Breast disease and mammography
10.1
Mammographic features of breast lesions
| Lesion characteristics | Benign | Malignant |
| Opacity | Smooth margin | Ill-defined margin, stellate |
| Speculate, comet tail | ||
| Low density | High density | |
| Homogeneous | Inhomogeneous | |
| Thin ‘halo’ | Wide ‘halo’ | |
| Calcification (see 10.3) | ± | ± |
| Surrounding parenchyma | Normal | Disrupted |
| Nipple/areola | ± Retracted | ± Retracted |
| Skin | Normal | ± Thickened |
| Cooper ligaments | Normal | May be thickened/increased |
| Ducts | Normal | Focal dilatation |
| Subcutaneous/retromammary space | Normal | ± Obliterated |
10.2
Calcification
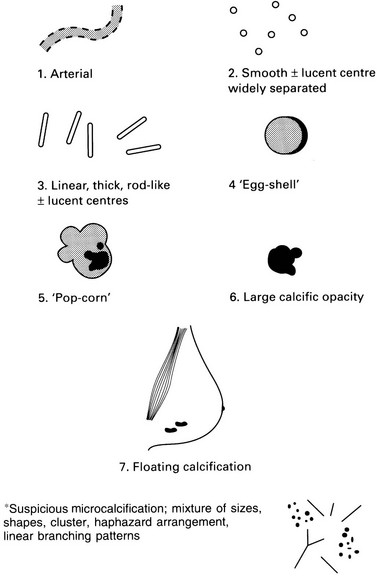
| Definitely benign (see figure, p. 247) | Probably benign | Suspicious of malignancy |
| Arterial, tortuous, tramline (1) Smooth, widely separated radiolucent centre (2) Linear, thick, rod-like ± radiolucent centre (3) Egg-shell, curvilinear margin of cyst, fat necrosis (4) Pop-corn (fibroadenoma) (5) Large individual > 2 mm (6) Floating, seen on lateral oblique, milk of calcium cysts (7) |
Widespread, both breasts Macrocalcification of one size Symmetrical distribution Widely separated Superficial distribution Normal breast parenchyma |
Microcalcification, segmental* Pleomorphic, linear, branching, punctuate* Associated suspicious soft-tissue opacity Eccentrically located in soft-tissue mass Deterioration on serial mammography |
Examples of definitely benign calcification:
10.4
Benign lesions with typical appearances
1. Fibroadenoma – rounded, lobulated, well-defined homogeneously dense soft-tissue opacity with eccentrically sited ‘pop-corn’ calcification.
2. Intramammary lymph node – well-defined, approximately 1.0 cm in diameter soft-tissue opacity, often with an eccentric radiolucency situated most often in the upper outer quadrant of the breast.
3. Lipoma – large, rounded, radiolucent, well-defined.
4. Lipid cyst – well-defined, multiple, lucent, ‘egg-shell’ calcification.
5. Hamartoma/fibroadenolipoma – ‘breast within a breast’ appearance on a mammogram.
10.5
Single well-defined soft-tissue opacity
Benign
3. Intramammary lymph node – intramammary lymph nodes occur in up to 40% of breasts. Causes include breast cancer, lymphoma, melanoma, regional inflammation/dermatitis, fungal infection, tuberculosis/granulomatous disease, foreign body reactions, e.g. gold injections for rheumatoid arthritis, silicone adenopathy, HIV and sinus histiocytosis.
Malignant
1. Cystosarcoma phylloides – usually large, may be benign but have malignant potential (5–10%), calcification rare, median age 45–49, rare < 30 or > 60. High tendency to recur, both in benign and malignant. Malignant lesions metastasize to lung and bone and may invade chest wall.
2. Carcinoma – a small group of carcinomas looks ‘benign’ on mammography; medullary, encephaloid, mucoid, papillary.
10.6
Multiple well-defined soft-tissue opacities
2. Fibroadenomas – 10–20% are multiple.
3. Skin lesions – e.g. neurofibromas.
5. Metastases – melanoma most common, lymphoma second most common non-mammary breast tumour, then lung, ovarian, soft-tissue sarcomas, gastrointestinal/genitourinary malignancy, carcinoid and sporadically thyroid, osteosarcoma, cervical, vaginal and endometrial. Mean survival after diagnosis of metastasis within the breast is < 1 year.
