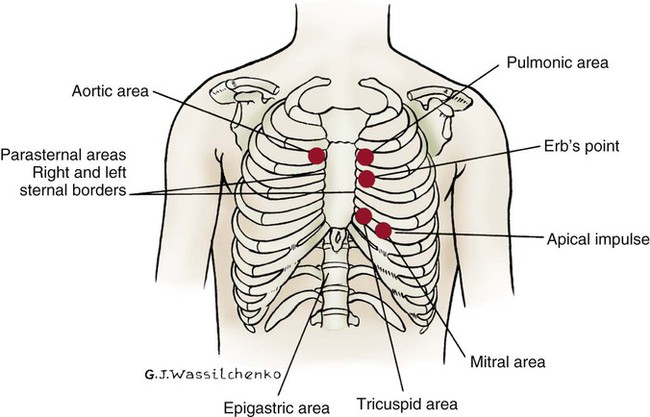
Thoracic Reference Points
The thoracic cage is divided with imaginary vertical lines (sternal, midclavicular, axillary, vertebral, and scapular), and the intercostal spaces are divided by imaginary horizontal lines to serve as reference points in locating or describing cardiac findings (Fig. 13-4). The ribs are numbered from 1 (the first rib below the clavicle) to 12. The intercostal space below each rib is given the same number as the rib that lies above it. The second rib is the easiest to locate because it is attached to the sternum at the angle of Louis. This angle (also called the sternal angle) is the bony ridge on the sternum that lies approximately 2 inches below the sternal notch (see Fig. 13-4A). After the second rib has been located, it can be used as a reference point to count off the other ribs and intercostal spaces.
Apical Impulse
The anterior thorax is inspected for the apical impulse, sometimes referred to as the point of maximal impulse (PMI). The apical impulse occurs as the left ventricle contracts during systole and rotates forward, causing the left ventricular apex of the heart to hit the chest wall. The apical impulse is a quick, localized, outward movement normally located just lateral to the left midclavicular line at the fifth intercostal space in the adult patient (Fig. 13-5). The apical impulse is the only normal pulsation visualized on the chest wall. In the patient without cardiac disease, PMI may not be noticeable (see Fig. 13-5).
Palpation
Arterial Pulses
Seven pairs of bilateral arterial pulses are palpated. The examination incorporates bilateral assessment of the carotid, brachial, radial, ulnar, popliteal, dorsalis pedis, and posterior tibial arteries. The pulses are palpated separately and compared bilaterally to check for consistency.13 Pulse volume is graded on a scale of 0 to 3+ (Box 13-5). The abdominal aortic pulse can also be palpated.
Brachial, Ulnar, and Radial Pulses
The brachial pulse is assessed by gently palpating the inner aspect of the slightly bent elbow with the fingers. The radial pulse is palpated in the medial area of the wrist (thumb side). The ulnar artery is palpated at the opposite side of the wrist (little finger side). The radial and ulnar arterial pulses must be assessed before an arterial line is inserted; this test, known as the Allen test, is described in Box 13-6.
Capillary Refill
Capillary refill assessment is a maneuver that uses the patient’s nail beds to evaluate arterial circulation to the extremity and overall perfusion. The nail bed is compressed to produce blanching, after which release of the pressure should result in the return of blood flow and baseline nail color in less than 2 seconds.14 The severity of arterial insufficiency is directly proportional to the amount of time required to re-establish blood flow and color.
Edema
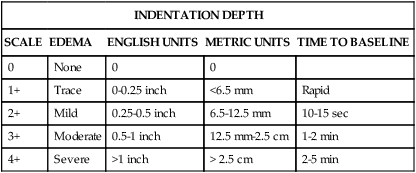
Edema is fluid accumulation in the extravascular spaces of the body. The dependent tissues within the legs and sacrum are particularly susceptible. Edema may be dependent, unilateral or bilateral, and pitting or nonpitting. The amount of edema is quantified by measuring the circumference of the limb or by pressing the skin of the feet, ankles, and shins against underlying bone. Edema is a symptom associated with several diseases, and further diagnostic evaluation is required to determine the cause. Although no universal scale for pitting edema exists, typical scales use a 0-to-4+ system (Table 13-3).
