Campylobacter, Arcobacter, and Helicobacter
1. List the Campylobacter species most often associated with infections in humans, and explain how they are transmitted.
2. Identify the culture methods for optimum recovery of Campylobacter jejuni and Campylobacter coli, including agar, temperatures, oxygenation, and length of incubation.
3. Describe how to isolate Campylobacter from blood, including special stains, atmospheric conditions, and length of incubation.
4. List the colonial morphology, microscopic, and biochemical reactions of Campylobacter and Helicobacter.
5. List the key biochemical test to identify Helicobacter pylori in specimens.
6. Describe how H. pylori colonize in the stomach and how motility plays an important role in the pathogenesis of the organism.
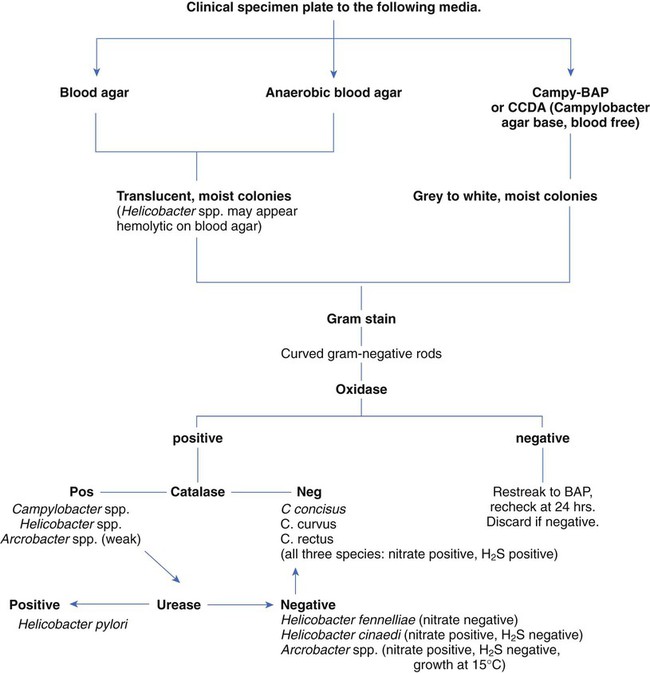
Because of morphologic similarities and an inability to recover these organisms using routine laboratory media for primary isolation, the genera Campylobacter, Arcobacter, and Helicobacter are considered in this chapter (Figure 34-1). All organisms belonging to these genera are small, curved, motile, gram-negative bacilli. With few exceptions, most of these bacteria also have a requirement for a microaerobic (5% to 10% O2) atmosphere.
Campylobacter and Arcobacter
General Characteristics
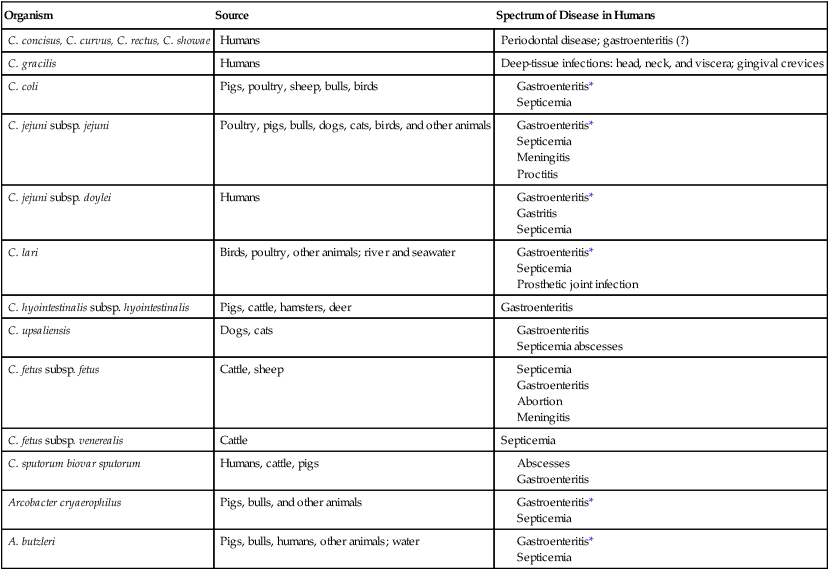
Campylobacter and Arcobacter spp. are relatively slow growing, fastidious, and, in general, asaccharolytic; organisms known to cause disease in humans are listed in Table 34-1.
