Cardiovascular and Pulmonary Pathophysiology
Because cardiovascular and pulmonary conditions are among the leading causes of morbidity and premature mortality, they often present clinically to the physical therapist as secondary as well as primary diagnoses. In addition, patients commonly have one or more risk factors for one or both of these broad categories of conditions. Thus physical therapists need a thorough understanding of these conditions, their prevention, their presentation, and their management. Lifestyle and environmental factors are primary causes and contributors (see Chapter 1); thus prevention and reversal of symptoms are aimed at modifying these factors wherever possible.
Smoking is a principal contributor to cardiovascular disease and the primary cause of chronic obstructive pulmonary disease (COPD). Abstinence from smoking is the only intervention that can prevent the majority of cases of COPD, and smoking cessation is the only intervention to retard its progression. Thus cessation of smoking is a primary health care goal at the community, societal, and individual levels,1,2 and it should be a primary intervention by the physical therapist in any patient who smokes (see Chapter 1).
Recent advances in understanding the pathophysiology of cardiovascular and pulmonary conditions have highlighted a common denominator: inflammation of the endothelium of the blood vessels and the epithelium of the airways. With increasing severity of the condition, proteins alter their structure, and repair is required to maintain their essential structure and function with the upregulation of reparative proteins.3 Activation of this defense system is triggered by ischemia, hypoxemia, and inflammation.
Myopathic changes have been observed in the peripheral muscles of people with chronic cardiovascular conditions and lung conditions (see Chapters 24 and 31). People with COPD, for example, have increased muscle fibrosis compared with age-matched people without the disease, and the cross-sectional area of type IIX muscle fibers is smaller.4
Some common causes underlying COPD have been proposed. The Dutch hypothesis explaining the development of both asthma and chronic obstructive lung disease proposes that environmental factors (e.g., smoking and air pollutants) are superimposed upon and interact with allergic and airway hyperresponsiveness components (genetic components). Smoking and airway hyperresponsiveness are the major risk factors for these conditions.5
It has been reported that gender plays a role in susceptibility to cardiovascular and pulmonary dysfunction, as well as in the severity of the dysfunction and the response to its management. This is reflected in the finding that women with COPD have an almost three-fold greater death rate than men. Specifically, women are more susceptible to the long-term adverse effects of smoking. Compared with men, they develop pathological changes more readily and have more severe symptomatology for a given long-term exposure to tobacco.6
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) has proposed universal guidelines for the classification of COPD7 on the basis of both spirometry and clinical symptoms to define stage of disease (Table 5-1). Serum or tissue markers are being sought to provide a basis for an objective diagnosis and refine intervention strategies.8
Table 5-1
Universal Guidelines for the Classification of COPD
| Predicted FEV1/FVC | FEV1 | |
| Mild COPD | <70% | ≥80% |
| Moderate COPD | <70% | <50% to 80% |
| Severe COPD | <70% | <30% |
From Crapo RO, Morris AH, Gardner RM: Reference spirometric values using techniques and equipment that meet ATS recommendations, Am Rev Respir Dis 123:659-664, 1981.
Objective measures of limitations of structure and function or impairments associated with cardiovascular and pulmonary conditions are not necessarily closely associated with health-related quality of life. Outcome measures of health-related quality of life and life satisfaction are supplemental to structure and function outcomes and are now being included in the overall clinical assessment of people with these conditions.9 Thus management programs focus on interprofessional rehabilitation consisting of multiple components to address the complexity of the limitations associated with these conditions rather than traditional primary focus on limitations of functions and structures (see Chapters 1 and 17).10
The two classic types of lung pathophysiology (obstructive and restrictive) are then presented. Interdependent pathophysiology of these lung pathologies, which invariably affects the heart as well as the lung, is described in Chapters 6 and 31 (also in Chapters 16, 34, and 36, which are related to critical care). The structure and function of the cardiovascular and pulmonary systems are interdependent (see Chapters 2, 3, 4, 5 and 6).11 Dysfunction in one system can affect the function of the other.
Coronary Artery Disease
Hypercholesterolemia
Although hypercholesterolemia is a primary risk factor for the development of atherosclerosis, increased cholesterol affects cardiac function independently in the absence of atherosclerosis and primary ischemic heart disease.12 Elevated cholesterol levels change the structure and function of cell membranes, which in turn, affects myocardial contractility, excitability, and conduction properties. In addition, smooth muscle and endothelial dysfunction occurs, and enzyme activity and cation transporters are disrupted throughout the cardiovascular system. Thus the consequences of hypercholesterolemia are pervasive and warrant assessment even in the absence of overt atherosclerosis and ischemic heart disease.
Atherosclerosis
Pathophysiology
Atherosclerosis is triggered by trauma to the intima of the arterial wall. The trauma may be related to various primary cardiac risk factors, such as high blood pressure and cigarette smoking. Oxidative stress has been identified as the common denominator for atherogenesis, acute myocardial infarction (MI), and heart failure.13 Healthy endothelium is central to optimal vascular control. High blood pressure has been identified as a trauma inducer because increased pressure and turbulence can damage the endothelial cells of the intima of the blood vessel wall, thus exposing the media to the circulation. The media, which consists primarily of smooth muscle, is thought to be the origin of the atherosclerotic lesion.
Diabetes is another risk factor for cardiovascular disease of the myocardium and of the peripheral arteries.14 The reduced contractility of the heart in people with diabetes is attributed to reduced calcium handling in the sarcoplasmic reticulum of muscle.
Atherothrombosis is a generalized and diffuse progressive process affecting multiple vascular beds.15,16 The clinical consequences include the acute coronary syndromes, ischemic stroke, and peripheral arterial disease. Thus these conditions can be viewed as diverse manifestations of a common underlying pathology. The time course of these conditions is unpredictable; yet they can be life-threatening.
Thyroid is an important regulator of cardiac function and cardiovascular hemodynamics.17 The effects of the physiologically active form of thyroid hormone triiodothyronine (T3) on the systemic vasculature include relaxation of the vascular smooth muscle, hence reduced resistance and diastolic blood pressure. In hypothyroidism, cardiac contractility and cardiac output are decreased and systemic vascular resistance is increased, whereas the opposite occurs in hyperthyroidism. Cardiac dysfunction is associated with low levels of T3.
Because of the current appreciation and understanding of the involvement of inflammation and endothelial injury in cardiovascular dysfunction, endothelial function biomarkers have been proposed as a sensitive means of evaluating cardiovascular disease.18,19
Risk Factors
As described earlier, high blood pressure, cigarette smoking, and hyperlipidemia are direct or primary risk factors for atherosclerosis. Secondary risk factors include age, gender, race, obesity, stress, and activity level. Modifiable risk factors include hypertension, hyperlipidemia, smoking, obesity, abnormal glucose tolerance and diabetes, stress level, and activity level. Homocysteine levels have a strong relationship with atherothrombotic disease and venous thromboembolism, and elevated levels may indicate thrombotic tendencies in individuals, particularly those who are younger, in the absence of other established risk factors.20 Homocysteine levels can be reduced and regulated through diet by increasing fruit and vegetable intake. Recently, the tendency to panic has been implicated as a risk factor for cardiovascular disease,21 and depression can result in a worse outcome.22
Vascular calcification is an established marker of atherosclerosis, which leads to increased arterial stiffness and reduced compliance and increased pulse pressure. Vascular calcification is highly correlated with mortality resulting from cardiovascular disease, particularly if diabetes or renal disease complicates the clinical presentation.23,24 The three primary risk factors—diet, hypertension, and smoking—are modifiable by the individual, and altering the diet alone can reduce the probability of CAD five- to ten-fold.25,26,27
A proposed and potentially underestimated risk factor for cardiovascular disease is related to circadian rhythms.28 It has been well established that people with heart failure have a higher rate of mortality in the early morning hours. This probably reflects diurnal variations in neurohumoral factors, including the activity of the sympathetic nervous system. The cardiac circadian clock synchronizes the response of the heart to the diurnal variations in the environment. Impairment of this mechanism could contribute to the pathogenesis of cardiovascular disease.
Aging has been implicated in lowering the threshold for the manifestation of cardiovascular disease.29 Stiffening of the arteries increases afterload and alters left ventricular architecture. Left ventricular diastolic function changes, whereas systolic function remains unchanged.
Angina Pectoris
Angina pectoris is defined as chest pain that is related to ischemia of the myocardium. Ischemic pain, however, may be referred to the left shoulder, neck, jaw, or between the shoulder blades. In fact, pain anywhere above the umbilicus could be related to coronary ischemia. Angina can be classified as stable, unstable, or variant. People with chest pain but normal coronary arteries on angiography tend to be women, and this presentation is not as benign as previously believed.30 With the advent of the ability to assess endothelial function, those at risk may be more readily identified and managed.
Prognosis of Angina
Individuals do not die of angina per se. The progression of atherosclerosis of the coronary arteries is reflected in the clinical changes that occur between the experience of angina and an MI. Even though there is no risk of mortality as a result of angina, an individual’s lifestyle can change drastically. People with angina may be fearful of being active and may deny that they are having exertional chest pain. Denial, depression, anger, and hostility are common psychosocial correlates.31 Depression and further reduction in physical activity can be associated with angina (diagnosed or undiagnosed and denied). Although restricted activity is an important component of initial treatment, low levels of activity can modify several risk factors and arrest the progression of atherosclerosis.32 In addition, diet and exercise have been documented to reverse atherosclerosis.25,26,33
Obstructive Sleep Apnea Syndrome
Obstructive sleep apnea (OSA) has a greater incidence in individuals with atherosclerosis, cardiac dysrhythmias, and hypertension than in those without.34 This has been explained in part by the presence of proinflammatory and prothrombotic factors.35 OSA is characterized by the repetitive closing and opening of the posterior pharynx that are synchronized with breathing while sleeping, usually when recumbent. Apneic periods and arterial desaturation are also common to the syndrome. Additional pathologies common to OSA and atherosclerosis include endothelial dysfunction, increased C-reactive protein, fibrinogen, reduced fibrinolytic activities, and increased platelet activity and aggregation. OSA is now considered a risk factor for cardiovascular disease. The complications of sleep apnea syndrome are exacerbated by autonomic dysfunction as well. Sleep apnea often coexists undiagnosed in people with cardiovascular disease, activates mechanisms known to aggravate and advance cardiovascular injury, and contributes to resistance to therapeutic interventions.34
Myocardial Infarction
Silent ischemia is particularly prevalent in patients with high cardiac risk and is associated with a poor outcome.36 Silent MIs can be detected when a patient has undergone ECG investigation or imaging for other problems.
