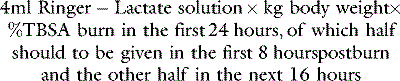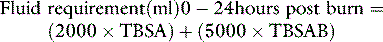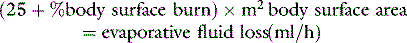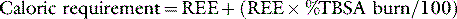Chapter 73 Burns
The last half of the twentieth century witnessed a sustained improvement in the survival of patients suffering thermal injury. Arguably, the single most important development has been the establishment of centralised burn care which made possible advances in fluid resuscitation, life support techniques and the prevention of infection. With optimal care, children and young adults with burns of more than 80% of total body surface area (TBSA) now stand a reasonable chance of survival.1
Improvements in survival have gradually led to a shift of emphasis in burn care towards qualitative aspects, such as rehabilitation and quality of life. The complexity of care has led to the concept of the multidisciplinary burn team, in which all aspects of care are coordinated in an integrated approach to clinical management.1
PATHOPHYSIOLOGY
LOCAL EFFECTS
Thermal injury produces complex local and systemic responses. The local inflammatory response results in vasodilatation and an increase in vascular permeability. The changes are immediate and combine to produce extravasation of fluid and plasma protein at the site of injury. In extensive burns, oedema becomes generalised. The greatest rate of oedema formation occurs in the first few hours, but further extravasation occurs up to 24 hours post burn.2 The total amount of oedema formed depends on the extent of injury and the volume and rate of fluid administration. Without fluid replacement, hypovolaemic shock occurs, limiting the extent of extravasation. On the other hand, excessive fluid administration will produce excessive oedema. By 24 hours post burn, oedema formation is largely complete and vascular integrity restored.
CIRCULATORY EFFECTS
Circulatory effects of burn injury become significant in burns of over 15% TBSA. Changes are rapid and the magnitude is roughly proportional to the extent of burn injury. Cardiac output is reduced immediately following injury. Secretion of adrenaline (epinephrine), noradrenaline (norepinephrine), vasopressin and angiotensin cause an increase in systemic and pulmonary vascular resistance. Circulating levels of agents with myocardial depressant properties, such as interleukin-1 and TNF-α, are increased following burn injury, but developing hypovolaemia and increased blood viscosity may be equally important.3
Cardiac output recovers gradually during the second post-burn day, reaching supranormal levels by day 3 as the hypermetabolic response to burn injury becomes manifest. Circulatory dynamics are complicated by resorption of oedema fluid and by continuing evaporative fluid loss from the wounds. Prolonged elevation of renin/angiotensin and antidiuretic hormone (ADH) has been well documented and circulating blood volume may remain subnormal into the second week post burn.4
METABOLIC EFFECTS
The hypermetabolic state, which ensues from around the third post-burn day until the wounds are substantially healed, is a manifestation of the systemic inflammatory response syndrome. The state is partly sustained by evaporative and radiant heat loss through the wounds, and energy expenditure can be reduced by increasing the ambient temperature and by the use of occlusive dressings.5,6 Other factors known to influence the metabolic rate include pain, fear and anxiety. There is also a suggestion that bacterial colonisation may aggravate hypermetabolism.7,8 Burn patients often develop moderate pyrexia, even when all microbial cultures are negative.
PHARMACOLOGICAL EFFECTS
The pharmacokinetics and pharmacodynamics of many drugs are markedly altered in burn patients. During the first 24 hours, when the cardiac output is depressed, absorption and distribution of administered drugs are delayed. Thereafter, increased cardiac output leads to accelerated drug absorption and distribution, while oedema fluid acts as an ill-defined third space. At the same time, renal blood flow and creatinine clearance are increased, particularly in younger patients. Drugs excreted via this route, such as the quinolone and aminoglycoside antibiotics, may therefore fail to reach effective levels at conventional dosages.9 On the other hand, toxic levels may ensue if renal failure supervenes. If possible, therefore, antibiotic administration should be guided by measurement of plasma concentrations.
Serum albumin levels are low in burn patients, and drugs bound to this protein, including some benzodiazepines, will show increased bioavailability. On the other hand, α1-glycoprotein levels, which bind fentanyl, are increased. Detoxification via redox pathways, such as cytochrome P-450, is depressed, lengthening the half-life of drugs such as diazepam.10 Accumulation of benzodiazepine derivatives may be increased.
The pharmacodynamics of muscle relaxants are significantly altered due to an increase in peri-junctional acetylcholine receptors.11 Patients become relatively insensitive to non-depolarising agents, while administration of succinylcholine may give rise to excessive release of potassium, and cardiac arrest.
