Burns
Since the late 1980s, trends of burn incidence, hospitalization, and death have all decreased.1,2 These decreases are attributed to fire and burn prevention education, management of burn patients in specialized burn centers, regulation of consumer products, and implementation of occupational safety standards. Many programs and organizations are dedicated to the prevention of burn injury. Societal changes involving decreased smoking and alcohol abuse, changes in home cooking practices, and reduced industrial employment also have contributed to the decline in burn incidence. However, in spite of a decline in overall burn incidence, because of an increase in child maltreatment (3.3 million referrals in 2010), burn injury as a result of physical abuse remains a differential diagnosis in pediatric burn patients.3
Burn injuries that require medical treatment annually number 450,000 per year.2 Burn injuries requiring hospitalization total approximately 45,000 per year, and 55% of these patients are admitted to one of the 125 specialized burn centers in the United States.2
Great advances have been made in the care of burn patients. In the mid-twentieth century, burn shock claimed many patients’ lives. If shock did not cause death, infection or respiratory insufficiency did. With improvements in fluid resuscitation, better critical care management, and the trend toward early excision and grafting, mortality rates have decreased. Focusing management on early eschar excision in patients with deeper, larger burns decreases wound sepsis, hypercatabolism, number of operations, and lengths of hospital stay.4
Anatomy and Functions of the Skin
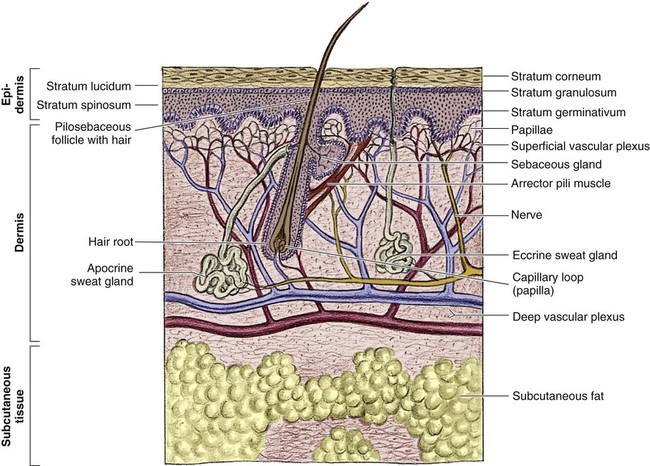
The skin is the largest organ of the human body, ranging from 0.2 meter squared (m2) in the newborn to more than 2 m2 in the adult. The integumentary system consists of two major layers: (1) epidermis and (2) dermis (Fig. 36-1).
The outermost layer of the epidermis is 0.07 to 0.12 millimeters (mm) thick, with the deepest layer found on the soles of the feet and the palms of the hands. The epidermis is composed of dead, cornified cells that act as a tough protective barrier against the environment. It serves as a barrier to bacteria and moisture loss.5 From the surface inward, its five layers are (1) stratum corneum, (2) stratum lucidum, (3) stratum granulosum, (4) stratum spinosum, and (5) stratum germinativum. The deepest layer of the epidermis contains fibronectin, which adheres the epidermis to the basement membrane. The epidermis regenerates every 2 to 3 weeks. The second, thicker layer, the dermis, is 1 to 2 mm thick and lies below the epidermis and regenerates continuously. The dermis is composed of two layers: (1) the more superficial, papillary layer next to the stratum germinativum and (2) the deeper, reticular layer. The dermis, composed primarily of connective tissue and collagenous fiber bundles made from fibroblasts, provides nutritional support to the epidermis. The dermis contains blood vessels; sweat and sebaceous glands; hair follicles; nerves to the skin and capillaries that nourish the avascular epidermis; and sensory fibers that detect pain, touch, and temperature. Mast cells in the connective tissue perform the functions of secretion, phagocytosis, and production of fibroblasts. Beneath the dermis is the hypodermis, which contains fat, smooth muscle, and areolar tissues. The hypodermis acts as a heat insulator, shock absorber, and nutritional depot.6
Pathophysiology and Etiology of Burn Injury
A burn injury results in tissue loss or damage. Injury to tissue can be caused by exposure to thermal, electrical, chemical, or radiation sources. The temperature or causticity of the burning agent and duration of tissue contact with the source determine the extent of tissue injury. Tissue damage can occur at various temperatures, usually between 40° C (104° F) and 44° C (111.2° F). The burn wound itself is responsible for the local and systemic effects seen in the burned patient.7 Tissue damage is caused by enzyme malfunction and denaturation of proteins. Prolonged exposure or higher temperatures can lead to cell necrosis and a process known as protein coagulation. The areas extending outward from this central area of injury sustain various degrees of damage and are identified by zones of injury.8
Zones of Injury
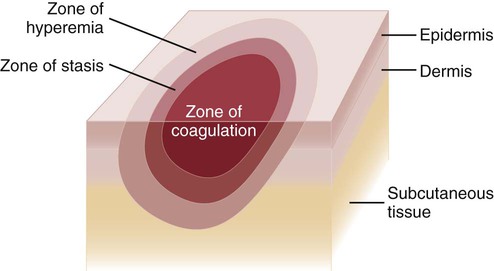
Three concentric zones are present in burn injury: (1) zone of coagulation, (2) zone of stasis, and (3) zone of hyperemia (Fig. 36-2). The central zone, or zone of coagulation, is the site of most severe damage, and the peripheral zone is the least. The central zone is usually the site of greatest heat transfer, leading to irreversible skin death. This area is surrounded by the zone of stasis, which is characterized by impaired circulation that can lead to cessation of blood flow caused by a pronounced inflammatory reaction. This area is potentially salvageable; however, local or systemic factors can convert it into a full-thickness injury. Some of the factors that can lead to deeper wound conversion are toxic mediators of the inflammatory process, infection, inappropriate volume resuscitation, malnutrition, chronic illness, or the local wound care provided. It may take 48 to 72 hours to determine the full extent of injury in this area. The outermost area, the zone of hyperemia, has vasodilation and increased blood flow but minimal cell involvement. Early spontaneous recovery can occur in this area.7,8
Classification of Burn Injury
Burns are classified primarily according to the size and depth of injury. However, the type and location of the burn and the patient’s age and medical history are also significant considerations. Recognition of the magnitude of burn injury, which is based on the above-mentioned factors, is of crucial importance in the overall plan of care and in decisions concerning patient management and appropriate referral to a burn center (Box 36-1).2 The patient’s age, burn size, and inhalation injury are the cardinal determinants of survival.9 A recent study of a large number of burn patients found the following factors to be the biggest predictors of patient mortality: age, percent total body surface area (TBSA), inhalation injury, coexistent trauma, pre-existing disease, and acute organ system failure.9,10
Size of Injury
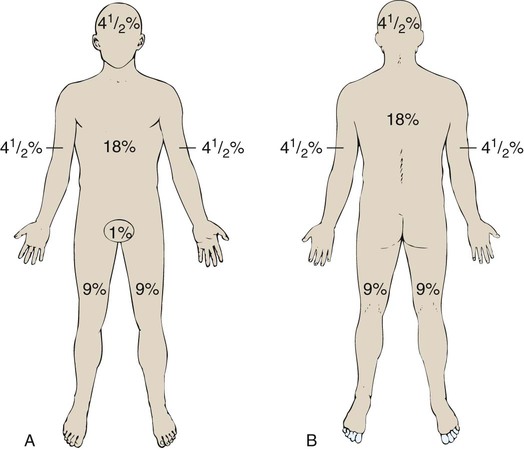
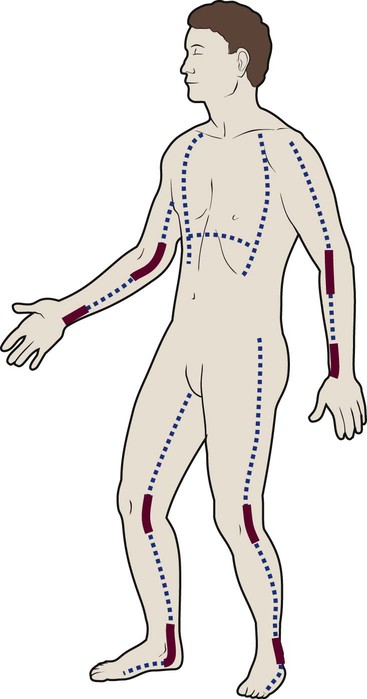
Several different methods can be used to estimate the size of the burn area. A quick and easy method is the rule of nines (or Berkow formula), which often is used in the prehospital setting for initial triage of the burn patient (Fig. 36-3). In this method, the adult body is divided into different surface areas of 9% per area. This method is modified for assessing infants and very small children and accounts for proportionate growth. For example, a 1-year-old has a head that is proportionately larger than the rest of the body, unlike in adults, so the child’s head would account for 19% of his or her TBSA, whereas the head of an adult would account for 7% of TBSA.5 Designated burn centers have access to Berkow formula charts, but local hospitals may not.
