Chapter 26 Bundle Branch Reentrant Ventricular Tachycardia
Pathophysiology
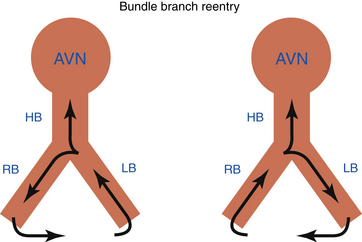
Bundle branch reentrant (BBR) ventricular tachycardia (VT) is a reentrant VT with a well-defined reentry circuit, incorporating the right bundle branch (RB) and left bundle branch (LB) as obligatory limbs of the circuit, connected proximally by the His bundle (HB) and distally by the ventricular septal myocardium (Fig. 26-1).
Single BBR beats can be induced in up to 50% of patients with normal intraventricular conduction undergoing electrophysiological (EP) study. The QRS during BBR can display either left bundle branch block (LBBB) or right bundle branch block (RBBB) when anterograde ventricular activation occurs over the RB or LB, respectively. The vast majority have LBBB configuration. BBR with an LBBB pattern can also occur occasionally during right ventricular (RV) pacing. This requires that the effective refractory period of the LB be longer than that of the RB, or that retrograde conduction over the RB be resumed after an initial bilateral block in the His-Purkinje system (HPS) (i.e., gap phenomenon). Left ventricular (LV) pacing does not seem to increase the yield of induction of BBR with RBBB morphology.1
In patients with normal intraventricular conduction, BBR is a self-limited phenomenon. The rapid conduction and long refractory period of the HPS prevent sustained BBR in normal hearts. Spontaneous termination of BBR most commonly occurs in the retrograde limb between the ventricular muscle and the HB.1 Sometimes, anterograde block can also occur, making refractoriness in the RB-Purkinje system the limiting factor. Continuation of BBR as a tachycardia is critically dependent on the interplay between conduction velocity and recovery of the tissue ahead of the reentrant wavefront. Two changes from normal physiology must occur for BBR to become sustained: (1) an anatomically longer reentrant pathway caused by a dilated heart, providing sufficiently longer conduction time around the HPS; and (2) slow conduction in the HPS caused by HPS disease.1 These two factors are responsible for sufficient prolongation of conduction time to permit expiration of the refractory period of the HPS ahead of the propagating reentrant wavefront.
Clinical Considerations
Epidemiology
Sustained BBR VT usually occurs in patients with structural heart disease, especially dilated cardiomyopathy. Idiopathic dilated cardiomyopathy is the anatomical substrate for BBR VT in 45% of cases, and BBR VT accounts for up to 41% of all inducible sustained VTs in this population. BBR VT can also be associated with cardiomyopathy secondary to valvular or ischemic heart disease, and has been reported with Ebstein anomaly, hypertrophic cardiomyopathy, and even in patients without structural heart disease other than intraventricular conduction abnormalities.2
In patients with spontaneous sustained monomorphic VT, the incidence of inducible BBR VT ranges from 4.5% to 6% in patients with ischemic heart disease to 16.7% to 41% in patients with nonischemic cardiomyopathy. BBR VT accounts for up to 6% of all forms of induced sustained monomorphic VT. Importantly, in patients with BBR VT, additional myocardial VTs occur in 25%.3 Of note, BBR VT is more frequently found in patients with VT clusters (up to 12.5%) than in patients with less frequent episodes of VT.4
Principles of Management
Associated myocardial VT occurs in approximately 25% of patients post ablation, and these patients continue to be at a high risk of sudden cardiac death. Therefore, implantable cardioverter-defibrillator (ICD) therapy is indicated for secondary prevention, and additional antiarrhythmic therapy is required for some patients. ICD implantation will also provide back-up pacing, which is frequently required post ablation secondary to the development of atrioventricular (AV) block or an excessively prolonged His bundle–ventricular (HV) interval. Implantation of a dual-chamber or biventricular ICD should be considered in these patients.