Uncomplicated Myocardial Infarction
An uncomplicated MI is described as a small infarction with no complications during recovery. Usually the result is full recovery without a significant decrease in cardiac performance at rest and during minimal to moderate activity.37 Location and the extent of the MI are critical with respect to outcome. MIs located in the inferior portion of the heart are considered the least clinically significant, and partial-wall-thickness MIs are less significant than transmural MIs.
Treatment
Because the clinical course is uncomplicated, a patient’s stay in the coronary care unit may be only a couple of days, with a total hospital stay of 3 to 5 days. Once the patient’s condition is stabilized, management is oriented toward increasing physical activity and educating the patient and family with respect to risk factor reduction (see Chapters 29, 30, and 31).38 This process is described as cardiac rehabilitation, phase I (see Chapter 30).
Complicated Myocardial Infarction
Dysrhythmias
Dysrhythmias occur in 95% of patients with MIs. The type and severity of the dysrhythmia is dependent on the location and extent of the myocardial damage. Imbalance in autonomic regulation has been implicated in dysrhythmogenesis and sudden cardiac death.39 Blunted heart rate variability has been established as a marker of sympathovagal imbalance and can serve as an indicator of cardiac risk.
Dysrhythmias may be present in the absence of overt myocardial ischemia or heart damage. Common dysrhythmias are presented in Chapter 4, and their clinical implications are presented in Chapter 12. One conduction abnormality that is receiving increasing attention is atrial fibrillation.40 This dysrhythmia warrants management, given its association with thromboemboli and stroke (see Chapters 6 and 32). Furthermore, atrial fibrillation is the most common dysrhythmia associated with cardiac surgery (25% to 60%), and it leads to increased postoperative morbidity and mortality and to associated health care costs.41,42 Atrial fibrillation has been reported to be more common in men—and also to be better tolerated by them than by women.43
Heart Failure
Recent terminology regarding the classification of heart failure differentiates diastolic and systolic heart failure.44–47 Diastolic heart failure refers to the presence of the symptoms of heart failure in the absence of left ventricular dysfunction, the hallmark of systolic heart failure. Diastolic heart failure in which the left ventricle is stiff (reduced compliance and impaired relaxation resulting in increased end diastolic pressure) has been estimated to account for 40% to 50% of all cases of heart failure.48 Diastolic dysfunction has been of increasing interest in nonprimary heart disease such as systemic sclerosis, and it appears to be more common than previously thought.49 The two types of heart failure must be distinguished on the basis of Doppler echocardiography because their signs and symptoms are comparable. Both types are associated with marked morbidity and mortality.
Immediately post-MI, cardiac output is markedly reduced. The compensatory response of the body is to increase sympathetic and renin-angiotensin-aldosterone stimulation, resulting in increased heart rate and myocardial contractility. The result of this compensation is to normalize cardiac output to normal resting values. If myocardial damage is extensive, the kidneys compensate by retaining sodium and water to improve circulatory volume and venous return. Depending on the amount of myocardial tissue death, the individual may survive, but with resulting chronic congestive heart failure through persistent fluid retention and hypotension. If more than 40% of the left ventricle is infarcted, the result is usually cardiogenic shock followed by the death of the individual. Renin-angiotensin-aldosterone activation contributes to left ventricular remodeling, which is further augmented by vascular endothelial dysfunction, resulting in decreased nitric oxide bioavailability.50
Diabetic cardiomyopathy leads to heart failure independent of underlying coronary artery disease.51 Both structural and functional abnormalities associated with diabetic cardiomyopathy have been linked to an underlying metabolic disorder. Other factors include myocardial fibrosis due to an inflammatory process, small blood vessel pathology, cardiac autonomic neuropathy, and insulin resistance.52
Thrombosis
Deep vein thrombosis (DVT) and related pulmonary emboli are largely preventable clinical complications, and when they do occur, their diagnosis may be missed in hospitalized patients.53,54 These life-threatening complications are serious and warrant early detection. Mortality resulting from thrombi that migrate to the lungs (pulmonary emboli) is greatest initially after an acute MI. DVTs can be challenging to detect because of the lack of specificity of their clinical presentation. Another complication is increased incidence of thrombosis originating in deep leg veins and in the damaged heart itself. Thrombosis that starts in deep leg veins occurs because of lower limb inactivity and circulatory stasis. This is a complication that can be observed in patients after surgery. Emboli from a deep leg vein thrombus usually cause pulmonary complications. If the emboli are large or numerous, the result can be pulmonary tissue infarction and death. The incidence of pulmonary emboli has been reduced because patients now are usually ambulated soon after a medical event or surgery. Nonetheless, a pulmonary embolus must be considered a distinct possibility in all patients after an MI, surgery, or major trauma.
Structural Damage
Risk factors for cardiac rupture include being female, being older, having hypertension, and experiencing the first cardiac event.55 Clinical signs of rupture include syncope, chest pain, and jugular venous distention. In addition, defective ventricular remodeling may predispose the heart to rupturing.
Treatment
Patients with complicated MIs require longer stays in the coronary care unit, and their total hospital stay times are longer than those of patients with uncomplicated MIs. The time in coronary care and total hospital stay are dependent on the complications that occur after the MI. Individuals with heart failure, thrombolytic events, or structural damage requiring surgery may be in the coronary care unit for a couple of weeks. Total hospital-stay times for patients with complicated MIs may exceed this length of time. Treatment after discharge from the intensive care unit, however, is similar to that of the patient with an uncomplicated MI; the goal is to increase physical activity and educate the patient and family in risk-factor reduction. The major difference in phase I cardiac rehabilitation for patients with complicated rather than uncomplicated MIs is the intensity, duration, and frequency of the initial exercise workload (i.e., a much lighter workload is prescribed for patients with complicated MI).56 These patients also require closer monitoring. Progression is more conservative because patients are at higher risk after a complicated MI than after an uncomplicated MI.56
Prognosis after a Myocardial Infarction
After an MI, the prognosis depends on many factors. Compared with the patient’s premorbid status, cardiovascular performance is reduced, unless the structural damage to the ventricle is minor (as in the case of many patients with uncomplicated MIs). The most important factor is the extent of ventricular damage. With early detection of transmural infarction and improvement in surgical intervention and coronary care, however, the number of acute post-MI deaths has been reduced.38 Other critical factors include remaining cardiac capacity and cardiac status and risk factors. Even though CAD mortality has declined in the United States, the disease remains the leading cause of death in adults.
Severe infarction can necessitate emergency or elective revascularization surgery. Emergency surgery for an acute MI complicated by cardiogenic shock is associated with satisfactory long-term survival; however, perioperative risk is high.57
Congestive Heart Failure
Congestive heart failure (CHF) is a leading cause of hospitalization and death. It is characterized by the inability of the heart to maintain adequate cardiac output. The incidence of congestive heart failure appears to be increasing because of the prevalence of injurious lifestyle behaviors, aging, and improved survival after acute cardiac episodes.58
Almost half of all patients with congestive heart failure are women.59 Smoking, diabetes, and high blood pressure are stronger risk factors for CHF in women than in men. Peripartum cardiomyopathy, which is unique to women in their childbearing years, occurs either in the late stages of pregnancy or within several months after giving birth.
Dyspnea is the primary complaint of people with congestive heart failure. It can be challenging to distinguish between dyspnea resulting from CHF and dyspnea resulting from pulmonary causes. B-type natriuretic peptide (BNP) is synthesized, stored, and released in the ventricular myocardium, and it is stimulated by changes in ventricular wall tension and stretch.60 The use of BNP holds some promise as a marker for congestive heart failure and a guide to clinical management.
Congestive heart failure is a major contributor to progressive renal dysfunction and anemia.61 Anemia is observed in one-third of all patients with CHF. Conversely, chronic renal dysfunction can cause severe cardiac injury and is often associated with anemia. Thus congestive heart failure, chronic renal insufficiency, and anemia create a vicious circle that warrants aggressive medical management to attenuate the progression of the three conditions.
Chronic Heart Failure
Gas transfer across the alveolar-capillary membrane is impeded in CHF. This may be explained by the pressure and volume overloading, which injures the alveolar blood-gas barrier, hence impairing the diffusion of blood across it.62 In the short term these changes may be reversible. If the membrane is chronically challenged, however, the anatomic and physiological integrity of the membrane is remodeled. These changes have been associated with worsened symptoms and exercise tolerance. Further, these changes may be prognostic of patient outcome.
Cardiac remodeling is a central feature of heart-failure progression.63 Remodeling refers to the alteration of the structure and geometry of the heart in response to myocardial insult or pressure or volume overload. Such remodeling reflects the adaptation that is needed to maintain adequate heart function with changing conditions. Increased muscle mass is one of the primary adaptations, and it usually involves left ventricular hypertrophy.64 Adaptive hypertrophy of the left ventricle is clinically important in that it is associated with increased morbidity and mortality rates.65 A further consequence of adaptive cardiac hypertrophy is the potential for reduced responsiveness to the metabolic and functional effects of insulin, which further contributes to the heart’s hypoeffectiveness.66 Pharmacological studies have been conducted to examine the role of drugs on the remodeling process in individuals with heart failure. Studies of the role of nonpharmacological interventions, including exercise, in cardiac remodeling have not been made.
A reciprocal relationship between CHF and diabetes has been well established.67 People with CHF may be at increased risk for diabetes due to reduced physical activity, cellular metabolic defects, reduced muscle perfusion, and poor nutrition. The increased sympathetic stimulation associated with CHF increases insulin resistance and decreases insulin release from the beta cells of the pancreas. Both factors contribute to glucose intolerance and diabetes, which in turn lead to hyperglycemia and increased risk for cardiovascular and metabolic complications.
Even in patients with chronic heart failure, regular exercise may be associated with a protective metabolic phenotype.68 This effect of exercise could explain why fit people have less severe MIs than nonfit people.
