Chapter 44 Calcium-Regulating Hormones and Other Agents Affecting Bone
| Abbreviations | |
|---|---|
| EDTA | Ethylenediamine tetraacetic acid |
| GI | Gastrointestinal |
| OPG | Osteoprotegerin |
| PTH | Parathyroid hormone |
| RANK | Receptor activator of nuclear factor-κB |
| RANKL | Receptor activator of nuclear factor-κB ligand |
| SERM | Selective estrogen receptor modulator |
Therapeutic Overview
The primary sites of regulation of Ca++ levels are the kidney, gastrointestinal (GI) tract, and bone (Fig. 44-1). The GI tract can normally absorb 10% to 20% of dietary Ca++, and effectiveness is directly dependent on vitamin D levels. Renal tubular reabsorption is highly efficient (99%) and recovers 10 to 20 gm of Ca++ filtered per day. Skeletal bone is the major site of Ca++ storage, containing approximately 1 kg in a 70-kg human. Of this, more than 99% is normally in a stable state, and 1% is in an exchangeable pool that turns over at a rate of approximately 20 g of Ca++ per day.
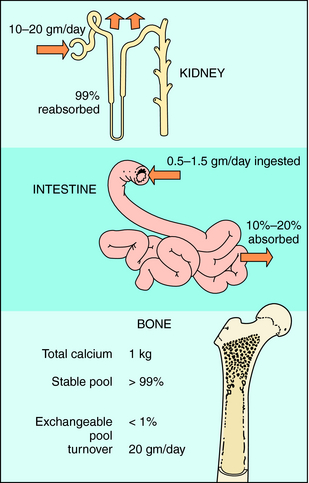
FIGURE 44–1 Sites of Ca++ regulation. Bone is the primary storage site, containing approximately 1 kg of Ca++.
associated with Ca++ or bone metabolism are dependent on the cause and severity of the disease and are summarized in the Therapeutic Overview Box (See page 501).
Hypercalcemia can result from a variety of diverse disorders including primary hyperparathyroidism, hyperparathyroidism caused by chronic renal disease, PTH-secreting parathyroid carcinoma, PTH-related protein producing-malignancy (bronchogenic carcinoma), and bone-wasting neoplasia. Management of mild hypercalcemia (10.5 to 11.4 mg/dL) usually involves dietary restriction of Ca++ and maintenance of hydration. Moderate hypercalcemia (11.5 to 14 mg/dL) has the same considerations as the mild form but requires a more aggressive and timely management plan. Specifically, it is necessary to rapidly reduce blood Ca++ levels using saline infusion and a diuretic, if renal function is intact, or dialysis, if renal function is impaired. The loop diuretics such as furosemide increase both Ca++ and Na+ excretion (see Chapter 21). Severe hypercalcemia (> 14 mg/dL) is a life-threatening condition often involving serious bone or renal pathology and requires immediate and intensive treatment. High Ca++ levels, dehydration, and volume depletion must be addressed. To rapidly reduce Ca++, intravenous administration of bisphosphonates with or without calcitonin is the safest option. Other agents and approaches have been used but are associated with frequent and severe side effects that reduce their desirability.
| Therapeutic Overview |
|---|
| Hypocalcemia |
| Disorders: |
| Hypoparathyroidism; pseudohypoparathyroidism; renal failure; inadequate calcium intake or absorption; abnormal vitamin D metabolism, ingestion, or absorption; tissue resistance |
| Management: |
| Soluble Ca++ salts and/or vitamin D or its analogs |
| Hypercalcemia |
| Disorders: |
| Hyperparathyroidism, hypervitaminosis D, neoplasia, sarcoidosis, hyperthyroidism |
| Management (based on cause and severity): |
| Mild hypercalcemia: dietary restriction of calcium |
| Moderate hypercalcemia: loop diuretics and intravenous saline |
| Severe hypercalcemia: intravenous bisphosphonates, replace phosphate as needed, calcitonin, glucocorticoids |
| Abnormal Bone Remodeling |
| Disorders: |
| Paget’s disease of bone, rickets (osteomalacia), drug-induced, osteopenia, osteoporosis |
| Management: |
