Cardiac Tamponade
Fundamentals of Tamponade
Cardiac tamponade is a condition characterized by an increase in pressure external to the heart resulting in impaired filling of the cardiac chambers. In the typical scenario, as fluid in the pericardium accumulates, cardiac output falls. The diagnosis represents a continuum from mild tamponade with subtle diagnostic findings to a critical clinical setting with imminent mortality.1 The variability in presentation, including diagnostic findings and course, and the morbidity and mortality associated with treatment make this a particularly challenging clinical problem in critical care medicine.
Pericardial Anatomy
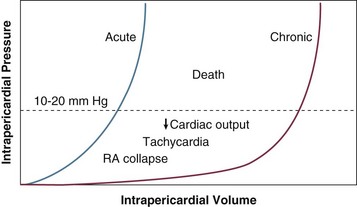
The pericardium consists of a visceral and a parietal pericardial segment, the former being composed of a single layer of cells that adhere to the cardiac epicardial surface.2,3 The parietal pericardium is the structure responsible for the clinically relevant features of tamponade; it is a relatively noncompliant structure composed of collagen and elastin and normally is less than 2 mm thick. The mechanical properties of the pericardium—in particular, those reflected by its pressure-volume curve (Fig. 24.1)—are responsible for the clinical features seen in cardiac tamponade.
The pericardium extends from the lower third of the superior vena cava to the apex of the heart. It is attached to the sternum, the diaphragm, and the great vessels. Because it extends beyond the heart border, trauma to not only the heart but also the great vessels approaching the heart borders can lead to cardiac tamponade.4
Physiology
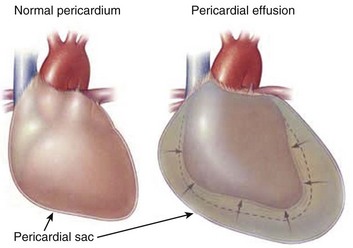
Cardiac tamponade is a result of decreased transmural pressure, typically from the accumulation of fluid in the pericardial space (Fig. 24.2). Other causes of “tamponade-like physiology” are related to extrinsic compression of the heart,5 although these pathologic processes should be separated from those causing constriction rather than true tamponade. Differentiating these two distinct physiologic entities, constriction and tamponade, is essential to diagnosis and management.
The fluid accumulating in the pericardial space can be blood, serous fluid, purulent material, clot, or rarely gas. As fluid accumulates, the pericardium stretches, until it reaches a point (see Fig. 24.1) at which its degree of compliance is exhausted, so that it has become largely inelastic. At this point, any further increase in intrapericardial fluid is associated with a decrease in intracardiac chamber volume, because the total volume of pericardial fluid, heart muscle, and the cardiac chambers becomes fixed by pericardium no longer able to stretch. This in turn results in decreased filling of the heart and consequently decreased stroke volume. To maintain cardiac output, an early compensatory mechanism is an increase in heart rate. Subsequent adaptations to maintain blood flow to central end organs (heart, brain, kidneys) are venous pressure rise, peripheral vasoconstriction, increase in ejection fraction, and selective shunting of blood to preserve flow to the essential end organs. Venous pressure increase is accomplished by fluid retention and peripheral venoconstriction. In severe tamponade, equalization of right atrial, right ventricular diastolic, pulmonary diastolic, pulmonary artery wedge, and intrapericardial pressures occurs. Both pulmonary and systemic arterial and pulse pressures fall. Tamponade is a continuum, ranging from a primarily echocardiographic finding of right-sided chamber collapse to shock and pulseless electrical activity.
Pericardial Pressure
The normal parietal pericardium, because of its limited compliance, limits abrupt expansion of the heart as a whole.6 Thus, in the setting of right ventricular infarction, for example, acute dilation of the right ventricle is at the cost of left ventricular volume decrease—a ventricular interdependence phenomenon similar to that illustrated in Figure 24.3. Acute chamber enlargement resulting from other etiologic conditions or disorders, such as abrupt volume loading or sudden onset of severe valvular regurgitation, is impeded by the constraint of pericardium that has reached the limits of its intrinsic elasticity. Because right-sided heart filling occurs preferentially with inspiration, when negative intrathoracic pressures result in increased venous return and higher right-sided chamber volume, left ventricular stroke volume and hence systolic blood pressure tend to fall as left atrial and left ventricular volumes decrease. This exaggerates the normal respiratory variation, and systemic pressure falls with inspiration to a level at which pulsus paradoxus, defined by convention as a greater than 10 mm Hg decrease in systolic pressure, is seen. In addition, decreased intrathoracic pressure has a disproportionate effect on the pulmonary venous circulation, which is not exposed to the high pericardial pressures; hence, a disproportionate fall during inspiration in the pulmonary vein to left atrial gradient further exacerbates the decrease in left atrial filling.7 The fall in systolic pressure is not in fact a paradox but rather an exaggeration of normal respiratory variation of approximately 3%8 with associated inspiratory decrease in left ventricular stroke volume. With severe tamponade, total elimination of pulse pressure can be seen with individual heartbeats, as in the example in Figure 24.4.
In contrast with acute tamponade physiology, in which hemodynamic decompensation may occur after only a modest accumulation of fluid, generally in the range of 100 to 200 mL, chronic pericardial effusion results in a gradual increase in distensibility of the pericardium. With increasing compliance, large amounts of fluid can accumulate at low pressure without decreasing transmural pressure or compressing the cardiac chambers (see Fig. 24.1). Even in chronic low pressure–high volume tamponade, a limit of distensibility is eventually reached that results in similar pathophysiology as with acute tamponade, in some cases only after a liter or more of fluid has accumulated. Once the steep portion of the pressure-volume curve is reached, it is important to appreciate that with accumulation of another 50 to 100 mL of pericardial fluid, hemodynamic decompensation can occur rapidly, with similar outcomes as in patients who suffer from acute tamponade, such as is seen with penetrating trauma, coronary artery perforation, aortic dissection, or cardiac rupture.
Etiology
Pericardial effusions generally can be characterized as transudate or exudate, infectious or bloody, with tamponade occurring with variable frequency depending both on the rapidity of fluid accumulation and, to a somewhat lesser degree, on characteristics of the effusion and physiology determined by its etiology. Transudates are characteristic of congestive heart failure, radiation, and uremia; exudates are characteristic of infections, malignancy, and connective tissue disorders. Laboratory testing may help differentiate exudates from transudates; pericardial and serum protein, albumin, lactate dehydrogenase (LDH), cholesterol, and glucose should be obtained,9 although some of the differentiating characteristics applicable to pleural effusions may not be discriminatory in pericardial effusions.10 In addition, pericardial fluid total and differential cell count, Gram stain, aerobic and anaerobic culture, and mycobacterial and fungal evaluation should be performed along with cytology when malignancy is considered. Molecular techniques can be useful in diagnosing otherwise occult infections.11 Amylase is reserved for cases where pancreatic disease or esophageal rupture are part of the differential diagnosis.
Conditions predisposing to slow accumulation such as heart failure, myxedema, chronic renal failure, and connective tissue disorders in general are less likely to cause acute tamponade, whereas those associated with rapid development, such as malignancy, infection (including bacterial, fungal, and human immunodeficiency virus related), or particularly hemorrhage, commonly result in abrupt hemodynamic deterioration. The effect of inflammation in decreasing compliance of the pericardium exacerbates the hemodynamic effects of effusions associated with pericarditis.12 The potential etiologic disorders are highly variable, with significant influence of demographics and geography, so that tamponade secondary to tuberculous pericardial effusion in immune-compromised patients is a not uncommon presentation in Africa,13 whereas in industrialized nations malignant effusions are a far more common cause.14 Large and usually benign pericardial effusions are seen frequently after heart transplantation, possibly immune mediated.15 Large pleural effusions can cause sufficient compression of the heart that tamponade physiology occurs.16 The most common etiologic disorders are listed in Table 24.1; more detailed discussion of the various causes is presented later in the chapter, organized by the hospital setting in which presentation typically is seen.
