Brucella
1. Identify the primary routes of transmission for Brucella spp.
2. Name the populations at risk for developing brucellosis.
3. Identify the signs and symptoms associated with brucellosis.
4. State two reasons the microbiology laboratory should be notified when Brucella infection is suspected.
5. Describe the principle and procedure for the rapid test for presumptive identification of Brucella spp.
6. Describe the differential characteristics of Brucella abortus, Brucella melitensis, Brucella suis, and Brucella canis.
Epidemiology and Pathogenesis
Each of the four Brucella spp. that are pathogenic for humans has a limited number of preferred animal hosts (Table 36-1). In the host, Brucella tend to localize in tissues rich in erythritol (e.g., placental tissue), a four-carbon alcohol that enhances their growth. Humans become infected by four primary routes:
TABLE 36-1
Brucella spp. and Their Respective Natural Animal Hosts
| Organism | Preferred Animal Host |
| B. abortus | Cattle |
| B. melitensis | Sheep or goats |
| B. suis | Swine |
| B. canis | Dogs |
| B. ovis | Rams (not associated with human infection) |
| B. neotomae | Desert and wood rats (not associated with human infection) |
• Ingestion of infected unpasteurized animal milk products (most common means of transmission)
• Inhalation of infected aerosolized particles (laboratory-acquired infection is the most important source of transmission)
• Direct contact with infected animal parts through ruptures of skin and mucous membranes
• Accidental inoculation of mucous membranes by aerosolization
Survival and multiplication of Brucella organisms in phagocytic cells are features essential to the establishment, development, and chronicity of the disease. The mechanisms by which brucellae avoid intracellular killing are not completely understood. Brucella spp. can change from a smooth to a rough colonial morphology based on the composition of their cell wall lipopolysaccharide O-side chain (LPS); those with a smooth LPS are more resistant to intracellular killing by neutrophils than those with a rough LPS. The smooth phenotype has been identified in B. abortus and B. melitensis. Brucellae ensure intracellular survival by interfering with the phagosome-lysosome fusion in macrophages and epithelial cells. In addition, as do Legionella spp. (see Chapter 35), brucellae use a type IV secretion system, VirB, for intracellular survival and replication. Unlike Legionella spp., however, brucellae modulate phagosome transport to avoid being delivered to lysosomes. Essentially, VirB is involved in controlling the maturation of the Brucella vacuole into an organelle that allows replication. In the mouse model, if mutations occur in this gene region, B. abortus is unable to establish chronic infections. In addition, Brucella spp. produce urease, which provides protection during passage through the digestive system when the organism is ingested in food products. Urease breaks down urea, producing ammonia, and neutralizes the gastric pH. Despite our current knowledge, many questions remain about the pathogenesis of disease caused by Brucella spp.
Laboratory Diagnosis
Specimen Collection, Transport, and Processing
• To ensure that specimens are cultivated in an appropriate manner for optimum recovery from clinical specimens
• To avoid accidental exposure of laboratory personnel handling the specimens, because Brucella spp. are considered class III pathogens (specimen labels should indicate that Brucella spp. are a potential pathogen)
Blood for culture can be collected routinely (see Chapter 68) into most commercially available blood culture bottles and the lysis-centrifugation system (Isolator; Alere, Waltham, MA). For other clinical specimens, no special requirements must be met for collection, transport, or processing.
Approach to Identification
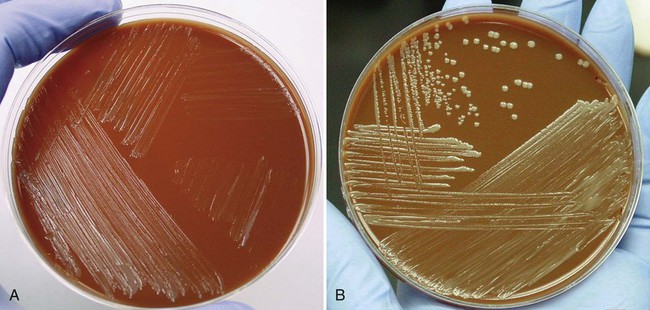
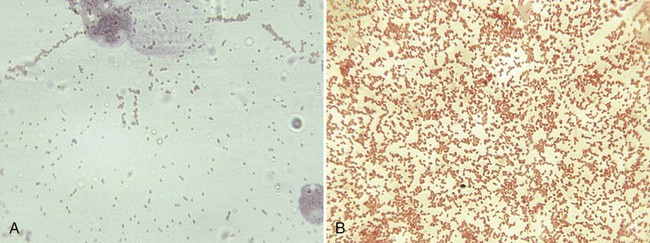
Because brucellosis is the most commonly reported laboratory-acquired bacterial infection, all handling and manipulations of suspected Brucella spp. should be performed in a class II or higher biologic safety cabinet. On Gram stain, the organisms are small coccobacilli that resemble fine grains of sand (Figure 36-2). Brucella spp. are catalase and urease positive, and most strains are oxidase positive. Other nonfermentative gram-negative coccobacilli that may be confused with brucellae are Bordetella, Moraxella, Kingella, and Acinetobacter spp. Brucella spp., however, are nonmotile, urease and nitrate positive, and strictly aerobic. The most rapid test for presumptive identification of Brucella spp. is the particle agglutination test with anti–smooth Brucella serum (Difco Laboratories, Detroit, Michigan).
Brucella spp. are differentiated by the rapidity with which an organism hydrolyzes urea, its relative ability to produce hydrogen sulfide (H2S), its requirements for CO2, and its susceptibility to the aniline dyes thionine and basic fuchsin (Table 36-2). For determination of the CO2 requirement, identical plates of Brucella agar or brain-heart infusion agar should be given equal inocula (e.g., with a calibrated loop) of a broth suspension of the organism to be tested. One plate should be incubated in a candle jar and the other plate in air in the same incubator. Most strains of B. abortus do not grow in air but show growth in the candle jar. Presumptive identification can be reported based on the colony’s morphology and a positive catalase, oxidase, urease, and slide agglutination reaction (see next section). Brucella isolates should be sent to state or other reference laboratories for confirmation or definitive identification, because most clinical laboratories lack the necessary media and containment facilities.
TABLE 36-2
Characteristics of Brucella spp. That Are Pathogenic for Humans
| INHIBITION BY DYE | |||||
| Species | CO2 Required for Growth | Time to Positive Urease | H2S Produced | Thionine* | Fuchsin* |
| B. abortus | ± | 2 hr (rare 24 hr) | + (most strains) | + | − |
| B. melitensis | − | 2 hr (rare 24 hr) | + | − | − |
| B. suis | − | 15 min | ± | − | + (most) |
| B. canis | − | 15 min | − | − | + |
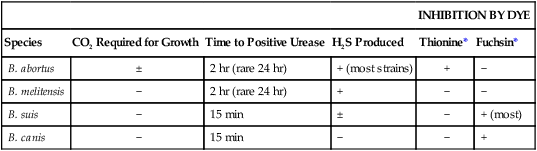
+, >90% of strains positive; − >90% of strains negative; ±, variable results.