CHAPTER 39 Cancer of the uterine cervix
Epidemiology
Cervical cancer is the most common gynaecological malignancy in the world with an estimated 493,000 new cases diagnosed each year (Ferlay et al 2004). It accounts for nearly 10% of all cancers in women. Although the incidence of cervical cancer has decreased in industrialized countries in the past 20 years, it still remains a major problem in the developing world. In most parts of Africa, South-Central Asia and Central America, cervical cancer accounts for 7–13% of all newly diagnosed cancers, with an incidence of 30–40 new cases per 100,000 population (Cancer Research UK, 2005).
In the UK, 2800 patients are diagnosed with cervical cancer per year, which accounts for approximately 2% of all female cancer cases, and 950 patients die from the disease (Cancer Research UK, 2005). With the introduction of the cervical screening programme in the UK, the incidence of cervical cancer has decreased from 16/100,000 in 1985 to 8/100,000 in 2005, while the incidence of carcinoma in situ has risen because of early detection with screening (Cancer Research UK, 2005). Carcinoma in situ is most common in women aged 35–39 years, while the incidence of cervical cancer is highest in women aged 30–34 years (17/100,000). The distribution of cervical cancer is bimodal, with a second peak reached in women aged over 85 years (14/100,000) (Cancer Research UK, 2005). Only 30% of cervical cancers are detected by screening, and the majority of cases occur in women who have never had a smear test or who have not been regular participants in the screening programme.
Aetiology
Epidemiological studies have long indicated a positive correlation between sexual activity and cervical cancer (Schiffman and Brinton 1995). Promiscuity, a sexual partner with promiscuous sexual behaviour and early age at first sexual intercourse have all been associated with a high risk of cervical cancer. It has been well demonstrated that human papilloma virus (HPV) infection is the major and probably a necessary causal factor, enhanced by several cofactors for both squamous cell carcinoma and adenocarcinoma of the cervix (Muñoz et al 1992, Schiffman et al 1993, Bosch et al 2002). HPV viral particles have been detected in almost all cases of cervical cancer (99.7%) (Walboomers and Meijer 1997, Franco et al 1999, Walboomers et al 1999).
Human papilloma virus
To date, more than 200 subtypes of HPV have been identified. According to oncogenic potential, HPVs can be classified as low-risk or high-risk types (Table 39.1) (Duenas-Gonzalez et al 2005, Smith et al 2007). Approximately 75% of cervical cancers are related to HPV 16 and 18 in Europe, and 90% of genital warts are caused by HPV 6 and 11 (Smith 2003). However, the distribution of HPV subtypes varies in other parts of the world (Smith et al 2007).
| High-risk HPV | 16, 18, 31, 33, 35, 45, 51, 52, 58, 59, 68, 73, 82 |
| Low-risk HPV | 6, 11, 40, 42, 43, 44, 54, 61, 70, 72, 81 |
It is estimated that nearly 80% of sexually active women will acquire an HPV infection during their lifetime, while oncogenic subtypes are identified in 15% of the population (Brown et al 2005, Dunne et al 2007). Ninety percent of HPV infections will be eradicated by the host immune system, and only a minority of women will develop preinvasive disease or cancer (Pagliusi and Teresa Aguado 2004). The prevalence of HPV infection decreases with age, from approximately 20% in women aged 20–25 years to approximately 5% in women aged 50 years (Woodman et al 2001).
Cofactors
Cigarette smoking
Cigarette smoking has been linked with a higher risk of cervical cancer and has been demonstrated to be an independent risk factor (Winkelstein 1990, Kapeu et al 2009). High concentrations of nicotine metabolites have been detected in cervical mucus, and their molecular interaction with fragile histidine triad (FHIT) tumour suppressor gene has been investigated (Schiffman et al 1987, Holschneider et al 2005). The loss of function of FHIT plays an important role in early carcinogenesis (Pichiorri et al 2008). In comparison with non-smokers, smokers have impaired immune responses in the cervical epithelium and produce reduced levels of HPV 16/18 antibodies (Simen-Kapeu et al 2008).
A recent meta-analysis confirmed that smoking is an independent risk factor for squamous cell cervical cancer, but failed to demonstrate the same for adenocarcinoma (Berrington de González et al 2004).
Oral contraceptives
The association between long-term oral contraceptive (OC) use and cervical cancer is unclear. Recent analyses have shown an increased risk (relative risk 1.3–1.9) of cervical cancer amongst OC users (Smith 2003, Appleby et al 2007, Hannaford et al 2007). In contrast, others have reported that sexual behaviour differs between OC users and non-users, and that OC use is not an independent risk factor (Miller et al 2004, Syrjänen et al 2006).
Immunodeficiency
It has been observed that patients with immunodeficiency disorders, those on immunosuppressive therapy and those with human immunodeficiency virus (HIV) infection are at higher risk of developing anogenital HPV-related premalignant changes and cancers. Cervical cancer is an acquired-immunodeficiency-syndrome-defining disease. One meta-analysis estimated a six-fold increased risk of developing cervical cancer for patients with HIV infection, and a two-fold increased risk for those with previous transplant surgery (Grulich et al 2007).
HPV vaccination
Cervical cytology
Two types of HPV vaccine have been developed: the bivalent Cervarix targeting HPV 16 and 18, and the quadrivalent Gardasil for HPV 6, 11, 16 and 18. The efficacy of Gardasil and Cervarix has been demonstrated in large, prospective, randomized trials (FUTURE II and PATRICIA) (FUTURE II Study Group 2007, Paavonen et al 2007). The vaccines are prophylactic and are not effective in patients with existing HPV infection. There is some cross-protection against other high-risk types of HPV.
Clinical Presentation
The positive predictive value of postcoital and intermenstrual bleeding for the diagnosis of cervical cancer in younger women is very low (Shapley et al 2006). Only 2% of patients with postcoital bleeding will be diagnosed with cervical cancer (Shapley et al 2006).