EP testing should be considered in patients with repetitive episodes of VT and dilated cardiomyopathy, history of cardiac valve repair or replacement, or QRS morphology during VT similar to sinus rhythm QRS. If sustained BBR VT is inducible during programmed stimulation, catheter ablation is recommended.4
Electrocardiographic Features
Baseline ECG
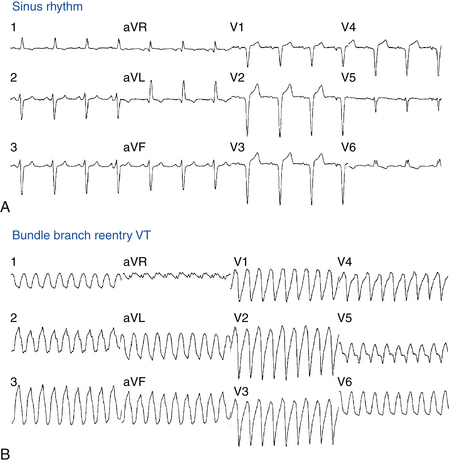
The baseline rhythm is usually NSR or atrial fibrillation (AF). Almost all patients with BBR VT demonstrate intraventricular conduction abnormalities. The most common ECG abnormality is nonspecific intraventricular conduction delay (IVCD) with an LBBB pattern and PR interval prolongation (Fig. 26-2). Complete RBBB is rare but does not preclude BBR as the mechanism of VT. Although total interruption of conduction in one of the bundle branches would theoretically prevent occurrence of BBR, an ECG pattern of complete BBB may not be an accurate marker of complete conduction block; a similar QRS configuration can be caused by conduction delay, rather than block, in the bundle branch. Occasionally, complete AV block may be observed.2
ECG during Ventricular Tachycardia
Twelve-lead ECG documentation of BBR VT is usually unavailable because the VT is rapid and hemodynamically unstable. The VT rate is usually 180 to 300 beats/min. QRS morphology during VT is a typical BBB pattern and can be identical to that in NSR. BBR VT with an LBBB pattern is the most common VT morphology, and it usually has normal or left axis deviation (see Fig. 26-2). In contrast to VT of myocardial origin, BBR with an LBBB pattern characteristically shows a rapid intrinsicoid deflection in the right precordial leads, suggesting that initial ventricular activation occurs through the HPS and not ventricular muscle. BBR VT with an RBBB pattern usually has a leftward axis, but it can have a normal or rightward axis, depending on which fascicle is used for anterograde propagation.1
Electrophysiological Testing
Baseline Observations during Normal Sinus Rhythm
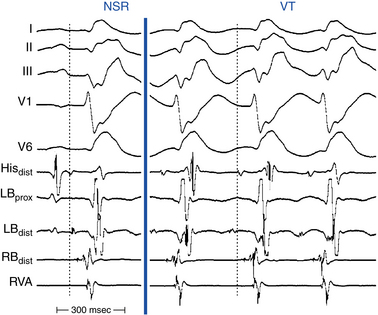
Conduction abnormalities in the HPS are almost invariably present and are a critical prerequisite for the development of sustained BBR, regardless of the underlying anatomical substrate (Fig. 26-3).5 The average HV interval is about 80 milliseconds (range, 60 to 110 milliseconds). Although some patients can have the HV interval in NSR within normal limits, functional HPS impairment in these patients manifests as HV interval prolongation or split HB potentials, commonly becoming evident during atrial programmed stimulation or burst pacing. Nonspecific IVCD with an LBBB pattern and PR interval prolongation are the most common abnormalities.2
Induction of Tachycardia
During RV pacing at a constant cycle length (CL) and during introduction of VES at relatively long coupling intervals, retrograde conduction to the HB occurs via the RB. At shorter VES coupling intervals, retrograde delay and block occur in the RB when its relative and effective refractory periods are encountered, respectively. When retrograde block occurs in the RB, the impulse propagates across the septum and retrogradely up the LB to the HB, producing a long V2-H2 interval. The LB would still be capable of retrograde conduction because of its shorter refractoriness and because of the delay associated with transseptal propagation. Further shortening of the coupling intervals is associated with increasing delay in LB conduction (i.e., increasing V2-H2 interval). Within a certain range of coupling intervals, increasing retrograde LB delay allows for the recovery of anterograde conduction via the RB, and another ventricular activation ensues, displaying a wide QRS with an LBBB pattern. This beat is called the “BBR beat” or “V3 phenomenon.”2
BBR is more likely to occur when the VES is delivered following pacing drives incorporating long to short CL changes as compared with constant CL drives, because of CL dependency of the HPS refractoriness. An abrupt change in CL (i.e., long to short) can result in a more distal site of retrograde block, and less concealment, along the myocardium-Purkinje-RB axis, which can allow sufficient recovery of excitability in the anterograde limb of the circuit (i.e., the RB-Purkinje-myocardium axis) for reentry to develop. In addition, earlier recovery of excitability along this axis, because of the more distal site of block and less concealment, is associated with a shorter H2-V3 interval in this reentrant beat.1