Compensated and Decompensated Heart Failure
Decompensated heart failure affects 5 million Americans and is associated with a 5-year mortality rate of almost 50%.69 Decompensated failure occurs when the heart is so severely damaged or weakened that normal cardiac output cannot be attained. This type of failure is defined as a sustained deterioration of at least one New York Heart Association functional class, usually with evidence of sodium retention.70 Cardiac output is insufficient to maintain normal renal function. Fluid continues to accumulate so the heart is stretched and weakened further, permitting only moderate to low quantities of blood to be pumped. In unilateral heart failure, the left ventricle may fail while the right ventricle continues to pump vigorously. Blood volume and pulmonary capillary pressure increase. If this occurs, fluid filters into the interstitial spaces of the alveoli, resulting in pulmonary edema, impaired gas exchange, and suffocation. As the heart weakens, not only is systemic blood flow compromised, so is the coronary system. The area most affected is the subendocardial region. As these cells become infarcted, the heart weakens further until other regions of the heart also become ischemic and infarcted.
Prognosis
End-stage cardiac disease without effective pharmaceutical or surgical intervention, including revascularization or heart transplantation, results in death. Intermediate measures to avert deterioration have emerged, including cardiac resynchronization therapy through biventricular pacing.71 The addition of implantable cardioverter defibrillators may help to minimize the occurrence of sudden death in people with CHF. Left-ventricle assistance devices may be used to support the function of the failing heart until it responds to conservative management or until surgery is scheduled.72 Surgical ventricular restoration holds some promise for reversing inappropriate remodeling of the myocardium after infarction and restoring its normal elliptical shape.73 The primary pharmaceutical interventions used to mitigate heart failure include diuretics to reduce fluid overload and cardiac glycosides such as digitalis to improve myocardial contractility. These interventions are combined with modification of salt and fluid intake. Other factors can predict a poor outcome. Sleep apnea syndrome, for example, is common in people with CHF and is associated with a poor prognosis. As ejection fraction is decreased, the risk for thrombus formation and stroke increases.74
Valvular Heart Disease
Heart valve incompetence is classified as being either congenital or acquired (i.e., after a bacterial or viral infection of the heart valves) and can affect any one of the four heart valves. Surgical repair or replacement of defective mitral valves, tricuspid valves, and aortic valves constitutes a significant proportion of cardiac surgeries. Mitral and aortic valve disease is particularly common in people over 65 years of age.75,76 In this age group, symptoms can be masked by such comorbidities as cardiovascular disease, pulmonary disease, and hypertension. Surgical repair is associated with favorable short- and long-term outcomes. Some valve defects are benign and treatment is not indicated. Some people can tolerate heart valve defects as children, but they become symptomatic with age. Currently, these types of valve defects are often corrected at birth or early in life.
Athletic Heart Syndrome
Although physical fitness is associated with innumerable health benefits (see Chapters 1 and 18), cardiovascular adaptation to intense exercise in athletes (athletic heart syndrome) can mimic disease processes associated with cardiovascular disease.77 Sudden cardiac death in athletes is often associated with cardiac hypertrophy, dysrhythmias, or both. In addition, cardiac episodes in athletes may reflect the manifestation of congenital abnormalities in electrical activity or mechanical function of the heart. Thus screening athletes for cardiovascular adaptation is warranted. Physical therapists involved with sports teams need to be vigilant regarding risk factors associated with the athletic heart syndrome to detect and avoid untoward events.
Systemic Hypertension
Systemic hypertension, or wide pulse pressure hypertension, has become increasingly prevalent and is implicated in multiorgan dysfunction, not only in cardiac dysfunction and failure (see Chapter 1). Systolic hypertension syndrome refers to a complex of hemodynamic maladaptations, including stiff central arteries, normal peripheral arteries, arteriolar constriction, metabolic abnormalities, cardiac hypertrophy, and increased blood pressure variability.78 In addition to the conventional measures of hemodynamic status, measures of arterial mechanics, including arterial compliance, elastic modulus, impedance, pulse wave velocity, and pulse pressure amplification, are used for diagnosis and management.
Hypertension is strongly implicated in cardiovascular disease and stroke.79 When systemic hypertension syndrome advances to heart disease, the prognosis is poor.80
Obesity is considered a primary risk factor for hypertension, as well as for heart disease, stroke, and renal dysfunction. Mechanisms that have been proposed for obesity-related hypertension include insulin resistance, hyperinsulinemia, dyslipidemia, increased sympathetic activity, sodium and water retention, cardiac dysfunction, and endothelial dysfunction.81 To adapt to an increasing workload, the heart enlarges. When it can no longer adequately compensate, the heart begins to fail, and that is usually coupled with respiratory failure. Myocardial hypertrophy is considered an independent risk factor for cardiovascular disease in people who are obese and is a strong predictor of heart failure.
The occurrence and clinical implications of myocardial fibrosis in people with hypertension are well established.82 The rennin-angiotensin-aldosterone system and contributions of mineralocorticoids and endothelin have been implicated in the development of myocardial fibrosis.
Thyroid hormone has well-documented effects on cardiovascular function, including blood pressure.17 In hyperthyroidism, pulse pressure is increased, whereas in hypothyroidism, pulse pressure is narrowed. Adaptations of the cardiovascular system alter blood pressure to accommodate new demands on the system. The effects of thyroid hormone on blood pressure, therefore, are mediated both directly and indirectly.
Pulmonary Hypertension
Pulmonary hypertension is increasingly recognized as a pathology that can occur secondary to some other pulmonary condition or with no apparent cause (idiopathic pulmonary hypertension). By definition, pulmonary hypertension exists when the mean pulmonary pressure is greater than 25 mm Hg at rest and greater than 30 mm Hg during exercise.83 Pulmonary blood pressure can increase in response to increased pulmonary vascular resistance, blood flow, and pulmonary artery wedge pressure. The cause of pulmonary hypertension has shifted from being attributed to vasoconstrictive dysfunction to being attributed to angioproliferative dysfunction.84 Endothelial dysfunction has been implicated.85 Although the prognosis is variable, with some people surviving months and others decades, guidelines have been recommended to assess the prognosis for individuals with pulmonary artery hypertension and to institute appropriate intervention expeditiously.86
Obstructive Lung Disease
COPD is also known as chronic obstructive lung disease (COLD), chronic obstructive airway disease (COAD), and chronic airway or airflow obstruction (CAO).87 Exacerbations characterize these conditions and contribute largely to their associated morbidity and mortality.88 COPD is common worldwide and contributes to major disability, as well as to economic and social burdens.
More than 30 million Americans have COPD.89 The first part of this chapter describes chronic bronchitis, emphysema, asthma, and bronchiectasis. A patient usually presents with more than one of these conditions. Most people with COPD have a combination of chronic bronchitis, emphysema, and airway hyperactivity. The typical presentation includes episodic wheezing along with a variable degree of chronic bronchitis and emphysema.90 A radiograph typically shows hyperinflated lungs, flattened diaphragms, and an enlarged right ventricle as a result of hypoxemia and increased pulmonary artery pressure. Other findings vary from patient to patient, depending on the predominant disease process contributing to the COPD.
Inflammation of the bronchial wall is typically present through the course of the disease, with increasing inflammation during exacerbations. In addition to destruction of the lung parenchyma in emphysema, small airways are affected (obstructive bronchiolitis). Chronic inflammation leads to the remodeling and narrowing of the small airways. The destruction of lung parenchyma and the inflammation cause loss of elasticity. The two principal theories about the pathophysiological causes of COPD include oxidative stress and an imbalance between proteinases and antiproteinases. Chronic inflammation of the lung parenchyma associated with COPD can be associated with systemic inflammation and further chronic degenerative dysfunction. Because of the prominence of inflammation in the underlying pathophysiology in COPD, interest in the use of inflammatory markers in clinical assessment is considerable.91,92
The symptoms of individuals with COPD can be accentuated during sleep. The mechanisms can originate centrally, as well as peripherally (i.e., in lower airways and the chest wall).93 Adverse effects include hypoventilation, cardiac dysrhythmias, and pulmonary hypertension, all of which can predispose an individual to nocturnal death. Evaluation of sleep is therefore an essential component of the overall assessment.
Chronic Bronchitis
Chronic bronchitis is a condition associated with chronic swelling and inflammation of the bronchi and bronchioles. The diagnosis is based on report of a cough producing sputum on most days for 3 months during 2 consecutive years when other conditions have been ruled out.94 The degree of airway narrowing is assessed with spirometry.
Pathologically, an increase in the size of the tracheobronchial mucous glands (increased Reid index) occurs, along with goblet cell hyperplasia.95 Mucous cell metaplasia of bronchial epithelium results in a decreased number of cilia. Ciliary dysfunction and disruption of the continuity of the mucous blanket are common. In the peripheral airways, bronchiolitis, bronchiolar narrowing, and increased amounts of mucus are observed.96
Chronic bronchitis results from long-term irritation of the tracheobronchial tree. The most common cause of irritation is cigarette smoking.97 Regular exposure to the inhaled toxic particles and gases in cigarette smoke causes inflammation in the epithelium of the central airways (larger than 4 mm in internal diameter). This inflammatory process is associated with an increased production of mucus by the goblet cells and mucous glands. Smoking inhibits ciliary action and destroys cilia. The hypersecretion of mucus and the loss and impairment of cilia lead to a chronic productive cough. The fact that smokers secrete an abnormal amount of mucus predisposes them to respiratory infection and prolonged recovery from such infection. The irritation of smoke in the tracheobronchial tree causes bronchoconstriction. Although smoking is the most common cause of chronic bronchitis, other triggers include air pollution, bronchial infections, and occupations in which air quality is affected.
Patients with chronic bronchitis often appear blue as a result of hypoxemia. Although many patients have a high arterial partial pressure of carbon dioxide (PaCO2), the pH is normalized by renal retention of bicarbonate (HCO3). The bone marrow compensates for chronic hypoxemia by increasing the production of red blood cells, leading to polycythemia.90 Polycythemia increases blood viscosity and the work required by the heart to pump and circulate the blood through the lungs and systemic vasculature. Long-term hypoxemia leads to hypoxic pulmonary vasoconstriction, increased pulmonary artery pressure and, potentially, right ventricular hypertrophy.
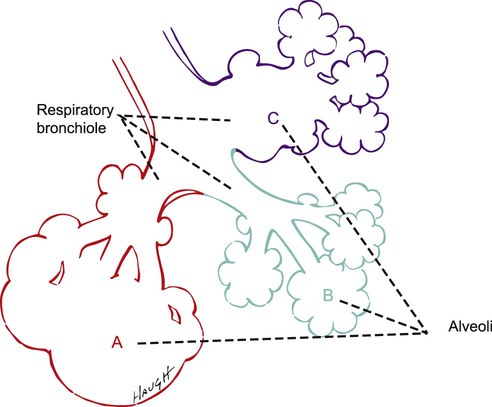
Emphysema
Emphysema is defined as “a condition of the lung characterized by abnormal permanent enlargement of airspaces distal to the terminal bronchiole, accompanied by destruction of their walls.”98 There are two main types of emphysema: centrilobular and panlobular.99 Centrilobular emphysema is 20 times more common than panlobular emphysema, although both types often coexist in the same patient.