Another method uses the measure of the palmar surface of the victim’s hand as a gauge for estimating burn area. The palmar surface (fingertip to wrist), which represents 1% of the TBSA, also can be useful in making burn estimates in the prehospital setting or for estimating the percentage of involvement in small and scattered areas of burn. Some studies have shown the adult hand’s TBSA is calculated to be closer to 0.8% versus 1% in a child.5
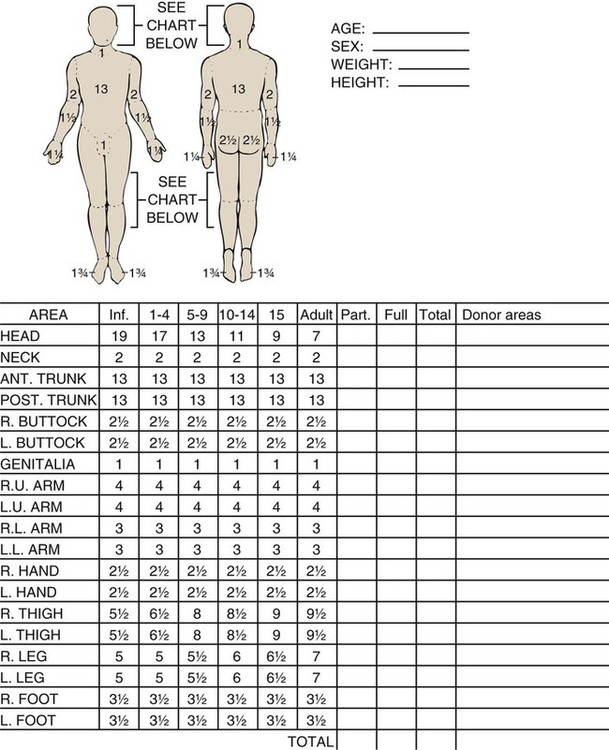
In the hospital setting, the Lund and Browder method (Fig. 36-4) is the most accurate and accepted method for determining the percentage of burn. Surface area measurements are assigned to each body part in terms of the age of the patient. This method is highly recommended for use with children younger than 10 years because it corrects for smaller surface areas of the lower extremities. It is also recommended for adult burn victims because of its accuracy.
Depth of Burn Injury
Traditionally, burn depth has been classified in degrees of injury based on the amount of injured epidermis, dermis, or both: first-degree, second-degree, third-degree, or fourth-degree burns. However, these terms are not descriptive of the burn surface. The depth of the burn is defined by how much of the skin’s two layers are destroyed by the heat source.10
Burns are classified as superficial, partial-thickness, deep partial-thickness, or full-thickness burns. These descriptions are based on the surface appearance of the wound. Superficial burns include first-degree burns. Partial-thickness wounds include various stages of second-degree burns, and full-thickness burns include third-degree burns. Four-degree burns extend through the skin to the subcutaneous fat, muscle, and even bone.10 Some authorities further separate partial-thickness burns as superficial, mid-partial, or deep-partial-thickness burns. Wound assessment involves recognition of the depth of injury and the size of burn, and it can be challenging even for experienced caregivers. Because the management of burn wounds is closely tied to the correct assessment of wound severity, some newer strategies, other than observation, are being investigated. Some of these techniques have taken into account the presence of denatured collagen, wound edema, and altered blood flow. One such technique is noncontact laser Doppler imaging, which can give a color perfusion map of the burn wound.11 Other techniques of assessing burn depth include use of vital dyes, ultrasonography, thermography, and magnetic resonance imaging.8 Research with these techniques is ongoing, but they are currently limited by their clinical usefulness at the bedside.
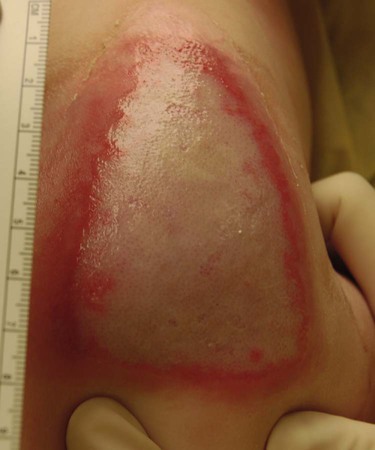
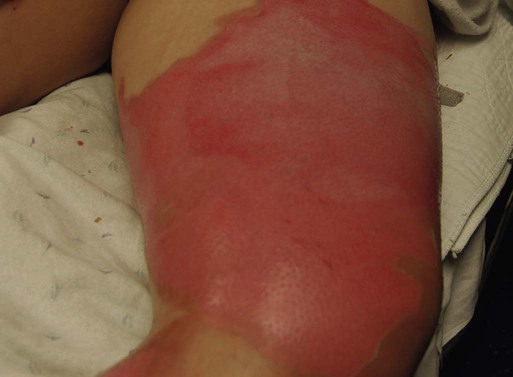
A partial-thickness (second-degree) burn involves all of the epidermis and part of underlying dermis.5 These burns usually are caused by brief contact with flames, hot liquid, or exposure to dilute chemicals (Fig. 36-5). A light to bright red or mottled appearance characterizes superficial second-degree burns. These wounds may appear wet and weeping, may contain bullae, and are extremely painful and sensitive to air currents. These burns blanch painfully.5 The microvessels that perfuse this area are injured, and permeability is increased, resulting in leakage of large amounts of plasma into the interstitium. This fluid lifts off the thin, damaged epidermis, causing blister formation. Despite the loss of the entire basal layer of the epidermis, a burn of this depth will heal in 7 to 21 days. Minimal scarring can be expected. Mid-dermal partial-thickness wounds commonly take 4 to 6 weeks to heal.
Deep-dermal partial-thickness (second-degree) burns involve the entire epidermal layer and deeper layers of the dermis.5 These burns often result from contact with hot liquids or solids or with intense radiant energy. A deep-dermal partial-thickness burn usually is not characterized by blister formation. Only a modest plasma surface leakage occurs because of severe impairment in blood supply. The wound surface usually is red with patchy white areas that blanch with pressure. The appearance of the deep-dermal wound changes over time. Dermal necrosis and surface coagulated protein turn the wound from white to yellow (Fig. 36-6). These wounds have a prolonged healing time. They can heal spontaneously as the epidermal elements germinate and migrate until the epidermal surface is restored, or they may require a skin substitute or surgical excision and grafting for wound closure. This process of healing by epithelialization can take up to 6 weeks. Left untreated, these wounds can heal primarily with unstable epithelium, late hypertrophic scarring, and marked contracture formation.5 Partial-thickness injuries can become full-thickness injuries if they become infected, if blood supply is diminished, or if further trauma occurs to the site. The treatment of choice is surgical excision and skin grafting.
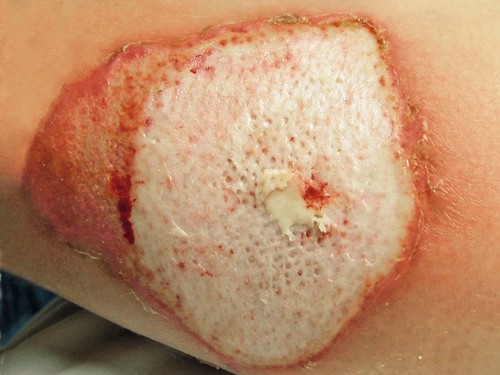
A full-thickness (third-degree) burn involves destruction of all the layers of the skin down to and including the subcutaneous tissue (Fig. 36-7). The subcutaneous tissue is composed of adipose tissue, includes the hair follicles and sweat glands, and is poorly vascularized. A full-thickness burn appears pale white or charred, red or brown, and leathery. The surface of the burn may be dry, and if the skin is broken, fat may be exposed. Full-thickness burns usually are painless and insensitive to palpation. Because all of the epithelial elements are destroyed, the wound will not heal by re-epithelialization. Wound closure of small full-thickness burns (<4 centimeters squared [cm2] area) can be achieved with healing by contraction. All other full-thickness wounds require skin grafting for closure. Extensive full-thickness wounds leave the patient extremely susceptible to infections, fluid and electrolyte imbalances, alterations in thermoregulation, and metabolic disturbances.