TABLE 13-3
| INDENTATION DEPTH | ||||
| SCALE | EDEMA | ENGLISH UNITS | METRIC UNITS | TIME TO BASELINE |
| 0 | None | 0 | 0 | |
| 1+ | Trace | 0-0.25 inch | <6.5 mm | Rapid |
| 2+ | Mild | 0.25-0.5 inch | 6.5-12.5 mm | 10-15 sec |
| 3+ | Moderate | 0.5-1 inch | 12.5 mm-2.5 cm | 1-2 min |
| 4+ | Severe | >1 inch | > 2.5 cm | 2-5 min |
Auscultation
Blood Pressure Measurement
Blood pressure measurement is an essential component of every complete physical examination. Hypertension is diagnosed as a systolic blood pressure (SBP) of 140 mm Hg or higher, or a diastolic blood pressure (DBP) of 90 mm Hg or above.15 Prehypertension is defined as SBP in the range of 120 to 139 mm Hg in association with a DBP between 80 to 89 mm Hg.15 The incidence of hypertension in the United States has increased dramatically as a result of an aging population and an increase in the prevalence of obesity. The risk of hypertension increases with older age. More than 90% of people who have a normal blood pressure at 55 years eventually develop hypertension, according to findings from the Framingham Heart Study.15
In the critical care setting, systemic blood pressure can be measured directly or indirectly. Arterial monitoring devices (see Chapter 14) that directly measure arterial pressure by means of an invasive technique, which requires placement of an arterial catheter, are considered the gold standard. Correct use of a stethoscope and sphygmomanometer or electronic measuring devices can produce indirect blood pressure values that closely reflect direct measurements (within 1 to 3 mm Hg). The following discussion reviews the essential elements of noninvasive blood pressure monitoring.
Noninvasive Blood Pressure Monitoring.
The most common peripheral locations for blood pressure monitoring are the bilateral brachial arteries. The pressure is measured in both arms to rule out subclavian arterial stenosis. Normally, the difference in pressure between the arms is less than 10 mm Hg. A finding of more than 15 mm Hg difference between the bilateral arm pressures suggests arterial obstruction on the side with the lower pressure.16 Asymmetry is documented so that all subsequent measurements can be made on the arm with the higher pressure.
Orthostatic Hypotension.
When a healthy person stands, 10% to 15% of the blood volume is pooled in the legs; this reduces venous return to the right side of the heart, which decreases CO and lowers arterial blood pressure.17 Transient orthostatic intolerance is part of daily life for many people, but acute orthostatic hypotension is a cause for concern.18 The fall in blood pressure activates baroreceptors; the subsequent reflex increases in sympathetic outflow and parasympathetic inhibition lead to peripheral vasoconstriction, with increased heart rate and contractility.17 Postural (orthostatic) hypotension occurs when the SBP or BP drops by 10 to 20 mm Hg, or the diastolic BP drops by 5 mm Hg, after a change from the supine posture to the upright posture. This is usually accompanied by dizziness, lightheadedness, or syncope. If a patient experiences these symptoms, it is important to complete a full set of postural vital signs before increasing the patient’s activity level (Box 13-7). Orthostatic hypotension can have many causes.18 The three most common causes of orthostatic vital sign changes (i.e., drop in blood pressure and rise in heart rate) observed in critical care are as follows:
1. Intravascular volume depletion or fluid loss caused by bleeding, excessive diuresis, or fever
2. Inadequate vascular vasoconstrictor mechanisms to constrict the arterial bed, which can occur in older patients after prolonged immobility or as a result of spinal cord injury
3. Autonomic insufficiency caused by administration of pharmacologic agents such as beta-blockers, angiotensin-converting enzyme (ACE) inhibitors, and calcium-channel blockers
Blood Pressure Cuff Size.
Correct size and placement of the inflatable bladder (inside the nondistensible cuff) are crucial to obtaining an accurate blood pressure measurement. The bladder should be long enough to encircle at least 80% of the width of the upper arm (or leg) in adults.19 Cuffs that are too small can give falsely high readings, and cuffs that are too large can give falsely low readings. Box 13-8 lists the key points to observe when obtaining blood pressure readings.
Auscultatory Gap.
In older patients with systolic hypertension, the presence of an auscultatory gap is not uncommon.19 It is important to inflate the cuff to greater than the patient’s normal systolic pressure to avoid this gap and underestimation of SBP. Use of an initial palpation estimate of the SBP taken before auscultation with a stethoscope is one recommended method to accurately determine the upper SBP.