Procainamide, which increases conduction time within the HPS, especially in the diseased HPS, and, potentially, isoproterenol can facilitate induction of sustained BBR. In some patients, the arrhythmia can be inducible only with atrial pacing.2
Tachycardia Features
BBR VT can only be diagnosed using intracardiac recording (Table 26-1). AV dissociation is typically present, during the tachycardia, but 1:1 ventriculoatrial (VA) conduction can occur. BBR VT is characterized by inscription of the His potential before the QRS complex, with the HV interval during BBR with an LBBB pattern generally being similar to or longer than that during baseline rhythm (the HV interval is usually 55 to 160 milliseconds; see Fig. 26-3). However, in rare cases, the HV interval during BBR VT can be slightly shorter (by less than 15 milliseconds) than the HV interval in NSR, because during BBR the HB is activated in the retrograde direction simultaneously (in parallel) with the proximal part of the bundle branch serving as the anterograde limb of the reentry circuit, whereas during NSR, activation of the HB and activation of the bundle branch occur in sequence.1
TABLE 26-1 Diagnostic Criteria of Bundle Branch Reentrant Ventricular Tachycardia
BBR = bundle branch reentry; HB = His bundle; HPS = His–Purkinje system; HV = His bundle–ventricular; LB = left bundle branch; LBBB = left bundle branch block; LB-V = left bundle–ventricular; RB = right bundle branch; RBBB = right bundle branch block; RB-V = right bundle–ventricular.
The relative duration of the HV interval recorded during VT as compared with NSR would depend on two factors: the balance between anterograde and retrograde conduction times from the upper turnaround point of the reentry circuit, and the site of HB recording relative to the upper turnaround point (i.e., the HB catheter electrode positioned at the proximal versus distal HB). Conduction delay in the bundle branch used as the anterograde limb of the circuit tends to prolong the HV interval during VT, whereas retrograde conduction delay to the HB recording site, as well as the use of a relatively proximal HB recording site (far from the turnaround point), tend to shorten the HV interval. The right bundle–ventricular (RB-V) interval during VT with LBBB morphology must always be longer than that recorded in sinus rhythm, emphasizing the importance of recording the RB potential during VT.1 The HV interval during BBR with an RBBB pattern can be significantly different from that during NSR (HV interval, 65 to 250 milliseconds). During NSR, the HV interval is usually determined by conduction over whichever bundle branch conducts most rapidly, whereas during BBR it is determined by conduction over the typically diseased LB.
During BBR VT, spontaneous variations in the V-V intervals are preceded and dictated by similar changes in the H-H (and RB-RB or LB-LB) intervals. In other words, the VT CL is affected by variation in the V-H (V-RB or V-LB) intervals. These changes can occur spontaneously, most commonly immediately after induction of the VT, or be demonstrated by ventricular stimulation during VT. However, oscillations in the V-V intervals can occasionally precede those of the H-H intervals during BBR VT because of conduction variations in the anterograde, rather than the retrograde, conducting bundle branch.3
Recording from both sides of the septum can help to identify the BBR mechanism. Documentation of a typical H-RB-V-LB activation sequence (during VT with LBBB morphology), or of an H-LB-V-RB activation sequence (during VT with RBBB morphology), supports the diagnosis of BBR (see Fig. 26-3).1 Unfortunately, the RB potentials, LB potentials, or both are not always recorded, so that the typical activation sequences (LB-H-RB-V or RB-H-LB-V) are not available for analysis. Even if either activation sequence is present, the HPS (usually the LB) could be activated passively in a retrograde fashion to produce an H-RB-V sequence during a VT with LBBB pattern without reentry requiring the LB. In these cases, other diagnostic criteria for BBR should be used. In addition, during VT with LBBB morphology, RV activation must precede the LV activation. The opposite is true for the VT with RBBB morphology.