CLINICAL MANAGEMENT
FIRST AID
Immediate aid comprises stopping the burn process, followed by the removal of clothing and cooling the wound, preferably with tepid, running water, for 10–20 minutes. This provides pain relief and may prevent deepening of the wound.12 Hypothermia should be avoided. Oxygen should be given, if available, and patients with burns to head and neck should be kept in a semi-upright position. Burn injury can only be assessed properly in hospital conditions, and priority should be given to early evacuation of the victim.
GENERAL MANAGEMENT: 0–24 HOURS
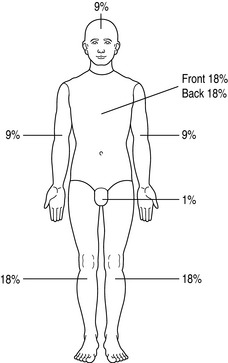
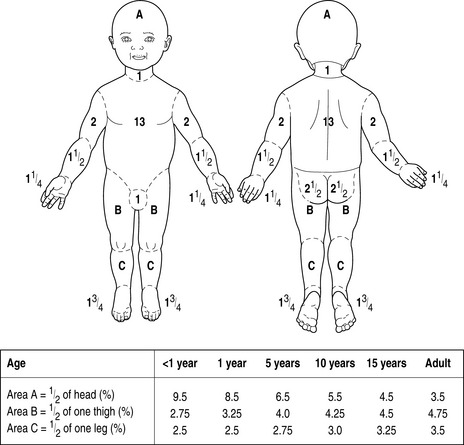
On admission, a careful history should be taken of the circumstances of the injury, and to elucidate past medical history. The patient should be undressed, weighed, and carefully examined to exclude additional traumatic injury. The extent and depth of injury is assessed with the aid of printed Lund and Browder charts, or by using the ‘rule of nines’ (Figure 73.1). The rule of nines is modified for children (Figure 73.2). A nasogastric tube and urinary catheter should be inserted in patients requiring resuscitation therapy. Escharotomies may be required for circular burns of the trunk and limbs.
FLUID THERAPY: 0–24 HOURS
Fluid therapy is required for injuries exceeding 15% of body surface area (10% in children and the elderly), preferably via a wide-bore peripheral i.v. cannula (preferably not in a burned area). The aim is to provide sufficient salt and water to preserve normal organ function, while minimising oedema formation. Excessive fluid administration increases the risk of circulatory overload in the days following the resuscitation period. Potentially fatal complications of excessive fluid administration include the abdominal compartment syndrome in adults13 and the occurrence of cerebral oedema in children.14 An increasing tendency in recent years to overresuscitate burn patients has been signalled.15,16 In contrast to other traumatic injuries, burn hypovolaemia is gradual, obligatory and predictable. Aggressive fluid administration will not restore the circulating volume17 and in the absence of frank shock, bolus fluids should not be given.
Various resuscitation formulae have been published in the past to guide initial fluid therapy. These formulae are entirely experience-based and many are of historical interest only, but all comprise a fluid intake of 2–4 ml/kg body weight per % burn in 24 hours, and a sodium intake of approximately 0.5 mmol/kg per % burn.18 These findings have led some to employ resuscitation regimens based on the administration of hypertonic sodium solutions, which require a smaller volume of fluid. However, the solute load may be excessive, requiring extra water administration in subsequent days, with an increased risk of fluid overload. The use of isotonic saline solutions is therefore preferred by those without experience of burns resuscitation.
The formula thus incorporates a faster rate of administration if initial treatment has been delayed.
Children require extra fluid to compensate for basal needs. For children under 30 kg, the resuscitation formula of Carvajal19 is useful:
Thirst is common, but unrestricted oral fluids will increase oedema formation. Controlled quantities of nutritional liquids are recommended to protect gut integrity.20 In patients with extensive injuries, tube feeding at a low rate can be commenced within a few hours of injury.
PAIN THERAPY
During the first 24 hours, pain management is best achieved by incremental doses of i.v. morphine, or equivalent opiate. Thereafter, the pain suffered by burn patients may be divided into background pain, which is continuous, and procedural pain caused by interventions.21 The recurring ordeal of dressing changes, physiotherapy exercises and surgical procedures generates apprehension and anxiety, which compound distress.
Regular pain therapy is required to counter background pain, topped up by extra analgesics prior to procedures. In recent years, the advent of slow-release oral opiate medication has greatly improved the administration of analgesia. Procedural pain is best treated with continuous i.v. morphine or fentanyl, with titrated incremental boluses as required. Patient-controlled analgesia is effective, although modification of the control button may be necessary for those with bandaged hands. Ketamine in subanaesthetic doses is extremely useful for procedural pain in children. The influence of anxiety and depression on pain perception suggests additional avenues of therapy.22 Non-pharmacological interventions, such as hypnosis and other distraction techniques, are an effective adjunct in susceptible patients.23 Many burn patients may develop posttraumatic distress syndrome, and the emergence of symptoms may require additional support. Above all, an attitude of reassurance and understanding by all carers is indispensable.