Pulsus Paradoxus.
In normal physiology, the strength of the pulse fluctuates throughout the respiratory cycle. When the “pulse” is measured using the SBP, the pressure is observed to decrease slightly during inspiration and to rise slightly during respiratory exhalation. The normal difference is 2 to 4 mm Hg. In some clinical conditions such as cardiac tamponade, the blood pressure decline is abnormally large during inspiration. In general, an inspiratory decline of SBP greater than 10 mm Hg is considered diagnostic of pulsus paradoxus.20–22 The traditional technique for measuring pulsus paradoxus using a sphygmomanometer and a blood pressure cuff and pulse oximetry is described in Box 13-9. If the patient is hypotensive, pulsus paradoxus is more accurately assessed in the critical care unit by monitoring a pulse oximetry waveform or an indwelling arterial catheter waveform.20–22
Normal Heart Sounds
Auscultation of the heart is the most challenging part of the cardiac physical examination, and, in an era of increasing technologic demands, it is daunting to new clinicians. To summarize the advice given by most experts, the examiner must do the following:23–25
1. Auscultate systematically across the precordium.
2. Visualize the cardiac anatomy under each point of auscultation, expecting to hear the physiologically associated sounds.
3. Memorize the cardiac cycle to enhance the ability to hear abnormal sounds.
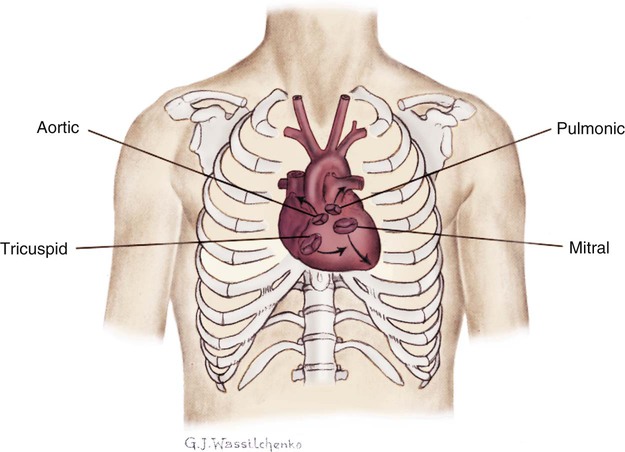
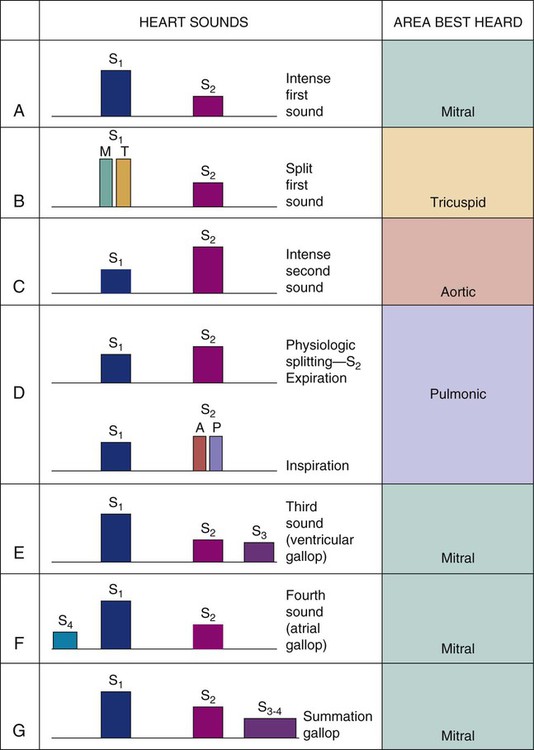
First and Second Heart Sounds.
Normal heart sounds are referred to as the first heart sound (S1) and the second heart sound (S2). S1 is the sound associated with mitral and tricuspid valve closure and is heard most clearly in the mitral and tricuspid areas. S2 (aortic and pulmonic closure) can be heard best at the second intercostal space to the right and left of the sternum (see Fig. 13-5). Both sounds are high pitched and heard best with the diaphragm of the stethoscope (Box 13-10). Each sound is loudest in an auscultation area located downstream from the actual valvular component of the sound, as shown in Figure 13-6.