Table 24.1
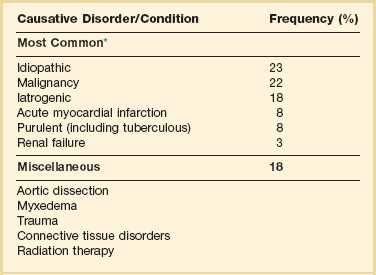
*Frequency of common etiologies of tamponade is based on 119 cases in Barcelona, Spain. Other relatively common causes of effusion include congestive heart failure, hypoalbuminemia, coagulopathy, and postcardiotomy and Dressler’s syndromes. Etiologies of tamponade will be dependent on geography and patient demographics and also will be strongly influenced by the presence of oncology, trauma, or dialysis units. For a comprehensive list of tamponade etiologies based on prior data, see Box 6.1.
Data from Sagrista-Sauleda J, Merce J, Permanyer-Miralda G, et al: Clinical clues to the causes of large pericardial effusions. Am J Med 2000;109:95-101.
History and Physical Examination
Dyspnea is the most common symptom of tamponade, although its etiology sometimes is unexplained and it usually is not associated with significant concomitant pulmonary vascular congestion. It is likely to be secondary to decreased cardiac output and encroachment on lung volume by the expanding pericardium as well as any simultaneous pleural effusions. The patient may describe a sensation of fullness in the chest or abdomen and dysphagia, associated with venous engorgement and passive congestion, stretching of the richly innervated pericardium, and occasionally vagal stimulation.17 Because most patients with tamponade have comorbid conditions accounting for their effusion, additional signs and symptoms are likely to be related to pericarditis, malignancy, or other concomitant conditions.
The hallmarks of cardiac tamponade on physical examination relate to features associated with venous hypertension, low cardiac output, and effects of the layer of fluid between the heart and the chest wall. In patients with tamponade, the general appearance changes substantially during progressive increase in pericardial pressure. Because tamponade represents a continuum, some patients with early tamponade physiology look well, whereas patients with more advanced tamponade show features of a low-output state, with clinical manifestations reflecting the high catecholamine levels required to maintain cardiac output. They become progressively more anxious and agitated and less communicative and may be struggling to breathe. Patients may complain of chest pain associated with pericardial irritation, not infrequently radiating to the neck, jaw, or shoulder18; if venous congestion is acute, patients may experience pain from stretching of Glissen’s capsule around the liver.
Pulsus Paradoxus
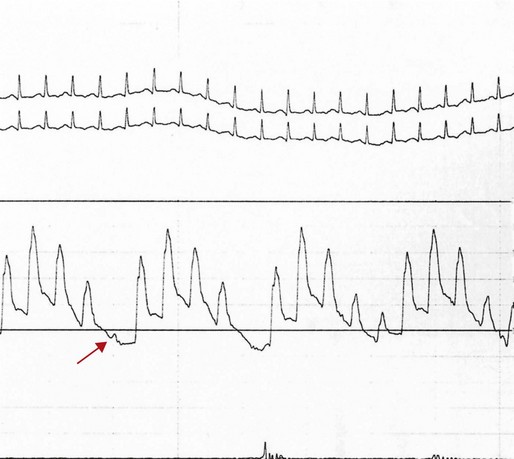
The physical examination in significant tamponade can include Beck’s triad, described in 1935 by the surgeon C. S. Beck.19 This entity features jugular venous distention, decreased arterial pressure, and a small, quiet heart. As described earlier, pulsus paradoxus is the result of cardiac chamber interdependence and a decrease in left ventricular chamber volume with inspiration. It can be detected at the bedside by auscultation of Korotkoff sounds, identifying the highest and lowest pressures at which sounds are first heard during inspiration and expiration; alternatively, a simpler and more useful technique is to palpate the radial artery pulse with the cuff inflated to the maximum pressure at which the pulse appears and then to lower the cuff pressure in increments of 10 mm Hg to detect the pressure at which pulses are continuously noted throughout the respiratory cycle. In the patient whose systolic pressure is shown in Figure 24.4, simple palpation of the radial pulse would detect the loss of pulse pressure in the beat flagged by the red arrow. It is important to recognize that although pulsus paradoxus is a classic feature of severe tamponade, as a diagnostic feature it is of limited sensitivity and specificity.
Various thresholds other than the relatively arbitrary 10 mm Hg threshold have been proposed to increase specificity, including a 10% decrease, rather than a 10 mm Hg fall.20 A drop in systolic pressure greater than 50% of the pulse pressure also has been proposed.1 Use of a 15 or 20 mm Hg fall in pressure by physical examination is a less sensitive but far more specific finding for tamponade but may result in delayed diagnosis. Pulsus paradoxus may be present in other conditions that result in an exaggerated decrease in systolic pressure with inspiration, such as massive pulmonary embolism, severe chronic obstructive pulmonary disease (which also can feature constrictive physiology because of limited expansion of the heart in the setting of hyperexpanded lungs),20 and right ventricular infarction.21 Furthermore, other features of the systemic blood pressure and pulse are important to consider. With a progressive decrease in cardiac filling overall, a decline in systemic pressure (regardless of phase of the respiratory cycle) as well as a decrease in pulse pressure (the difference between systolic and diastolic pressures) occurs, reflecting decreasing stroke volume and decreasing cardiac output. Thus, it may be impossible to palpate radial artery pulsations; with severe tamponade, the patient is likely to feel cool and clammy, a finding consistent with severe peripheral vasoconstriction.
Tachycardia is almost invariable, except for comorbid conditions associated with a decrease in heart rate, such as electrical conduction disturbances, severe hypothyroidism, or aggressive β-blockade. Tachycardia is a compensatory mechanism for decreased stroke volume, is also caused by high catecholamine levels, and may result from pericardial irritation of the sinus node that stimulates a higher heart rate. On occasion, acute bradycardia may be seen in tamponade, sometimes the first finding after hemorrhage into the pericardial sac. Although a well-preserved systolic pressure and a wide pulse pressure are uncommon in tamponade, neither a low pressure nor a narrow pulse pressure is completely specific. Further, early in the course of tamponade, acute surge in catecholamine levels may result in hypertension rather than shock; in addition, patients with tamponade in the setting of renal disease and chronic hypertension appear to present more often with elevated systolic pressures.22
The pulsus paradoxus may be absent in conditions in which ventricular interdependence is masked23 (Box 24.1), such as a nonrestrictive atrial septal defect, in which inspiration also increases left atrial filling, or aortic insufficiency, in which left ventricular filling in diastole is increased by regurgitation from a high-pressure source: the aorta. Localized tamponade may have some general tamponade features (such as decreased stroke volume) but may not result in ventricular interdependence—hence, the clinical picture may be that of a sick patient with tamponade but without pulsus paradoxus. Markedly elevated left-sided heart diastolic pressures in severe left ventricular hypertrophy and other disease states may exceed the elevated right atrial and intrapericardial pressures in tamponade, decreasing the effect of inspiration on the interdependence of the right and left sides of the heart. An example in which both pulse pressure and pulsus paradoxus would be insensitive markers of tamponade is aortic dissection that combines aortic insufficiency with tamponade, in which a wide pulse pressure may be seen in some patients with partial compensation, and in which pulsus paradoxus, as discussed, may be masked. By contrast, conditions resulting in impaired right ventricular filling, such as right ventricular hypertrophy in severe pulmonary hypertension, also may result in the absence of a pulsus paradoxus24; furthermore, in settings such as cor pulmonale, the dramatic elevation in right-sided diastolic pressures will delay onset of the otherwise highly sensitive and early finding of right atrial and right ventricular diastolic collapse until tamponade is severe.25 A substantial number of case reports describe physiologic conditions in which the classic findings for tamponade are not seen or are attributable to other etiologic disorders.26
Venous Pressure
Because impairment of right-sided filling is usually the first manifestation of increasing pericardial pressure, high jugular venous pressure manifested by prominent venous pulsations may be the earliest finding on physical examination, occurring with increasing intrapericardial and right atrial pressures. Jugular venous distention may, however, be simultaneously absent because venoconstriction, a common finding with acute tamponade, makes detection of elevated venous pressures difficult. It also will be masked by low-volume tamponade, including volume-depleted states such as trauma, when, in addition to hemorrhage into the pericardium, significant blood loss has occurred. Other settings in which venous distention may not be observed are post-dialysis in patients with uremic pericardial effusions, and excessive diuresis, sometimes as part of treatment for symptoms of congestive heart failure when the cause of elevated filling pressures has not been appreciated.27 In general, lack of venous engorgement in tamponade, particularly when the latter is acute, is not uncommon. Thus, prominent jugular venous distention may suggest an alternative diagnosis, such as severe right-sided heart failure. Increased venous pressure with inspiration, Kussmaul’s sign, is a feature of constriction, reflecting increased venous return to the thorax without increased right atrial filling, because the latter is constricted by the pericardium. This is in contrast with tamponade, in which negative intrathoracic pressure is transmitted through the pericardial effusion, and results in increased right-sided heart filling with decrease in venous pressure. Because these findings are difficult to differentiate in most acutely ill patients, much of the subsequent description about right atrial pressure is based on catheter based hemodynamic findings rather than the physical examination.