10.8
Benign conditions that mimic malignancy
2. Suspicious soft-tissue opacity
(a) Summation of normal tissues.
(b) Fibroadenoma/cyst – when one margin ill-defined.
(c) Fat necrosis – ill-defined, sometimes with radiolucent centre.
(e) Radial scar – 22% of excised radial scars show invasive cancer or DCIS not diagnosed at core biopsy and therefore excision generally recommended. Carries a 2-fold higher risk of developing a subsequent breast cancer in either breast; increases to 4–5-fold higher risk if associated atypical ductal hyperplasia.
10.9
Risk factors for breast cancer
1. Gender – 100 times more common in women.
2. Age – majority of cases occur after age of 50.
3. Family history and genetic factors – see below.
4. Personal history of breast cancer – 3–4-fold higher risk of developing a new cancer in the other breast or another part of the same breast.
5. Previous chest irradiation – women who received mantle radiotherapy for Hodgkin’s and non-Hodgkin’s lymphoma have significantly higher risk and should undergo annual MRI screening.
6. Early menarche, late menopause, nulliparity and having first child after the age of 30 associated with slightly raised risk.
Family and genetic factors
1. First-degree relative (mother, sister, daughter) approximately doubles risk, two first-degree relatives increase risk 5-fold; however, 70–80% of women who develop breast cancer do not have a family history.
2. Genetic factors – 5–10% of all breast cancers, of which BRCA1 mutation accounts for 20–40%, chromosome 17, AD, lifetime risk of breast cancer is 50–85%. BRCA2 mutation accounts for 10–30%, chromosome 13, AD, lifetime risk of breast cancer is 50–85%. P53 mutation accounts for < 1%. Li Fraumeni syndrome: rare cause of breast cancer but individuals have a greater than 90% lifetime risk of developing breast cancer and at a young age. PTEN mutations account for < 1%, Cowden syndrome, 25–50% lifetime risk of breast cancer. 30–70% due to other gene mutations.
10.11
Ultrasound in breast disease
Uses and indications
1. Assessing a palpable abnormality
(a) In combination with mammography 97% sensitivity, 98.6% negative predictive value.
2. Assessing a mammographic abnormality.
3. Assessment of nipple discharge, diagnosis of papilloma; DCIS may be diagnosed with US.
4. Biopsy guidance, aspiration of cysts/breast abscesses.
5. Lesion localization for surgery, skin marking and wire localization of impalpable tumours.
6. Preoperative staging of the axilla. May preclude sentinel node imaging and avoid unnecessary second surgery for nodal clearance.
7. Distinguishing local recurrence from surgical scar.
8. Targeted/second-look US for MRI-detected abnormality. 60% of MRI-detected lesions are seen on targeted US. Masses are more likely to be found than non-masses; increasing size increases US conspicuity. Malignant lesions are more likely to be seen.
9. US vacuum-assisted biopsy guidance for the removal of benign lesions.
| Typical appearances of simple cysts | Typical appearances of carcinoma |
| Round/oval in shape | Irregular mass |
| Well-defined | Ill-defined |
| Anechoic | Heterogeneous internal echo pattern |
| Posterior wall enhancement | Absent ‘far wall’ echoes |
| Posterior acoustic enhancement | Posterior acoustic shadowing |
10.12
Appearances and features of fibroadenomas
1. Most common benign breast tumour and most common solid mass in those aged < 35 years. Multiple in 10–20% and bilateral in 4%.
3. On US generally oval, but may be round, homogeneous echotexture hypoechoic/isoechoic. 2–4% contain small cystic foci. Posterior enhancement variable and can have posterior acoustic shadowing when hyalinized or contain calcium.
4. Tend to regress with age, undergo myxoid degeneration giving pathognomonic ‘pop-corn’ calcification on mammography.
5. Juvenile fibroadenomas occur most commonly between 10 and 20 years, but majority of teenage fibroadenomas are of adult type. Juvenile fibroadenomas usually solitary.
6. Giant fibroadenomas are > 5 cm, may grow to 15 cm, more common in African-American women.
7. Fibroadenomas are well described on treatment with cyclosporin A following renal transplantation, and may be single, multiple or giant in type.
10.13
Gynaecomastia
1. 85% of all breast masses in males.