Diagnostic Maneuvers during Tachycardia
Entrainment
BBR VT can be entrained by ventricular pacing. Entrainment with manifest QRS fusion during ventricular pacing is classic for BBR VT. Entrainment with concealed fusion can be demonstrated by pacing at the LB or RB (i.e., within the reentrant circuit). During entrainment from the RV apex, a post-pacing interval (PPI) – VT CL difference of greater than 30 milliseconds excludes a BBR mechanism, provided that the RV apex catheter is correctly positioned. The greater this value is, the more reliable is the certainty of exclusion. Conversely, a p[PPI – VT CL] difference of less than 30 milliseconds is consistent with, but not diagnostic of, a BBR mechanism.3
Other Pacing Maneuvers
The ability to dissociate the HB or, particularly, a bundle branch (RB or LB) potential strongly argues against a BBR mechanism. An atrial extrastimulus (AES) that blocks below the HB deflection should terminate BBR. Additionally, simultaneous LV and RV pacing should prevent BBR.2
Exclusion of Other Arrhythmia Mechanisms
Myocardial Ventricular Tachycardias
Myocardial VTs are rarely associated with classic LBBB (or RBBB) QRS morphology, as is expected with BBR VT. Additionally, in myocardial VTs, the His potential is often obscured within the local ventricular electrogram. Occasionally, the His potential may precede the onset of the QRS, thus resembling BBR VT; however, the HV interval in myocardial VTs is usually shorter than that during NSR. In contrast to BBR VT, spontaneous or induced tachycardia CL variations during myocardial VTs usually precede and dictate H-H interval changes.3
Supraventricular Tachycardia With Aberrancy
The atrium is not part of the circuit in the BBR VT and can be dissociated by atrial pacing, which excludes AT and AVRT as the mechanism of wide complex tachycardia. Moreover, during entrainment from the RV apex, a [PPI – tachycardia CL] difference of greater than 30 milliseconds excludes a BBR mechanism. Conversely, a [PPI – tachycardia CL] difference of less than 30 milliseconds excludes atrioventricular nodal reentrant tachycardia (AVNRT). Additionally, entrainment with manifest fusion during ventricular pacing excludes AVNRT and AT and should be expected in BBR VT. Furthermore, the ability to terminate or reset the tachycardia with a VES introduced when HB is refractory excludes AT and AVNRT.
Antidromic Atrioventricular Reentrant Tachycardia Using An Atriofascicular Bypass Tract
During antidromic AVRT, the impulse propagates retrogradely over the RB to the HB, a sequence inconsistent with BBR with an LBBB pattern. The AV relationship is always 1:1 during AVRT and rarely so during BBR VT. Because the atrium is not part of the circuit in BBR VT, it can be dissociated by atrial pacing, which excludes AVRT. Moreover, resetting the tachycardia with an AES from the right atrial (RA) free wall, timed when the AV junctional atrium is refractory, excludes VT. Similarly, the ability of an AES to terminate the tachycardia without conduction to the ventricle or to retard (delay) the following ventricular activation excludes VT (see Figs. 18-28 and 19-4).
Ablation
Target of Ablation
The ablation target is either the RB or the LB; however, RB ablation is easier and usually is the method of choice. In most patients with BBR VT, although diffuse conduction system disease is present, the conduction abnormality in the LB is more severe than that in the RB. Nonetheless, preserved slow conduction over the LB can be demonstrated in the majority of patients, and the LB can still maintain 1:1 AV conduction during NSR.6 For the occasional patients in whom anterograde conduction down the LB is known to be inadequate for maintaining AV conduction, ablation of the RB will commit the patient to a permanent pacemaker. Therefore, ablation of the LB in these patients is preferable because it can prevent BBR and still preserve anterograde conduction.