Physiologic Splitting of S1 and S2.
Each normal heart sound has two components: right and left. Mitral valve closure and tricuspid valve closure are both responsible for S1, and aortic valve and pulmonic valve closure are responsible for S2. All the components of the split sounds are high pitched and best heard with the diaphragm of the stethoscope (Fig. 13-7). Normally, sounds emitted from the left side are louder than those from the right because left ventricular contraction occurs milliseconds before that of the right ventricle. Physiologic splitting is accentuated by inspiration and usually disappears on expiration. This splitting is most easily detected on inspiration because there is an increased blood return to the right side of the heart and a decreased amount of blood return to the left side of the heart. As a result, pulmonic valve closure is delayed because of the extra time needed for the increased blood volume to pass through the pulmonic valve, and aortic valve closure is early because of the relatively smaller amount of blood ejected from the left ventricle. The resulting heart sound is a split S2 (the closure of each valve is audible because there is more time between left and right contractions) (see Fig. 13-7).
Abnormal Heart Sounds
Third and Fourth Heart Sounds.
Abnormal heart sounds are known as the third heart sound (S3) and the fourth heart sound (S4); they are referred to as gallops when auscultated during an episode of tachycardia. These low-pitched sounds occur during diastole and are best heard with the bell of the stethoscope positioned lightly over the apical impulse. The characteristics of S3 and S4 are detailed in Box 13-11. The presence of S3 may be normal in children, young adults, and pregnant women because of rapid filling of the ventricle in a young, healthy heart. However, an S3 in the presence of cardiac symptoms is an indicator of heart failure in a noncompliant ventricle with fluid overload.26 Not unexpectedly, the development of an S3 heart sound is strongly associated with elevated levels of brain natriuretic peptide (BNP or proBNP).26
Auscultation of an S4 also leads the examiner to suspect heart failure and decreased ventricular compliance.27 An S4, also referred to as an atrial gallop, occurs at the end of diastole (just before S1), when the ventricle is full. It is associated with atrial contraction, also called atrial kick.
Heart Murmurs
Heart valve murmurs are prolonged extra sounds that occur during systole or diastole. Murmurs are produced by turbulent flood flow through the chambers of the heart, from forward flow through narrowed or irregular valve openings, or backward regurgitant flow through an incompetent valve.28 Murmurs occur in both systole and diastole. Most murmurs are caused by structural cardiac changes. The steps to effectively and accurately auscultate for cardiac murmurs are listed in Box 13-12. Murmurs are characterized by specific criteria:
Timing: place in the cardiac cycle (systole/diastole)
Location: where it is auscultated on the chest wall (mitral or aortic area)
Radiation: how far the sound spreads across chest wall
Quality: whether the murmur is blowing, grating, or harsh
Pitch: whether the tone is high or low
Intensity: the loudness is graded on a scale of 1 through 6; the higher the number, the louder is the murmur (Box 13-13).
The four most common valvular murmurs auscultated in adults are briefly discussed in the following paragraphs. For more information on valvular anatomy, refer to Chapter 12.
Aortic Stenosis.
The term aortic stenosis describes narrowing of the aortic valve orifice. As a result, the left ventricle faces increasing difficulty in ejecting blood to the aorta. The ventricle responds by increasing intraventricular pressure and adding muscle mass (left ventricular hypertrophy), but over time, because of the pressure load and the stenotic aortic valve, the left ventricle will fail and lose contractile force. The decreased blood volume entering the aorta during systole means that the coronary arteries do not fill efficiently, and chest pain is a common symptom of aortic stenosis. This chest pain can be difficult to differentiate from angina caused by CAD, especially in the older adult.29 Other symptoms include dizziness, syncope, and breathlessness caused by left-sided heart failure. After symptoms occur, the clinical course is poor unless the aortic valve is replaced. Two years after onset of aortic stenosis symptoms, survival is poor without aortic valve replacement.29 The murmur of aortic stenosis occurs during systole. It is auscultated at the aortic area (second intercostal space, right sternal border). Aortic stenosis produces a low-pitched murmur that does not radiate, although the tone of the murmur varies, depending on the degree of valvular obstruction. Because a strong correlation does not exist among the loudness of the murmur, clinical symptoms, and the severity of the stenosis, it is advisable to perform an ECG to visualize the valve after an aortic stenosis murmur is detected.