Centrilobular emphysema is characterized by inflammation, edema, thickened bronchiolar walls, and destruction of the respiratory bronchioles. These changes are common and are marked in the upper lobes and the superior segments of the lower lobes.100 Centrilobular emphysema is more common in men than in women, is rare in nonsmokers, and is common in patients with chronic bronchitis.
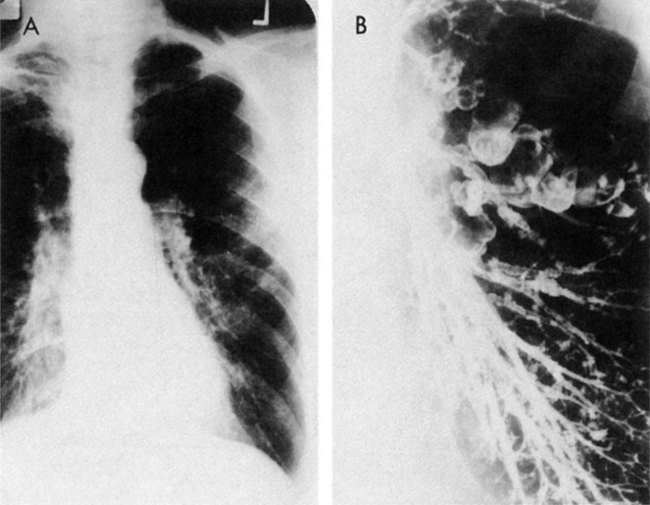
Panlobular emphysema, characterized by destructive enlargement of the alveoli, affects primarily the lower lobes (Fig. 5-1). This type of emphysema is characteristic of alpha1-antitripsin deficiency, which involves an imbalance between the elastin and elastase in the lung parenchyma. The emphysematous lung develops secondary to loss of radial traction on the bronchioles and loss of the elastic recoil of the alveoli. When individuals without pulmonary dysfunction inhale, the airways are stretched open by the enlarging elastic lung. In exhalation, the airways are narrowed as a result of the decreasing stretch of the lung. The lungs of people with panlobular emphysema have decreased elasticity because of the destruction of surrounding alveolar walls. Thus the bronchioles lack tethering and support, which exposes them to collapse even during normal exhalation.
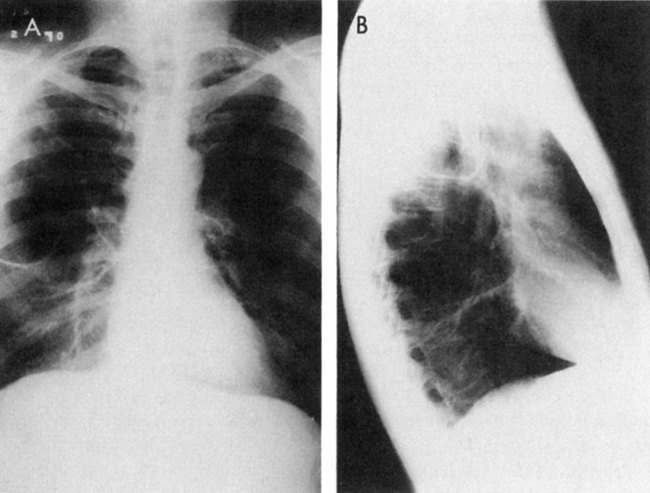
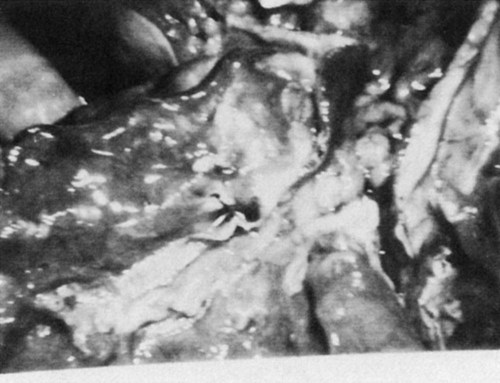
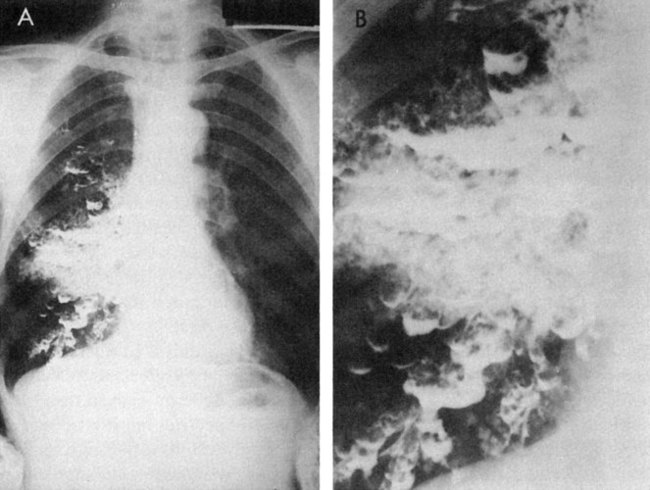
Bullae, emphysematous spaces larger than 1 cm in diameter, are commonly present in patients with emphysema (Fig. 5-2).101,102 Bullae develop from a coalescence of adjacent areas of emphysematous lung or because of an obstruction of the conducting airways that permits the flow of air into the alveoli during inspiration but impedes the outflow of air during expiration. The alveoli become hyperinflated and eventually the walls are damaged, leading to an enlarged air space in the lung parenchyma. These bullae can exceed 10 cm in diameter and they compromise the function of the remaining lung tissue (Fig. 5-3). Surgical intervention to remove bullae may be indicated. Pneumothorax, a serious complication, can result from their rupture.
Shortness of breath is the most common complaint of a person with emphysema, and it reflects psychological factors, as well as pulmonary impairment.103 Dyspnea is persistent (present daily) and worsens with exertion and during respiratory infections. These patients appear thin and have elevated shoulders and an increased anteroposterior chest diameter. They tend to breathe with the accessory muscles of respiration. These patients tend to lean forward when distressed, resting their forearms on their knees or sitting with their arms extended at their sides and pushing down against the bed or chair, to elevate their shoulders and improve the efficiency of the accessory muscles to support breathing. They may breathe through pursed lips during the expiratory phase of breathing.
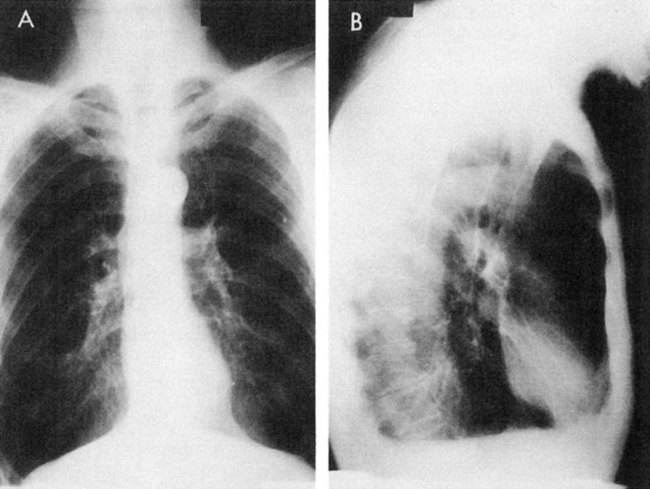
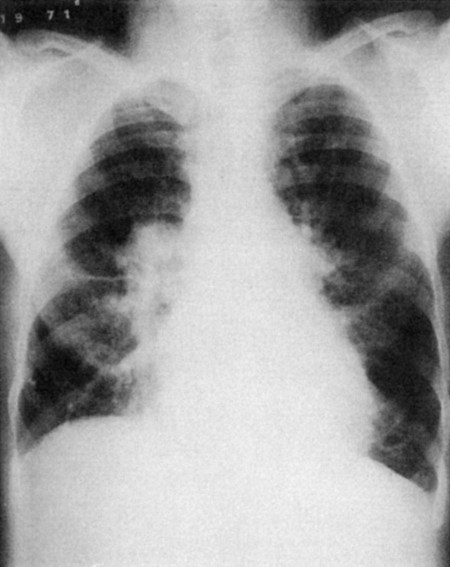
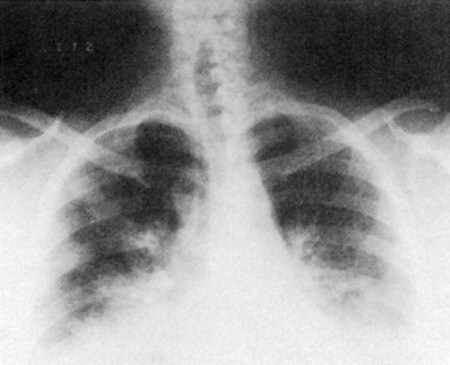
On auscultation, breath sounds are reduced throughout most or all lung fields. Radiographs show overinflated lungs, flattened hemidiaphragms, and elongated hearts (Fig. 5-4). Pulmonary function tests show decreased vital capacity, FEV1, maximum voluntary ventilation, and diffusing capacity. The total lung capacity increases as a result of greater anatomic dead space caused by hyperinflation, whereas the residual volume and functional residual capacity increase even more. Arterial blood gases reflect a mildly or moderately low PaO2, a normal or slightly elevated PaCO2, and a normal pH. At the end stage of disease, these patients develop heart failure (Fig. 5-5).
COPD is often associated with sodium retention, leading to massive edema and the development of right-sided heart failure.104 Cardiac output can remain remarkably normal, suggesting that the pathology is related to fluid overload. Underfilling of the arterial side of the circulation has been proposed for stimulating the sodium-retaining mechanism in right-sided heart failure.