The Y descent, in contrast with pericardial constriction, in which it is prominent, typically is limited or absent (Fig. 24.5). Unlike in pericardial constriction, filling of the right ventricle (and hence emptying of the right atrium) is impaired throughout the cardiac cycle, because extrinsic compression by pericardial fluid results in elevated diastolic pressures in the right ventricle when rapid filling would otherwise occur. Because the pericardium allows additional atrial expansion during ventricular ejection (when ventricular volume decreases), the X descent is typically the more prominent negative pressure wave seen. The classic square root sign seen in the right ventricular pressure tracing in constriction is absent in tamponade.
Cardiac and Chest Examination
The quiet heart in Beck’s triad relates to several features that tend to muffle the intensity of heart sounds. First, the insulating effects of pericardial fluid on sound waves tend to decrease the sound volume transmitted to the chest wall. Second, low stroke and filling volumes tend to decrease the forces that cause the sounds generated by heart valve closure, with low pulse pressure in both the aortic and the pulmonary arteries. Because the S3 gallop is created by rapid ventricular filling, a phenomenon absent in tamponade, it would not be expected, nor would an S4, because the hemodynamic characteristics of late diastolic atrial emptying that causes the fourth heart sound are not seen.1 A pericardial friction rub, if pericarditis is involved, may be heard. Except in severe tamponade, the pericardium around the apex of the heart may contain relatively little fluid, and left ventricular contraction typically is vigorous unless underlying left ventricular dysfunction also is present. Hence, a palpable point of maximal impulse may be felt.
Diagnostic Tests
Echocardiography
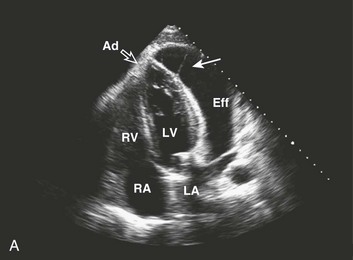
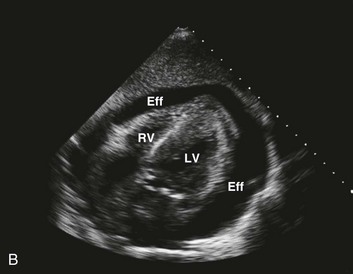
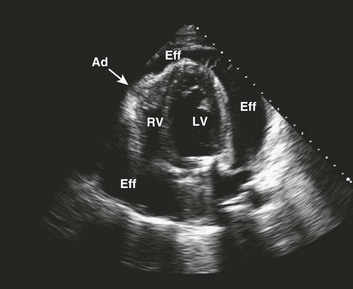
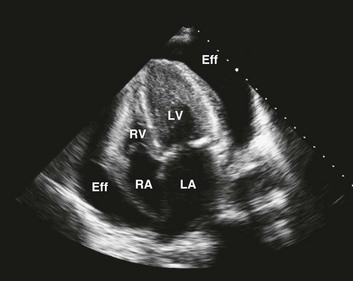
Echocardiography is the most easily accessible and accurate means for the timely diagnosis of tamponade and remains the diagnostic modality of choice.28 Besides evaluating presence and degree of chamber collapse, the echocardiogram is useful for assessing the amount and location of effusion in the pericardial space, for characterizing the fluid, and for judging the hemodynamic effect. Cardiac tamponade represents an echocardiographic continuum, from a small accumulation of fluid with at most subtle clinical findings, to large, usually circumferential29 accumulations with resultant cardiogenic shock. Until effusions are at least moderate in size, fluid may be shifted by gravity to a location primarily posterior to the heart, because most patients are recumbent during echocardiography (Fig. 24.6).
Several echocardiographic features have been described in tamponade that result from an increase in intrapericardial pressure. These include exaggerated late diastolic right atrial collapse, early diastolic right ventricular collapse, significant respiratory variation in Doppler inflow velocities across the tricuspid and mitral valves, a plethora of the inferior vena cava, exaggerated expiratory flow reversal of the hepatic veins, and blunted diastolic flow of the inferior vena cava (IVC) during expiration.30 Because the right-sided cardiac chambers are more compliant, thin walled, and at relatively low pressure, the increase in intrapericardial pressure results in the collapse of these chambers first.
Immediately after atrial contraction, the right atrial volume is at its lowest, whereas right ventricular volume is at its maximum; total pericardial volume in cardiac tamponade is relatively fixed. The increase in ventricular filling during diastole increases pericardial pressure, which prevents expansion of the right atrium during atrial relaxation (late ventricular diastole).31 Persistence of right atrial collapse for more than one third of the cardiac cycle is a useful marker for early tamponade physiology. This phenomenon is best appreciated from the apical or subcostal four-chamber views (see Fig. 8.43 in Chapter 8).