Aortic Insufficiency.
The term aortic regurgitation, also commonly known as aortic insufficiency, describes an incompetent aortic valve. It is often described in layperson’s terms as a “leaking valve.” After the left ventricle has ejected blood into the aorta, the valve normally closes, maintains a tight seal, and prevents blood from moving back into the left ventricle. If the valve cusps do not maintain this seal, the sound of blood flowing back into the left ventricle during diastole is heard as a decrescendo, high-pitched, blowing murmur. This early diastolic murmur is initially audible at the aortic area (second intercostal space, right sternal border), but as the aortic regurgitation progresses, it can be auscultated along the length of the left sternal border. As with all valvular murmurs, the pitch and intensity vary with the degree of regurgitation. Box 13-14 provides expected abnormal findings at each of the key auscultatory areas; Table 13-4 compares the features of the most common valvular murmurs.
TABLE 13-4
CHARACTERISTICS OF SOME MURMURS
| DEFECTS | TIMING IN THE CARDIAC CYCLE | PITCH, INTENSITY, QUALITY | LOCATION, RADIATION |
| Systolic Murmurs | |||
| Mitral regurgitation |
 |
High Harsh Blowing |
Mitral area May radiate to axilla |
| Tricuspid regurgitation |
 |
High Often faint, but varies Blowing |
Tricuspid RLSB, apex, LLSB, epigastric areas Little radiation |
| Ventricular septal defect |
 |
High Loud Blowing |
Left sternal border |
| Aortic stenosis |
 |
Chhhh hh Medium Rough, harsh |
Aortic area to suprasternal notch, right side of neck, apex |
| Pulmonary stenosis |
 |
Low to medium Loud Harsh, grinding |
Pulmonic area No radiation |
| Diastolic Murmurs | |||
| Mitral stenosis |
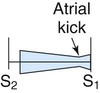 |
Low Quiet to loud with thrill Rough rumble |
Mitral area Usually no radiation |
| Tricuspid stenosis |
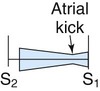 |
Medium Quiet; louder with inspiration Rumble |
Tricuspid area or epigastrium Little radiation |
| Aortic regurgitation |
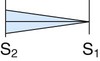 |
High Faint to medium Blowing |
Aortic area to LLSB and aorta Erb’s point |
| Pulmonic regurgitation |
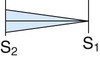 |
Medium Faint Blowing |
Pulmonic area No radiation |
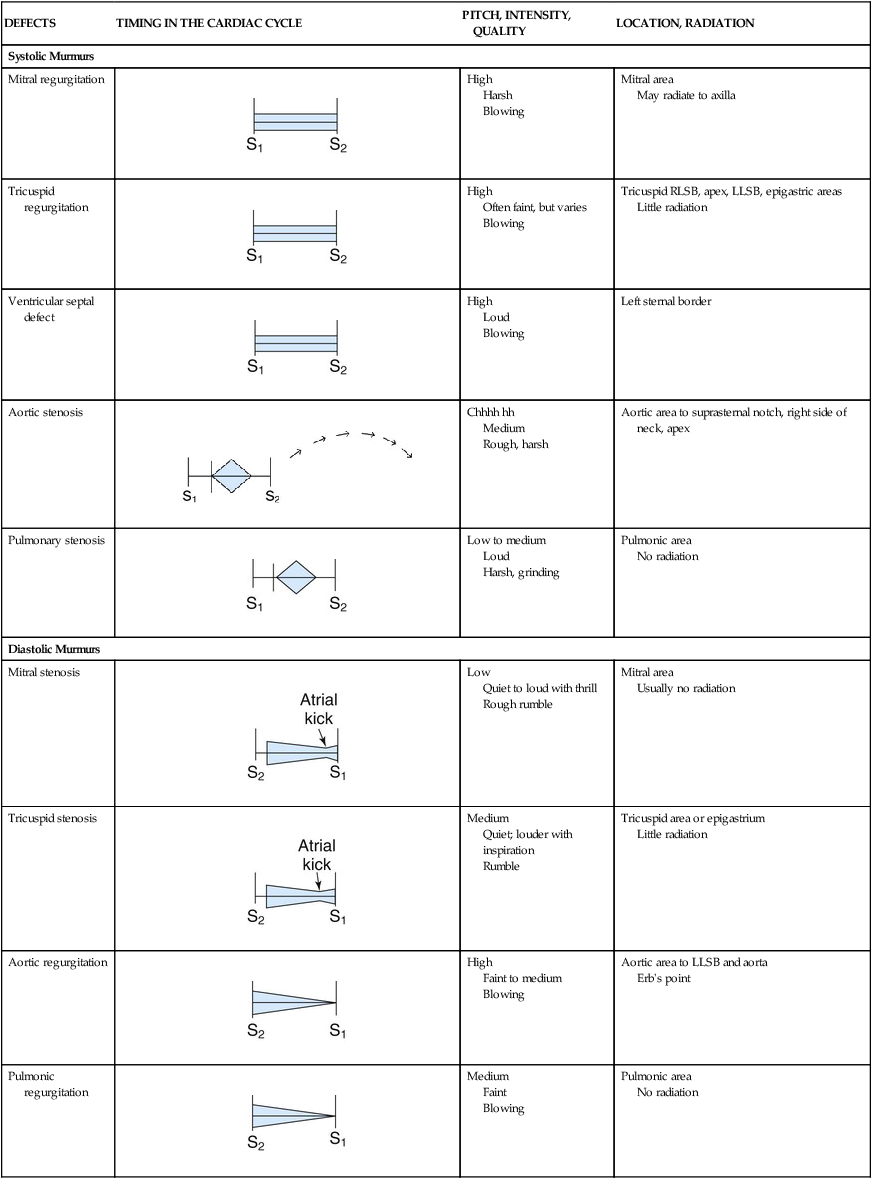
LLSB, Left lower sternal border; RLSB, right lower sternal border.
Innocent Murmurs
In children, adolescents, and healthy young adults, systolic “high-flow” murmurs are common and are a result of vigorous ventricular contraction. These nonpathologic murmurs are termed innocent murmurs. They are always systolic, have a low-to-medium pitch (heard best with the bell of the stethoscope), and are of grade 1 to 2 intensity with a blowing quality. They are often heard best in the tricuspid area and do not radiate (Box 13-15).