TABLE 34-1
Campylobacter and Arcobacter spp., Their Source, and Spectrum of Disease in Humans
| Organism | Source | Spectrum of Disease in Humans |
| C. concisus, C. curvus, C. rectus, C. showae | Humans | Periodontal disease; gastroenteritis (?) |
| C. gracilis | Humans | Deep-tissue infections: head, neck, and viscera; gingival crevices |
| C. coli | Pigs, poultry, sheep, bulls, birds | |
| C. jejuni subsp. jejuni | Poultry, pigs, bulls, dogs, cats, birds, and other animals | |
| C. jejuni subsp. doylei | Humans | |
| C. lari | Birds, poultry, other animals; river and seawater | |
| C. hyointestinalis subsp. hyointestinalis | Pigs, cattle, hamsters, deer | Gastroenteritis |
| C. upsaliensis | Dogs, cats | |
| C. fetus subsp. fetus | Cattle, sheep | |
| C. fetus subsp. venerealis | Cattle | Septicemia |
| C. sputorum biovar sputorum | Humans, cattle, pigs | |
| Arcobacter cryaerophilus | Pigs, bulls, and other animals | |
| A. butzleri | Pigs, bulls, humans, other animals; water |
Spectrum of Disease
As previously mentioned, Campylobacter species are the causative agent of gastrointestinal or extraintestinal infections. An increase in extraintestinal disease, including meningitis, endocarditis, and septic arthritis has been reported in patients with acquired immunodeficiency syndrome (AIDS) and other immunocompromised individuals. The different campylobacters and the associated diseases are summarized in Table 34-1. Gastroenteritis associated with Campylobacter spp. is usually a self-limiting illness and does not require antibiotic therapy. Most recently, postinfectious complications with C. jejuni have been recognized and include reactive arthritis and Guillain-Barré syndrome, an acute demyelination (removal of the myelin sheath from a nerve) of the peripheral nerves. Studies indicate that 20% to 40% of patients with this syndrome are infected with C. jejuni 1 to 3 weeks prior to the onset of neurologic symptoms.
Laboratory Diagnosis
Specimen Collection, Transport, and Processing
Direct Detection
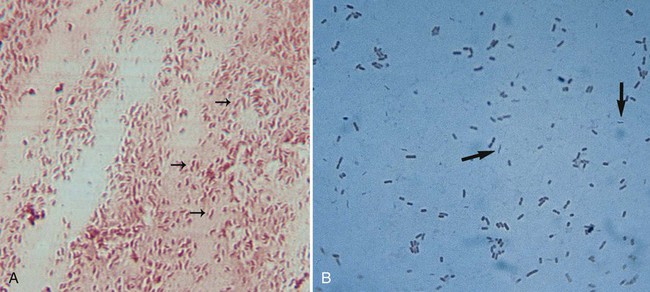
Upon gram staining, Campylobacter spp. display a characteristic microscopic morphology as small, curved or seagull-winged, faintly staining, gram-negative rods (Figure 34-2). Polymerase chain reaction (PCR) amplification may provide an alternative to culture methods for the detection of Campylobacter spp. from clinical specimens. The detection of Campylobacter DNA in stools from a large number of patients with diarrhea suggests that Campylobacter spp. other than C. jejuni and C. coli may account for a proportion of cases of acute gastroenteritis in which no etiologic agent is identified.
Cultivation
Stool.
Successful isolation of Campylobacter spp. from stool requires selective media and optimum incubation conditions. Recommended inoculation of two selective agars is associated with increased recovery of the organisms. Because Campylobacter and Arcobacter spp. have different optimum temperatures, two sets of selective plates should be incubated, one at 42° C and one at 37° C. Extended incubation may be required, 48 to 72 hours, before there is evidence of visible growth. Table 34-2 describes the selective plating media and incubation conditions required for the recovery of Campylobacter spp. from stool specimens.