Exacerbations of the disease are more frequent and severe with increased severity of lung pathology and impairment of baseline pulmonary function.105 The principal cause of an acute exacerbation of COPD is infection, which may be of viral or bacterial origin. The infection causes an inflammatory response, which causes an increased production of mucus and airflow obstruction. If hospitalization is required, treatment usually includes IV fluids, antibiotics, and low-flow oxygen.90 Some patients may receive bronchodilators, corticosteroids, diuretics, and digitalis. Theophylline is often prescribed as a bronchodilator in COPD, but it also has other important benefits. Theophylline has been shown to improve respiratory muscle strength and endurance, mucociliary clearance, respiratory drive, and cardiac function.106,107 Pursed-lip breathing can relieve dyspnea and improve arterial blood gases in some people with COPD.108 The majority of people with COPD report symptoms consistent with asthma, bronchitis, or both, which explains why inhaled corticosteroids reduce symptoms and improve survival in some patients.106,109,110
Individuals with COPD who are hypoxemic when awake become more hypoxemic during sleep, with desaturation most marked during REM sleep.111 The mechanism is thought to reflect alveolar hypoventilation and ventilation-perfusion mismatch. Diminished ventilation during sleep also increases the risk for cardiac dysrhythmias.93 If COPD is associated with obstructive sleep apnea, patients have increased risk for respiratory insufficiency and failure. During acute exacerbations, these effects can be augmented, thus contributing to nocturnal death.
Risk factors for emphysema include smoking, a history of chronic bronchitis, and increasing age.94 The risk for developing COPD is 30 times greater in smokers than in nonsmokers.99,100 The relatively rare form of inherited emphysema, alpha1-antitrypsin deficiency, develops fairly early in life and is unrelated to smoking history. Repeated lower respiratory tract infections can play a role.
COPD is associated with clinically important extrapulmonary effects, including peripheral skeletal muscle dysfunction, muscle wasting, and osteoporosis.91 Recent literature supports the theory that chronic lung and heart conditions share a common myopathy (see Chapters 24 and 31).112–114 Both muscle wasting and altered muscle protein metabolism have been implicated in the muscle adaptation to COPD.115 This finding has important implications for rehabilitation.
Respiratory muscle weakness and fatigue are associated with the severity of COPD.116 Muscle weakness results from structural ventilatory impairments, adaptations to the chronic mechanical load imposed by the underlying pathology, and deconditioning. A delicate balance exists between respiratory muscle overload and adaptation. Respiratory muscle strength and endurance are fundamental to assessment, evaluation, treatment prescription, and prognosis.
Venous thromboembolism poses major risk to individuals with COPD, particularly during acute exacerbations, when blood volume may be relatively reduced in the presence of normally elevated hematocrit and, during an exacerbation, these patients are less active.117 Even though pharmacologic prophylaxis has been advocated for high-risk patients, the physical therapist should institute prophylactic measures for all patients.
For comprehensive evaluation of the functional status of individuals with chronic lung disease, exercise testing provides an integrated perspective of organ-system function and can be used to prescribe physical activity and a structured exercise program (see Chapter 24). Exercise testing data provide supplemental information in the assessment because resting pulmonary function is not necessarily closely associated with functional capacity.
COPD progresses from respiratory insufficiency to failure. The two categories of failure are lung failure, resulting in hypoxemia, and pump failure, resulting in alveolar ventilation and hypercapnia.118 These two conditions are managed differently based on their underlying pathophysiological mechanisms and varying clinical manifestations.
Prognosis of Chronic Bronchitis and Emphysema
Without cessation of smoking and with chronic respiratory irritation, progressive loss of lung function and worsening of symptoms will progress in people with chronic bronchitis and emphysema.119
Smoking cessation is the only effective intervention for preventing or reducing the life-threatening effects of COPD.120 The decline in pulmonary function (FEV1) is decelerated, supporting the theory that inflammation and remodeling of the airways are positively affected. Histopathological studies, however, support the idea that inflammation can persist after cessation of smoking. Longitudinal studies are needed to identify the time course and other factors that may contribute to this negative sequela and its remediation.
Individuals with COPD are at risk for tissue catabolism and associated weight loss. Poor nutrition is associated with increased morbidity. Diets are optimized, and nutritional supplements are recommended on the basis of consultation with a nutritionist. The physical therapist pays particular attention to nutrition because interventions that include exercise impose energetic demands. Diet and exercise confer considerable benefits to the functional status of people with COPD that cannot be duplicated by pharmacological interventions.121
The life-threatening complications of COPD are associated with prolonged hypoxemia, leading to oxidative stress on the individual, which is manifested by ischemia affecting the organ systems. This causes dysfunction of the brain, heart, kidneys, and lungs. The most common causes of death in patients with COPD are congestive heart failure, respiratory failure, pneumonia, bronchiolitis, and pulmonary embolism. The basic mechanism underlying acute respiratory dysfunction is ventilation-perfusion mismatching with increased anatomical and physiological dead space that leads to hypercapnia and acidosis.122
With increasing severity of disease, the load on the heart increases as a result of several factors. COPD leads to sodium retention, volume overload, edema, and right-sided heart dysfunction.97 Cardiac output, however, can remain relatively normal. Total peripheral vascular resistance falls, with reduced effective circulating blood volume.
An individual with end-stage COPD is severely compromised and may be unable to engage in much meaningful activity. Lung-volume reduction surgery has demonstrable long-term (at least 5 years) objective and subjective benefits in selected patients with disease of the upper lobes and impaired exercise tolerance.123,124
Asthma
Asthma is a chronic inflammatory condition of the airways that is characterized by an increased responsiveness of the airway smooth muscle to various stimuli (due to a lowering of the threshold of airway smooth muscle reactivity). It is manifested by widespread narrowing of the airways that reverses either spontaneously or as a result of treatment.125–127 During an asthma attack, the lumens of the airways are narrowed or occluded by a combination of bronchial smooth muscle spasm, inflammation of the mucosa, and overproduction of viscous mucus.128–130 Specifically, eosinophilic inflammation is prevalent, and airway remodeling of the bronchial airways occurs over time.131,132 Clinically, peak expiratory flow rates are sensitive to subtle changes in airway status, which may be detected before the onset of symptoms.133 This early objective sign can be useful in anticipating an asthma attack.
Asthma is a widespread condition that affects 5% to 10% of the population of the United States.134 Asthma is prevalent in individuals under 25 years of age, where estimates of prevalence vary from 5% to 15%.135 Approximately 80% of children with asthma do not have asthma after 10 years of age.
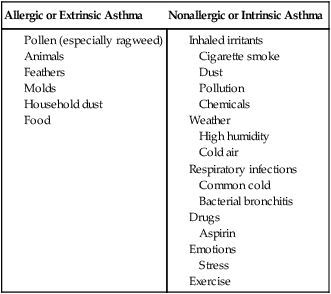
Asthma that begins before the age of 35 is usually allergic or extrinsic. Asthma attacks are precipitated when an individual comes into contact with a substance to which she or he is sensitive, such as pollens or household dust (Box 5-1). Commonly, people with asthma are allergic to multiple allergens.126
Exercise-induced asthma (EIA) is prevalent in school children as well as in competitive athletes. EIA appears to result from hyperosmolar changes or exposure to temperature changes in the airways.136 The condition can often be well controlled medically.
If a person’s first asthma attack occurs after 35 years of age, there is usually evidence of chronic airway obstruction with intermittent episodes of acute bronchospasm. These individuals whose attacks are not triggered by specific substances are referred to as having nonallergic or intrinsic asthma (see Box 5-1). Chronic bronchitis is commonly found in this group, and this type of individual is often seen in the hospital setting.
Individuals with acute asthma attacks often report being awakened at night or early in the morning with one or more symptoms, including cough, dyspnea, wheezing, and chest tightness.95 Waking at night is so common in the patient with asthma that its absence from the history may cast doubt on its diagnosis. The patient has a rapid rate of breathing and is using the accessory respiratory muscles (Fig. 5-6). The expiratory phase of breathing is prolonged with audible wheezing. As the lungs become increasingly hyperinflated, however, the breath sounds diminish. The patient may cough often, though unproductively, and may complain of chest tightness. Radiologically, the lungs may appear hyperinflated or may show small atelectatic areas caused by retained secretions. Tachypnea, hyperinflation, accessory muscle use, sitting upright, and pulsus paradoxus (difference in systolic blood pressure during inspiration and expiration) are useful guides for determining the severity of airway obstruction present.129,137 Early in the attack, arterial blood gases reflect slight hypoxemia and a low PaCO2 (from hyperventilation). As the attack progresses, the PaO2 continues to fall and the PaCO2 increases. As obstruction becomes severe, deterioration of the patient occurs, evidenced by a high PaCO2, a low PaO2, and a pH below 7.30. Optimal monitoring of the patient’s status is based on flow-volume loops and clinical judgment.138
The goals of medical management of acute asthma include maintenance of adequate arterial oxygen saturation, relief of airway obstruction, and reduction of airway inflammation.129 Patients who are hospitalized usually require intravenous fluids, bronchodilators, supplemental oxygen, and corticosteroids.
A severe asthma attack that persists for hours and is unresponsive to medical management is referred to as status asthmaticus. The patient may appear dehydrated, cyanotic, and near exhaustion due to labored breathing. In contrast to the audible wheezing heard early in the attack, the lung sounds can be greatly diminished or absent. Status asthmaticus has a significant death rate, so it is a medical emergency. Bilateral manual lower chest compression can assist expiration and may have value as an emergency treatment of asthma. Patients in respiratory failure require mechanical ventilation. Averting the adverse consequences of dynamic hyperinflation is the goal.139
On autopsy, the lungs of people with asthma are hyperinflated and fail to deflate when the thorax is opened.140 The airway mucosa is inflamed and edematous, and the basement membrane is thickened. The mucous glands are enlarged, and the number of goblet cells is increased. Bronchospasm is evidenced by airway smooth muscle hypertrophy. The lumens of the bronchioles are filled with viscous, sticky mucus, which can precipitate death by asphyxiation (Fig. 5-7). Secretions in the tracheobronchial tree of a patient with asthma are a combination of mucus, secreted by the mucous glands, and an exudate from the dilated capillaries beneath the basement membrane.141 Cilia fail to sweep the mucoserous fluid as effectively as they sweep mucus alone. In addition, sheets of ciliated epithelium can be shed into the bronchial lumens, further contributing to airway obstruction. Although the alveoli are overinflated, the permanent destructive changes associated with emphysema are not present.