Ablation Technique
Ablation of the Right Bundle Branch
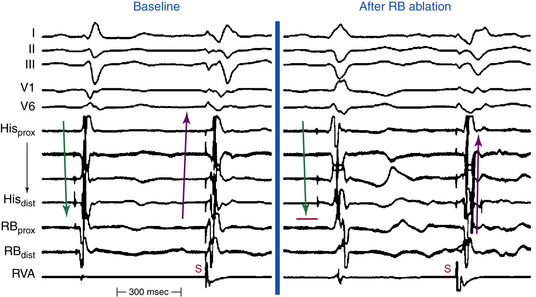
The RB potential can be distinguished from the HB potential by the absence of or minimal atrial electrogram on the recording and presence of a sharp deflection inscribed at least 15 to 20 milliseconds later than the His potential (Fig. 26-4). An RB-V interval value of less than 30 milliseconds may not be a reliable marker of the RB potential in these patients because disease of the HPS can cause prolongation of the RB-V conduction time.
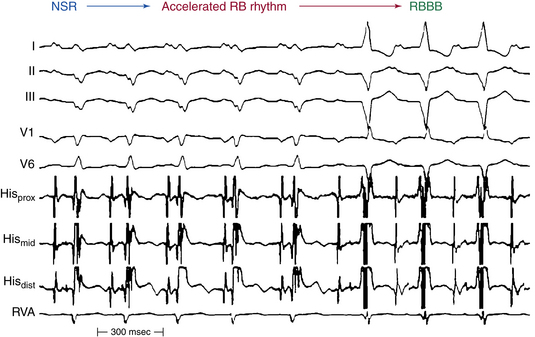
A 4-mm-tip ablation catheter is typically used for RB ablation. RF application is usually started at low levels (5 W) and gradually increased every 10 seconds, targeting a temperature of 60°C. In general, RBBB develops at 15 to 20 W. Successful ablation will result in clear development of RBBB in lead V1 (Fig. 26-5; and see Fig. 26-4). Occasionally, an accelerated rhythm from the RB is observed during ablation (analogous to accelerated junctional rhythm with HB ablation; see Fig. 26-5).
Ablation of the Left Bundle Branch
Whereas the RB is anatomically a continuation of the HB, the LB arises as a broad band of fibers from the HB in a perpendicular direction toward the inferior septum. The main LB penetrates the membranous portion of the interventricular septum under the aortic ring and then divides into several fairly discrete branches. The LPF arises more proximally than the LAF, appears as an extension of the main LB, and is large in its initial course. It then fans out extensively posteriorly toward the papillary muscle and inferoposteriorly to the free wall of the LV. The LAF crosses the LV outflow region and terminates in the Purkinje system of the anterolateral wall of the LV. In the RAO view, the LPF extends from the HB region toward the inferior diaphragmatic wall and the LAF extends toward the apex of the heart. However, considerable variability exists.
Endpoints of Ablation
Endpoints of RB ablation include the development of an RBBB pattern (see Fig. 26-5), noninducibility of BBR, and reversal of the direction of HB and RB activation during RV pacing. Prior to ablation, HB electrodes are depolarized during RV pacing from distal to proximal (RB-HB); after RB ablation, HB activation is delayed and reversed (HB-RB; see Fig. 26-4).