Diagnosis and Staging
Imaging in cervical cancer
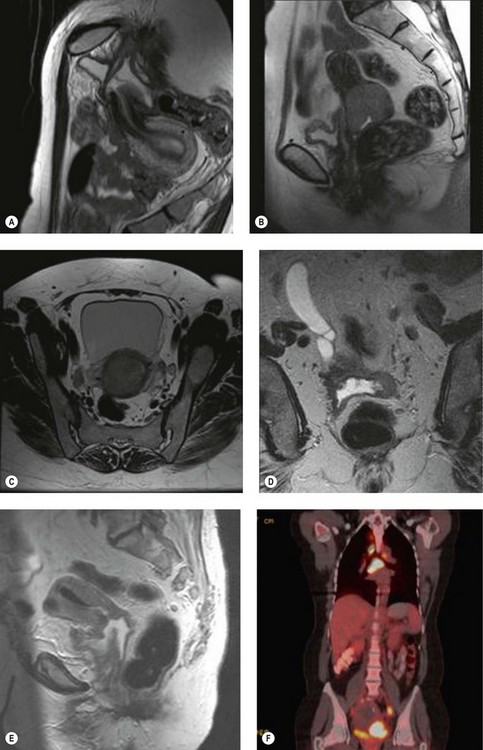
Positron emission tomography (PET) is a functional scan that assesses the increased uptake of fluorodeoxyglucose (FDG). Organs with high metabolic activity (liver, brain, neoplastic tissue) have high FDG uptake. Addition of CT to PET (PET–CT) provides improved assessment of tumour localization. PET–CT has been used to assess para-aortic nodal status to facilitate planning of radiotherapy fields (Figure 39.1F) (Grigsby et al 2001). A further role of PET–CT is to assess the response to primary chemoradiotherapy, as well as early detection of cancer recurrences (Figure 39.2D).
Laparoscopic para-aortic lymph node sampling
Laparoscopic para-aortic lymph node sampling has been performed to assess the para-aortic lymph nodes; however, the only prospective randomized study failed to confirm therapeutic benefit (Lai et al 2003). The detection of micrometastasis in the para-aortic nodes has been used to modify radiation treatment fields (Leblanc et al 2007). Superiority of lymphadenectomy over PET–CT in assessment of the para-aortic nodes has not been established.
Staging
The International Federation of Obstetricians and Gynecologists (FIGO) staging of cervical cancer is based on clinical findings and, in the early stages, on the microscopic extent of the disease, and is not changed by intraoperative findings or the postoperative histological result (Table 39.2). When doubt exists regarding the stage of a particular cancer, the earlier stage should be chosen.
| Stage I | The carcinoma is strictly confined to the cervix (extension to the corpus would be disregarded) |
| IA | Invasive carcinoma which can be diagnosed only by microscopy, with deepest invasion ≤5.0 mm and largest extension ≤7.0 mm |
| IA1 | Measured stromal invasion of ≤3.0 mm in depth and horizontal extension of ≤7.0 mm |
| IA2 | Measured stromal invasion of >3.0 mm and not >5.0 mm with an extension of not >7.0 mm |
| IB | Clinically visible lesions limited to the cervix uteri or preclinical cancers greater than stage IA* |
| IB1 | Clinically visible lesion ≤4.0 cm in greatest dimension |
| IB2 | Clinically visible lesion >4.0 cm in greatest dimension |
| Stage II | Cervical carcinoma invades beyond the uterus, but not to the pelvic wall or to the lower third of the vagina |
| IIA | Without parametrial invasion |
| IIAl | Clinically visible lesion ≤4.0 cm in greatest dimension |
| IIA2 | Clinically visible lesion >4.0 cm in greatest dimension |
| IIB | With obvious parametrial invasion |
| Stage III | The tumour extends to the pelvic wall and/or involves lower third of the vagina and/or causes hydronephrosis or non-functioning kidney§ |
| IIIA | Tumour involves lower third of the vagina, with no extension to the pelvic wall |
| IIIB | Extension to the pelvic wall and/or hydronephrosis or non-functioning kidney |
| Stage IV | The carcinoma has extended beyond the true pelvis or has involved (biopsy proven) the mucosa of the bladder or rectum. A bullous oedema, as such, does not permit a case to be allotted to stage IV |
| IVA | Spread of the growth to adjacent organs |
| IVB | Spread to distant organs |
* All macroscopically visible lesions, even with superficial invasion, are allotted to stage IB carcinomas. Invasion is limited to a measured stromal invasion with a maximal depth of 5.0 mm and a horizontal extension of not >7.0 mm. Depth of invasion should not be >5.0 mm taken from the base of the epithelium of the original tissue — superficial or glandular. The depth of invasion should always be reported in mm, even in those cases with ‘early (minimal) stromal invasion’ (∼1.0 mm). The involvement of vascular/lymphatic spaces should not change the stage allotment.
§ On rectal examination, there is no cancer-free space between the tumour and the pelvic wall. All cases with hydronephrosis or non-functioning kidney are included, unless they are known to be due to another cause.
Surgical staging in cervical cancer is an issue that continues to generate discussion. Should lymph node status influence the staging? Should further substages be included? Should the histological findings and cross-sectional imaging influence the process of staging? A consensus statement from the International Gynecological Cancer Society meeting in 2006 reported as follows (Odicino et al 2007).
Histology
Adenocarcinoma is the second most common histological type and represents 20% of cervical cancers. The proportion of adenocarcinomas compared with squamous cell carcinomas has increased significantly over the past three decades. The cause of this change is two-fold: relative, due to the gradual decrease in incidence of squamous cell carcinoma following the introduction of the cervical screening programme; and absolute, probably due to factors such as increased use of OCs (Ursin et al 1994, Smith et al 2000, Sasieni and Adams 2001). The gross appearance of adenocarcinomas is similar to squamous lesions; however, nearly 15% of patients present with clinically non-apparent lesions due to the endophytic expansion of the cancer (barrel-shaped cervix).
Neuroendocrine cervical carcinomas are rare histological subtypes, accounting for less than 5% of cervical cancers. They are characterized by highly aggressive clinical behaviour, manifesting as early nodal and distant diseases in more than half of patients. This results in poor survival despite multimodal treatment. Neuroendocrine cervical carcinomas are similar to the neuroendocrine cancers of the lung, both clinically and histologically. There are four subgroups: classical carcinoid, atypical carcinoid tumour, neuroendocrine large cell carcinoma and neuroendocrine small cell (oat cell) carcinoma (Albores-Saavedra et al 1997).