Prognosis of Asthma
Early detection of an exacerbation of asthma is imperative. Mortality has been attributed to the failure to detect severity early.129 As a chronic inflammatory condition, asthma may result in irreversible lung damage if untreated.142 Antiinflammatory medications such as inhaled corticosteroids have a role in controlling mild to moderate cases of asthma. In severe disease that requires heavier doses, the adverse effects of long-term corticosteroid use become prevalent— namely, osteoporosis and glaucoma.137 Guidelines for the prevention and management of asthma have been well established.143
Bronchiectasis
The airway deformities can be classified into three types.90 Cylindrical (or longitudinal) bronchiectasis is the most common type, with a uniform dilation of the airways. Varicose bronchiectasis refers to a greater dilation than in cylindrical bronchiectasis, causing the bronchial walls to resemble varicose veins (Fig. 5-8). Saccular (or cystic) bronchiectasis refers to airways that have intermittent spherical ballooning (Fig. 5-9).
Pathologically, the mucosa appears edematous and ulcerated. Destruction of the elastic and muscular structures of the airway walls is evident with resultant dilation and fibrosis. The walls are lined with hyperplastic, nonciliated, mucus-secreting cells that have replaced the normal ciliated epithelium. This change is significant because it interrupts the mucociliary blanket and causes the pooling of infected secretions, which further damages and irritates the bronchial walls.144
The cause of bronchiectasis is related to obstruction of the airways and respiratory infections.90,144 Some 60% of cases of bronchiectasis are preceded by acute respiratory infections. The infection involves the bronchial walls. Portions of the mucosa are destroyed and replaced by fibrous tissue. The radial traction of the lung parenchyma on the damaged bronchi causes the involved airways to become permanently dilated and distorted. These areas, devoid of normal ciliated cells, contain secretions that eventually become chronically infected.
Obstruction can cause bronchiectasis by collapsing lung tissue (atelectasis) distal to the obstruction.144 The increased negative pressure in the chest (resulting from the collapsed lung) increases the traction on the airways causing them to expand and become distorted. Secretions are retained and, if the obstruction is prolonged, infection may destroy the walls of the bronchi. Infection control with antibiotics has markedly decreased the incidence of bronchiectasis.
It is now relatively uncommon for people to have severe, diffuse, long-standing bronchiectasis. Those who do have it appear physically emaciated, and as many as 25% of them may have clubbed fingers. A chronic cough with expectoration of unpleasant-tasting purulent sputum is typical in these patients. Changes in body position while sleeping or on arising often stimulate coughing as secretions drain from the small peripheral to the large central airways. These individuals may have right-sided heart failure due to fibrosis that extends into and involves the pulmonary capillary bed. Patients with widespread bronchiectasis appear dyspneic and must work hard to breathe because of hypoxemia and hypercapnia due to ventilation-perfusion mismatching. Anastomosis of the bronchial and pulmonary vascular systems causes shunting of the systemic blood from the hypertrophied bronchial arteries.145
Most patients have a chronic productive cough. Some patients complain of relatively few symptoms except during a respiratory infection, when they have increased cough and sputum production. The amount of sputum expectorated and the severity of the cough vary from patient to patient according to the amount of involvement. Hemoptysis does occur in about half of older patients, evidently because of the erosion of enlarged bronchial arteries that accompany the dilated bronchi. Other common symptoms include dyspnea, wheezing, and pleuritic chest pain.144
Pulmonary function tests of patients with localized bronchiectasis show few or no abnormalities. In more widespread disease, however, there is a reduction in the FEV1, maximum midexpiratory flow rate, maximal voluntary ventilation (MVV), and diffusing capacity and an increase in the residual volume.90,95
Prognosis of Bronchiectasis
Before the era of antibiotics, the prognosis for individuals with bronchiectasis was poor. Infection was usually the precipitating cause of death. Today, with advances in medical management, the prognosis for patients is much better.144 Most patients can lead relatively normal lives. Right-sided heart failure, a complication of diffuse, long-standing bronchiectasis, can lead to death. Pneumonia and hemorrhage are less common causes of death. Repeated bronchopulmonary infections can contribute to worsening pulmonary function and an earlier death. Before the antimicrobial era, most patients with untreated, widespread, severe bronchiectasis died within 25 years. Prognosis depends on the extent of the disease process at the time of diagnosis and on optimal medical management. Patients with moderate, localized disease, with timely intervention, can expect a relatively normal life expectancy.
Restrictive Lung Disease
Environmental factors play a major role in the etiology of primary restrictive lung conditions. Recently, smoking, which has been largely implicated in COPD and has been associated with all-cause cancer, has been reported to have an associated restrictive pulmonary component in some people.146 Primary restrictive lung disease is characterized by stiffening of the lung parenchyma, which prevents the lungs from expanding fully. Normally, as the diaphragm descends during inspiration, the dimensions of the chest wall increase and the alveoli expand. A decrease in the compliance of the lung parenchyma, as in interstitial fibrosis, sarcoidosis, pneumoconiosis, and scleroderma, can produce this defect. Pleural abnormalities such as pleural effusion due to alveolar compression prevent the lungs from expanding fully. Thoracic changes such as kyphoscoliosis and ankylosing spondylitis restrict lung expansion by restricting chest wall movement. Obesity and ascites restrict lung expansion by limiting diaphragmatic displacement.
The pulmonary function of individuals with interstitial lung dysfunction has characteristic features.147 Vital capacity, inspiratory capacity, and total lung capacity are reduced. Residual volume can be normal or reduced. If the restriction is pulmonary in origin, the lung compliance and diffusing capacity are reduced. Reduced exercise tolerance is associated with marked arterial desaturation.
Common restrictive lung conditions are described below. Multisystem conditions that can predispose an individual to restrictive lung dysfunction are common and are presented in Chapter 6.
Diffuse Interstitial Pulmonary Fibrosis
Pulmonary fibrosis is a common response to various types of lung injuries.148 Numerous acute and chronic lung conditions are associated with fibroproliferation, inflammation, or both, predisposing an individual to fibrosis. These are collectively known as interstitial lung disease.
The hypothesis that inflammation causes the development of interstitial pulmonary fibrosis is being challenged by a growing body of evidence suggesting that an abnormal wound-healing response to multiple microscopic sites of acute alveolar injury occurs, progressing to fibrosis.135,149 The resolution of the fibroproliferation response to irritation and injury is essential to patient survival. More than 130 types of interstitial lung disease have been described. Diffuse interstitial pulmonary fibrosis represents a common histological response to a wide variety of insults.150 Initially, an insult or injury to the pulmonary parenchyma causes an influx of inflammatory and immune cells, resulting in a diffuse inflammatory process distal to the terminal bronchiole (alveolitis).151 This can progress to subacute interstitial disease with the presence of acute and chronic inflammatory cells. Chronic disease is manifest by thickened alveolar walls and progression to fibrosis and scarring. A role for circulating cells of hematopoietic origin has been proposed as a causal factor.150 In addition, apoptosis has been implicated in the pathophysiology of pulmonary fibrosis.152
The known causes of pulmonary fibrosis include occupational or environmental exposure to inorganic dusts (e.g., silica and coal), toxic gases, and certain drugs or poisons.153,154 In addition, interstitial lung disease is associated with rheumatoid arthritis and systemic sclerosis.155 Twins, siblings, and other family members have been reported to have a higher incidence of diffuse interstitial pulmonary fibrosis, which supports the idea that there is a genetic link. Less commonly, interstitial pulmonary fibrosis has been reported in individuals with Raynaud’s phenomenon and ulcerative colitis.
Idiopathic pulmonary fibrosis (IPF), or cryptogenic fibrosing alveolitis, is a chronic, progressive, irreversible disease of unknown cause.148,149 The median survival time after diagnosis is approximately 3 years. Idiopathic interstitial pneumonia is associated with idiopathic pulmonary fibrosis and is a type of chronic fibrosing interstitial pneumonia with the histiologic appearance of usual interstitial pneumonia (UIP).87 It is a condition that occurs primarily in individuals over 50 years of age. Risk factors include cigarette smoking, use of antidepressants, chronic aspiration, and exposure to metal and wood dusts.
Pulmonary function tests show reduced lung volumes and capacities, maintained flows, and abnormal gas exchange.156 Compliance is markedly reduced to less than half of the predicted value. Reduced diffusing capacity is the earliest and most consistent change. Initially, arterial PaO2 may be normal at rest but may decrease markedly with exercise. PaCO2 is reduced as a result of hyperventilation, and pH is maintained by renal compensation. Later, the PaO2 is greatly reduced because of the thickened alveolar membrane and ventilation-perfusion mismatching.
Bronchoalveolar lavage (BAL) may be used to assess the amount of inflammation and the accumulation of immune effector cells and proteins in the alveoli.157 The technique consists of wedging a fiberoptic bronchoscope into a sublobar airway, then infusing 20 to 50 mL aliquots of saline into the peripheral airway. The saline is immediately aspirated by syringe. A total of 150 to 300 mL is instilled and recovered. The fluid and cells are analyzed. High-intensity alveolitis is defined by the presence of 10% or more polymorphonuclear granulocytes (PMNs) in BAL cell differential counts; low-intensity alveolitis consists of 10% PMNs or less.158
Lung biopsies that demonstrate an abundance of inflammatory cells suggest early disease, whereas a prevalence of fibrosis is indicative of advanced disease. A distinct category of IPF has been described and may be clinically useful.154 Desquamous interstitial pneumonitis (DIP) is characterized by the intraalveolar accumulation of mononuclear cells, relatively intact alveolar walls without destruction or fibrinous exudates (numerous inflammatory cells with little or no fibrosis). This pattern has correlated with a more benign course and a better response to corticosteroids. Because a pattern of DIP is commonly seen with UIP and IPF, DIP may represent an earlier, more easily reversible stage of IPF.
Corticosteroids are the mainstay of treatment for diffuse interstitial pulmonary fibrosis,154,159 although their efficacy is being questioned for treatment of IPF because the favorable response rate is only 25% to 30% and there is a high incidence of adverse effects.160,161 Regardless, a trial of immunosuppressive therapy is usually prescribed for a short period. Objective measures such as blood counts, erythrocyte sedimentation rate, pulmonary function testing, exercise tests with measurement of arterial oxygen desaturation and diffusing capacity, BAL fluid analysis, and the patient’s symptoms should demonstrate improvement; otherwise, the medication is likely to be discontinued. Individuals who are not responsive to corticosteroids may be placed on antiinflammatory medications such as colchicines160 or immunosuppressive drugs such as cyclophosphamide, azathioprine, or penicillamine.148,161,162 Penicillamine is more effective in patients with connective tissue diseases and interstitial fibrosis other than IPF.