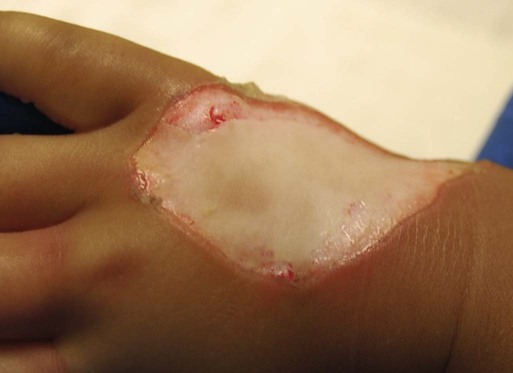
The exact depth of many burn wounds cannot be clearly defined on the first inspection, and many burn wounds may contain superficial, mid-dermal, and deep-dermal wounds. A major difficulty is distinguishing deep-dermal partial-thickness from full-thickness injury. It is important to identify the depth of injury for appropriate treatment. Deep, partial-thickness wounds that will not heal within a relatively short time are treated with wound excision and grafting. Burn wounds can evolve over time, and they require frequent reassessment. Special consideration must always be given to very young and older patients because of their thin dermal layer. Older adults may also have reduced sensation and blood supply, causing them to be more susceptible to a full-thickness injury. Burn injuries in these age groups may be more severe than they initially appear.12
Types of Injury
Thermal Burns
The most common type of burn is a thermal burn caused by steam, scalds, contact with heat, and fire injuries. About 80% of burns in children are caused by scalds (i.e., contact with hot objects or liquids).5 Toddlers are most often affected by scalding burns. Contact burns are also common. The length of time the hot object is in contact with the skin determines the depth of injury. Flame injuries are often associated with other trauma to the patient. The highest incidence of flame injuries are more common in patients older than 6 years of age.5 Experimentation with lighters, lighter fluid, fire, firecrackers, and gasoline is more common in children age 6 to 16 years.5 Contact and flame burns tend to be deep-dermal or full-thickness injuries.
Electrical Burns
Electrical and lightning injuries result in 1000 deaths per year in the United States. Electrical injuries count for 4% of burn patient admissions to burn centers.2 Low-voltage (alternating) current or high-voltage (alternating or direct) current, which is greater than 1000 volts, can cause electrical burns.4 Children have the highest incidence of electrical injury. These accidents occur as a result of insertion of an object into an outlet or by biting or sucking on an electrical cord. Pediatric electrical burns occur most often in the home, whereas in adults, electrical injuries occur in the workplace.13 Common situations that may increase the risk for electrical injuries include occupational exposure and accidents involving household current.
Radiation Burns
Burns associated with radiation exposure are uncommon. Radiation burns usually are localized and indicate high radiation doses to the affected area. Radiation burns may appear identical to thermal burns.14 The major difference is the time between exposure and clinical manifestation; it can be days to weeks, depending on the level of the radiation dose. Radiation injury can occur with exposure to industrial equipment such as accelerators and cyclotrons and to equipment used for medical treatment.
Location of Injury
Location of injury can be a determining factor in differentiating the level of care required. According to triage criteria from the American College of Surgeons, burns on the face, hands, feet, genitalia, major joints, and perineum are best treated in a burn center. These burns involve functional areas of the body and often require specialized intervention (Fig. 36-8). Injuries to these areas can result in significant long-term morbidity from impaired function and altered appearance.
Patient Age and History
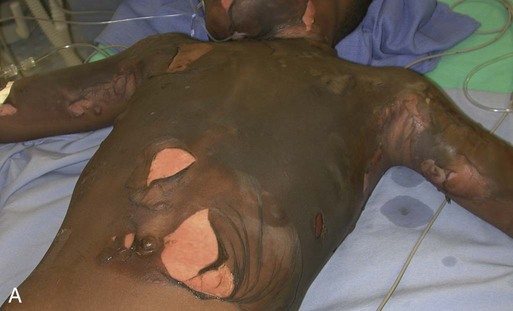
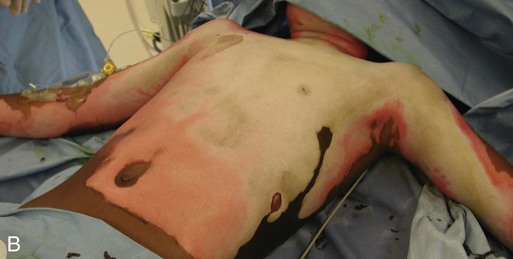
Patient age and history are significant determinants of survival. Patients considered most at risk are those younger than 2 years and those older than 60 years. History of inhalation injury and electrical burns and all burns complicated by trauma and fractures (considered major injuries) significantly increase the risk for death. Obtaining the patient’s medical history is important, particularly a history related to cardiac, pulmonary, or kidney dysfunction; diabetes; and central nervous system (CNS) disorders. In evaluating the pediatric patient, it is essential to obtain a thorough history, especially for the nonverbal patient. Attention should be paid to the description of the burn event to rule out nonaccidental trauma (Fig. 36-9). Social services such as child protective services and the police should be consulted if abuse or neglect is suspected.
Child Abuse
According to the U.S. Department of Health and Human Services Child Maltreatment 2010 report, 3.3 million referrals were made for child maltreatment, and of those, 436,321 were investigated and substantiated for abuse.15 According to this report, 1560 children died from abuse and neglect. Child abuse or maltreatment should be a differential diagnosis when caring for children with burn injury, since greater than 15% of children suffering from maltreatment suffer from physical abuse.15 Most often burns in children are accidental but are related to failure of the caregiver or parent to provide adequate supervision. However, the developmental age and size of the child are of key importance in ruling out accidental and nonaccidental burn injuries. The patterns of burns, that is, inflicted burns consistent with immersion or dunking, can be diagnostic of child abuse regardless of history.16
Initial Emergency Burn Management
The goals of acute care of the patient with thermal injuries are to save life, minimize disability, and prepare the patient for definitive care. The burn injury may involve multiple organ systems, and the approach to the injured patient should be expeditious and methodical in identifying problems and establishing priorities of care.17
Airway Management
The first priority of emergency burn care is to secure and protect the airway. If there is any possibility of underlying cervical instability, cervical precautions must be initiated.17 In patients with facial burns, exposure to fire in an enclosed space, or both, inhalation injury should be suspected. Carbon monoxide (CO) poisoning is associated with high mortality rates. Carboxyhemoglobin (HbCO) levels are obtained, and oxygen therapy is initiated. All patients with major burns or suspected inhalation injury are initially administered 100% oxygen.17 The nurse should continue to observe the patient for clinical manifestations of impaired oxygenation such as tachypnea, agitation, anxiety, and upper airway obstruction (e.g., hoarseness, stridor, wheezing). Early intubation may save the life of the patient who has an inhalation injury because it may be impossible to perform this procedure later, when edema has obstructed the larynx. The need for frequent blood sampling and the benefit of continuous blood pressure monitoring may necessitate placement of an arterial line.
Circulatory Management
The extent and depth of the burn are assessed. The extent of TBSA of the burn is calculated for estimation of fluid resuscitation requirements (Table 36-1); the Parkland formula is the most widely used method of calculation (see Box 36-5, Case Study: Patient with Burns at the end of the chapter). Burn shock is caused by the loss of fluid from the vascular compartment into the area of injury resulting in hypovolemia. The larger the percentage of burn area, the greater is the potential for development of shock. Lactated Ringer solution is infused through a large-bore cannula in a peripheral vein. Lactated Ringer solution, an isotonic crystalloid, is the resuscitation fluid used most often. Given in large amounts, it can restore cardiac output to normal in most patients. It is preferred over normal saline because it most closely matches extracellular fluid. Because isotonic salt solutions generate no difference in osmotic pressure between plasma and the interstitial space, the entire extracellular space must be expanded to replace intravascular losses. Diuretics should not be given during the resuscitative phase of burn care.
TABLE 36-1
FORMULAS FOR FLUID REPLACEMENT OR RESUCITATION IN FIRST 24 HOURS
| FLUID AND DOSE RATE | ABA CONSENSUS | PARKLAND | MODIFIED BROOKE | BROOKE | HYPERTONIC |
| Electrolyte solution | Ringer lactate | Ringer lactate | Ringer lactate | Ringer lactate | Hypertonic lactated saline (sodium, 250 mEq/L) |
| Dose: mL/kg/% burned* | 2-4 50% of fluid over first 8 hr; 50% of fluid over next 16 hr |
4 | 2 | 1.5 | Rate based on urine output of 30-50 mL/hr |
| Examples using the ABA consensus formula: an 85-kg patient with 35% TBSA burn | ||
| 2 mL × 85 kg × 35% = 5950 mL in first 24 hours | 3 mL × 85 × 35% = 8925 mL | 4 mL × 85 × 35% = 11,900 mL |
| 2975 mL in first 8 hr = 372 mL/hr | 4462 mL in first 8 hr = 558 mL/hr | 5950 mL in first 8 hr = 744 mL/hr |
| 2975 mL in next 16 hr = 186 mL/hr | 4462 mL in next 16 hr = 279 mL/hr | 5950 mL in next 16 hr = 372 mL/hr |
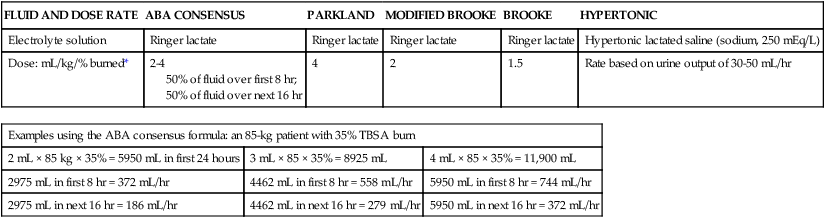
*Adjust these rates to maintain a urine output >30 mL/hr in adults or 1 mL/kg/hr in children.