The right ventricle, conversely, is more vulnerable to collapse during early diastole, when its pressure is at its lowest as it expands following closure of the pulmonic valve. The right ventricular outflow tract is the more compressible area of the right ventricle and hence tends to collapse first.30 With increasing intrapericardial pressure, the collapse extends to the right ventricular free wall, which is best appreciated on parasternal long or short axis views (see Figure 8.44 in Chapter 8). This is a more specific finding for tamponade than right atrial collapse, as the latter can occur in the setting of hypovolemia alone. However, right ventricular diastolic collapse may not be seen in conditions that result in significantly elevated right ventricular diastolic pressure or in right ventricular hypertrophy. Collapse of the higher-pressure left-sided chambers, particularly the left ventricle, is uncommon, but, when seen, is highly specific for tamponade. Collapse of the left atrium is not uncommon in the immediate postcardiac surgery period, frequently a result of loculated effusion causing regional tamponade.32
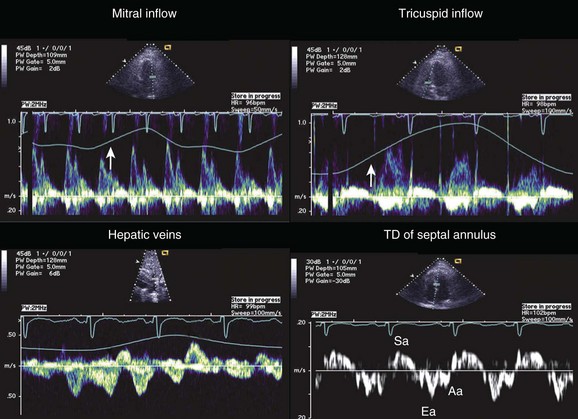
Although cardiac chamber collapse is a highly sensitive marker and typically seen before clinical manifestations of tamponade, a number of other findings are more specific. As discussed previously, pulsus paradoxus results from exaggerated ventricular coupling. The ventricular interdependence (Fig. 24.7) can be appreciated on echocardiography by demonstration of marked respiratory variation of Doppler flow across the atrioventricular or semilunar valves (see also Figs. 8.43 and 8.44 in Chapter 8). During inspiration, the drop in intrathoracic pressure results in increased right ventricular (RV) filling. As right ventricular cavity size increases during diastole, the increased intrapericardial pressure impairs expansion of the right ventricular free wall, and continued RV filling is accomplished only by diastolic expansion of the interventricular septum. The consequent bulging of the interventricular septum into the left ventricular (LV) cavity during diastole results in marked reduction in LV compliance and inspiratory filling.12,33 These filling changes are reflected in a marked inspiratory increase in tricuspid valve Doppler inflow velocities with simultaneous inspiratory decrease in mitral valve inflow velocities. Reciprocal changes are noted during expiration. A corollary to these respiratory changes is noted in the hepatic vein and vena cava flow patterns. Normal vena cava flow into right atrium occurs during both ventricular systole and diastole. In tamponade, already impaired diastolic flow is further compromised in expiration by the exaggerated ventricular interdependence. This hemodynamic effect manifests on Doppler as blunted diastolic caval flow during expiration with increased expiratory flow reversal in the Doppler flow pattern of hepatic veins34 (Fig. 24.8). Although it is expected that in tamponade the IVC will be dilated and nonreactive,35 a plethora of the IVC can be seen in any condition causing elevation of right atrial pressure, including positive pressure ventilation in an intubated patient.
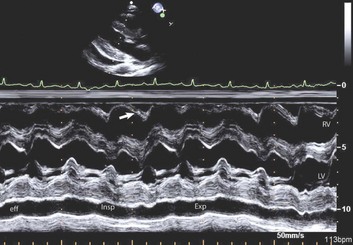
Figure 24.7 Classic findings for ventricular interdependence in cardiac tamponade. See the legend for Figure 8.45 in Chapter 8.
Portable echocardiography, performed at the bedside, in the emergency room, or in critical care units, is highly reliable in cardiac tamponade.36,37 However, adequate training in basic technique and fundamental anatomy is essential to avoid misdiagnosis. Epicardial fat can be confused with effusion,38 and isolated or coexistent pleural effusions, mediastinal masses, and atelectasis can confound the diagnosis as well.39 It is important to assess the heart from as many echo windows as possible for small but opportunistic loculated accumulations of fluid, for the presence of clot, fibrinous “strandlike” material or masses, and for compression from processes outside the pericardium, such as a mediastinal hematoma and pleural effusion, all of which will significantly impact management.
X-Ray Studies
The classic “water bottle” configuration of the heart is due to a large pericardial effusion and therefore occurs only if the pressure-volume curve has altered sufficiently through chronic accumulation to allow significant increase in pericardial volume. It is a misconception that a normal-sized heart excludes tamponade; in acute tamponade the heart size can be expected to appear normal or minimally enlarged.12 With marked enlargement of the cardiac silhouette, the finding is nonspecific and not readily distinguishable from cardiomegaly, although in tamponade pulmonary vascular congestion is usually not seen, whereas prominence of the vena cava may be noted. Other modalities such as computed tomography (CT) scanning and magnetic resonance imaging (MRI) show large effusions and can demonstrate chamber collapse. In addition, characterization of pericardial thickness and of the pericardial fluid to differentiate between blood and fluid of different densities can be useful. Although CT and MRI show primarily anatomy rather than physiology, they can identify chamber collapse, characterize soft tissue, and demonstrate reflux into the azygous vein, a useful sign of tamponade.40 CT scanning can provide information supplemental to that seen on echocardiography, including identification of extracardiac disease lesions,41 and can provide additional sensitivity for the detection of loculated effusions.42 Coronary sinus compression on CT is an early and specific indicator of tamponade.43
Electrocardiography
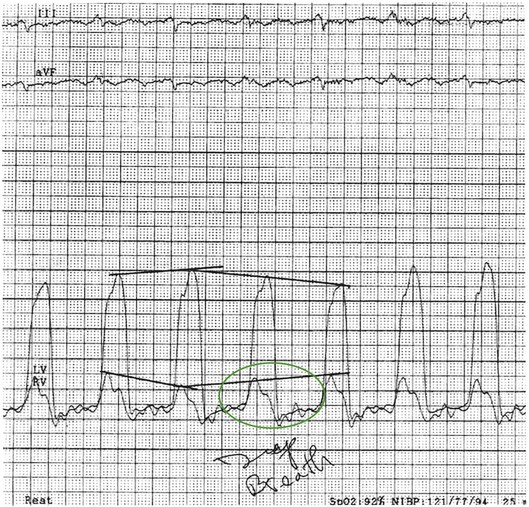
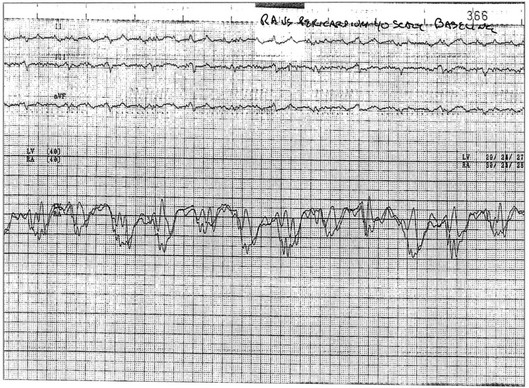
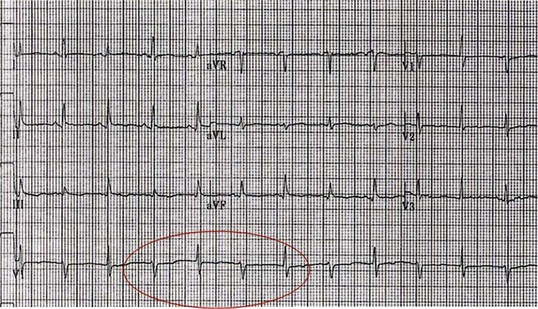
The classic electrocardiographic features include low voltage, a result of poor transmission of electrical activity across the fluid in the pericardial space, and electrical alternans, an insensitive but relatively specific finding for tamponade generated by swinging of the heart in the fluid-filled chamber (Fig. 24.9). Electrical alternans typically is seen only with large effusions in the later stages of tamponade, although the finding is related more to fluid volume and the ability of the heart to swing within the pericardial space. It may involve alternans of both QRS complexes and P waves. Thus, tamponade with adhesions (Fig. 24.10), loculation, or masses that restrict heart motion may not manifest the alternans phenomenon.