Patients should stop smoking. Supplemental oxygen is important, along with exercise, because there is a characteristic significant fall in arterial oxygen tension (PaO2).162 Individuals who require more than a 4-L flow per minute by nasal prongs may prefer direct administration of oxygen into the trachea. In addition to supplying higher concentrations of oxygen to the lungs, many patients prefer not to wear conspicuous nasal prongs.
Patients who have refractory restrictive lung disease may be candidates for single or double lung transplantation. Outcomes of transplantation surgery have been improving (see Chapter 40).
Most individuals with acute interstitial pneumonia and diffuse alveolar damage die within 6 months.148 Patients with the chronic form who are treated with steroids can survive for years. Untreated, these patients commonly die within 1 to 4 years. The cause of death is usually related to respiratory or heart failure, although some die of adenocarcinoma and undifferentiated or alveolar cell carcinoma of the lung.
Pulmonary Infiltrates with Eosinophilia
Eosinophils are commonly present in lung tissue as part of the body’s cellular response to a variety of agents and systemic immunological diseases.95 They are present in the airways and lung tissue of patients with idiopathic pulmonary fibrosis. In interstitial lung disease that appears to have an allergic component (e.g., hypersensitivity pneumonitis, drug-induced lung syndromes, and sarcoidosis), eosinophils are minor components of the tissue reaction. In certain primary or systemic diseases, however, eosinophils can be the most conspicuous inflammatory cell present in the lung. These conditions can be grouped together and referred to as eosinophilic syndromes. Considerable overlapping exists among these syndromes because their causes and pathogenesis remain poorly understood.162
Simple Pulmonary Eosinophilia
Simple pulmonary eosinophilia is a self-limiting disease in which chest radiographs demonstrate migratory, fleeting areas of pulmonary infiltrates located in the periphery of the lungs, along with minimal respiratory symptoms and blood eosinophilia. Certain drugs such as sulfonamides have been implicated as a cause.162 This disease is also referred to as Loeffler pneumonia and as the PIE syndrome (peripheral infiltrates with blood eosinophilia). If the disease is related to an allergic response to microfilaria, human parasites (e.g., Ascaris lumbriocoides, Strongyloides stercoralis), or cat and dog parasites (ascarids) that produce visceral larva migrans, it is termed tropical eosinophilia.
Prolonged Pulmonary Eosinophilia
The condition is more common in women. Symptoms include an acute respiratory illness with fever, night sweats, weight loss, and dyspnea. Prolonged pulmonary eosinophilia can be confused with tuberculosis, but patients with prolonged pulmonary eosinophilia deteriorate when treated with antituberculosis drugs. This disease must also be differentiated from eosinophilic granuloma and the desquamative form of idiopathic interstitial pneumonitis. Dense infiltrates located in the periphery of the lung on chest radiographs provide an important clue. Often a lung biopsy is necessary to confirm the diagnosis. Corticosteroids markedly improve the patient’s symptoms and the appearance of the chest radiograph within weeks. Months or years of treatment may be required.154
Eosinophilic Granuloma
The course of the disease varies.154 Spontaneous regression with residual symptoms occurs in 10% to 25% of cases. In many patients, the disease stabilizes or “burns out,” leaving them with a moderate pulmonary impairment as a result of fibrosis, cystic lung changes, and a restrictive defect on pulmonary function tests. Dyspnea on exertion is common. Some patients have persistent bronchitis. Corticosteroids are not particularly effective. Treatment is mainly symptomatic, with judicious use of antibiotics and bronchodilators. Occasionally, progressive pulmonary disease leads to right-sided heart failure and respiratory failure.
Pulmonary Alveolar Proteinosis
Pulmonary alveolar proteinosis (PAP) is an uncommon condition of unknown origin that is characterized by alveoli filled with lipid-rich “proteinaceous” material (i.e., surfactant phospholipids) in the absence of defects of the alveolar wall, interstitial spaces, conducting airways, or pleural surfaces. Most commonly, men between the ages of 30 and 50 are affected, although people of all ages and both sexes can be affected. There are three clinical types of PAP. The most common type (>90%) is acquired PAP, which is considered an autoimmune response. Hematopoietic growth factor (granulocyte-macrophage colony-stimulating factor [GM-CSF]) is essential for local regulation of surfactant homeostasis in the lungs. People with acquired PAP have a circulating, neutralizing GM-CSF antibody. The remaining two types are congenital PAP and secondary PAP; the latter is associated with environmental factors, immunological disorders, malignancies, and hematopoietic disorders.163
The most common symptoms are progressive dyspnea and weight loss, with cough; hemoptysis and chest pain are reported less frequently. Chest radiographs reveal diffuse bilateral (commonly perihilar) opacities (Fig. 5-10). Physical findings may include fine inspiratory crackles, dullness to percussion, and in the later stages, cyanosis and finger clubbing. Pulmonary function studies show reduced vital capacity, functional residual capacity, and diffusing capacity. Arterial blood gases indicate a low PaO2, especially during exercise, with normal PaCO2, and pH.95
The treatment of choice for patients with moderate to severe dyspnea on exertion due to alveolar proteinosis is whole-lung lavage.164 In the operating room, after general anesthesia and the placement of a double-lumen tube (which isolates each lung), the patient is positioned in the lateral decubitus position, with the lung that is to be lavaged in the lowermost position. The double-lumen tube enables the patient to be ventilated to functional residual capacity by the uppermost lung while the lower lung is filled with saline. An additional 300 to 500 mL saline is alternately allowed to run in and out of the lung in response to gravity. As the saline flows out, manual percussion is performed over the lavaged lung, which increases the amount of proteinaceous material washed from the affected lung. The effectiveness of mechanical percussion, manual vibration, and manual percussion in removing material from involved lungs has been compared, and manual percussion has been found to be superior.165,166 In this procedure the affected lung is lavaged with 20 to 70 L saline. The patient may be positioned prone during the administration of the final 10 to 15 L of the lavage. After the procedure, the patient is transported to the recovery room and, once stabilized, to the ward. After a few days, the procedure may be repeated in the opposite lung.
Most patients show significant clinical improvement after whole-lung lavage, including improved PaO2, diffusing capacity, and vital capacity.164,166 Many also show an improved radiograph. After lavage, patients must be followed up, because the material may reaccumulate over time. Some patients do have spontaneous remissions without undergoing lavage.
The use of limited lobar lavage through a bronchoscope has shown some benefit.167 Half of patients who have acquired PAP with subcutaneous GM-CSF show improvement, but they show a lower rate of improvement than is seen after whole-lung lavage.164
Before whole-lung lavage became standard treatment, almost all children with alveolar proteinosis died and 20% to 25% of adults died within a few years, usually because of respiratory failure, right-sided heart failure, or uncontrolled infection. The majority of patients now improve greatly or recover.164
Sarcoidosis
Sarcoidosis is one of the most common causes of interstitial pulmonary disease.95,168 It is a granulomatous disorder of unknown origin that can affect multiple organ systems.169 Initial findings can include bilateral hilar adenopathy, pulmonary infiltration, and skin or eye lesions.170 The lungs are the organs most often involved, and in such cases, 20% to 50% of patients seek medical attention because of respiratory symptoms. African Americans are affected 10 to 20 times more often than Caucasians, and women are affected twice as often as men. It usually occurs in the third or fourth decade of life.
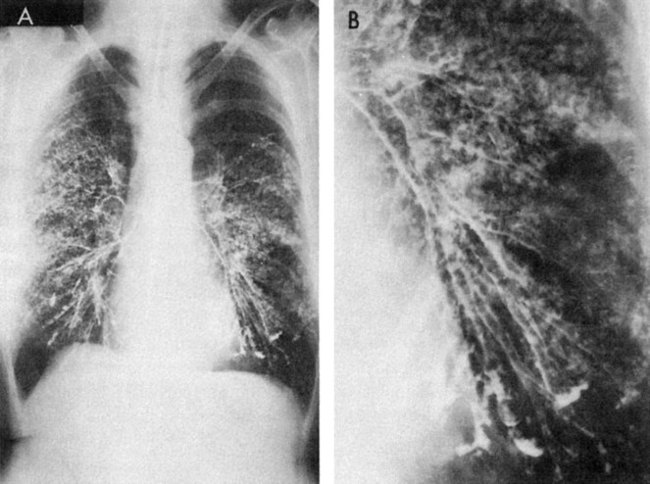
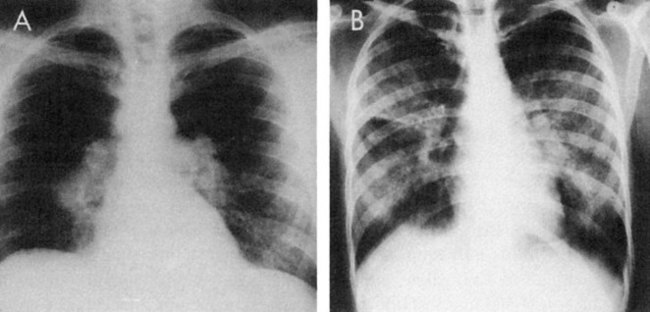
The intrathoracic changes can be classified into four stages.95 In the first stage, the patient is asymptomatic, with the chest radiograph showing bilateral hilar adenopathy and right paratracheal adenopathy (Fig. 5-11, A). In the second stage, a diffuse pulmonary infiltration is found, along with the bilateral hilar adenopathy. Interstitial infiltration or fibrosis, without hilar adenopathy, characterizes the third stage (Fig. 5-11, B). In the fourth stage, emphysematous changes, cysts, and bullae occur.
Rheumatoid Arthritis
Rheumatoid arthritis (RA) is a systemic disease that involves the joints but can affect the lungs, pleurae, and heart. The most common thoracic complication in RA is pleuritis, with or without pleural effusions. Although RA occurs twice as often in women, pleuritis has a striking predilection for men.162 Pleural disease is one manifestation of RA, occasionally causing fibrothorax and restrictive lung disease that requires decortication.