After ablation, aggressive ventricular stimulation should be performed to evaluate the inducibility of VT. Also, decremental atrial pacing should be performed to evaluate the conduction properties of the HPS and the propensity for infra-Hisian AV block. It is advisable to stress the HPS with intravenous procainamide and ensure that anterograde conduction is preserved.3
Interfascicular Reentrant Ventricular Tachycardia
Of the two types of macroreentry in the HPS (i.e., BBR and interfascicular reentry), BBR is by far, the most common mechanism of VT.7 VT secondary to interfascicular reentry is extremely rare; when it does occur, it is most commonly seen in patients with coronary artery disease, specifically those with anterior myocardial infarction (MI) with LAF or LPF block. In these patients, RBBB is complete and bidirectional, so true BBR cannot occur. Additionally, there is slow conduction in the apparently blocked fascicle.2 Of note, interfascicular reentrant VT can develop in patients following ablation of the RB for the treatment of BBR VT.7
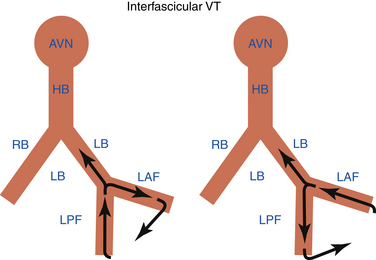
Interfascicular reentry incorporates the LAF and LPF as obligatory limbs of the circuit, connected proximally by the main trunk of the LB and distally by the ventricular myocardium (Fig. 26-6). The tachycardia usually has an RBBB morphology. The orientation of the frontal plane axis is variable and depends on the direction of propagation in the reentrant circuit. Anterograde activation over the LAF and retrograde through the LPF would be associated with right axis deviation, whereas the reversed activation sequence shows left axis deviation (see Fig. 26-6).1,2
In contrast to BBR VT, the HV interval during interfascicular VT is usually shorter by more than 40 milliseconds than that recorded in NSR.1 This is because the upper turnaround point of the circuit, the distal end of the LB bifurcation point, is relatively far from the retrogradely activated HB. During interfascicular VT, the LB potential should be inscribed before the His potential. In contrast, during BBR VT with RBBB morphology, the His potential usually precedes the LB potential, although the reverse is theoretically possible if the retrograde conduction time to the HB recording point is significantly prolonged. Interfascicular reentry also demonstrates variations in the V-V interval preceded by similar changes in the H-H interval.
Atrial pacing, AES, VES, and ventricular pacing can initiate interfascicular reentry by producing transient anterograde block in the slowly conducting fascicle (LAF or LPF), with subsequent impulse anterograde conduction over the healthy fascicle, giving rise to a QRS morphology identical to that during NSR, and then retrogradely in the initially blocked fascicle to initiate reentry. Compared with BBR VT, interfascicular VT can be more difficult to induce by ventricular pacing, because of the inability to create the necessary EP conditions for this type of reentry during ventricular stimulation (i.e., retrograde block in the distal LAF and slow conduction via the LPF, or vice versa), although this may occur during anterograde penetration of the HPS by supraventricular impulses (i.e., anterograde block in the LPF and slow conduction in the LAF, or vice versa).7
Successful ablation of the arrhythmia can be performed by targeting the diseased fascicle (LAF or LPF), guided by fascicular potentials.2 When interfascicular reentry occurs in the setting of an anterior MI, complete cure by ablation is usually not possible because other myocardial VTs are almost always present, and the LV ejection fraction is usually poor, thereby mandating ICD implantation for improved survival. When interfascicular reentry occurs without coronary artery disease, in association with degenerative disease of the conduction system, LV systolic function is usually normal and cure of the VT is possible by ablation of the diseased fascicle, although implantation of a permanent pacemaker will likely be required.
1. Josephson M.E. Recurrent ventricular tachycardia. In: Josephson M.E., editor. Clinical cardiac electrophysiology. ed 4. Philadelphia: Lippincott Williams & Wilkins; 2008:446-642.
2. Daoud E.G. Bundle branch reentry. In: Zipes D., Jalife J., editors. Cardiac electrophysiology: from cell to bedside. ed 4. Philadelphia: WB Saunders; 2004:683-688.
3. Balasundaram R., Rao H.B., Kalavakolanu S., Narasimhan C. Catheter ablation of bundle branch reentrant ventricular tachycardia. Heart Rhythm. 2008;5(6 Suppl):S68-S72.
4. Sakata T., Tanner H., Stuber T., Delacretaz E. His-Purkinje system re-entry in patients with clustering ventricular tachycardia episodes. Europace. 2008;10:289-293.
5. Lopera G., Stevenson W.G., Soejima K., et al. Identification and ablation of three types of ventricular tachycardia involving the His-Purkinje system in patients with heart disease. J Cardiovasc Electrophysiol. 2004;15:52-58.
6. Schmidt B., Tang M., Chun K.R., et al. Left bundle branch–Purkinje system in patients with bundle branch reentrant tachycardia: lessons from catheter ablation and electroanatomic mapping. Heart Rhythm. 2009;6:51-58.
7. Blanck Z., Sra J., Akhtar M. Incessant interfascicular reentrant ventricular tachycardia as a result of catheter ablation of the right bundle branch: case report and review of the literature. J Cardiovasc Electrophysiol. 2009;20:1279-1283.