NUTRITION
The hypermetabolic response is roughly proportional to the extent of injury. In young adults and children with extensive burns, energy expenditure may be doubled. In addition, protein and substantial amounts of trace elements, such as zinc, copper and selenium are lost in wound exudate.24
There is no doubt that burn patients require additional calories and proteins, but traditional formulae for calculating requirements produce overestimates in the context of current burn care.25 In the absence of direct metabolic measurements we currently use an empirical formula for estimating caloric intake:
where REE is the resting energy expenditure calculated from the Harris and Benedict equation.
Patients with extensive burns will require tube feeds, which are generally well tolerated. If gastric emptying is problematic, a post-pyloric tube is usually effective. A high-protein proprietary feed is usually adequate, but should be supplemented by extra trace elements and vitamins. The possible benefit of glutamine supplements in burns has recently been reviewed.26 Adequacy of feeding is best assessed by monitoring body weight. Adjunctive therapy aimed at promoting anabolism includes the use of insulin to maintain normoglycaemia, the administration of oxandrolone (10 mg twice daily for adults),27 and the use of β-blockers to control tachycardia and extensive catabolism.28
WOUND HEALING
Treatment of extensive, full-thickness burn wounds by early excision and grafting has been firmly linked to survival.29 Wound excision should be completed within the first week, before bacterial colonisation and neovascular infiltration of the wound bed develop. These operations are therefore urgent. Successfully grafted wounds will heal within 5 weeks, reducing the time available for bacterial infection to develop, and shortening the period of physiological disturbance. Wounds covered with widely meshed autografts lose large amounts of fluid, unless protected by a semipermeable layer, such as allograft skin. Autograft donor sites are a further source of fluid loss.
For wounds treated conservatively, the main effort is devoted to the prevention of infection. Topical antimicrobial agents are commonly used, but may have potential side-effects (Table 73.1). These compounds change the appearance of the wound and should never be applied until expert wound inspection is complete. A number of biosynthetic materials are currently available, which are designed to improve cosmetic and functional outcome. Despite the use of antiseptic dressings or biosynthetic coverings there is still a risk of microbial infection developing and unexplained signs of sepsis may necessitate urgent wound revision.
| Agent | Comments |
|---|---|
| Silver sulfadiazine (SSD) | The most widely used agent with broad spectrum cover. Hypersensitivity (rarely) and transient leucopenia have been reported |
| Cerium nitrate 0.5% | Often added to SSD, and forms a stable eschar. It is reported to bind ‘burn toxins’. Methaemoglobinaemia has been reported |
| Silver nitrate 0.5% | Applied as a soak, and is especially effective against Pseudomonas. However, it may increase sodium loss, and potentially can cause methaemoglobinaemia |
| Mafenide acetate 5–10% | Effective but short-lived antimicrobial, requiring repeated application. It has good penetration, and its side-effects (pain and metabolic acidosis) are less evident with 5% solution |
| Chlorhexidine | Aqueous solution (0.2%) or 1% gel provides broad spectrum cover, but is rapidly inactivated, and may cause local pain, and rarely causes hypersensitivity |
| Nitrofurazone | In polyethylene glycol (PEG) solution it is effective against S. aureus, but resistance develops early. Side-effects include hypersensitivity (common), hyperosmolarity, and renal failure due to PEG absorption has been reported |
| Povidone iodine | In PEG solution provides broad spectrum cover, but is rapidly inactivated. It prevents wound maceration. Side-effects include occasional hypersensitivity, renal dysfunction due to excessive PEG, metabolic acidosis and rarely dysfunction |
| Antibiotics | Have been used in solutions, creams, gels and sprays, but selection and development of resistant strains is inevitable, with a risk of systemic toxicity through absorption. Their usage is generally discouraged |
PREVENTION OF WOUND INFECTION
Bacterial infection is still the most common cause of death in burns. Depression of the immune system is well documented.30 At the same time, the burn wound presents a favourable medium for bacterial growth.
PATIENT ISOLATION
In an effort to reduce wound colonisation and contamination from cross-infection, barrier nursing of patients with extensive injuries is mandatory. The importance of isolation measures has been stressed,31 but a significant proportion of patients still become colonised by micro-organisms from endogenous reservoirs, which cannot be controlled by barrier nursing alone.32,33 Positive experiences have been reported with selective decontamination of the digestive tract,8,34 but large scale prospective trials have not been performed.