TABLE 34-2
Modified Skirrow’s medium: Columbia blood agar base, 7% horse-lysed blood, and antibiotics (vancomycin, trimethoprim, and polymyxin B)
Campy-BAP: Brucella agar base with antibiotics (trimethoprim, polymyxin B, cephalothin, vancomycin, and amphotericin B) and 10% sheep blood
Blood-free, charcoal-based selective medium: Columbia base with charcoal, hemin, sodium pyruvate, and antibiotics (vancomycin, cefoperazone, and cycloheximide)
Modified charcoal cefoperazone deoxycholate agar (CCDA)
Semisolid motility agar: Mueller-Hinton broth II, agar, cefoperazone, and trimethoprim lactate
Campy-CVA: Brucella agar base with antibiotics (cefoperazone, vancomycin, and amphotericin B) and 5% sheep blood

*Atmosphere can be generated in several ways, including commercially produced, gas-generating envelopes to be used with plastic bags or jars. Evacuation and replacement in plastic bags or anaerobic jars with an atmosphere of 10% CO2, 5% O2, and the balance of nitrogen (N2) is the most cost-effective method, although it is labor intensive.
†All these organisms are susceptible to cephalothin.
‡C. upsaliensis will grow at 42° C but not on cephalothin-containing selective agar.
Approach to Identification
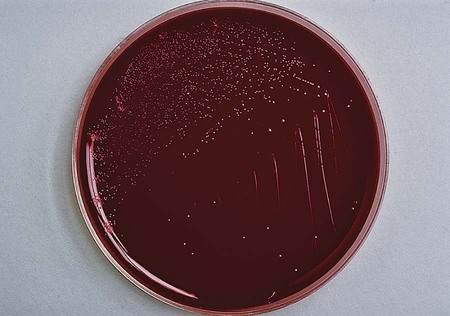
Plates should be examined for characteristic colonies, which are gray to pinkish or yellowish gray and slightly mucoid looking; some colonies may exhibit a tailing effect along the streak line (Figure 34-3). Colony morphology varies with the type of medium used for isolation. Suspicious-looking colonies observed on selective media incubated at 42° C may be presumptively identified as Campylobacter spp., usually C. jejuni or C. coli, with a few basic tests. A wet preparation of the organism in broth may be examined for characteristic darting motility and curved morphology on Gram stain. Both organisms are cephalothin resistant, nalidixic acid sensitive, and sensitive to lysis by complement. C. fetus is incapable of growth at 42°, and optimal growth is 37°; it is cephalothin sensitive, nalidixic acid resistant, and resistant to complement lysis.
Most Campylobacter spp. are asaccharolytic, unable to grow in 3.5% NaCl, although strains of Arcobacter appear more resistant to salt and, except for Arcobacter cryaerophilus, unable to grow in ambient air. Growth in 1% glycine is variable. Susceptibility to nalidixic acid and cephalothin, as previously described (Table 34-3), is determined by inoculating a 5% sheep blood or Mueller-Hinton agar plate with a McFarland 0.5 turbidity suspension of the organism, placing 30-mg disks on the agar surface and incubating in 5% to 10% CO2 at 37° C. Other tests useful for identifying these species are the rapid hippurate hydrolysis test, production of hydrogen sulfide (H2S) in triple sugar iron agar slants, nitrate reduction, and hydrolysis of indoxyl acetate. Indoxyl acetate disks are available commercially. Cellular fatty acid analysis is useful for species identification. This method is not available in routine clinical microbiology laboratories. Several commercial products are available for species identification, including particle agglutination methods and nucleic acid probes.
TABLE 34-3
Differential Characteristics of Clinically Relevant Campylobacter, Arcobacter, and Helicobacter spp.