Overall Assessment
In general, the severity of tamponade can be judged by the extent of hypotension, tachycardia, and pulsus paradoxus on physical examination and confirmed by findings on echocardiography.44 Mild tamponade features no hypotension or tachycardia, and no pulsus, with mild RV collapse by echo. Patients with moderate tamponade have preservation of systemic pressure, but have tachycardia, some degree of pulsus paradoxus, and clear RV collapse on echo. Severe tamponade is associated with tachycardia, shock, profound pulsus paradoxus, and chamber collapse with a swinging heart on ultrasound.21 Hemodynamic findings correlate with increasing pericardial pressure at each stage of tamponade,45 initially less than right atrial or pulmonary wedge pressure, then equilibrating with right but less than left atrial pressure, and in severe tamponade equilibrating with both.
Special Syndromes in Tamponade
Although most cases of tamponade have at least some of the classic features, there are several important variants. Loculated effusions that compress the heart primarily in one region are typically postsurgical, although they may be due to neoplasms or a number of other etiologies; they are discussed in the section on postcardiac surgery cases that follows. There are also a number of conditions where pulsus paradoxus does not occur or is masked, summarized in Box 24.1.
Effusive Constrictive Disease
This phenomenon is an important-to-recognize condition occurring in less than 10% of patients with tamponade,46 but up to 40% in some series. Hospitals with disproportionately high populations of oncology patients, because of tumor metastases or postradiation pericardial involvement, or tuberculosis, will have a higher percentage of tamponade patients with this diagnosis. The syndrome can occur after acute pericarditis of multiple etiologies and may even be transient.47 The classic features of tamponade are seen on presentation, and a history of malignancy should increase the suspicion of prepericardiocentesis.
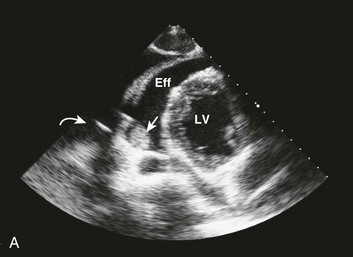
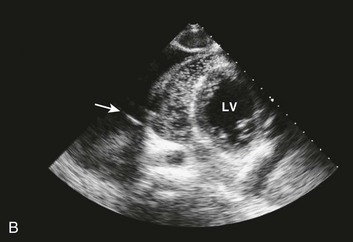
Effusive constrictive disease is a setting in which careful hemodynamic monitoring is very helpful for accurate diagnosis. Monitoring of intrapericardial and right atrial or wedge pressure during pericardiocentesis demonstrates findings as seen in Figure 24.11. In general, with relief of tamponade intrapericardial hypertension resolves, and classic respiratory variation is seen in most patients, whereas right atrial pressure remains elevated though lower than prior to the pericardiocentesis. The Y descent becomes prominent because of the elimination of high transmural pressures that restrict filling during early diastole in tamponade, unmasking classic constrictive physiology. Figure 24.12 shows echocardiographic images in a typical patient with this syndrome.
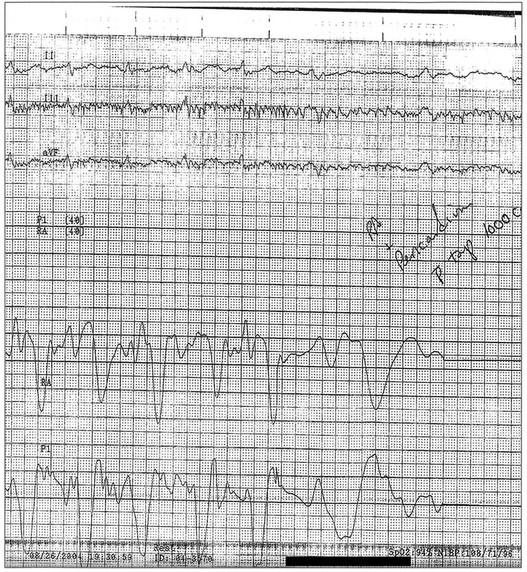
Figure 24.11 Hemodynamic tracings (40–mm Hg scale) obtained 1 hour after those seen in Figure 24.5. After pericardiocentesis of 1 L of fluid, the pericardial pressure has achieved a mean equivalent to atmospheric pressure (0 mm Hg by convention), while right atrial pressure remains elevated near 22 mm Hg. This is physiology consistent with effusive-constrictive disease. On removal of the effusion, the hemodynamics, including the newly seen steep Y descent, are classic for constriction. This patient had a malignant pericardial effusion. P, intrapericardial pressure; RA, right atrium.
Low- and High-Pressure Tamponade
Low-pressure tamponade has been defined as featuring hypotension secondary to pericardial effusion but with low venous and intrapericardial pressures, most commonly in the setting of hypovolemia resulting from dehydration or blood loss. A more formal definition, based on a single site experience, was described by Sagrista-Sauleda and colleagues48 as an intrapericardial pressure less than 7 mm Hg, with a post-pericardiocentesis right atrial pressure less than 4 mm Hg and equalization of intrapericardial and right atrial pressures before pericardiocentesis. Importantly, 20% of their patients with cardiac tamponade met these criteria (and 10% of their patients with large pericardial effusions), suggesting that low-pressure tamponade, previously the subject primarily of case reports and small series, may be more common than previously appreciated. Because of low pressures, there is a lack of features such as pulsus paradoxus or jugular venous distention, with only 24% demonstrating these classic findings, making the diagnosis significantly more difficult. Fluid challenge may result in more typical findings.
Patients with chronic hypertension in whom tamponade develops occasionally have high blood pressure despite tamponade physiology, presumably because of an exaggerated systemic pressure response to the catecholamine storm associated with tamponade.49 In this setting, injudicious use of the usual medications to lower blood pressure can result in profound hemodynamic compromise.