Treatment of Cervical Cancer
Surgical management
The method of surgical treatment of cervical cancer depends on the stage of disease, tumour volume, histological features, patient’s desire for future fertility and performance status. Treatment options for different stages of cervical cancer are shown in Table 39.3. The Wertheim-Meigs radical hysterectomy with pelvic lymphadenectomy has been the standard treatment for over a century in patients with stage IA2–IIA cervical cancer. Critical analysis of the data on the role of parametrectomy and pelvic lymphadenectomy has changed the practice significantly, and narrowed the indication for the radical operation. In stage IA2 and low-volume stage IB1 (<500 m3) cervical cancer, the risk of parametrial involvement is low (<2%); therefore, simple hysterectomy is recommended (Stegeman et al 2007). Novel developments in surgical technique, such as fertility-sparing surgery (trachelectomy), laparoscopic radical hysterectomy, pelvic lymphadenectomy and para-aortic lymphadenectomy, have been developed and are practised increasingly. Sentinel node biopsy in cervical cancer is also being explored (Levenback et al 2002).
| Stage | Treatment | LND |
|---|---|---|
| IA1 | Cone biopsy/simple trachelectomy/simple hysterectomy | No |
| IA2 | Cone biopsy/simple trachelectomy/simple hysterectomy | +/− (variable practice) |
| IA1/IA2 + LVSI | Cone biopsy/simple trachelectomy/simple hysterectomy | + |
| IB1: low volume (<500 mm3) | Simple trachelectomy/simple hysterectomy | + |
| IB1: <2 cm diameter | Radical trachelectomy/radical hysterectomy | + |
| IB1: 2–4 cm diameter | Radical hysterectomy | + |
| IB2/IIA | Chemoradiation/radical hysterectomy | + |
| IIB–IVA | Chemoradiation/palliative surgery | |
| IVB | Chemotherapy +/− palliative radiotherapy |
LND, lymph node dissection.
Stage IA1
The presence of LVSI is generally considered to be a poor prognostic factor in cervical cancer (relative risk of recurrence 2.0) (Marchiole et al 2005). Therefore, for stage IA1 cervical cancer with extensive LVSI, pelvic lymphadenectomy might be considered in addition to simple hysterectomy or simple trachelectomy. However, this approach does not have a robust evidence base.
Stage IA2
The reported incidence of nodal involvement in stage IA2 cervical cancer varies from 0.5% to 10% due to the lack of uniformity in measurement of tumour dimensions (Hasumi et al 1980, van Nagell et al 1983, Simon et al 1986, Maiman et al 1988, Creasman et al 1998). The accepted surgical treatment for stage IA2 cervical cancer is pelvic lymphadenectomy with simple hysterectomy. However, in the presence of extensive LVSI, modified radical hysterectomy or RVT with pelvic node dissection has been recommended. It is debateable whether lymphadenectomy should be performed routinely in this substage as, by strict histological subgrouping of stage IA2 cervical cancer (i.e. depth of invasion 3–5 mm and horizontal dimension up to 7 mm), the true incidence of positive lymph node metastasis is 0.5% (1/205 histologically confirmed stage IA2) (Rogers and Luesley 2009).
Stage IB1 and IIA cervical cancer
Both surgery and chemoradiotherapy are thought to be equally effective for the management of stage IB1 and IIA cervical cancer. Surgical treatment which involves radical hysterectomy with pelvic lymphadenectomy has potential advantages in younger women by preserving ovarian function and avoiding radiotherapy-related late complications (e.g. vaginal stenosis, radiation cystitis and radiation-induced bowel damage). Complications related to radical surgery are usually temporary and short term (e.g. autonomic dysfunction of the bladder and rectum, shortening of the vagina, lymphoedema and lymphocysts). Careful preoperative selection of patients for radical surgery avoids subjecting them to double treatment (surgery followed by adjuvant treatment). If adverse histological factors are found in the surgical specimen, postoperative chemoradiotherapy is required (Table 39.4). Approximately 20% of patients with stage IB1 and IIA cervical cancer will require adjuvant chemoradiotherapy, which results in increased treatment-related morbidity.
Table 39.4 Adverse histological prognostic factors in cervical cancer
| Major risk factors | Minor risk factors |
|---|---|
| Positive pelvic nodes | Presence of lymphovascular space invasion |
| Parametrial invasion | Deep cervical stromal invasion (>1/3) |
| Positive or close (<5 mm) surgical margins | Large tumour size (≥4 cm) |
The reported incidence of parametrial involvement in stage IB1 cervical cancer with tumour size less than 2 cm, depth of invasion less than 10 mm, negative pelvic lymph nodes and absent LVSI is low (0.4–0.6%) (Stegeman et al 2007, Wright et al 2007). This suggests that it may be advisable to avoid parametrectomy in this subgroup.
Stage IB2 cervical cancer
The rate of pelvic lymph node metastasis in patients with bulky cervical cancer (>4 cm) is approximately 44% (Finan et al 1996). Although primary surgery is feasible and has some advantages (e.g. removal of tumour bulk, removal of bulky lymph nodes, proper assessment of tumour extension), due to the high likelihood of requiring double treatment, the logical option is primary chemoradiotherapy (NIH Consensus Development Conference Statement on Cervical Cancer 1997). The rate of pelvic relapse is significantly higher in patients with stage IB2 disease who had radiation alone compared with patients who had surgery followed by adjuvant radiotherapy (30% vs 20%) (Landoni et al 1997).
Neoadjuvant chemotherapy has been used with a rationale of reducing tumour bulk prior to surgery or radiotherapy, but no survival benefit has been demonstrated over conventional radiotherapy (Sananes et al 1998, Benedetti-Panici et al 2002).
Surgical techniques
Radical hysterectomy
Radical hysterectomy was classified into five types by Piver et al in 1974 based upon the site of ligation of the uterine vessels and the radicality of parametrial resection. The Surgery Committee of the Gynecological Cancer Group of the European Organization for Research and Treatment of Cancer have produced, approved and adopted a revised version of the original Piver classification (Table 39.5) (Trimbos et al 2004). The salient steps of radical hysterectomy are (Figure 39.3):
Table 39.5 Modified Piver/European Organization for Research and Treatment of Cancer classification of radical hysteretomy
| Class | Description |
|---|---|
| Type I | Simple hysterectomy. |
| Type II | Modified radical hysterectomy: the uterus, paracervical tissues and upper vagina (1–2 cm) are removed after dissection of the ureters to the point of their entry to the bladder. The uterine arteries are ligated at the site of crossing the ureters, and the medial half of the parametria and proximal uterosacral ligaments are resected. |
| Type III | Radical hysterectomy: en-bloc removal of the uterus with the upper third of the vagina along with the paravaginal and paracervical tissues. The uterine vessels are ligated at their origin, and the entire width of the parametria is resected bilaterally. Removal of as much of the uterosacral ligaments as possible. |
| Type IV | Extended radical hysterectomy: the difference from the type III procedure is that three-quarters of the vagina and paravaginal tissue are excised. |
| Type V | Partial exenteration: the terminal ureter or a segment of the bladder or rectum is removed, along with the uterus and parametria (supralevator exenteration). |
Landoni et al (2001) conducted a prospective randomized study comparing survival, relapse and morbidity between type II and type III radical hysterectomy for patients with stage IB and IIA cervical cancer. They found similar outcomes but a higher rate of late morbidity in the group with type III radical hysterectomy.