| Genus and Species | Growth at 25° C | Growth at 42° C | Hippurate Hydrolysis | Catalase | H2S in Triple Sugar Iron Agar | Indoxyl Acetate Hydrolysis | Nitrate to Nitrite | Susceptible to 30-µg Disk Cephalothin | Nalidixic Acid (30 µg) |
| C. coli | − | + | − | + | − | + | + | − | + |
| C. concisus | − | + | − | − | + | − | + | − | − |
| C. curvus* | − | + | − | − | + | + | + | ND | + |
| C. fetus subsp. fetus | + | −/+ | − | + | − | + | + | + | − |
| C. hyointestinalis | +/− | − | − | + | + | − | + | + | − |
| C. jejuni subsp. jejuni | − | + | + | + | − | + | + | − | + |
| C. jejuni subsp. doylei | − | +/− | + | +/− or weak + | − | + | − | + | + |
| C. lari | − | + | − | + | − | − | + | − | − |
| C. rectus* | − | Slight + | − | − | + | + | + | ND | + |
| C. sputorum | − | + | − | −/+ | + | − | + | + | −/+ |
| C. upsaliensis | − | + | − | −/weak + | − | + | + | + | + |
| A. butzleri† | + | − | − | −/weak + | − | + | + | −/+ | +/− |
| A. cryaerophilus‡ | + | − | − | +/− | − | + | + | −/+ | +/− |
| H. cinaedi | − | −/+ | − | + | − | −/+ | + | +/− | + |
| H. fennelliae | − | − | − | + | − | + | − | + | + |
| H. pylori§ | − | + | − | + | − | − | +/− | + | − |
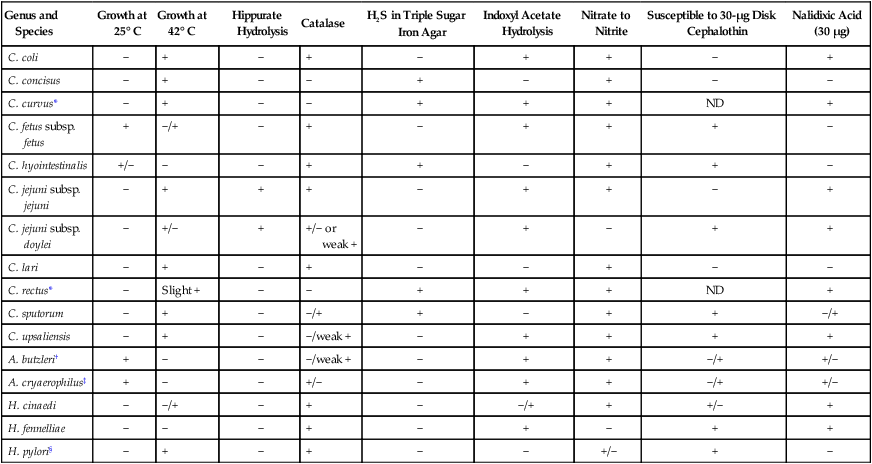
ND, Test not done; +, most strains positive; −, most strains negative; +/−, variable (more often positive); −/+, variable (more often negative).
‡Aerotolerant, not microaerobic; except for a few strains, A. cryaerophilus cannot grow on MacConkey agar, whereas A. butzleri grows on MacConkey agar.
Helicobacter
General Characteristics
Epidemiology and Pathogenesis
H. pylori is capable of colonizing the mucous layer of the antrum and fundus of the stomach but fails to invade the epithelium. Motility allows H. pylori to escape the acidity of the stomach and burrow through and colonize the gastric mucosa in close association with the epithelium. In addition, the organism produces urease that hydrolyzes urea-forming ammonia (NH3) significantly increasing the pH around the site of infection. The change in pH protects the organism from the acidic environment produced by gastric secretions. H. pylori also produces a protein called CagA and injects the protein into the gastric epithelial cells. The protein subsequently affects host cell gene expression inducing cytokine release and altering cell structure, and interactions with neighboring cells enabling H. pylori to successfully invade the gastric epithelium. Individuals who demonstrate positive antibody response to cag protein are at increased risk of developing both peptic ulcer disease and gastric carcinoma. Other possible virulence factors include adhesins for colonization of mucosal surfaces, mediators of inflammation, and a cytotoxin capable of causing damage to host cells (Table 34-4). Although H. pylori is noninvasive, untreated colonization persists despite the host’s immune response.
TABLE 34-4
Genes and Their Possible Role in Enhancing Virulence of H. pylori
| Gene | Possible Role |
| VacA | Exotoxin (VacA) Creates vacuoles in epithelial cells, decreases apoptosis, and loosens cell junctions |
| CagA | Pathogenicity island Encodes a type IV secretion system for transferring CagA proteins into host cells |
| BabA | Encodes outer membrane protein: mediates adherence to blood group antigens on the surface of gastric epithelial cells |
| IceA | Presence associated with peptic ulcer disease in some populations |
Spectrum of Disease
Laboratory Diagnosis
Specimen Collection, Transport, and Processing
Approach to Identification
Colonies of Helicobacter spp. may require 4 to 7 days of incubation before small, translucent, circular colonies are observed. Organisms are identified presumptively as Helicobacter pylori by the typical cellular morphology and positive results for oxidase, catalase, and rapid urease tests. H. pylori, H. cinaedi, and H. fennelliae are definitively identified by using a similar approach to Campylobacter spp. (see Table 34-3).