| Oral/intravenous bisphosphonates, calcitonin, Ca++, vitamin D, selective estrogen receptor modulators (SERMs) teriparatide |
Mechanisms of Action
Vitamin D, Metabolites, and Analogs
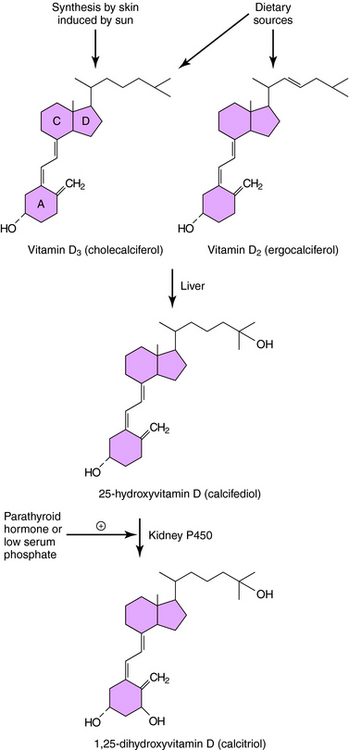
The structure and metabolism of vitamin D is shown in Figure 44-2. Vitamin D is a secosteroid, a steroid in which the B ring is cleaved and the A ring rotated. Vitamin D3, cholecalciferol, is the natural form of vitamin D in humans and is synthesized from cholesterol in the skin in response to solar ultraviolet light. Vitamin D2, ergocalciferol, is the plant-derived form of vitamin D; both vitamins D2 and D3 are present in the diet and equally effective in adults. The activation of vitamin D requires enzymatic hydroxylation by the liver and the kidney. In the endoplasmic reticulum and mitochondria of the liver, vitamin D is hydroxylated to form 25-hydroxyvitamin D (calcifediol), which becomes the primary circulating metabolite. Renal metabolism of 25-hydroxyvitamin D to 1,25-dihydroxyvitamin D (calcitriol) involves mitochondrial P450-catalyzed hydroxylation by the enzyme 1α-hydroxylase (CYP27B1), whose activity is stimulated by PTH and low plasma PO4 concentrations.
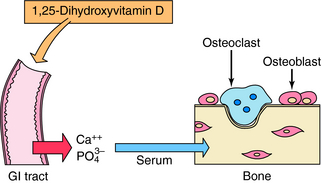
Calcitriol and active vitamin D analogs bind primarily to nuclear receptors in target cells and act as ligand-activated transcription factors by binding to response elements on genes and modulating synthesis of specific proteins. Among the protein products resulting from actions of vitamin D on the intestine are two high-affinity Ca++-binding proteins, the calbindins, which play a role in stimulation of intestinal Ca++ transport. Vitamin D metabolites increase absorption of dietary Ca++ and PO4−3 by stimulating uptake across the GI mucosa, leading to an increase in serum Ca++ concentration (Fig. 44-3). The antirachitic effect of vitamin D on bone mineralization is an indirect result of this increased Ca++ and PO4−3 absorption, which also results in deposition of more mineral in bone.
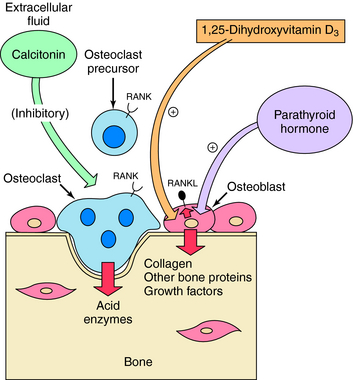
Vitamin D metabolites, especially at higher concentrations, stimulate the release of Ca++ from bone. The synthesis of a membrane-associated cytokine, receptor activator of nuclear factor-κB ligand (RANKL), is activated. Interaction of RANKL with receptor activator of nuclear factor-κB (RANK) receptors on osteoclasts stimulates osteoclast differentiation, survival, and activity, resulting in Ca++ release (Fig. 44-4). A decoy receptor, osteoprotegerin (OPG), is produced by bone marrow stromal cells and can competitively antagonize the effects of RANKL. Increased RANKL is a general mechanism by which many factors, including PTH, prostaglandins, and inflammatory cytokines, stimulate bone resorption. Vitamin D metabolites inhibit PTH synthesis and secretion. Vitamin D also affects differentiation of other cell types, including keratinocytes.
Several synthetic vitamin D analogs have unique clinical utility. 1α-Hydroxyvitamin D2 and dihydrotachysterol (in which the A ring is not rotated) are active even in the absence of renal 1α-hydroxylase. 19-Nor-1α, 25-hydroxyvitamin D2 (paricalcitol), and calcipotriene have less effect on Ca++ metabolism and can therefore be used for other therapeutic indications to decrease the risk of hypercalcemia. Paricalcitol is used to suppress elevated PTH secretion in chronic renal disease, and calcipotriene is used to promote normal skin cell differentiation in psoriasis.