Interstitial lung disease, indicated by abnormal pulmonary function tests demonstrating a restrictive ventilatory impairment and a reduced ventilatory capacity, is evident in about 40% of people with RA. It is also more prevalent in men. The chest radiograph shows diffuse interstitial infiltrates, especially in the lung bases. Pulmonary nodules, which are pathologically identical to the subcutaneous nodules found in RA, may also occur and may cavitate.155
Systemic Lupus Erythematosus
Chronic interstitial pneumonitis is found in 3% to 13% of people with SLE, and acute lupus pneumonitis occurs in 1% to 4%.171 Patients with pulmonary involvement have dyspnea on exertion and a cough productive of mucoid sputum.155 Although rare, complaints of supine dyspnea suggest diaphragmatic paresis or a diffuse myopathy of the diaphragm.171 The chest radiograph usually indicates patchy, nonspecific densities, basilar linear or plate-like atelectasis, or both. Pleural effusions and pulmonary infiltrates are common, whereas diffuse interstitial fibrosis is rare. Pulmonary function tests often indicate a restrictive pattern with a decreased diffusing capacity and reduced arterial oxygen saturation.
Progressive Systemic Sclerosis (Scleroderma)
Progressive systemic sclerosis, also called scleroderma, is an uncommon condition that causes thickening and fibrosis of the connective tissue throughout the body and replacement of many elements of the connective tissue by colloidal collagen. Although the skin is most often involved, the lungs, heart, kidney, bones, and other parts of the body can also be affected. Approximately two-thirds of people with progressive systemic sclerosis have pulmonary involvement.155,162
The form of treatment for pulmonary involvement in patients with progressive systemic sclerosis is controversial, but usually involves corticosteroids, cyclophosphamide, or both.155
Tuberculosis
Tuberculosis (TB), a highly infectious bacterial disease, was prominent in the first half of the 1900s. With improved sanitation and medical care, TB was largely controlled. A recent resurgence of the disease has been documented, however, probably resulting from the migration patterns of people globally and the poor becoming poorer in high-income countries. TB affects many organ systems, including the lungs, and is more prevalent in people living in poor sanitary conditions with poor health. Mycobacterium tuberculosis is highly contagious but responds well to medication.87,172 The resurgence of tuberculosis in high-income countries has become a significant public health concern.
Lung Cancer
Lung cancer is by far the leading cause of death due to cancer in both men and women.173 The growth and division of cells is regulated by the DNA within every cell. When the DNA is damaged, which is estimated to be the result of lifestyle behaviors and environmental factors at least 50% of the time, cell division becomes uncontrolled. Cancer-causing agents, or carcinogens, related to lung disease include oxygen radicals and toxins in cigarette smoke. Although lung cancer is associated primarily with smoking, the occurrence of many other cancers is also higher in smokers. Thus the risk for cancer in general is increased in smokers. A physical therapist needs to understand the pathophysiology of all cancers, particularly lung cancer, and their medical and surgical management, as well as the impact of the natural history of a given cancer and the stage of a particular cancer on an individual’s health-related quality of life. Lung cancer, as all cancers, tends to have multisystem consequences that must be understood by the physical therapist and considered during management. The physical therapist has a primary role in cancer prevention, in its early detection (by recognizing the seven warning signs of cancer), and in its management during conservative and surgical phases of care and over the long term.
Anemia
Anemia usually results from blood loss or from acute or chronic dysfunction of red blood cell production. The adaptive responses of the oxygen transport system involve the cardiovascular system and an increase in cardiac output and redistribution of blood flow to favor coronary and cerebral circulations at the expense of the splanchnic vascular beds.175 These adaptations can maintain tissue oxygenation with hematocrit levels as low as 21%.176
Anemia is also commonly seen clinically in patients after orthopedic surgery, in renal failure, and in patients undergoing chemotherapy for cancer. Patients may be treated with blood transfusion or erythropoietin. Mild anemia may be managed by dietary measures and over-the-counter vitamin and mineral tablets alone. Moderate anemia may require mineral supplements. Anemia compromises recovery. Optimal management augments responses to rehabilitation and recovery, reduces fatigue, and improves exercise capacity, muscle strength, and performance of the activities of daily living.177 Whether there is a critical hemoglobin level that allows for optimal participation and activity performance is unclear. The presence of CAD may mitigate hemodynamic compensation to blood loss and other causes of anemia and may attenuate treatment responses.178
Sickle cell anemia is a genetic condition that is common in African Americans and characterized by an abnormal shape of the red blood cell, which impedes its movement through the capillary beds. Patients with sickle cell anemia have a low anaerobic threshold in the presence of a high heart rate reserve, but they have no gas exchange abnormalities.179 Patients may have restrictive lung pathology, increased alveolar dead space, and hypoxemia. Increased dead space may reflect impaired pulmonary capillary perfusion due to the sickle cells. Exercise hyperventilation is thought to be associated with increased anaerobiosis.
Type 2 Diabetes Mellitus
Type 2 diabetes mellitus is increasing in prevalence worldwide (see Chapter 1). Insulin resistance and glucose intolerance result from the additive effect of poor diets and inactivity. Diabetes is a multisystem condition; however, cardiovascular disease is the leading cause of morbidity and mortality in people with diabetes.180 Organ system dysfunction results from hyperglycemia and the deposition of sorbitol on cell membranes. The toxicity of sorbitol leads to the vascular and neurological complications of this life-threatening condition. Recent evidence supports the theory that the metabolic defects associated with diabetes (impaired glucose tolerance, insulin resistance, and proinflammatory and prothrombotic states) lead to endothelial dysfunction and acceleration of atherosclerosis.181 Hyperglycemia is associated with changes in the myocardium that lead to diabetic cardiomyopathy and heart failure. These changes can occur even in the absence of hyperglycemia, which has been linked with cardiovascular remodeling.182 Erectile dysfunction in men has been associated with atherosclerosis and with high fasting blood glucose.15 A diagnosis of erectile dysfunction can lead to the detection of diabetes. Adequate control of diabetes is essential to minimize these consequences.
Metabolic Syndrome
People with metabolic syndrome are at increased risk for cardiac, pulmonary, and metabolic dysfunction and diabetes. The syndrome results from deleterious body weight, inactive lifestyle, atherogenic diet, or some combination thereof.183 Assessment is based on elevated triglycerides, low high-density lipoprotein cholesterol, elevated fasting blood glucose, elevated blood pressure, and increased abdominal circumference. At least three of these findings must be present for a diagnosis of metabolic syndrome.
Syncope
Syncope is a syndrome hallmarked by a short period of loss of consciousness resulting from a transient reduction of blood flow to the brain. Although systemic hypotension is the most common cause, it may result from a combination of factors. Syncope occurs mostly in people with cardiovascular dysfunction and in older people. Cardiovascular-related causes include orthostatic factors related to standing upright, cardiac dysrhythmia, and structural cardiac and pulmonary causes.184 Less common forms of syncope include neurally mediated reflex syncope and syncope resulting from cerebrovascular disorders. A patient complaining of light-headedness, dizziness, grayouts, blackouts, or fainting episodes needs a thorough multisystem assessment to establish and manage the precise causes.
Cardiac Dysfunction in Noncardiac Conditions
Cardiovascular and pulmonary consequences of noncardiovascular and pulmonary conditions are described in Chapter 6. Although their effects can be subtle, cardiovascular and pulmonary manifestations of these conditions can be serious and even life-threatening. These consequences need to be addressed by the physical therapist during the assessment to guide and modify management. Tools such as ECG are essential in detecting cardiovascular abnormalities at rest and during exercise in patients with noncardiovascular and pulmonary conditions such as electrolyte disturbance, pulmonary embolism, esophageal disorders, drug-related conditions, and neuromuscular conditions.185
The connective tissue disorders, including systemic sclerosis, have cardiovascular consequences.49 Because the heart is significantly involved, scleroderma can manifest as myocardial dysfunction, conduction abnormalities, and pericardial disease. The cardiovascular system can also be involved secondarily because of involvement of the lungs and kidneys.
Myocardial and cardiovascular dysfunction is well recognized as a leading cause of morbidity and mortality in people with neuromuscular conditions, and it can be a major complication in their management.186,187 Early detection and management of such dysfunction are keys to lifelong health, reduced incidence and severity of associated morbidity, and modification of rehabilitation in this cohort of individuals.
Obesity is an independent risk factor for heart failure.188 The cellular changes reflect the increased metabolic strain mediated by increased mechanical strain and work of the heart.
Renal disease can lead to heart failure or result from it. In addition, anemia is implicated in both conditions. The triad of these conditions has been termed the cardiorenal anemia syndrome.189 Anemia increases the severity of CHF and mortality, and renal disease causes anemia. Both renal dysfunction and anemia worsen heart complications. Epoetin (recombinant human erythropoietin), which is used to correct anemia associated with renal dysfunction, also decreases the compensatory increase in left ventricular muscle mass, thereby reducing associated cardiac morbidity and mortality.190
Summary
This chapter first distinguishes the pathophysiology related to the structure and function of obstructive and restrictive lung conditions. Obstructive conditions are characterized by decreased airflow during expiration (as a result of increased airway resistance), airway obstruction, and hyperinflation of the lungs. Restrictive conditions are characterized by increased lung stiffness or chest wall rigidity that limits inspiration. Both types of lung pathology progressively limit functional capacity. Second, the essential pathophysiological features of atherosclerosis, ischemic heart disease, coronary artery disease, and related syndromes are described. The pathophysiological consequences of hypertension, diabetes mellitus, anemia, and syncope on oxygen transport are highlighted. Finally, cardiac dysfunction secondary to noncardiac conditions is addressed and linked to discussion of this topic covered in Chapter 6.
1. Describe the role of smoking in the incidence of cardiovascular and pulmonary conditions. Or consider, if smoking had never existed, what would be the epidemiological impact on cardiovascular and pulmonary disease?
2. Describe how lung dysfunction can lead to cardiac dysfunction and provide examples.
3. Describe how cardiac dysfunction can lead to lung dysfunction and provide examples.
4. Distinguish obstructive from restrictive pulmonary pathology and how each manifests clinically.
5. Obstructive and restrictive lung pathology seldom exist alone. Describe how these types of pathology can coexist.
6. Describe tuberculosis and explain why it must be a concern to the contemporary physical therapist.
7. Describe the process of atherosclerosis and the ways in which it is injurious to life.
8. Evaluate the relative contributions of acquired cardiovascular disease and congenital cardiovascular disease to your role as a physical therapist.
9. Describe the pathophysiological progression of cardiac insufficiency to failure and death. Describe how lifestyle changes recommended by the physical therapist as part of comprehensive management could stall such progression.
10. Describe the implications of hypertension, anemia, diabetes mellitus, and syncope on your practice as a physical therapist across clinical settings, including working in the community and working with healthy seniors.
11. What is meant by the term athletic heart syndrome? What are the implications in regard to your role as a physical therapist with a sports team?