According to the Parkland formula (see Table 36-1), half of the calculated amount of fluid is administered to the patient in the first 8 hours after injury; 25% is given in the second 8 hours, and 25% is given in the third 8 hours. It is important to remember that calculated fluid requirements are guidelines. Fluid resuscitation is a dynamic process. The rate of fluid administration is adjusted according to the individual’s response, which is determined by monitoring urine output, heart rate, blood pressure, and level of consciousness. Meticulous attention to the patient’s intake and output is imperative to ensure that he or she is appropriately resuscitated. Under-resuscitation may result in inadequate cardiac output, leading to inadequate organ perfusion and the potential for wound conversion from a partial-thickness to full-thickness injury. Over-resuscitation may lead to moderate to severe pulmonary edema, to excessive wound edema causing a decrease in perfusion of unburned tissue in the distal portions of the extremities, or to edema inhibiting perfusion of the zone of stasis, resulting in wound conversion.11 Fluid requirements may be much higher than estimated by using the Parkland formula. The recommendations for these situations are included later in this chapter. For pediatric burn patients, add dextrose containing intravenous fluid at a maintenance rate along with Parkland resuscitation fluid. Pediatric patients need continuous dextrose infusions for vital organs.
Pathophysiology of Burn Shock
Burn injuries greater than 20% of TBSA can result in burn shock.4 Shock is defined as inadequate cellular perfusion. Significant burn injury results in hypovolemic shock and tissue trauma. Both cause the production and release of several local and systemic mediators. Burn shock can occur even when hypovolemia is corrected.
Burn edema has been attributed to several factors. Barrier property changes of the capillary wall occur by direct injury and indirect mediator-modulated changes. Increases in permeability of protein and water occur, resulting in edema. In most forms of shock, capillary pressure decreases as a result of arteriolar vasoconstriction. However, after burn injury, an increase in capillary pressure has been found in burned tissue in the first minutes to hours after injury, causing capillary leak for first 24 hours.4 Coupled with this increase in capillary pressure is a negative interstitial hydrostatic pressure that occurs in the dermis layer of burned skin after thermal injury. This negative interstitial hydrostatic pressure represents an edema-generating mechanism that occurs for approximately 2 hours after injury. Plasma colloid osmotic pressure is decreased as a result of protein leakage into the extravascular space. Plasma is then further diluted with fluid resuscitation. The osmotic pressure is decreased, and further fluid extravasation can occur.
In addition to leaking capillaries, local and systemic mediators cause edema and the cardiovascular problems seen in burn patients. These mediators include histamine, prostaglandins, kinins, and oxygen radicals, they increase arteriolar vasodilation.4 Manipulation of these mediators to stop the cascade of burn edema and burn shock is being researched.
Kidney Management
If fluid resuscitation is inadequate, acute kidney injury (AKI) may occur. An indwelling urinary catheter should be placed for burns greater than 15% to 20% of TBSA in the emergency department to monitor urine output and the effectiveness of fluid resuscitation. A catheter may be necessary if the burn extends into the perineal area because of the presence or development of edema. Urinary catheters with temperature probes should be used whenever possible. The nurse measures urine output hourly. Adequate urine output for adults is 0.5 to 1 milliliter per kilogram per hour (mL/kg/hr), or 30 to 50 mL/hr; in children, it is 1 mL/kg/hr.4
Gastrointestinal System Management
Patients with burns of more than 20% of TBSA are prone to gastric dilation as a result of paralytic ileus. Nasogastric or orogastric tubes are placed in these patients to prevent abdominal distention, emesis, and potential aspiration. This decrease in gastrointestinal (GI) function is caused by the effects of hypovolemia and the neurologic and endocrine response to injury. GI activity usually returns in 24 to 48 hours. Gastric prophylaxis with histamine blockers or sucralfate is initiated because burn patients are prone to Curling stress ulcers. These acute ulcerations of the duodenum are caused by sloughing of the gastric mucosa resulting from loss of plasma volume after severe burns. Enteral nutrition has been shown to be protective of gastric mucosal integrity and to improve intestinal flow, gastric motility, and intestinal blood flow in burned patients.18 Enteral nutrition should be started as soon as possible. Severely burned patients can be safely fed within 6 hours of burn injury in the duodenum or jejunum.18 Care should be taken to start enteral feeds for burn patients via nasoduodenal or nasojejunal tube promptly.
Extremity Pulse Assessment
Edema formation may cause neurovascular compromise to the extremities; frequent assessments are necessary to evaluate pulses, skin color, capillary refill, and sensation. Arterial circulation is at greatest risk with circumferential burns. If not corrected, reduced arterial flow causes ischemia and necrosis. The Doppler flow probe is one of the best ways to evaluate arterial pulses. An escharotomy may be required to restore arterial circulation and to allow for further swelling. The escharotomy can be performed at the bedside with a sterile field and scalpel. Care must be taken to avoid major nerves, vessels, and tendons. The incision extends through the length of the eschar, over joints, and down to the subcutaneous fat. The incision is placed laterally or medially on the extremity. If a single incision does not restore circulation, bilateral incisions are required (see Fig. 36-8). If escharotomy is required before the patient is transferred to a burn center, consultation with the receiving physician is advised.
Wound Care
After the wounds have been assessed, topical antimicrobial therapy is not a priority during emergency care. However, the wounds must be covered with clean, dry dressings or sheets. Every attempt must be made to keep the patient warm because of the high risk of hypothermia. The administration of tetanus prophylaxis is recommended for all burns covering more than 10% of the TBSA and for patients with a questionable immunization history.10
Burn Center Referral
After initial treatment and stabilization at an emergency department, referral to a burn center is considered (see Box 36-1). A burn center must be able to deliver all therapy required, including rehabilitation, and must perform personnel training and burn research.17 Patients meeting the criteria for referral need the expertise of a multidisciplinary team. Referring hospitals must always contact the burn center in their region.2 Providers in the field should cover the burns with clean, dry cloth until arrival at the burn center. Early communication between the initial provider and the burn center is encouraged by the American Burn Association (ABA).19
Special Management Considerations
Inhalation Injury
Inhalation injury can occur in the presence or absence of cutaneous injury. Inhalation injuries are strongly associated with burns sustained in a closed space, and they constitute the leading cause of fire-related deaths.20 Inhalation injury appears in three basic forms, alone or in combination: CO poisoning, direct heat injury, and chemical damage. The three types of inhalation injury are CO poisoning, upper airway injury, and lower airway injury.
Immediate measures to save the life of the burn patient include management of the airway. The burn patient may exhibit few or no signs of airway distress; however, thermal injury to the airway must be anticipated if facial burns, singed eyebrows and nasal hair, carbon deposits in the oropharynx, and carbonaceous sputum are present or if the history suggests confinement in a burning environment. Any of these findings indicates acute inhalation injury and requires immediate and definitive care. To prevent the necessity of tracheostomy or cricothyrotomy, the use of early intubation and respiratory support must be considered before tracheal edema occurs. Inhalation injury predisposes the patient to the development of pneumonia and acute respiratory distress syndrome (ARDS).20 Management of ARDS necessitates mechanical ventilatory support and, in extreme cases, high-frequency oscillatory ventilation or extracorporeal membrane oxygenation. The occurrence of inhalation injury with cutaneous burns increases the fluid requirements during resuscitation to a higher level than would be predicted by the cutaneous burn alone.
Carbon Monoxide Poisoning
Persons found dead at the scene of a fire, who may have few or no cutaneous thermal injuries, would have died of CO poisoning. CO is a colorless, odorless, and tasteless gas. Inhalation of CO, a byproduct of the incomplete combustion of carbon, results in its bonding to available hemoglobin, producing HbCO, which effectively decreases oxygen saturation of hemoglobin. The affinity of hemoglobin molecules for CO is approximately 200 to 250 times greater than that for oxygen.9 HbCO binds poorly with oxygen, reducing the oxygen-carrying capacity of blood and causing hypoxemia. The shortage of oxygen at the tissue level is worsened by a shift to the left of the oxyhemoglobin dissociation curve, reflecting the fact that the oxygen in the hemoglobin is not readily given up to the cells.
Arterial oxygen saturation measurement is of limited value because oxygen saturation may be quite high despite dangerously low levels of oxygen content. Because the pulse oximeter cannot distinguish between oxyhemoglobin and HbCO, it is an unreliable predictor of tissue oxygenation during the initial stages of CO poisoning.9 Arterial blood gas determinations and oxygen saturation are used to accurately assess the hemoglobin oxygen saturation level. A serum HbCO level is obtained the diagnosis. Normal HbCO levels are less than 2%. HbCO levels of 40% to 60% often produce unresponsiveness or obtundation; levels of 15% to 40% may result in various degrees of CNS dysfunction. Levels of 10% to 15%, which can be found in cigarette smokers, rarely produce serious symptoms but may cause a headache.