Okabayashi’s nerve-sparing radical hysterectomy preserves both the hypogastric nerves and the splanchnic nerves in order to reduce bladder and bowel dysfunction (Fujii et al 2007). Laparoscopic radical hysterectomy is a novel approach with similar efficacy and recurrence rates to open radical hysterectomy, but with reduced blood loss and wound-related complications, and a shorter recovery period (Abu-Rustum et al 2003, Ghezzi et al 2007).
Removal of the tubes and ovaries is not routinely part of a radical hysterectomy. The risk of ovarian metastasis is 0.5% in early squamous cell carcinomas and 1.7% in adenocarcinomas (Sutton et al 1992). Therefore, for women under 45 years of age with cervical cancer, the ovaries can usually be preserved and can be transposed into the paracolic gutters out of the pelvis (outwith the potential radiation field). Conventionally, patients with adenocarcinoma are offered salpingo-oophorectomy; however, isolated ovarian metastasis in the absence of adverse pathological features is rare. The ovarian failure rate after transposition is 50% (Anderson et al 1993, Feeney et al 1995).
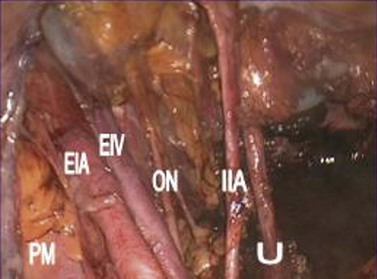
Type II–V radical hysterectomies are completed with systematic bilateral pelvic lymphadenectomy. The anatomical borders of the lymphadenectomy are the bifurcation of the common iliac vessels cranially, the deep circumflex iliac vein and the bladder caudally, the obturatour internus muscle and the genitofemoral nerve laterally, the internal iliac artery medially, and the obturator nerve inferiorly (Figure 39.4). Any bulky para-aortic nodes should also be resected, given that radiation therapy cannot sterilize metastatic nodes larger than 2 cm in diameter (Hacker et al 1995).
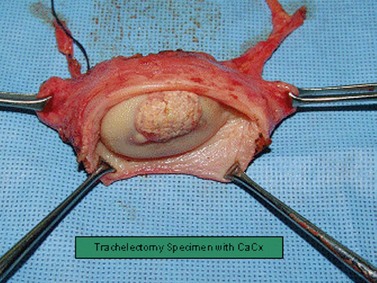
Trachelectomy
In the UK, the peak incidence of cervical cancer is in women aged 35–39 years. As maternal age at first childbirth has increased progressively, it is not uncommon to find women with cervical cancer who have not yet started or completed their families (Cancerstats 2003). RVT is a fertility-preserving operation for the treatment of invasive cervical cancer, and was first reported by Dargent et al in 1994.
Procedure and technique
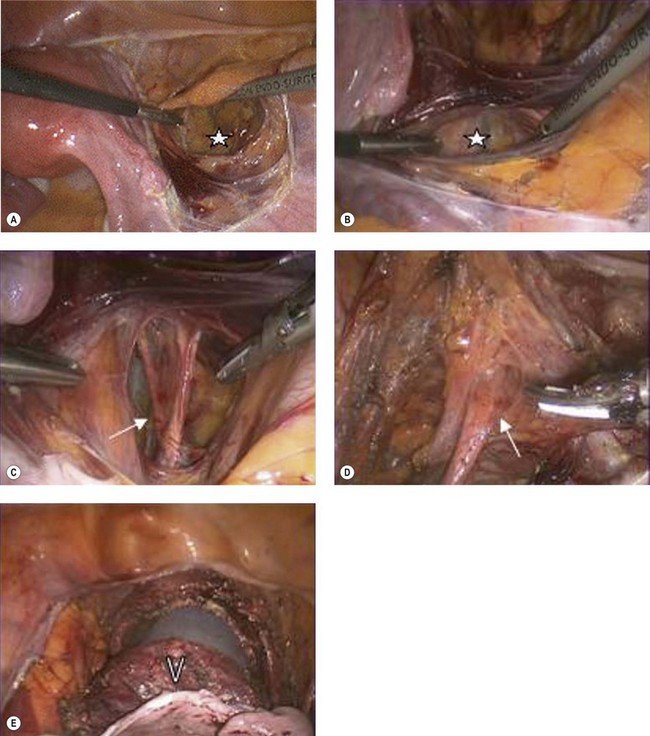
RVT is performed in two steps. Firstly, the pelvic lymph nodes are assessed laparoscopically to exclude metastasis. The second step is radical resection of the cervix along with a vaginal cuff and the paracervical tissue (Figure 39.5). Both procedures can be performed on the same day if a reliable frozen section facility is available. If not, laparoscopic lymphadenectomy can precede the radical trachelectomy by a few days. A circumferential incision is made on the upper vagina creating a 1–2 cm vaginal cuff. The vesicovaginal and paravesical spaces are dissected anteriorly and the pouch of Douglas is opened posteriorly. The knee of the ureter is palpated in the bladder pillar, which is reflected upwards and medially. The bladder pillars are divided inferiorly, further releasing the bladder and ureters superiorly. The cardinal–uterosacral complex is clamped and divided just below the ureters. The cervix is then amputated leaving 0.5–1.0 cm of normal cervical tissue below the internal os. Cervical cerclage suture is inserted, the vagina is sutured in a circular fashion around the cervix and a new, vagino–isthmic junction is created.
Radical trachelectomy can also be performed abdominally and laparoscopically (Cibula et al 2005). The abdominal approach is suitable in women with poor vaginal access, when the cervix is flush with the vault or in the presence of a large, exophytic cervical growth (Cibula et al 2008). Pelvic lymphadenectomy is usually performed laparoscopically, but can also be performed by an extraperitoneal approach.
Outcome after radical vaginal trachelectomy
Potential morbidities after RVT are bladder and ureteral injury, bladder dysfunction, haematoma, lymphoedema and cervical stenosis with haematometra (Plante et al 2005). The reported recurrence rate after RVT is 4% (Milliken and Shepherd 2008). Common sites of recurrence are the vagina, parametrium, pelvic sidewall and para-aortic lymph nodes. Central recurrences may be attributable to either persistent disease in the isthmus or persistent high-risk HPV infection (Morice et al 2004). In the presence of poor histological prognostic factors, additional treatment may be recommended, including completion radical hysterectomy if the margins of clearance are less than 1 cm, or chemoradiation if more than one poor prognostic factor is present (Table 39.4).