Settings in Which Tamponade is Seen
The Emergency Room
The primary cause of tamponade in the emergency room relates to hemopericardium, although the complete range of medical etiologies can be seen in this setting. Trauma50 includes gunshot and stab wounds, as well as penetrating and crush wounds to the chest, including those related to automobile accidents. Penetrating wounds are significantly more likely to result in tamponade than crush injuries.51 Medical presentations with acute hemopericardium include aortic dissection with tamponade, postmyocardial infarction rupture, or leaking thoracic aneurysm, situations in which pericardiocentesis may lead to further hemodynamic decompensation,52,53 as well as hemopericardium secondary to overanticoagulation. In general, acute hemopericardium may be associated with continued hemorrhage as well as clot in the pericardium; the latter makes complete drainage difficult. Clot alone can compress the heart and cause tamponade-like physiology. The clinical presentation of acute intrapericardial hemorrhage is typically shock, and jugular venous distention may or may not be present because of venoconstriction and hypovolemia as previously described. Use of echocardiography in the emergency room in patients with unexplained dyspnea is an important diagnostic tool.54
The Cardiac Catheterization and Electrophysiology Laboratories
Perforation of the heart, including coronary arteries and cardiac chambers, during invasive cardiac procedures has become an increasingly important cause of pericardial tamponade.55 Common scenarios include electrophysiology procedures, where relatively stiff catheters are placed in the relatively thin-walled structures of the right heart, including the right atrium, right ventricle, and the coronary venous system including the coronary sinus. Anticoagulation, when required for electrophysiology procedures, such as those involving left atrial ablation, raises the risk substantially. Perforation of coronary arteries occurs in particular with atheroablation devices such as those used for rotational atherectomy, directional atherectomy and lasers, with guidewires used in chronic total occlusions or injudiciously manipulated distal to the target lesion, and with oversized balloons and stents.56 Even with normal sizing, settings such as myocardial bridges or ruptured balloons are associated with vessel perforation or rupture. A third setting is transseptal puncture, a resurgent technique because of burgeoning technologies requiring left atrial access57 but associated with a significant risk of tamponade.58 Besides left-sided ablations, mitral valvuloplasties, and percutaneous circulatory bypass (with its intake source in the left atrium), transseptal puncture is required for a number of techniques under development, including left atrial appendage occlusion and percutaneous mitral valve repair. The risk of pericardial effusion and tamponade with these interventions contributes significantly to their overall morbidity and mortality.55 However, and somewhat counterintuitively, electrophysiologists now frequently anticoagulate prior to transseptal puncture or perform the procedure in patients already on full oral anticoagulation.59 This practice is driven by fear of thromboembolism rather than tamponade, the former an important complication of left-sided ablations in particular, which can be long procedures, and associated with clot inducing char formation. Other endovascular procedures associated with tamponade include myocardial biopsy and central venous line placement, pacemaker lead extraction, as well as erosion of devices implanted across the atrial septum or in the left atrial appendage.60
The potentially catastrophic setting of free perforation during coronary interventions is usually readily diagnosable during the procedure, with free flow in the pericardium seen during coronary injection, sometimes before the patient shows any signs of hemodynamic deterioration. This is readily treatable with balloon occlusion of the coronary artery at the perforation site, pericardiocentesis if necessary, and excluding the fenestration with a covered stent. However, lower-grade perforations that result in pooling of contrast or small adventitial craters can be more subtle. It is essential to discontinue anticoagulation on such patients and consider heparin reversal, and to ensure that personnel responsible for postprocedure management monitor the patient closely for hypotension and tachycardia. With transseptal punctures, hypotension at any time during the case, but especially after anticoagulation is administered, should raise the possibility of tamponade. A quick and relatively specific finding in the catheterization laboratory is straightening and lack of motion of the left heart border on anteroposterior fluoroscopy associated with hypotension (and sometimes reflex bradycardia); rapid intervention is essential in these patients. Hypotension after the procedure should raise the possibility that left atrial access involved a “stitch perforation” whereby the needle exited the right atrium and then entered the left atrium through the pericardial space; this mishap is possible with low punctures and frequently manifests only when catheters are removed from the fenestration at the end of the procedure. In patients who have had pacemaker placement, tamponade can develop acutely or after considerable delay as in the case of lead erosion.61
Critical Care Units and Medical Wards
Tamponade in the critical care unit typically is due to complications of myocardial infarction, concomitant sepsis with purulent pericarditis, or hemorrhage into the pericardium because of anticoagulation (either iatrogenic or endogenous) or, most commonly, is secondary to neoplasms. In myocardial infarction, tamponade can be acute, secondary to massive or limited rupture, or somewhat more insidious in onset as part of Dressler’s syndrome, with hemodynamic compromise sometimes exacerbated by anticoagulation. Tamponade also can be iatrogenic, in particular, secondary to placement of various lines through the venous or arterial circulation. Uncommon causes of tamponade, even with pericardial effusions of sometimes significant size, are viral pericarditis, congestive heart failure, uremia, myxedema (Fig. 24.13), and connective tissue disorders.
In patients with malignancy, several possible causes of tamponade are seen, including tumor involvement in the pericardium, postradiation pericarditis, graft-versus-host disease, and direct or indirect complications of therapy.62 Patients with malignancies also are at increased risk for infectious pericarditis, coagulopathies or thrombocytopenia with secondary hemorrhage, and hypothyroid- and hypoalbuminemia-related effusions. AIDS and other immunosuppressed patients similarly are predisposed to infectious tamponade, as well as pericardial tumor involvement, including that from lymphoma. The mechanisms of pericardial effusion in cancer are varied but include direct spread to the pericardium from primary tumors such as lung, mediastinal, and esophageal cancer; hematogenous spread such as with lymphomas; and obstruction of the lymphatic drainage of the heart by tumors in the mediastinum.
Tamponade after cardiac surgery is an important phenomenon, usually occurring as a result of hemorrhagic effusion. Early postoperative tamponade is not uncommon and is important to recognize as a cause of hypotension because it has been reported to occur with a frequency of 5% to 10%, although this generally is thought to be in the 1% range in the modern era.63 Moderate to large pericardial effusions that do not cause hemodynamic compromise have been much more common.64 Delayed tamponade after heart surgery, with onset on average at 2 to 3 weeks postoperatively but as late as 6 months or more, occurs in less than 1% of patients but is disproportionately more common in patients undergoing valve surgery, probably related to postoperative anticoagulation in this cohort.65,66 Delayed tamponade probably is a variant of the more common postcardiotomy syndrome.
Late tamponade typically is due to loculated effusion resulting from formation of adhesions (see Figs. 24.6 and 24.10) during recovery from surgery. Such adhesions can cause decreased cardiac output if they compress the left side of the heart, and in some cases pulmonary edema occurs if obstruction to inflow results. A variety of clinical problems related to increased right-sided heart pressure occur when tamponade involves the right side, including a clinical picture that resembles superior vena cava syndrome; several case reports have described right-to-left shunting across a patent foramen ovale as well.67 Pericardiectomy to prevent postoperative tamponade has an uncertain risk-benefit ratio but does appear to decrease the incidence of hemodynamic compromise.68
Management
Pericardial tamponade is life threatening, requiring difficult diagnostic and, in particular, management decisions. When hemodynamics do not suggest significant compromise and effusion is small to moderate in volume, intervention frequently is not necessary and may involve increased risk to the patient, whereas late intervention may be fatal. Typically, the risk associated with pericardiocentesis is inversely related to the volume of accumulated fluid and also is related to the location of the effusion. Thus, relatively small effusions, early in the course of tamponade, usually are located primarily posteriorly because of gravity-dependent accumulation. Waiting until the effusion is large and occupies significant volume anteriorly decreases the risk of percutaneous drainage but increases the possibility of decompensation and death before intervention. Frequent monitoring of hemodynamics and the application of sound clinical judgment are essential, but an optimal algorithm does not exist. Comorbid conditions such as endogenous or extrinsic anticoagulation may substantially confound the situation.69 The clinical decision to drain the effusion percutaneously or to send the patient for thoracotomy will be a function of the clinician’s assessment of risk of the former versus the potential advantages of surgery in obtaining pericardial tissue and providing a pericardial window as well. If the percutaneous approach is chosen, performing pericardiocentesis in the cardiac catheterization laboratory has significant advantages. However, the benefit of positioning the pericardial effusion anteriorly, for increased safety during needle entry into the pericardial space, requires that the patient’s chest be elevated during the procedure, which typically can be accomplished only to a 20- or 30-degree angle on most catheterization tables because of the image intensifier unless the table has tilt capabilities. A moribund patient frequently needs pericardiocentesis performed at the bedside without fluoroscopic guidance, sometimes without adequate hemodynamic monitoring, and at times without echocardiographic monitoring—all of which are associated with increased risk of failed pericardiocentesis and adverse cardiac events. Patients with severe tamponade are often anxious and hypoxic; sedation is hazardous as is intubation, though the latter is at times necessary. As a result of associated positive intrathoracic pressure, intubation may cause abrupt hemodynamic deterioration because it decreases venous return to the heart.2 Cardiopulmonary resuscitation using chest compression is unlikely to be effective; immediate pericardiocentesis or thoracotomy are ordinarily the only possibilities for survival. Further discussion of percutaneous interventions for tamponade can be found in Chapter 6.