The major clinical manifestations of severe CO poisoning are related to the CNS and the heart. Symptoms associated with CO poisoning include headache, dizziness, nausea, vomiting, dyspnea, and confusion.9 In severe cases, CO poisoning may lead to myocardial ischemia and CNS complications caused by lowered oxygen delivery and the already compromised circulatory system. Early signs of CO poisoning may include tachycardia, tachypnea, confusion, and lightheadedness. As the CO level rises, patients exhibit a decreased level of responsiveness, which may progress to unresponsiveness and respiratory failure.
The treatment of choice for CO poisoning is high-flow oxygen administered at 100% through a tight-fitting nonrebreathing mask or endotracheal intubation. The half-life of CO in the body is 4 hours at room air (21% oxygen), 2 hours at 40% oxygen, and 40 to 60 minutes at 100% oxygen.9 The half-life of CO is 30 minutes in a hyperbaric oxygen chamber at three times atmospheric pressure. The use of hyperbaric oxygen is controversial in the care of the burn patient. Because of the rapid removal of CO with the administration of 100% oxygen, the time required to transport a patient who has received oxygen in the field should always be considered to avoid possible underestimation of inhalation injury.
Upper Airway Injury
Heat damage may be severe enough to cause upper airway obstruction at any time, beginning from moment of injury through the resuscitation period. Caution is taken for patients with severe hypovolemia because supraglottic edema may be delayed until fluid resuscitation is under way and third spacing occurs. Patients must be monitored for hoarseness, stridor, audible airflow turbulence, and the production of carbonaceous sputum. Maximal edema occurs 24 hours after injury with upper airway injuries, and these patients should be observed in the critical care unit for a minimum of 24 hours.10
Lower Airway Injury
Heated air rarely causes lower airway injury. If it does, it usually is associated with death at the scene. Lower airway injuries are typically caused by chemical damage to mucosal surfaces. Tracheobronchitis with severe spasm and wheezing may occur in the first minutes to hours after injury. Historically, the most accurate method of documenting lower airway injury is the xenon ventilation–perfusion lung scan. Prolonged retention or symmetry of washout of the radioisotope indicates pulmonary parenchymal injury on the side of the retained emissions. The onset of symptoms is unpredictable following smoke inhalation and patients at risk must be closely monitored for at least 24 to 48 hours after injury.9
Research into optimal ventilator management strategies for patients with inhalation injury is a high priority.21 In addition to thermal damage, secondary effects from massive fluid resuscitation and high-volume, high-pressure ventilator settings can precipitate ARDS.21 The use of lung protective ventilation with low tidal volumes, higher levels of positive end-expiratory pressure (PEEP), and plateau pressures maintained below 30 mm Hg are increasingly employed.21 Intrabronchial surfactant and inhaled nitric are other last resorts in dealing with burn patients with inhalation injuries that have not responded to other means of ventilator support.9 Benefits include decreased rates of pneumonia and increased survival.22 Treatment of lower airway injury is largely symptomatic. As with upper airway injury management, aggressive pulmonary hygiene, removal of secretions, ventilatory support, and careful fluid resuscitation as to avoid exacerbating pulmonary edema and ARDS when caring for burn patients with lower airway inhalation injuries.10
Nonthermal Burns
Chemical Burns
Electrical Burns
Electricity always travels toward the ground. The body conducts electrical current as a whole, as opposed to the earlier belief that it traveled most quickly through the nerves and circulatory system. Electrical burns often are much more serious than the surface appearance of the wound suggests.22 As the electrical current passes through the body, it damages the inner tissues and may leave little evidence of a burn on the skin surface.
The electrical burn process can result in a profound alteration in acid–base balance and rhabdomyolysis resulting in myoglobinuria, which poses a serious threat to kidney function. Myoglobin is a normal constituent of muscle. With extensive muscle destruction, it is released into the circulatory system and filtered by the kidneys. It can be highly toxic and can lead to AKI. Fluid resuscitation for the electrical burn patient does not correlate with the Parkland formula, and the fluid is adjusted according to the patient’s urine output. If myoglobin is present in the urine, a urine output greater than 100 mL/hr in adults and 2 mL/kg/hr in children is established until the urine is clear of all myoglobin pigment.4,22
If hemoglobinuria is identified, the clinician should assume that the patient has myoglobinuria and acidosis. Sodium bicarbonate may be administered to bring the pH level into the normal range, to correct a documented acidosis, or to alkalize urine to promote myoglobin excretion. Diuretics such as mannitol also may be administered intravenously until myoglobinuria resolves.4 Sodium bicarbonate infusions and diuretic therapy are called forced alkaline diuresis. A baseline ECG and myocardial biomarker levels are obtained while the patient is in the emergency department. The following criteria are used for monitoring the cardiac status of burned patients:
Burn Nursing Diagnoses and Management
The clinical course of a burn injury has three phases: (1) resuscitative, (2) acute care, and (3) rehabilitative. Each phase has a unique set of actual and potential problems. The resuscitative phase begins with initial hemodynamic response to the injury and lasts until capillary integrity is restored and the repletion of plasma volume by fluid replacement occurs. Spontaneous diuresis demonstrates that capillaries have regained their integrity. The acute phase begins with the onset of diuresis of fluid mobilized from the interstitial space and ends with the closure of the burn wound. The major focus of the acute phase is wound healing, wound closure, and prevention of infection. The rehabilitative phase begins when the patient is admitted to the hospital, with correction of functional deficits and scar management being major considerations. The rehabilitative phase may last months to years, depending on the severity of injury. The rehabilitation phase focuses on support for adequate wound healing, prevention of scarring and contractures, and psychologic support of the patient and the family.23
Resuscitation Phase
Life-threatening airway and breathing problems, cardiopulmonary instability, and hypovolemia characterize the resuscitation phase, or shock phase. Every organ is involved in the physiologic response that occurs with thermal injury of greater than 20% of TBSA.4 The magnitude of this pathophysiologic response is proportional to the extent of cutaneous injury. The goal of the resuscitation phase is to maintain vital organ function and perfusion. Emergent interventions for inhalation injury, airway management, and hypovolemia are concurrently addressed.
Oxygenation Alterations
• Assess breath sounds and the rate and quality of respirations.
• Administer oxygen, as prescribed.
• Elevate the head of the bed, and implement VAP precautions.
• Assess and assist with pulmonary secretion suctioning.
• Observe for signs of airway obstruction (e.g., increased respiratory rate and heart rate, increased work of breathing, use of accessory muscles, stridor, wheezing, hoarseness, crackles).
• Prepare for endotracheal intubation and mechanical ventilation.
Ineffective Airway Clearance.
Laryngeal swelling and upper airway obstruction may occur at any time during the first 24 hours after the burn injury. Endotracheal intubation must be accomplished early because this simple procedure can become extremely difficult in the presence of laryngeal edema. Usually, enough time is available to intervene after obtaining the history and transporting the patient to the primary hospital. Edema may continue to develop for 72 hours after the burn incident.4 The patient who has not initially undergone intubation must be carefully monitored during this critical period. When prolonged ventilatory failure is expected as a result of severe inhalation, a tracheostomy is performed.
Fluid Resuscitation
Current resuscitation protocols emphasize fluid delivery rates based on the extent of burn and the patient’s weight. The patient’s weight measured in kilograms must be obtained on admission to the hospital. The extent of the burn is calculated by using one of the methods previously described. Several formulas are available to guide fluid resuscitation and should be used to assist in the management of fluid replacement (see Table 36-1). The formulas differ primarily in terms of administration, volume, and sodium content. The actual amount of fluid given to any patient must be based on that individual’s response.