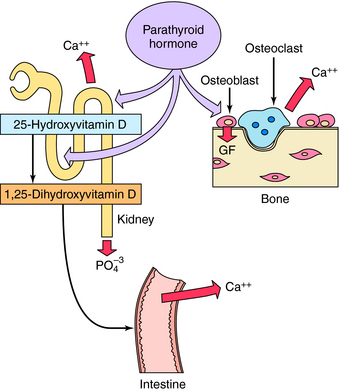
PTH is an 84-amino acid polypeptide formed from the cleavage of larger precursors in the parathyroid gland. The first 34 amino acids of PTH (PTH 1-34) possess the full effects of the peptide on bone and Ca++ metabolism. Calcium exerts negative feedback regulation of PTH secretion through a specific G-protein-linked Ca++ membrane receptor. PTH binding to target cell receptors stimulates protein phosphorylation by both protein kinase A and specific protein kinase C isozymes. PTH has multiple effects that influence Ca++ and PO4−3 metabolism and bone (Fig. 44-5). PTH acts directly at renal tubules to decrease reabsorption of PO4−3 and increase reabsorption of Ca++, resulting in decreased serum PO4−3 and increased serum Ca++ concentrations. PTH interaction with receptors on osteoblasts, analogous to vitamin D metabolites, increases production of RANKL, stimulating resorption and increasing serum Ca++ concentrations. When PTH is administered intermittently to attain subphysiological levels, an anabolic effect on bone has been reported, leading to approval by the United Sstates. Food and Drug Administration of teriparatide (recombinant human PTH 1-34), which is administered by injection to promote bone formation. This anabolic effect on bone may involve growth factors such as insulin-like growth factor-1 that promote interaction with osteoblasts. Teriparatide-injection is approved to treat severe osteoporosis for patients in whom antiresorptive agents have failed to prevent recurrent fractures. PTH enhances Ca++ absorption indirectly by stimulating the formation of 1,25-dihydroxyvitamin D (see Fig. 44-2). A recently discovered protein, the PTH-related protein, has significant amino acid sequence homology to PTH at the N-terminal region of the molecule. PTH-related protein is produced by several types of tumors, is important in malignancy-related hypercalcemias, in normal Ca++ metabolism in mammary gland and placenta, and in chondrocyte differentiation.
Calcitonin is a 32-amino acid polypeptide secreted by the parafollicular cells of the thyroid. It decreases postprandial absorption of Ca++ and increases excretion of Ca++, Na+, Mg++, Cl−, and PO4−3. At a cellular level, it inhibits the activity of osteoclasts by direct actions on G-protein-linked receptors in these cells (see Fig. 44-4). The inhibition of osteoclast activity results in a decrease in both serum Ca++ and PO4−3. The ability of calcitonin to decrease hypercalcemia diminishes with continued use. Calcitonin is effective in treatment of Paget’s disease of bone and is also approved for treatment of osteoporosis. Calcitonin and the neuronal calcitonin gene-related peptide arise from differential splicing in the parafollicular cells and in neural tissue.
Estrogens and Selective Estrogen Receptor Modulators
Estrogens (see Chapter 40) inhibit bone resorption and prevent fractures. The mechanism appears to involve decreased production of interleukins that activate and promote survival of osteoclasts. Consistent with this protective effect, reduction or inhibition of estrogen production can produce osteopenia and eventually osteoporosis. These situations commonly occur as a result of ovarian failure, ovariectomy, chronic suppression with long-acting gonadotropin-releasing hormone agonists, and natural menopause. Estrogen supplementation or replacement is indicated in premenopausal women to delay the onset of osteopenia and osteoporosis. Women undergoing normal menopause have also been considered candidates for estrogen supplementation to reduce vasomotor symptoms and delay osteopenia and osteoporosis. Women with an intact uterus are coadministered a progestin to reduce the incidence of endometrial cancer. Also, a personal or familial history of cardiovascular disease or estrogen-dependent breast or endometrial cancer usually leads to exclusion from estrogen treatment. The use of estrogen in postmenopausal women has drastically declined following problems observed in studies from the Women’s Health Initiative and Heart and Estrogen Replacement Study (see Chapter 40). Other studies have shown that low doses of conjugated estrogens and micronized estradiol are effective in maintaining bone mineral density. However, as a result of controversies related to estrogen supplementation, other agents are being used to delay bone loss and reduce vasomotor symptoms. Although SERMs such as raloxifene do not significantly reduce vasomotor symptoms, they do have estrogenic effects on bone and have been approved for prevention and treatment of osteoporosis. SERMs are partial antagonists of estrogen on the mammary gland and uterus, possibly because of differential interactions with tissue-specific stimulatory and inhibitory cofactors (see Chapter 40).