Obstetric outcome after radical trachelectomy
Out of 780 RVT procedures reported to date, there have been 302 pregnancies with 190 live births (Shepherd and Milliken 2008). Following RVT, a significant proportion (57%) of patients did not attempt any further pregnancy. In those who did, there was a 70% conception rate with a 30% pregnancy loss during the first and second trimesters, and a preterm delivery rate of 20%. Pregnancies after RVT should be accorded high-risk status because of the increased risk of miscarriage, ascending infection, premature membrane rupture and preterm labour. Regular screening for bacterial vaginosis, prophylactic antibiotics, antenatal steroid therapy and elective caesarean section are recommended.
Follow-up after radical vaginal trachelectomy
Follow-up practices are varied in terms of intervals, duration and modalities used. Shepherd and Milliken (2008) recommend 4-monthly visits for 3 years, and 6-monthly visits until 5 years. Endocervical and vaginal vault cytology and colposcopy are performed during follow-up. HPV testing may be recommended in the presence of persistent dyskariotic cytology on follow-up smears (Singh et al 2004).
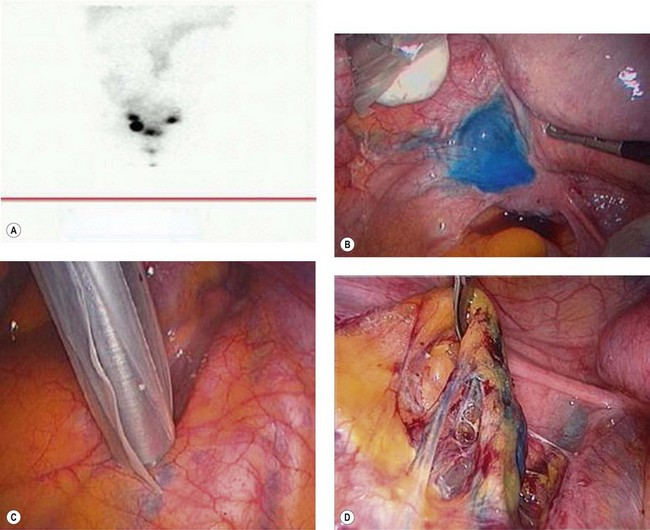
Sentinel lymph node biopsy in cervical cancer
Limitations of sentinel node dissection in cervical cancer are:
Technique of sentinel node biopsy in cervical cancer
Radionucleotide tracer 99m technetium sulphur colloid (1 ml) is injected around the cervical tumour in four quadrants, followed by lymphoscintigraphy performed a few hours prior to surgery. Immediately prior to surgery, 2 ml of blue dye diluted with 2 ml of normal saline is injected in four quadrants around the cervical tumour. Laparoscopic identification and retrieval of the sentinel node using a gamma probe is performed (Figure 39.6). Using a combination of radioactive colloid and blue dye increases the identification of sentinel nodes to 98%. Histological ultrastaging and immunostaining with pancytokeratin further increase the detection of micrometastases.
Radiotherapy
Recently, three randomized trials administering concurrent chemotherapy (cisplatin) and radiotherapy to patients with locally advanced cervical cancer demonstrated a significant benefit in progression-free and overall survival compared with radiotherapy alone (Keys et al 1999, Morris et al 1999, Rose et al 1999, Bekkers et al 2002). Since these seminal trials, chemoradiation therapy has become the standard treatment for medically fit patients with locally advanced cervical cancer. Concurrent chemotherapy enhances the effect of radiotherapy in two ways: certain chemotherapeutics (e.g. cisplatin, hydroxyurea) behave as radiation sensitizers, and the chemotherapy itself is cytotoxic.
Primary radiotherapy
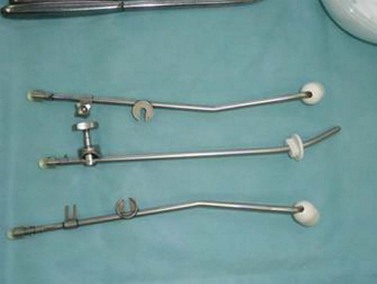
Brachytherapy
Placement of radioactive material into a body cavity is called ‘brachytherapy’. Various delivery techniques are used. The Manchester applicator consists of a uterine probe and a pair of intravaginal receptacles (Figure 39.7). The patient is isolated in a shielded room and remote control is used to deliver the radioactive particles into the applicators. There are two main forms of brachytherapy:
Vaginal brachytherapy is critically important to control the central tumour mass adequately. The omission of brachytherapy will result in a higher locoregional relapse rate (Lanciano et al 1991, Logsdon and Eifel 1999).
Adjuvant radiotherapy
Adjuvant radiation therapy is administered in combination with chemotherapy. Adjuvant therapy in the presence of one major risk factor or at least two of the three minor adverse factors (Table 39.4) reduces the risk of recurrence, and increases recurrence-free survival (Sedlis et al 1999).
Complications of radiation therapy
Late complications can occur many months or years after treatment and are usually chronic.
Recurrent Cervical Cancer
The risk of recurrence depends on the extent of the primary cancer at presentation. Approximately 10–20% of patients with stage IB–IIA cervical cancer with negative lymph nodes will recur, compared with up to 70% of patients with nodal metastasis or locally advanced disease (Table 39.6).
Table 39.6 Rate of pelvic and distant recurrences in cervical cancer
| Stage | Pelvic recurrence (%) | Distant metastasis (%) |
|---|---|---|
| IB | 10–14 | 16 |
| IIA | 17–20 | 31 |
| IIB | 23 | 26 |
| III | 42 | 39 |
| IVA | 74 | 75 |
Source: Chan YM, Ng TY, Ngan HY, Wong LC 2002 Monitoring of squamous cell carcinoma antigen levels in invasive cervical cancer: is it cost effective? Gynecological Oncology 84: 7–11.
Recurrences can be locoregional or distant (Table 39.7). Patients with pelvic recurrence usually present with vaginal bleeding, discharge, pelvic pain and sciatic pain. Patients with disseminated recurrence will develop systemic symptoms associated with cachexia.
Table 39.7 Distribution of distant metastases
| Site | Rate (%) |
|---|---|
| Lung | 21 |
| Para-aortic lymph nodes | 11 |
| Abdominal cavity | 8 |
| Supraclavicular lymph nodes | 7 |
| Bone (lumbar/thoracic) | 16 |
Source: Chan YM, Ng TY, Ngan HY, Wong LC 2002 Monitoring of squamous cell carcinoma antigen levels in invasive cervical cancer: is it cost effective? Gynecological Oncology 84: 7–11.