Risk for Infection
Preventing infection in the burn patient is a true challenge and involves complex decision making. Considerable debate has been going on about the infection control precautions to use with burn patients. The burn wound is the most common source of infection in the burn patient. The loss of the protective mechanism of the skin and contamination from the patient’s own bacterial flora can lead to bloodstream infections. Some centers advocate routine wound surveillance cultures and wound biopsy to identify infection early. Daily wound inspection for changes in appearance such as an increase in exudate, odor, or color is necessary to minimize the risk of bacteremia. Patients should not be treated with antibiotics prophylactically; rather, treatment should be tailored to positive culture results.10
Acute Care Phase
Inflammatory Phase
The inflammatory phase begins immediately after injury. Vascular changes and cellular activity characterize this period. Changes in the severed vessels occur in an attempt to wall off the wound from the external environment. Platelets, activated as a result of vessel wall injury, aggregate; blood coagulation is initiated; and in larger vessels, smooth muscle tissue contraction occurs, resulting in a reduction in the diameter of the vessel lumen. These brief but important compensatory mechanisms protect the individual from excessive blood loss and increased exposure to bacterial contamination. As vasodilation occurs, vascular permeability and blood supply to the wound site increase. Clinically, this is observed as erythema and exudates.24 Granulocytes invade the wound within 24 hours and initiate the phagocytosis of necrotic tissue and bacteria. Fibroblasts migrate to the wound and multiply, producing a bed of collagen. This phase of healing lasts from the moment of injury to day 3 to day 5.25
Proliferative Phase
The proliferative phase of healing occurs approximately 4 to 20 days after injury. The key cell in this phase of healing, the fibroblast, rapidly synthesizes collagen. Collagen synthesis provides the needed strength for a healing wound. Epithelial cells migrate across the wound bed. After these cells contact each other, the wound is covered. This process is known as epithelialization. The wound contraction process happens when specialized fibroblasts known as myofibroblasts, pull down the wound edges in an effort to close the wound.24
Impaired Tissue Integrity
Factors Affecting Healing of the Burn Wound
Sources of contamination include the patient’s endogenous flora found on the skin, the upper respiratory tract, and the GI tract. Exogenous flora found in the patient care setting include bacteria carried by staff members and present in the environment. Patient-specific factors that predispose the patient to infection include age, diabetes, steroid therapy, extreme obesity, severe malnutrition, and infections in remote sites. Because wound healing and clinical infection are inflammatory responses, it is essential to differentiate between normal wound inflammation in the presence of colonization of microorganisms and that of invading organisms. Most burn wounds are colonized with bacteria and usually does not signify infection.26 In diagnosing infection, the importance of microbiologic results must be evaluated in conjunction with clinical findings such as excessive erythema, edema, pain, and purulence. Clinical findings in conjunction with burn wound biopsy or culture results determine the diagnosis of wound infection. Other factors that affect wound healing are tissue hypoxia from low blood flow to the burn wound, presence of eschar that will require débridement, exudate on the wound that can be harmful to the granulating wound or consume oxygen in the wound, and trauma to the wound from daily dressing changes or lack of protection from the outside environment.
Wound Care
Although many options for burn wound care are available, the basic principle of maintaining a moist wound environment while preventing wound infection is the standard of care. Benefits include preventing wound desiccation, optimal function of local wound growth factors and proteolytic enzymes to remove dead tissue, increased re-epithelialization and collagen synthesis, and decreased wound fluid loss. The most common regimen for burn wound care continues to involve the application of a topical antimicrobial agent, followed by a primary gauze dressing to absorb burn wound drainage and an outer layer to provide increased absorption, compression, and occlusion.6
Topical Antibiotic Therapy
Burn injuries destroy the function of the skin’s protective mechanism, including that of sebaceous glands. Sebaceous glands normally secrete sebum, which contains fatty acids, including oleic acid. In addition to lubricating the skin, sebum is believed to help destroy some microorganisms, such as streptococci and some strains of staphylococci. Serum is lost from damaged capillaries, providing a rich nutritional medium for bacterial colonization. Topical antibiotic agents are used to control this colonization. Effective antibacterial agents should control colonization so that specimens for wound biopsy reveal fewer than 105 microorganisms per gram of tissue. More than 105 microorganisms per gram of tissue makes control of wound sepsis with topical antibiotics questionable. Oral or intravenous therapy may then be considered. Topical antibiotics selected must meet several criteria; side effects must be minimal; resistant strains must not develop with use; application must be easy and rapid; and use must be relatively economical. The most commonly used topical antibiotics are silver sulfadiazine (SSD), mafenide acetate cream (Sulfamylon), bacitracin ointment, and silver impregnated into the primary dressing (Table 36-2).
TABLE 36-2
PHARMACOLOGIC MANAGEMENT
Topical Antimicrobial Agents
| Agent | Advantages | Disadvantages | Implications |
| Silver sulfadiazine |
SSD (Silvadene cream) is a broad-spectrum antimicrobial agent with bactericidal action against many gram-negative and gram-positive bacteria associated with burn-wound infection. It is a thick white cream that is applied once or twice daily to a wound that has not been grafted, according to the burn unit protocol.27 To provide antimicrobial benefit, sustained release of silver is necessary. This is achieved with moisture provided by the wound exudate that may give the SSD cream layer a yellow-gray pseudo-eschar.27 The old SSD layer must be removed prior to a new application.27 The SSD cream removal is often painful for the patient, although reapplication of a new SSD layer is not. A common side effect of silver sulfadiazine is leukopenia resulting from bone marrow suppression, which may develop 24 to 72 hours after application. Rebound to normal leukocyte levels follows onset within 2 to 3 days, and it is not necessary to discontinue SSD use. SSD is indicated for use with partial and full-thickness wounds and is the most commonly used topical antimicrobial agent used to treat burn wounds.6
Mafenide acetate cream penetrates through burn eschar and is bacteriostatic against many gram-negative and gram-positive organisms.27 Its use is limited because the application is uncomfortable for the patient as it creates a burning sensation, and it is rapidly absorbed, requiring dressing changes two or three times daily. It is used routinely for coverage of small wounds involving anatomic areas that contain cartilage such as the ears and nose.27 Metabolic acidosis can result from the use of mafenide acetate. The patient must be observed closely for hyperventilation (see Table 36-2).
Silver has long been used for the treatment of wounds because of its broad-spectrum bacteriostatic properties. The wound moisture activates the silver and releases it into the wound. An advantage of silver dressings is that the dressing does not need to be changed daily because of the sustained release of silver. Silver dressings should be used judicious and limited to 4 to 6 weeks despite the current absence of negative systemic or local consequences.24
A Cochrane systematic review of dressing products for superficial and partial thickness burn wounds reported a shorter wound healing time, fewer dressing changes and consequently fewer pain-experience opportunities with hydrocolloid dressings, antimicrobial dressings with silver as the active ingredient, silicon dressings, nylon polyurethane film and biosynthetic dressings compared with SSD cream.28
Wound Débridement
Mechanical Débridement.
Mechanical débridement involves the use of scissors and forceps to gently lift and trim loose, necrotic tissue (Fig. 36-11A and B). Only experienced nurses and physicians perform this procedure. Sterile gauze may be used in the form of a wet-to-dry or wet-to-wet dressing to further débride the wound bed.
Skin Substitutes
Skin substitutes must possess properties that mimic the native epidermis and dermis. They are made from a variety of synthetic materials such as nylon, polyurethane, or solid silicone polymers. Skin barrier substitutes must possess several properties to accomplish their desired effect as a temporary wound covering to protect the granulating tissue and to preserve a clean, viable wound surface for future autografting (Box 36-2). The most important property of these materials is adherence so that the skin substitute can simulate the function of the skin. Adherence must be uniform to prevent fluid accumulation beneath the surface of the substitute, which can lead to bacterial proliferation.
Definitive Burn Wound Closure
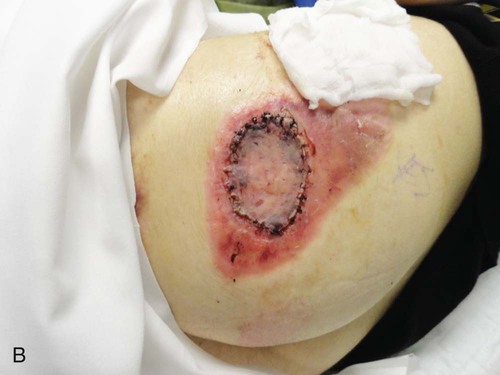
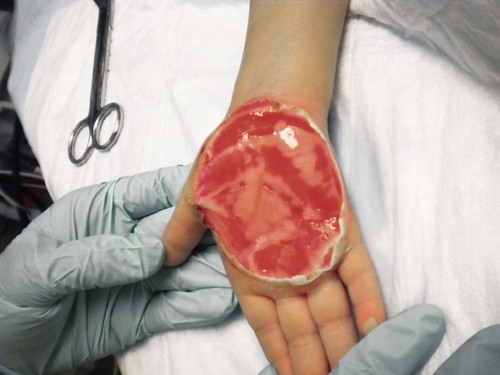
The primary goal of burn wound management is wound closure during the acute phase. Full-thickness (third-degree) burns are most often treated by excision and grafting.29 Some deep partial burns that have a prolonged healing time may also benefit from excision and grafting. If a burn wound does not heal in 10 to 14 days, excision should be undertaken to improve functional and cosmetic results, decrease infection risk, decrease in-hospital time, and to reduce the cost of burn care.6,29 Surgical débridement may begin as early as 3 to 5 days after the burn insult, as soon as hemodynamic stability has been achieved.27 Some physicians operate within 24 hours of admission if the patient is hemodynamically stable. To minimize contraction, burn wounds over joint surfaces should be excised and grafted as soon as possible.30 Typically, excision procedures are limited to 20% of the body surface or 2 hours of operating time. In patients with massive burns, excision procedures are commonly staged, requiring the patient to return to the operating room every 2 to 3 days until all wounds have been excised. This technique helps avoid excessive transfusions and limits the physiologic stress.27 Table 36-3 reviews advantages and disadvantages of different graft types.