THE GASTROINTESTINAL TRACT
Loss of gut integrity following burn injury has been well demonstrated.35 In addition to reactive damage following reperfusion of the ischaemic gut, mediators derived from the burn wound itself may also be involved.36 Clinical strategies aimed at protecting the gastrointestinal tract include optimal fluid therapy during the first hours following injury to prevent mesenteric hypoperfusion, and the institution of early enteral nutrition, which can be safely commenced within a few hours of injury.20 Diets enriched with glutamine may contribute to the maintenance of gut integrity.26,37 Whatever the merits of each approach, all are secondary to the maintenance of effective hygienic policies in all aspects of patient care.
ANTIBIOTIC THERAPY
This is chosen on the basis of bacteriological surveillance data, and should be given early, if possible. Fluid therapy in burn patients is complicated by evaporative fluid loss, which may increase if the wound surface degenerates. Supportive therapy for (multi-) organ failure should be implemented as indicated.
INHALATION INJURY
HEAT INJURY TO THE UPPER AIRWAY
INHALATION OF TOXIC GASES
Of the many toxic compounds38 in smoke (Table 73.2), carbon monoxide (CO) deserves special mention. The affinity of CO for haemoglobin is 240 times that of oxygen. The loss of oxygen transport capacity is dependent on the concentration of inhaled CO and the duration of exposure. In addition, CO binds to cytochrome systems, inhibiting cellular oxidative processes. The half-life of COHb is 4 hours when breathing air, compared with 45 minutes when breathing 100% oxygen.
| Gas | Source | Effect |
|---|---|---|
| Carbon monoxide | Organic matter | Tissue hypoxia, lipid peroxidation |
| Carbon dioxide | Organic matter | Narcosis, tachycardia, hypertension |
| Nitrogen dioxide | Wallpaper, wood | Bronchial irritation, dizziness, pulmonary oedema |
| Hydrogen chloride | Plastics | Severe mucosal irritation |
| Hydrogen cyanide | Wood, silk, nylons, polyurethane | Headache, coma, acidosis |
| Benzene | Petrol, plastics | Mucosal irritation, coma |
| Ammonia | Nylon | Mucosal damage, extensive lung injury |
| Aldehyde | Wood, cotton, paper | Mucosal irritation |
HYDROGEN CYANIDE TOXICITY
Hydrogen cyanide (HCN) toxicity frequently occurs in conjunction with CO inhalation. Inhaled concentrations of 200 ppm are rapidly fatal and HCN may account for many deaths at fire accident scenes.39 Conventional treatment is as for CO intoxication. Removal by chelating agents, such as hydroxycobalamin or by the administration of sodium thiosulphate is also possible, but treatment would have to be immediate, based on a presumptive diagnosis, and is therefore not generally recommended.
1 Herndon DN, Blakeney PE. Teamwork for total burn care: achievements, directions and hopes. In: Herndon DN, editor. Total Burn Care. 2nd edn. London: WB Saunders; 2002:11-15.
2 Demling RH, Mazess RB, Witt RM. The study of burn wound edema using dichromatic absorptiometry. J Trauma. 1978;18:124-128.
3 Cioffi WG, De Meules JE, Gamelli RL. The effects of burn injury and fluid resuscitation on cardiac function in vitro. J Trauma. 1986;26:638-643.
4 Cioffi WG, Vaughan GM, Heironimus JD. Disassociation of blood volume and flow in regulation of salt and water balance in burn patients. Ann Surg. 1991;214:213-219.
5 Wilmore DW, Mason AD, Johnson DW. Effect of ambient temperature on heat production and heat loss in burn patients. J Appl Physiol. 1975;38:593-597.
6 Caldwell FT, Bowser GH, Crabtree JH. The effects of occlusive dressings on the energy expenditure of severely burned children. Ann Surg. 1981;193:579-591.
7 Waymack JP. Antibiotics and the post-burn hypermetabolic response. J Trauma. 1990;30:S30-35.
8 Mackie DP, van Hertum WAJ, Schumburg T. Prevention of infection in burns: preliminary experience with selective decontamination of the digestive tract in patients with extensive injuries. J Trauma. 1992;32:570-575.
9 Alexander D, Richard K, Morris S. Application of newer antibiotic concepts in the use of ciprofloxacin for treatment of infections in the burn patient. J Burn Care Rehab. 2001;22:S137.