When such symptoms are present, the following investigations should be considered:
Pelvic recurrence after radiotherapy
Exenterations are significantly morbid surgical procedures with a major degree of psychosexual impact. Patients require extensive preoperative counselling and postoperative support. With careful selection, the 5-year survival rate after anterior and posterior exenteration is 30–60% and 20–40%, respectively (Friedlander and Grogan 2002).
Patients with sidewall recurrences of less than 5 cm in diameter can be considered for laterally extended endopelvic resection, and can achieve a 5-year survival rate of up to 60% (Hockel 2003).
Follow-up after Treatment of Cervical Cancer
Follow-up is aimed to detect recurrent disease, assess treatment-related morbidity, and analyse psychosocial and psychosexual morbidity. There is no standardized effective protocol for follow-up in cervical cancer. Vault cytology is not effective in detecting recurrences of cervical cancer and is not recommended (Bodurka-Bevers et al 2000). Symptom status at the time of recurrence is a significant predictor of survival; the median survival is 11 months for symptomatic recurrence and 42 months for asymptomatic recurrence. Routine clinical examination in asymptomatic patients appears to lack sensitivity, as a high proportion of recurrences are not detected until symptoms are apparent.
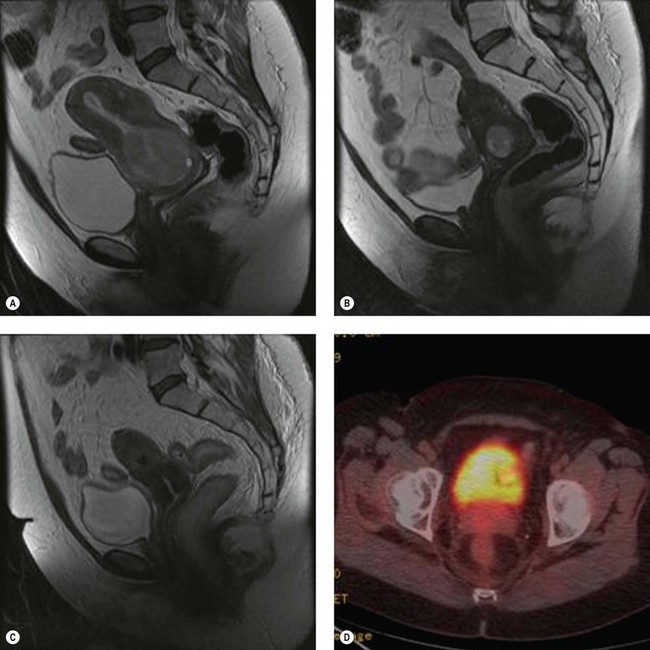
In an observational study of 993 cases of stage IB cervical cancer treated with surgery or radiotherapy, 63% of recurrences occurred before 24 months and 77% occurred before 36 months. The median time to relapse was 16 months (Bodurka-Bevers et al 2000). Patients should be followed-up every 4 months for at least 2 years. MRI and CT are performed whenever recurrence is suspected, but have limitations in differentiating postradiotherapy fibrosis from recurrent tumour. PET–CT scan has been demonstrated as a more sensitive modality for post-therapy surveillance for the detection of recurrent and persistent cervical cancer in both symptomatic and aymptomatic patients. There is limited evidence regarding the optimal timing and frequency of post-therapy scans (Figure 39.2). PET–CT scans should not be used within 3 months of the primary treatment to reduce false-positive results, as the therapeutic effect of radiation continues even after completion of treatment. PET–CT scan is able to identify persistent disease in patients following completion of chemoradiotherapy to select patients for salvage hysterectomy or exenteration.
The tumour marker squamous cell carcinoma antigen is neither sensitive nor cost-effective in the follow-up of cervical cancer and is not recommended (Chan et al 2002).
Management of Complications in Advanced Disease
Patients with advanced cervical cancer may develop the following distressing symptoms.
Conclusions
KEY POINTS
Abu-Rustum NR, Gemignani ML, Moore K, et al. Total laparoscopic radical hysterectomy with pelvic lymphadenectomy using the argon-beam coagulator: pilot data and comparison to laparotomy. Gynecological Oncology. 2003;91:402-409.
Albores-Saavedra J, Gersell D, Gilks CB, et al. Terminology of endocrine tumors of the uterine cervix: results of a workshop sponsored by the College of American Pathologists and the National Cancer Institute. Archives of Pathology and Laboratory Medicine. 1997;121:34-39.
Anderson B, LaPolla J, Turner D, Chapman G, Buller R. Ovarian transposition in cervical cancer. Gynecological Oncology. 1993;49:206-214.
Appleby P, Beral V, Berrington de González A, et al. Cervical cancer and hormonal contraceptives: collaborative reanalysis of individual data for 16,573 women with cervical cancer and 35,509 women without cervical cancer from 24 epidemiological studies. The Lancet. 2007;370:1609-1621.
Bekkers RL, Keyser KG, Bulten J, et al. The value of loop electrosurgical conization in the treatment of stage IA1 microinvasive carcinoma of the uterine cervix. International Journal of Gynecological Cancer. 2002;12:485-489.
Benedetti-Panici P, Greggi S, Colombo A, et al. Neoadjuvant chemotherapy and radical surgery versus exclusive radiotherapy in locally advanced squamous cell cervical cancer: results from the Italian multicenter randomized study. Journal of Clinical Oncology. 2002;20:179-188.
Berrington de González A, Sweetland S, Green J. Comparison of risk factors for squamous cell and adenocarcinomas of the cervix: a meta-analysis. British Journal of Cancer. 2004;90:1787-1791.
Bodurka-Bevers D, Morris M, Eifel PJ, et al. Posttherapy surveillance of women with cervical cancer: an outcome analysis. Gynecological Oncology. 2000;78:187-193.
Bosch FX, Lorincz A, Muñoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. Journal of Clinical Pathology. 2002;55:244-265.
Brown DR, Shew ML, Qadadri B, et al. A longitudinal study of genital human papillomavirus infection in a cohort of closely followed adolescent women. Journal of Infectious Diseases. 2005;191:182-192.
Cancer Research UK. www.cancerresearchuk.org, 2005.
Cancerstats. Cervical Cancer UK. Cancer Research UK; 2003.
Chan YM, Ng TY, Ngan HY, Wong LC. Monitoring of squamous cell carcinoma antigen levels in invasive cervical cancer: is it cost effective? Gynecological Oncology. 2002;84:7-11.
Cibula D, Ungar L, Palfalvi L, Bino B, Kuzel D. Laparoscopic abdominal radical trachelectomy. Gynecological Oncology. 2005;97:707-709.