Little in the way of medical treatment is clearly therapeutic for tamponade. Volume expansion in relatively acute tamponade will support right-sided filling in patients with low circulatory volumes, such as are seen in trauma.70 Patients with chronic tamponade typically have considerable fluid retention, and additional hydration is unlikely to be of benefit. Pressors may be modestly helpful, but for patients with preserved cardiac function, catecholaminergic drugs provide only modest augmentation of cardiac output; patients with hemodynamically important tamponade are usually already maximally catecholamine stimulated. Vasodilator therapy is less clearly beneficial, and drugs that decrease preload, such as nitrates and nitroprusside, should be avoided if the patient is hypotensive. Reversal of anticoagulation is essential, both to stop bleeding into the pericardial space and to decrease the risk of trauma to the heart or major blood vessels during pericardiocentesis (e.g., coronary arteries, hepatic vessels). The introduction of direct thrombin inhibitors may affect this portion of the management algorithm because they are not reversible with vitamin K, and there are anecdotal reports of tamponade occurring after the introduction of oral versions of these agents71 as well as failed pericardiocentesis during percutaneous coronary intervention associated with bivalirudin administration.72 Antibiotic therapy in the setting of purulent pericarditis and tamponade is not expected to acutely relieve hemodynamic decompensation in most settings; postdrainage treatment, including local infusion of drugs, is discussed in Chapter 6. A targeted approach for patients with malignant pericardial disease includes the use of sclerosing agents combined with antineoplastic drugs infused into the pericardial space, sometimes combined with radiation; a variety of such management strategies continue to be applicable for some cancer patients.73 Leaving an indwelling drain is controversial because of the associated risk of infection, although there is some evidence that it can decrease the risk of recurrent tamponade.74 An excellent review of the clinical approach to pericardial disease in general has been published by the European Society of Cardiology.75
Acknowledgment
The author wishes to acknowledge Priscilla Peters for kindly providing Figures 24.6, 24.7, 24.8, 24.10, 24.12, and 24.13.
References
1. Hancock, EW. Cardiac tamponade. Med Clin North Am. 1979; 63:223–237.
2. Little, WC, Freeman, GL. Pericardial disease. Circulation. 2006; 113:1622–1632.
3. Spodick, DH. Macrophysiology, microphysiology, and anatomy of the pericardium: A synopsis. Am Heart J. 1992; 124:1046–1051.
4. Brown, KT, Getrajdman, GI. Balloon dilation of the superior vena cava (SVC) resulting in SVC rupture and pericardial tamponade: A case report and brief review. Cardiovasc Intervent Radiol. 2005; 28:372–376.
5. Wynne, J, Markis, JE, Grossman, W. Extrinsic compression of the heart by tumor masquerading as cardiac tamponade. Cathet Cardiovasc Diagn. 1978; 4:81–85.
6. Watkins, MW, LeWinter, MM. Physiologic role of the normal pericardium. Annu Rev Med. 1993; 44:171–180.
7. Hancock, EW. Pericardial tamponade. In: Parrillo JE, Dellinger RP, eds. Critical Care Medicine: Principles of Diagnosis and Management in the Adult. 2nd ed. Philadelphia: C. V. Mosby; 2001:453–463.
8. Ruskin, J, Bache, RJ, Rembert, JC, Greenfield, JC, Jr. Pressure-flow studies in man: Effect of respiration on left ventricular stroke volume. Circulation. 1973; 48:79–85.
9. Light, RW. Clinical practice: pleural effusion. N Engl J Med. 2002; 346:1971–1977.
10. Ben-Horin, S, Bank, I, Shinfeld, A, Kachel, E, et al. Diagnostic value of the biochemical composition of pericardial effusions in patients undergoing pericardiocentesis. Am J Cardiol. 2007; 99:1294–1297.
11. Levy, PY, Fournier, PE, Charrel, R, et al. Molecular analysis of pericardial fluid: A 7-year experience. Eur Heart J. 2006; 27:1942–1946.
12. Spodick, DH. Acute cardiac tamponade. N Engl J Med. 2003; 349:684–690.
13. Gladych, E, Goland, S, Attali, M, et al. Cardiac tamponade as a manifestation of tuberculosis. South Med J. 2001; 94:525–528.
14. Keefe, DL. Cardiovascular emergencies in the cancer patient. Semin Oncol. 2000; 27:244–255.
15. Al-Dadah, AS, Guthrie, TJ, Pasque, MK, et al. Clinical course and predictors of pericardial effusion following cardiac transplantation. Transplant Proc. 2007; 39:1589–1592.
16. Kopterides, P, Lignos, M, Papanikolaou, S, et al. Pleural effusion causing cardiac tamponade: Report of two cases and review of the literature. Heart Lung. 2006; 35:66–67.
17. Krantz, MJ, Lee, JK, Spodick, DH. Repetitive yawning associated with cardiac tamponade. Am J Cardiol. 2004; 94:701–702.
18. Khandaker, MH, Espinosa, RE, Nishimura, RA, et al. Pericardial disease: Diagnosis and management. Mayo Clin Proc. 2010; 85:572–593.
19. Beck, CS. Two cardiac compression triads. JAMA. 1935; 104:714–716.
20. Meltser, H, Kalaria, VG. Cardiac tamponade. Catheter Cardiovasc Interv. 2005; 64:245–255.
21. Goldstein, JA. Cardiac tamponade, constrictive pericarditis, and restrictive cardiomyopathy. Curr Probl Cardiol. 2004; 29:503–567.
22. Argulian, E, Herzog, E, Halpern, DG, Messerli, FH. Paradoxical hypertension with cardiac tamponade. Am J Cardiol. 2012.
23. Klein, LW. Diagnosis of cardiac tamponade in the presence of complex medical illness. Crit Care Med. 2002; 30:721–723.
24. Akinci, SB, Gaine, SP, Post, W, et al. Cardiac tamponade in an orthotopic liver recipient with pulmonary hypertension. Crit Care Med. 2002; 30:699–701.
25. Gollapudi, RR, Yeager, M, Johnson, AD. Left ventricular cardiac tamponade in the setting of cor pulmonale and circumferential pericardial effusion: Case report and review of the literature. Cardiol Rev. 2005; 13:214–217.
26. Jairath, UC, Benotti, JR, Spodick, DH. Cardiac tamponade masking pulmonary embolism. Clin Cardiol. 2001; 24:485–486.
27. Shabetai, R. Pericardial effusion: Haemodynamic spectrum. Heart. 2004; 90:255–256.
28. Cheitlin, MD, Armstrong, WF, Aurigemma, GP, et al. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography—Summary article: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography). J Am Coll Cardiol. 2003; 42:954–970.
29. LeWinter, MM, Tischler, MD. Pericardial diseases. In: Bonow RO, Mann DL, Zipes DP, Libby P, eds. Braunwald’s Heart Disease. 9th ed. Philadelphia: Elsevier Saunders; 2012:1651–1671.
30. Armstrong, WF, Ryan, T. Pericardial Diseases: Feigenbaum’s Echocardiography, 7th ed. Philadelphia: Williams & Wilkins; 2010.
31. Gillam, LD, Guyer, DE, Gibson, TC, et al. Hydrodynamic compression of the right atrium: A new echocardiographic sign of cardiac tamponade. Circulation. 1983; 68:294–301.
32. Torelli, J, Marwick, TH, Salcedo, EE. Left atrial tamponade: Diagnosis by transesophageal echocardiography. J Am Soc Echocardiogr. 1991; 4:413–414.
33. Troughton, RW, Asher, CR, Klein, AL. Pericarditis. Lancet. 2004; 363:717–727.
34. Burstow, DJ, Oh, JK, Bailey, KR, et al. Cardiac tamponade: Characteristic Doppler observations. Mayo Clin Proc. 1989; 64:312–324.
35. Himelman, RB, Kircher, B, Rockey, DC, Schiller, NB. Inferior vena cava plethora with blunted respiratory response: A sensitive echocardiographic sign of cardiac tamponade. J Am Coll Cardiol. 1988; 12:1470–1477.
36. Price, AS, Leech, SJ, Sierzenski, PR. Impending cardiac tamponade: A case report highlighting the value of bedside echocardiography. J Emerg Med. 2006; 30:415–419.