10 Martyn JAJ, Greenblatt GS, Quinby WC. Diazepam kinetics following burn injury. Anesth Analg. 1983;62:293-297.
11 Martyn JAJ, Fukushima Y, Chon JY, et al. Muscle relaxants in burns, trauma, and critical illness. Int Anesthesiol Clin. 2006;44:123-143.
12 Davies JWL. Prompt cooling of the burned area: a review of the benefits and the effector mechanisms. Burns. 1982;9:1-6.
13 Ivy ME, Atweh NA, Palmer J. Intra-abdominal hypertension and abdominal compartment syndrome in burn patients. J Trauma. 2000;49:387-391.
14 Prekop R, Bardosova G, Simko S. Brain oedema in burned children. Acta Chir Plast. 1984;26:184-192.
15 Pruitt BAJr. Protection from excessive resuscitation: ‘pushing the pendulum back’. J Trauma. 2000;49:567-568.
16 Friedrich JB, Sullivan SR, Engrav LH. Is supra-Baxter resuscitation in burns patients a new phenomenon? Burns. 2004;39:583-590.
17 Holm C, Mayr M, Tegeler J. A clinical randomised study on the effects of invasive monitoring on burn shock resuscitation. Burns. 2004;30:798-807.
18 Settle JAD. Principles of replacement fluid therapy. In: Settle JAD, editor. Principles and Practice of Burns Management. London: Churchill Livingstone; 1996:217-222.
19 Carvajal HF. A physiologic approach to fluid therapy in severely burned children. Surg Gynecol Obstet. 1980;150:379-384.
20 Alexander JW, Gottschlich MM. Nutritional immunomodulation in burn patients. Crit Care Med. 1990;18:S149-153.
21 Choinière M, Melzak R, Rondeau J. The pain of burns: characteristics and correlates. J Trauma. 1989;29:1531-1539.
22 Taal LA, Faber AW, van Loey NEE. The abbreviated burn-specific anxiety scale: a multi-centre study. Burns. 1999;25:493-497.
23 Miller AC, Hickman LC, Lemasters GK. A distraction technique for control of burn pain. J Burn Care Rehabil. 1992;13:576-580.
24 Berger MM, Cavadini C, Chiolero R. Influence of large intakes of trace elements on recovery after burns. Nutrition. 1994;10:327-334.
25 Wolfe RR. Caloric requirements of the burned patients. J Trauma. 1981;21:712-714.
26 Windle EM. Glutamine supplementation in critical illness: evidence, recommendations, and implications for clinical practice in burn care. J Burn Care Rehab. 2006;27:764-772.
27 Wolf S, Edelman L, Kemalyan N. Effects of oxandrolone on outcome measures in the severely burned: a multicenter prospective double-blind trial. J Burn Care Res. 2006;27:131-141.
28 Pereira CT, Murphy KD, Herndon DN. Altering metabolism. J Burn Care Rehabil. 2005;26:194-199.
29 Muller MJ, Ralston D, Herndon DN. Operative wound management. In: Herndon DN, editor. Total Burn Care. 2nd edn. London: WB Saunders; 2002:170-182.
30 Abraham E. Physiologic stress and cellular ischemia: relationship to immunosuppression and susceptibility to sepsis. Crit Care Med. 1991;19:613-618.
31 McManus AT, Mason WF, McManus WF. A decade of reduced Gram negative infections and mortality. Abstracts, 5th European Burns Association Congress. 1992, M2.
32 Burke JF, Quimby WC, Bondoc CC. The contribution of a bacterially isolated environment to the prevention of infection in seriously burned patients. Ann Surg. 1977;186:377-387.
33 Lee JJ, Marvin JA, Heimbach DM. Infection control in a burns centre. J Burn Care Rehabil. 1990;11:575-580.
34 Manson WL, Klasen HJ, Sauer EW. Selective intestinal decontamination for prevention of wound colonization in severely burned patients: a retrospective analysis. Burns. 1992;18:98-102.
35 Deitch EA. Intestinal permeability is increased in burn patients shortly after injury. Surgery. 1990;107:411-416.
36 Trop M, Schiffrin EJ, Carter EA. Effect of platelet activating factor on reticulo-endothelial system function. Burns. 1991;17:193-197.
37 Peng X, Yan H, You Z, et al. Glutamine granule-supplemented enteral nutrition maintains immunological function in severely burned patients. Burns. 2006;32:589-593.
38 Prien T, Traber DL. Toxic smoke compounds and inhalation injury – a review. Burns. 1988;14:451-460.
39 Purser DA, Grimshaw P, Berril KR. Intoxication by cyanide in fires: a study in monkeys using polyacrylonitrile. Arch Environmental Health. 1984;39:394-400.