Murmurs Associated with Myocardial Infarction
Papillary Muscle Rupture.
The auscultation of a new, high-pitched, holosystolic, blowing murmur at the cardiac apex heralds mitral valve regurgitation resulting from papillary muscle dysfunction. This murmur may be soft (grade 1 or 2) and may occur only during ischemic episodes when the papillary muscle contractility is impaired, but its presence is associated with persistent pain, ventricular failure, and higher mortality. If the murmur is loud (grade 5 or 6), harsh, and radiating in all directions from the apex, the papillary muscle or the chordae tendineae may have ruptured. The clinical auscultation of a new murmur should always be confirmed by transthoracic or transesophageal echocardiography (TEE).28,30 Papillary muscle rupture is an emergency situation requiring immediate medical and surgical intervention.
Ventricular Septal Rupture.
Ventricular septal rupture is a rare emergency situation that can occur after an acute MI. Ventricular septal rupture describes a new opening in the septum between the two ventricles. It creates a harsh, holosystolic murmur that is loudest (by auscultation) along the left sternal border.31 The clinical picture associated with acute ventricular septal rupture is that of acute ventricular failure and cardiogenic shock. Immediate diagnosis and treatment are necessary to prevent death.
Summary
• An accurate history from the patient, or from a family member or significant other who knows the individual’s current state of health, is essential. Signs and symptoms experienced by the patient offer clues as to the underlying causes of the cardiac condition. If the patient exhibits obvious signs of acute distress (shortness of breath, pink-frothy sputum, hypotension, tachycardia, pallor, sweating), or complains of chest pain or pressure, the questions are brief and focused to quickly identify the immediate problem.
• Inspection is used to determine whether the patient is anxious or relaxed. Inspection is also employed to identify serious conditions such as cyanosis, clubbing of fingernails, or significant peripheral edema.
• Palpation of the major pulses is a routine part of the cardiovascular physical examination. Normally, pulses are equal bilaterally. The loss of a pulse on one side may indicate the presence of atherosclerotic arterial vascular disease.
• Auscultation of heart sounds and murmurs is a skill that takes time and practice to master. When performed skillfully, the auscultation can reveal much about cardiac function and blood flow.