Cibula D, Slama J, Fischerova D. Update on abdominal radical trachelectomy. Gynecological Oncology. 2008;111(Suppl):S111-S115.
Creasman WT, Zaino RJ, Major FJ, DiSaia PJ, Hatch KD, Homesley HD. Early invasive carcinoma of the cervix (3 to 5 mm invasion): risk factors and prognosis. A Gynecologic Oncology Group study. American Journal of Obstetrics and Gynecology. 1998;178:62-65.
Dargent D, Burn JL, Roy M, Remi I. Pregnancies following radical trachelectomy for invasive cevical cancer. Gynecological Oncology. 1994;52:a14.
Duenas-Gonzalez A, Cetina-Perez L, Lopez-Graniel C, et al. Pathologic response and toxicity assessment of chemoradiotherapy with cisplatin versus cisplatin plus gemcitabine in cervical cancer: a randomized phase II study. International Journal of Radiation Oncology, Biology and Physics. 2005;61:817-823.
Dunne EF, Unger ER, Sternberg M, et al. Prevalence of HPV infection among females in the United States. JAMA: the Journal of the American Medical Association. 2007;297:813-819.
Feeney DD, Moore DH, Look KY, Stehman FB, Sutton GP. The fate of the ovaries after radical hysterectomy and ovarian transposition. Gynecological Oncology. 1995;56:3-7.
Ferlay J, et al. Cancer Incidence, Mortality and Prevalence Worldwide. IARC CancerBase No. 5, Version 20. IARC Press, Lyon, 2004.
Finan MA, DeCesare S, Fiorica JV, et al. Radical hysterectomy for stage IB1 vs IB2 carcinoma of the cervix: does the new staging system predict morbidity and survival? Gynecological Oncology. 1996;62:139-147.
Franco EL, Rohan TE, Villa LL. Epidemiologic evidence and human papillomavirus infection as a necessary cause of cervical cancer. Journal of the National Cancer Institute. 1999;91:506-511.
Friedlander M, Grogan M. Guidelines for the treatment of recurrent and metastatic cervical cancer. The Oncologist. 2002;7:342.
Fujii S, Takakura K, Matsumura N, et al. Anatomic identification and functional outcomes of the nerve sparing Okabayashi radical hysterectomy. Gynecological Oncology. 2007;107:4-13.
FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. New England Journal of Medicine. 2007;356:1915-1927.
Ghezzi F, Cromi A, Ciravolo G, et al. Surgicopathologic outcome of laparoscopic versus open radical hysterectomy. Gynecological Oncology. 2007;106:502-506.
Grigsby PW, Siegel BA, Dehdashti F. Lymph node staging by positron emission tomography in patients with carcinoma of the cervix. Journal of Clinical Oncology. 2001;19:3745-3749.
Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. The Lancet. 2007;370:59-67.
Hacker NF, Wain GV, Nicklin JL. Resection of bulky positive lymph nodes in patients with cervical carcinoma. International Journal of Gynecological Cancer. 1995;5:250-256.
Hannaford PC, Selvaraj S, Elliott AM, Angus V, Iversen L, Lee AJ. Cancer risk among users of oral contraceptives: cohort data from the Royal College of General Practitioner’s oral contraception study. BMJ (Clinical Research Ed.). 2007;335:651.
Hasumi K, Sakamoto A, Sugano H. Microinvasive carcinoma of the uterine cervix. Cancer. 1980;45:928-931.
Hockel M. Surgical treatment of locally advanced and recurrent cervical carcinoma: overview on current standard and new developments. Onkologie. 2003;26:452-455.
Holschneider CH, Baldwin RL, Tumber K, Aoyama C, Karlan BY. The fragile histidine triad gene: a molecular link between cigarette smoking and cervical cancer. Clinical Cancer Research. 2005;11:5756-5763.
Kapeu AS, Luostarinen T, Jellum E, et al. Is smoking an independent risk factor for invasive cervical cancer? A nested case–control study within Nordic biobanks. American Journal of Epidemiology. 2009;169:480-488.
Keys HM, Bundy BN, Stehman FB, et al. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. New England Journal of Medicine. 1999;340:1154-1161.
Lai CH, Huang KG, Hong JH, et al. Randomized trial of surgical staging (extraperitoneal or laparoscopic) versus clinical staging in locally advanced cervical cancer. Gynecological Oncology. 2003;89:160-167.
Lanciano RM, Won M, Coia LR, Hanks GE. Pretreatment and treatment factors associated with improved outcome in squamous cell carcinoma of the uterine cervix: a final report of the 1973 and 1978 patterns of care studies. International Journal of Radiation Oncology, Biology and Physics. 1991;20:667-676.
Landoni F, Maneo A, Colombo A, et al. Randomised study of radical surgery versus radiotherapy for stage Ib–IIa cervical cancer. The Lancet. 1997;350:535-540.
Landoni F, Maneo A, Cormio G, et al. Class II versus class III radical hysterectomy in stage IB–IIA cervical cancer: a prospective randomized study. Gynecological Oncology. 2001;80:3-12.
Leblanc E, Narducci F, Frumovitz M, et al. Therapeutic value of pretherapeutic extraperitoneal laparoscopic staging of locally advanced cervical carcinoma. Gynecological Oncology. 2007;105:304-311.
Levenback C, Coleman RL, Burke TW, et al. Lymphatic mapping and sentinel node identification in patients with cervix cancer undergoing radical hysterectomy and pelvic lymphadenectomy. Journal of Clinical Oncology. 2002;20:688-693.
Logsdon MD, Eifel PJ. Figo IIIB squamous cell carcinoma of the cervix: an analysis of prognostic factors emphasizing the balance between external beam and intracavitary radiation therapy. International Journal of Radiation Oncology, Biology and Physics. 1999;43:763-775.
Maiman MA, Fruchter RG, Dimaio TM, Boyce JG. Superficially invasive squamous cell carcinoma of the cervix. Obstetrics and Gynecology. 1988;72:399-403.
Marchiole P, Buenerd A, Benchaib M, Nezhat K, Dargent D, Mathevet P. Clinical significance of lymphovascular space involvement and lymph node micrometastases in early-stage cervical cancer: a retrospective case–control surgico-pathological study. Gynecological Oncology. 2005;97:727-732.
Miller K, Blumenthal P, Blanchard K. Oral contraceptives and cervical cancer: critique of a recent review. Contraception. 2004;69:347-351.
Milliken DA, Shepherd JH. Fertility preserving surgery for carcinoma of the cervix. Current Opinion in Oncology. 2008;20:575-580.