Other Agents Affecting Calcium Metabolism and Bone Formation
The mechanisms of action of compounds affecting Ca++ and bone metabolism are summarized in Table 44-1.
TABLE 44–1 Mechanisms of Action of Agents Altering Bone and Ca++ Metabolism
| Mechanism | Agents |
|---|---|
| Increase intestinal Ca++ absorption | Vitamin D metabolites |
| PTH (indirect) | |
| Increase renal Ca++ excretion | Na+ sulfate, EDTA* |
| Loop diuretics | |
| Calcitonin | |
| Increase bone reabsorption | PTH |
| Vitamin D metabolites | |
| Increase bone formation | Teriparatide (intermittent injection to achieve low PTH levels) |
| Second-generation bisphosphonates | |
| Decrease intestinal Ca++ absorption | Glucocorticoids |
| Calcitonin (postprandial) | |
| Decrease renal Ca++ excretion | PTH |
| Decrease bone resorption | Bisphosphonates |
| Calcitonin | |
| Estrogens* | |
| SERMs |
* Agent is less commonly used because of high risk-to-benefit ratio compared with other available drugs.
Pharmacokinetics
Vitamin D, 25-hydroxyvitamin D (calcifediol), and 1,25-dihydroxyvitamin D (calcitriol) are absorbed rapidly after oral administration (Table 44-2). Bile salts are required, however, and absorption is impaired in patients with biliary cirrhosis. Absorption is also decreased during steatorrhea or an excessive loss of fat in the feces. Vitamin D compounds circulate bound to a specific vitamin D-binding protein, a slightly acidic monomeric glycoprotein synthesized in liver, and are metabolized to inactive glucuronides. It has been proposed that 24,25-dihydroxyvitamin D may have unique mineralization properties, although this is not well established. Clearly, 1,24,25-trihydroxyvitamin D is less active than its precursor, 1,25-dihydroxyvitamin D. Vitamin D is stored for long periods in liver, fat, and muscle.
Estrogens and Selective Estrogen Receptor Modulators
The estrogens used for prevention of osteoporosis include conjugated estrogens and micronized estrogen. Their pharmacokinetics and those of the SERMs are discussed in Chapter 40.
Relationship of Mechanisms of Action to Clinical Response
Vitamin D and its active metabolites are used primarily for the treatment of rickets, osteomalacia, and hypocalcemia. The actions of vitamin D compounds to increase Ca++ absorption form the basis for their antirachitic activity. Their effects on Ca++ release from bone likely contribute to their hypercalcemic effect. Use of calcifediol and calcitriol is logical if a defect in the formation of these metabolites is present. Alternatively, larger doses of the precursor can be given to produce sufficient concentrations of active metabolites to increase serum Ca++. Therefore doses of vitamin D more than 10 times greater than those used for simple replacement therapy are used to treat hypoparathyroidism or vitamin D-resistant rickets. Calcipotriol, applied topically, can promote keratinocyte differentiation without affecting Ca++ metabolism and is used for the treatment of psoriasis. 19-Nor-1α,25-dihydroxyvitamin D2 can suppress the parathyroid glands with minimal hypercalcemic effects and is used to inhibit PTH secretion for the treatment of renal osteodystrophy, where impaired renal function and hydroxylation of vitamin D result in secondary hyperparathyroidism.
Anonymous. A once-yearly IV bisphosphonate for osteoporosis. Med Lett Drugs Ther. 2007;49:89-90.
Anonymous. Drugs for postmenopausal osteoporosis. Treat Guidel Med Lett Drugs Ther. 2008;6:67-74.
Anonymous. Teriparatide (forteo) for osteoporosis Treat Guidel Med Lett 2008; 6:67-74. Med Lett Drugs Ther. 2003;45:9-11.
Jackson RD, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354:669-683. 750
Khosla S, Melton LJ. Osteopenia. N Engl J Med. 2007;356:2293-2300.
Rosen CJ. Postmenopausal osteoporosis. N Engl J Med. 2005;353:595-603.
Seeman E, Delmas PD. Bone quality-the material and structural basis of bone strength and fragility. N Engl J Med. 2006;354:2250-2261.