37. Sagrista-Sauleda, J, Merce, AS, Soler-Soler, J. Diagnosis and management of pericardial effusion. World J Cardiol. 2011; 3:135–143.
38. Blaivas, M, DeBehnke, D, Phelan, MB. Potential errors in the diagnosis of pericardial effusion on trauma ultrasound for penetrating injuries. Acad Emerg Med. 2000; 7:1261–1266.
39. Come, PC, Riley, MF, Fortuin, NJ. Echocardiographic mimicry of pericardial effusion. Am J Cardiol. 1981; 47:365–370.
40. Harries, SR, Fox, BM, Roobottom, CA. Azygos reflux: A CT sign of cardiac tamponade. Clin Radiol. 1998; 53:702–704.
41. Yared, K, Baggish, AL, Picard, MH, et al. Multimodality imaging of pericardial diseases. . JACC Cardiovasc Imaging. 2010; 3:650–660.
42. Restrepo, CS, Lemos, DF, Lemos, JA, et al. Imaging findings in cardiac tamponade with emphasis on CT. Radiographics. 2007; 27:1595–1610.
43. Gold, MM, Spindola-Franco, H, Jain, VR, et al. Coronary sinus compression: An early computed tomographic sign of cardiac tamponade. J Comput Assist Tomogr. 2008; 32:72–77.
44. Fowler, NO. Cardiac tamponade: A clinical or an echocardiographic diagnosis? Circulation. 1993; 87:1738–1741.
45. Reddy, PS, Curtiss, EI, Uretsky, BF. Spectrum of hemodynamic changes in cardiac tamponade. Am J Cardiol. 1990; 66:1487–1491.
46. Sagrista-Sauleda, J, Angel, J, Sanchez, A, et al. Effusive-constrictive pericarditis. N Engl J Med. 2004; 350:469–475.
47. Sagrista-Sauleda, J, Permanyer-Miralda, G, Candell-Riera, J, et al. Transient cardiac constriction: An unrecognized pattern of evolution in effusive acute idiopathic pericarditis. Am J Cardiol. 1987; 59:961–966.
48. Sagrista-Sauleda, J, Angel, J, Sambola, A, et al. Low-pressure cardiac tamponade: Clinical and hemodynamic profile. Circulation. 2006; 114:945–952.
49. Ramsaran, EK, Benotti, JR, Spodick, DH. Exacerbated tamponade: Deterioration of cardiac function by lowering excessive arterial pressure in hypertensive cardiac tamponade. Cardiology. 1995; 86:77–79.
50. Tyburski, JG, Astra, L, Wilson, RF, et al. Factors affecting prognosis with penetrating wounds of the heart. J Trauma. 2000; 48:587–590.
51. Grove, CA, Lemmon, G, Anderson, G, McCarthy, M. Emergency thoracotomy: Appropriate use in the resuscitation of trauma patients. Am Surg. 2002; 68:313–316.
52. Huang, CH, Chang, Y, Chan, WS, et al. Acute cardiovascular collapse after pericardial drainage in a patient with aortic dissection. Acta Anaesthesiol Taiwan. 2005; 43:39–42.
53. Isselbacher, EM, Cigarroa, JE, Eagle, KA. Cardiac tamponade complicating proximal aortic dissection. Is pericardiocentesis harmful? Circulation. 1994; 90:2375–2378.
54. Blaivas, M. Incidence of pericardial effusion in patients presenting to the emergency department with unexplained dyspnea. Acad Emerg Med. 2001; 8:1143–1146.
55. Holmes, DR, Jr., Nishimura, R, Fountain, R, Turi, ZG. Iatrogenic pericardial effusion and tamponade in the percutaneous intracardiac intervention era. JACC Cardiovasc Interv. 2009; 2:705–717.
56. Ellis, SG, Ajluni, S, Arnold, AZ, et al. Increased coronary perforation in the new device era. Incidence, classification, management, and outcome. Circulation. 1994; 90:2725–2730.
57. Solomon, SB. The future of interventional cardiology lies in the left atrium. Int J Cardiovasc Intervent. 2004; 6(3-4):101–106.
58. Turi, ZG. Puncturing the septum: Resurgent technique with inherent risk. J Invasive Cardiol. 2004; 16:3–4.
59. Wazni, OM, Beheiry, S, Fahmy, T, et al. Atrial fibrillation ablation in patients with therapeutic international normalized ratio: Comparison of strategies of anticoagulation management in the periprocedural period. Circulation. 2007; 116:2531–2534.
60. Amin, Z, Hijazi, ZM, Bass, JL, et al. Erosion of Amplatzer septal occluder device after closure of secundum atrial septal defects: Review of registry of complications and recommendations to minimize future risk. Catheter Cardiovasc Interv. 2004; 63:496–502.
61. Eberhardt, F, Bode, F, Bonnemeier, H, et al. Long term complications in single and dual chamber pacing are influenced by surgical experience and patient morbidity. Heart. 2005; 91:500–506.
62. Retter, AS. Pericardial disease in the oncology patient. Heart Dis. 2002; 4:387–391.
63. Kuvin, JT, Harati, NA, Pandian, NG, et al. Postoperative cardiac tamponade in the modern surgical era. Ann Thorac Surg. 2002; 74:1148–1153.
64. Stevenson, LW, Child, JS, Laks, H, Kern, L. Incidence and significance of early pericardial effusions after cardiac surgery. Am J Cardiol. 1984; 54:848–851.
65. Meurin, P, Weber, H, Renaud, N, et al. Evolution of the postoperative pericardial effusion after day 15: The problem of the late tamponade. Chest. 2004; 125:2182–2187.
66. Mangi, AA, Palacios, IF, Torchiana, DF. Catheter pericardiocentesis for delayed tamponade after cardiac valve operation. Ann Thorac Surg. 2002; 73:1479–1483.
67. Sandifer, DP, Gonzalez, JL. Refractory postoperative hypoxemia associated with regional cardiac tamponade and patent foramen ovale. Crit Care Med. 1997; 25:1608–1611.
68. Asimakopoulos, G, Della, SR, Taggart, DP. Effects of posterior pericardiotomy on the incidence of atrial fibrillation and chest drainage after coronary revascularization: A prospective randomized trial. J Thorac Cardiovasc Surg. 1997; 113:797–799.
69. Maisch, B, Ristic, AD. Practical aspects of the management of pericardial disease. Heart. 2003; 89:1096–1103.
70. Kerber, RE, Gascho, JA, Litchfield, R, et al. Hemodynamic effects of volume expansion and nitroprusside compared with pericardiocentesis in patients with acute cardiac tamponade. N Engl J Med. 1982; 307:929–931.
71. Dy, EA, Shiltz, DL. Hemopericardium and cardiac tamponade associated with dabigatran use. Ann Pharmacother. 2012; 46:e18.
72. Al Shammeri, OM, Crowell, R, Stewart, K, Fort, S. Failed pericardiocentesis for acute cardiac tamponade: Two cases associated with bivalirudin administration during PCI. Catheter Cardiovasc Interv. 2010; 75:114–116.
73. Lestuzzi, C. Neoplastic pericardial disease: Old and current strategies for diagnosis and management. World J Cardiol. 2010; 2:270–279.
74. Rafique, AM, Patel, N, Biner, S, et al. Frequency of recurrence of pericardial tamponade in patients with extended versus nonextended pericardial catheter drainage. Am J Cardiol. 2011; 108:1820–1825.
75. Maisch, B, Seferovic, PM, Ristic, AD, et al. Guidelines on the diagnosis and management of pericardial diseases executive summary; the task force on the diagnosis and management of pericardial diseases of the European Society of Cardiology. Eur Heart J. 2004; 25:587–610.