Morice P, Dargent D, Haie-Meder C, Duvillard P, Castaigne D. First case of a centropelvic recurrence after radical trachelectomy: literature review and implications for the preoperative selection of patients. Gynecological Oncology. 2004;92:1002-1005.
Morris M, Eifel PJ, Lu J, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. New England Journal of Medicine. 1999;340:1137-1143.
Muñoz N, Bosch FX, de Sanjosé S, et al. The causal link between human papillomavirus and invasive cervical cancer: a population-based case–control study in Colombia and Spain. International Journal of Cancer. 1992;52:743-749.
NIH Consensus Development Conference Statement on Cervical Cancer. Gynecological Oncology. 1997;66:351-361.
Odicino F, Tisi G, Rampinelli F, Miscioscia R, Sartori E, Pecorelli S. New development of the FIGO staging system. Gynecological Oncology. 2007;107(Suppl):S8-S9.
Paavonen J, Jenkins D, Bosch FX, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. The Lancet. 2007;369:2161-2170.
Pagliusi SR, Teresa Aguado M. Efficacy and other milestones for human papillomavirus vaccine introduction. Vaccine. 2004;23:569-578.
Pichiorri F, Palumbo T, Suh SS, et al. Fhit tumor suppressor: guardian of the preneoplastic genome. Future Oncology. 2008;4:815-824.
Piver MS, Rutledge F, Smith JP. Five classes of extended hysterectomy for women with cervical cancer. Obstetrics and Gynecology. 1974;70:172-175.
Plante M, Renaud MC, Roy M. Radical vaginal trachelectomy: a fertility-preserving option for young women with early stage cervical cancer. Gynecological Oncology. 2005;99(Suppl 1):S143-S146.
Rogers LJ, Luesley DM. Stage 1A2 cervical cancer: how much treatment is enough? International Journal of Gynecological Cancer. 2009;19:1620-1624.
Rose PG, Bundy BN, Watkins EB, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. New England Journal of Medicine. 1999;340:1144-1153.
Sananes C, Giaroli A, Soderini A, et al. Neoadjuvant chemotherapy followed by radical hysterectomy and postoperative adjuvant chemotherapy in the treatment of carcinoma of the cervix uteri: long-term follow-up of a pilot study. European Journal of Gynaecological Oncology. 1998;19:368-373.
Sasieni P, Adams J. Changing rates of adenocarcinoma and adenosquamous carcinoma of the cervix in England. The Lancet. 2001;357:1490-1493.
Schiffman MH, Haley NJ, Felton JS, et al. Biochemical epidemiology of cervical neoplasia: measuring cigarette smoke constituents in the cervix. Cancer Research. 1987;47:3886-3888.
Schiffman MH, Bauer HM, Hoover RN, et al. Epidemiologic evidence showing that human papillomavirus infection causes most cervical intraepithelial neoplasia. Journal of the National Cancer Institute. 1993;85:958-964.
Schiffman MH, Brinton LA. The epidemiology of cervical carcinogenesis. Cancer. 1995;76:1888-1901.
Sedlis A, Bundy BN, Rotman MZ, Lentz SS, Muderspach LI, Zaino RJ. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: a Gynecologic Oncology Group study. Gynecological Oncology. 1999;73:177-183.
Shapley M, Jordan J, Croft PR. A systematic review of postcoital bleeding and risk of cervical cancer. British Journal of General Practice. 2006;56:453-460.
Shepherd JH, Milliken DA. Conservative surgery for carcinoma of the cervix. Clinical Oncology (Royal College of Radiologists). 2008;20:395-400.
Simen-Kapeu A, Kataja V, Yliskoski M, et al. Smoking impairs human papillomavirus (HPV) type 16 and 18 capsids antibody response following natural HPV infection. Scandinavian Journal of Infectious Diseases. 2008;40:745-751.
Simon NL, Gore H, Shingleton HM, Soong SJ, Orr JWJr, Hatch KD. Study of superficially invasive carcinoma of the cervix. Obstetrics and Gynecology. 1986;68:19-24.
Singh N, Titmuss E, Chin AJ, et al. A review of post-trachelectomy isthmic and vaginal smear cytology. Cytopathology. 2004;15:97-103.
Smith JS. Cervical cancer and use of hormonal contraceptives: a systematic review. The Lancet. 2003;361:1159-1167.
Smith JS, Lindsay L, Hoots B, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. International Journal of Cancer. 2007;121:621-632.
Smith HO, Tiffany MF, Qualls CR, Key CR. The rising incidence of adenocarcinoma relative to squamous cell carcinoma of the uterine cervix in the United States — a 24-year population-based study. Gynecological Oncology. 2000;78:97-105.
Stegeman M, Louwen M, van der Velden J, et al. The incidence of parametrial tumor involvement in select patients with early cervix cancer is too low to justify parametrectomy. Gynecological Oncology. 2007;105:475-480.
Sutton GP, Bundy BN, Delgado G, et al. Ovarian metastases in stage IB carcinoma of the cervix: a Gynecologic Oncology Group study. American Journal of Obstetrics and Gynecology. 1992;166:50-53.
Syrjänen K, Shabalova I, Petrovichev N, et al. Oral contraceptives are not an independent risk factor for cervical intraepithelial neoplasia or high-risk human papillomavirus infections. Anticancer Research. 2006;26:4729-4740.
Trimbos JB, Franchi M, Zanaboni F, Velden J, Vergote I. tate of the art’ of radical hysterectomy; current practice in European oncology centres. European Journal of Cancer. 2004;40:375-378.
Ursin G, Peters RK, Henderson BE, d’Ablaing G3rd, Monroe KR, Pike MC. Oral contraceptive use and adenocarcinoma of cervix. The Lancet. 1994;344:1390-1394.
van Nagell JRJr, Greenwell N, Powell DF, Donaldson ES, Hanson MB, Gay EC. Microinvasive carcinoma of the cervix. American Journal of Obstetrics and Gynecology. 1983;145:981-991.
Walboomers JM, Meijer CJ. Do HPV-negative cervical carcinomas exist? Journal of Pathology. 1997;181:253-254.
Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. Journal of Pathology. 1999;189:12-19.
Winkelstein W. Smoking and cervical cancer: current status — a review. American Journal of Epidemiology. 1990;131:945-957.
Woodman CB, Collins S, Winter H, et al. Natural history of cervical human papillomavirus infection in young women: a longitudinal cohort study. The Lancet. 2001;357:1831-1836.
Wright JD, Grigsby PW, Brooks R, et al. Utility of parametrectomy for early stage cervical cancer treated with radical hysterectomy. Cancer. 2007;110:1281-1286.