TABLE 36-3
| GRAFT | USE | ADVANTAGES | DISADVANTAGES |
| Autograft |
Autograft
An autograft is a skin graft harvested from a healthy, uninjured donor site on the burn patient and then placed over the patient’s burn wound to provide permanent coverage of the wound. Autografts are the only grafts that provide permanent wound coverage. Preferred sites for obtaining these grafts are the thighs, back, and abdomen; however, grafts can be harvested from almost anywhere on the body.9 Autografting with the patient’s own skin from a donor site is the preferred choice for wound closure. However, with large TBSA burns, availability of donor sites can be problematic. When an autograft is not available, many alternate methods are used to achieve this goal. Creative attempts have been initiated to establish a skin substitute that permanently closes the wound in a cosmetically and functionally acceptable fashion. Skin substitutes can be used until the patient’s own skin is available for harvesting. Previously used and healed donor sites can be used again in later return visits to the operating room.29
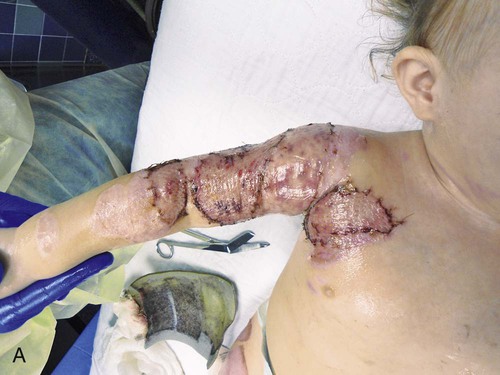
Surgical excision is performed to mechanically remove necrotic tissue from the burn wound; it may be performed tangentially or fascially. Tangential excision involves sequentially excising the eschar down to bleeding, viable tissue and then placing a split-thickness skin graft over the wound. Fascial excision is used when the wounds are deep and the fat does not appear viable.29 Sheets of the patient’s epidermis and a partial layer of the dermis are harvested with use of a dermatome. These grafts are referred to as split-thickness skin grafts and can be applied to the wound bed as a sheet or in meshed form (Fig. 36-12). The split-thickness skin grafts harvested are approximately twelve thousandths of an inch thick. The split-thickness skin graft can be meshed 1.5 : 1 to 4 : 1 in a mesher and then placed on the wounds.6 The size of the mesh is based on the areas requiring grafting and the availability of donor skin. This meshing prevents serum accumulation under the graft and permits coverage of a surface area larger than its original surface. Grafts that are placed on the face, neck, lower portions of the arms, and hands are sheet grafts, when possible. Grafts that are meshed can cover more area but may not produce the cosmetic appearance desired, and therefore, they are usually placed on areas covered by clothing.
The grafts can be secured with sutures, fibrin glue, or staples. The choice of dressing that is placed over the graft varies widely based on physician and institution preference. One choice is fine mesh gauze impregnated with an emollient. It is placed over the graft, covered with a heavy gauze dressing, and secured to the patient with or without a splint, depending on the anatomic area of the graft. A vacuum-assisted closure device provides a safe and effective method for securing split-thickness skin grafts, and it is associated with improved graft survival.31 Negative wound pressure therapy can be used to secure the graft in place. Trained nursing professionals or physicians remove the dressings on postoperative day 3 to 5 for assessment of adherence and graft survival. Nurses, in collaboration with the multidisciplinary team, provide proper positioning, splinting, and pain management in the postoperative period. Great care must be taken not to disturb the graft. Mobilization of the graft area usually occurs around the first dressing change on postoperative day 3 to 5, according to the surgeon’s preference.
Biosynthetic Skin Substitutes
After the pigskin is in place, it may be dressed with antibacterial-impregnated dressings or other forms of dressings. Pigskin usually is removed or dissolves because of lack of blood supply in 5 to 7 days. If sloughing or purulent drainage occurs, the xenograft is removed (see Table 36-3).
Synthetic Skin
The lack of available donor sites for major burn injury often delays wound closure.32 In an effort to minimize infection and to promote healing, many attempts have been made to develop skin substitutes that will seal the wound in a functional and cosmetically acceptable fashion.32 Integra has become a very popular dermal substitute with its ultrathin layer of epidermal autograft used successfully. Integra is untended to be placed on freshly excised, full-thickness burns, and the outer silicone membrane replaced with an ultrathin epithelial autograft 2 to 3 weeks later.33 Integra can also lead to more superficial donor sites.34 A technique that involves the growth and subsequent graft placement of cultured epithelial autograft (CEA) has also become an adjunct to the treatment of burn wounds. A complex process that allows for separation of keratinocytes is performed. The CEA is grown over a period of 2 to 3 weeks to achieve a graft size of 25 cm2. This represents an expansion of 50 to 70 times the original specimen. These confluent sheets of cultured epithelial cells are attached to a gauze backing and placed on the wound. The published results reveal that graft acceptance is unpredictable because the CEA lacks dermis. Even when grafts take initially, graft loss can occur later. Compared with other methods, the CEA also is more fragile, and the technique is quite costly. This therapy is being recommended as an adjunct for traditional split-thickness skin graft, and it continues to be investigated and combined with newer dermal skin substitutes.
Imbalanced Nutrition: Less Than Body Requirements
The basal metabolic rate of a burn patient may be elevated 40% to 100% above the normal rate, depending on the amount of TBSA involved. The metabolic rate is influenced by the amount of protein and albumin lost through the wounds, the catabolic response associated with stress, associated injuries, fluid loss, fever, infection, immobility, gender, and the height and weight of the patient before the injury.31 A 10% loss of total body mass leads to immune dysfunction; 20% leads to decreased wound healing; 30% leads to severe infections; and a 40% loss leads to death. Severely burned, catabolic patients can lose up to 25% of total body mass after acute severe burn injury.18
Because of the increased nutritional needs of the burn patient, oral feedings are usually inadequate, and supplemental enteral feedings are necessary. Caloric requirements are calculated on the basis of the size of the burn; the age, height, and weight of the patient; and stress factors. Protein and caloric requirements for the burn patient are elevated by a negative nitrogen balance. The daily protein requirement may increase to two to four times the normal 0.8 g/kg of body weight. Carbohydrates and fat are used for energy and to spare proteins required for wound healing. Daily caloric intake can be 2 to 20 times higher than normal. Vitamins and minerals usually are given in doses higher than normal. Serum albumin, prealbumin, iron, zinc, calcium, phosphate, and potassium values are monitored, and supplements given as indicated by results.31
Pain Management
Burn injuries are very painful. Pain management must be addressed early and frequently reassessed. Pain is an individualized and subjective phenomenon, and it has physiologic and psychologic components. Pain results from the acute burn injury and throughout the phases of healing. Background pain is related to the physiologic changes associated with the burn injury and includes the damage or exposure of the nerve endings within partial-thickness burns and donor sites. Range of motion of the affected limbs and routine activities contribute to background pain. Breakthrough pain is described as episodes of pain more severe than background pain, and it is not relieved by routine pain medications. Procedural pain includes pain caused by interventions such as daily wound care, arterial punctures, physical and occupational therapy, and the use of splints.35 Pruritus (itch) may replace pain as a stressful symptom in the rehabilitation phase.
Pain continues even after healing; some patients describe the itching, tingling, and paresthesias as being as uncomfortable as the initial injury.35 Paresthesias are abnormal neurologic sensations of numbness, tingling, or burning that can last 1 year or more after injury. Inadequate pain management is an issue in many burn units because of the fear of opioid side effects and opioid addiction and the lack of pain evaluation or treatment protocols.
Nonpharmacologic techniques such as imagery, hypnosis, and distraction and methods adapted from some of the popular childbirth techniques can be effective in reducing anxiety and the pain experience. Giving the patient some management control also can reduce anxiety and the perception of pain. The perception of pain often is increased in the patient who is anxious and lacks control of the situation.29
Rehabilitation Phase
The rehabilitation phase is one of recuperation and healing physically and emotionally. The patient is not acutely ill but may or may not be ready for discharge. This phase can last several years. Psychologic rehabilitation is equally important in the burn patient. The patient may require extensive reconstructive surgery. Psychologically, the patient focuses on attaining specific personal goals related to achieving as much preburn function as possible.23 Minor and major accomplishments must be praised. This phase is characterized by scar management techniques and by physical and occupational therapies.
Impaired Physical Mobility
Tremendous advances have been made in the physical care of the burn patient. The survival rate of patients with full-thickness burns greater than 40% of TBSA has increased significantly. Survival of patients with burns greater than 90% of TBSA is not impossible. As patients with larger and deeper burns survive, the challenge to maintain their optimal mobility and cosmetic appearance has been met with increased success. Despite the advances in other areas of burn care, contractures still develop after a burn injury. A contracture is the shortening of a scar over a joint surface and is the primary cause of functional deficits in the burn patient (Fig. 36-13). Contractures develop because of a variety of factors: the extent, depth, location, and configuration of the burn; the position of comfort the patient most frequently assumes; the relative underlying muscle strength; and the patient’s motivation and compliance. The affected body parts should be positioned to prevent long-term deformity. Frequent change of position also is important and may need to be performed as often as every hour. Burn patients are at greater risk for pressure sores than the general hospital population.
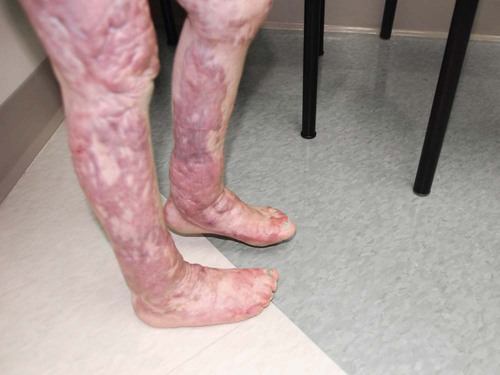
Active exercise is encouraged and is preferred, although active-assisted or gentle-passive exercises also may be an important part of the rehabilitation program. Active exercise maintains muscle mass, aids in restoring protein structures within the muscle tissue, aids in venous and lymphatic return, and reduces the risk of pulmonary embolus and deep vein thrombosis. The patient’s tolerance must be carefully evaluated. The number of repetitions of an exercise is proportional to the degree of anticipated contracture and the patient’s tolerance. Anticipation of the patient’s pain also must be carefully considered. Before range-of-motion exercises and activities of daily living are performed, the need for pain medication must be assessed.12 Nursing diagnoses for the burn patient are summarized in the Nursing Diagnoses features on the three phases of burn injury (Box 36-3 and Box 36-4).
Scar Management
Another concern in burn wound healing is the prevention or reduction of scarring. A person’s skin response to a burn injury can be barely noticeable such as slight color change or lead to cosmetic disfigurement and dysfunction. The goal of scar management is to minimize scarring, and it is important to inform the patient that the tissue may not return to the preburn texture or appearance. Hypertrophic scars are red, raised, and lack elasticity, and they are painful and itchy.24 Deep partial and full-thickness burns are at the highest risk for scar tissue development because of their depth and increased risk of infection. Areas of the skin that required skin grafting also leave a visible scar (Fig. 36-14). Scar maturation occurs 6 months to 2 years from the time of the injury. One method to reduce scar formation is timely application of uniform pressure. Hypertrophic scarring can be controlled with the use of tubular support bandages. They are readily available for immediate use during the wait for the commercial manufacture of the customized elastic pressure garment for long-term use, which can take up to 3 or 4 weeks. Custom-made elastic pressure garments are worn for 6 months to 1 year, if needed (Fig. 36-15). These garments reduce scar blood flow and may provide force that helps developing collagen to organize.23 Although compression therapy is one of the main prophylaxis and treatment of burn scars, its mechanism is not totally understood.36 It is important to assess for pressure points under the garment as weight is gained and as growth occurs. It is necessary to assess the garment for elasticity over time because elasticity decreases with washing.
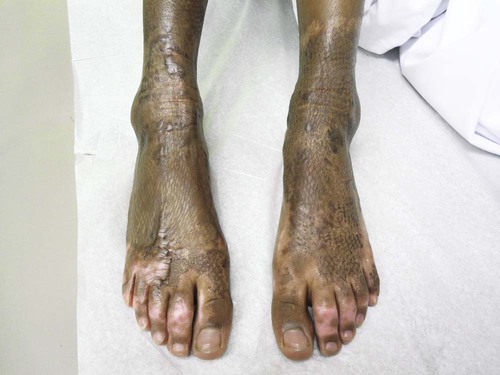
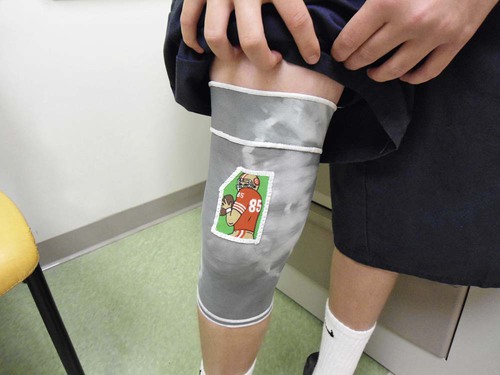
Additional approaches to reduce scarring are scar massage, high-SPF sun protection, silicone gel sheeting and steroid treatment. Scar massage works by stretching the scar and providing moisture and is most helpful in preventing contractures. Sun protection over the healed burn may decrease the long-term pigment change to the injured area. Silicone gel sheets are used alone over the scar or in conjunction with compression to soften the scar by maintaining scar hydration and tension reduction. Injectable or topical steroids are used for treatment of hypertrophic scars; however, they have some side effects that may make them undesirable. Corticosteroid injections inhibit fibroblast growth and enhance collagen breakdown leading to a flatter and softer scar.24 Topical steroid treatment poses less risk, however has been found to be less effective. If less invasive techniques have been unsuccessful for troublesome scars that cause pain or inhibit full range of motion, surgical excision may be recommended by the physician.
Itching
Pruritus is common in the maturing burn wound. One study reported itching as the most common skin-related problem described by 94% of patients.37 For 6 to 8 weeks, a mild, non–alcohol-based skin cream is applied every 4 hours to healed areas to lubricate the skin until natural lubrication occurs. Patients can be relieved of this discomfort by the administration of an antipruritic agent such as diphenhydramine hydrochloride, doxepin hydrochloride (5%), or hydroxyzine and by the application of moisturizing creams. Itching can be extremely uncomfortable for the patient and should be continually assessed.
Multidisciplinary Care
A multidisciplinary approach to burn treatment is integral in providing quality care. This approach involves considering all aspects of patient’s care when treatment decisions are made.38 The burn team should work together to address all needs of the patient and the family. The team should meet frequently to review patient care and maximize patient and family support. The multidisciplinary team includes, but is not limited to, physicians, nurses, social work, nutrition, physical and occupational therapy, respiratory therapy, psychology, pain service, child life services, and utilization review.
Support of the Burn Patient
Burn injuries are physically and psychologically traumatic and life altering for the patient. The patient’s preburn level of emotional functioning can greatly affect the course of recover.39 The family of the burn patient is also affected, and their needs should be remembered during the healing process. Often, guilt is associated with burn injuries, especially when the victim is a child. It is important for the clinical providers to support the burn patient in all aspects. As health care providers, we heal and support the patient throughout the hospital stay but often forget that the survivor’s biggest challenges may still lie ahead after hospitalization. Many survivors have stated that they wish they could take the hospital staff home with them to help them transition into the community.40
Many resources are available to help burn survivors cope after they are home and should be provided by the hospital team during their inpatient stay. Psychologic distress occurs in as many as 34% of burn patients and persists in severity long after discharge. 9 Research has shown that many patients have difficulty returning to work and desire a coordinator during the rehabilitation process to assess work capacity.41 Programs are available to help with such issues as social and school re-entry, support for sexual considerations, and dealing with scars. These resources are needed at different times of the recovery process.40 The clinical team and the community must collaborate to improve burn survivor transition and support.
Summary
• Burn care is highly complex, and decisions regarding appropriate management of burn injuries are best managed by certified burn centers, where the most accurate burn size estimates can be made.
• Burn size, types of injury, location of the burn, and the patient’s age and history are all determinants of survival.
• Size and depth of burns are divided into four main categories: (1) superficial (first-degree) burns, which are injuries to epidermis; (2) partial-thickness (second-degree) burns, which involve the epidermis and dermis of the skin; (3) deep-dermal, partial thickness (second-degree) burns, which involve the entire epidermal layer of the skin and part of the dermis; and (4) full-thickness (third-degree) burns, which involve destruction of skin layers from the epidermis down to and including the subcutaneous tissue.
• Accurate fluid resuscitation of a burn patient for more severe burn injuries (>10% of TBSA) is crucial to prevent acute kidney injury that may result in acute kidney failure, cardiovascular collapse, and death from burn shock. The Parkland formula is a guide for determining resuscitation fluid (Ringer lactate) volume.
• Careful assessment of the burn patient’s intake and output is critical to achieve the optimal patient response to fluid resuscitation.
• After the resuscitative phase of burn patients, the acute care phase of wound healing, wound closure, and prevention of infection begins. This hypermetabolic phase can be complicated by wound infection and sepsis.
• The gold standard of burn wound care includes the application of a topical antimicrobial agent, followed by a gauze dressing to absorb excess drainage, with an outer layer to provide increased absorption, compression, and occlusion. Silver dressings have shown positive results in the prevention of bacterial growth without silver toxicity.
• The rehabilitative phase of the burn patient care starts from admission of the burn-injured patient and may last years, depending on future surgical procedures, therapy needs, contracture prevention, and psychologic or emotional needs of the patient.
• All of the multidisciplinary needs of burn-injured patients must be addressed for them to be able to perform in and feel accepted back into society.