3. Trimodal distribution – neonatal, pubertal and senescence.
4. Differential diagnosis includes pseudogynaecomastia (fat rather than glandular tissue), diabetic mastopathy and male breast cancer.
Causes
1. Drugs – alcohol, amiodarone, alkylating agents, amphetamines, anabolic steroids, captopril, cimetidine, cocaine, diazepam, digoxin, haloperidol, heroin, izoniazid, marijuana, metronidazole, nifedipine, omeprazole, phenytoin, spironolactone, tricyclic antidepressants, thiazides, verapamil.
2. Systemic causes – chronic renal failure, cirrhosis, HIV, hypothyroidism and hyperthyroidism, refeeding gynaecomastia following nutritional deprivation.
3. Tumours – germ cell, Leydig, Sertoli tumours; adrenal, liver, lung and renal (secondary to ectopic hCG secretion).
10.14
MRI in breast disease
Indications
1. Evaluate local extent of cancer in those anticipating breast conservation when tumour size uncertain on conventional imaging.
2. Lobular carcinoma – typically mammographically occult and may be multifocal/bilateral.
3. Metastatic axillary adenopathy of unknown primary to identify occult breast cancer.
4. High-risk screening – those with history of mantle radiotherapy or genetic mutation.
10.15
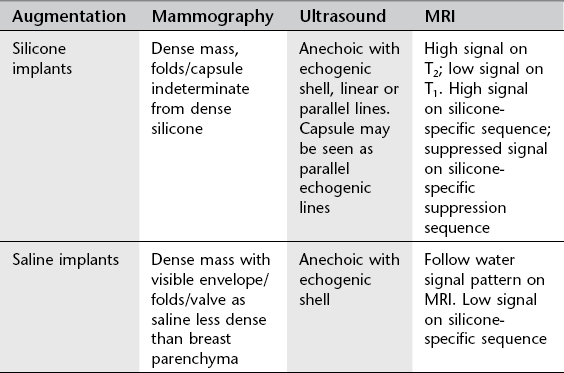
Breast augmentation
Types of breast augmentation
Key features of implants
1. Increased risk of rupture over time, median time to rupture 8–11 years for all implants, faster if subpectoral. Most silicone implant ruptures are asymptomatic. 80% of ruptures are intracapsular.
2. US findings of intracapsular rupture include the stepladder sign, stacked echogenic lines corresponding to the displaced envelope/shell within the anechoic silicone, which remains contained by the fibrous capsule.
3. Extracapsular rupture on US is seen as the ‘snowstorm’ appearance of extracapsular silicone. Snowstorm echogenic silicone nodes in the axilla.
4. Extracapsular silicone can be seen in patients with residual silicone from previous implant rupture, herniation of gel through focally weakened capsule where the implant shell remains intact, direct injections of silicone oil and with gel bleed alone without rupture (extremely rare).
5. MR findings include the linguine sign of layers of collapsed shell within the implant and the teardrop or keyhole sign with more focal separation of the shell in early implant rupture.
10.16
CT and pet CT in breast imaging
1. PET/CT has a higher sensitivity and specificity than conventional CT in identifying asymptomatic distant metastases.
2. PET ± CT is not reliable for the diagnosis of primary breast cancer and has a low sensitivity for detection of axillary node metastases, but it is highly specific for malignancy when positive axillary nodes are identified.
3. Body CT for indications other than breast carcinoma identifies incidental breast lesions. 24–32% of these will ultimately be proven to be malignant and referral for triple assessment is advocated. Spiculation, irregular margin, rim enhancement and associated axillary adenopathy are suggestive of malignancy. Associated calcification has not been shown to be discriminatory.
Berg, W. A., Birdwell, R. L., Gombos, E., et al. Diagnostic imaging: breast. Salt Lake City, UT: Amirsys; 2006.
Brennan, M. E., Houssami, N. Evaluation of the evidence on staging imaging for detection of asymptomatic distant metastases in newly diagnosed breast cancer. Breast. 2012; 21(2):112–123.
Morrow, M., Waters, J., Morris, E. MRI for breast cancer screening, diagnosis and treatment. Lancet. 2011; 378(9805):1804–1811.
Moyle, P., Sonoda, L., Britton, P., Sinnatamby, R. Incidental breast lesions on CT: what is their significance? Br J Radiol. 2010; 83:233–240.
Venkataraman, S., Hines, N., Slanetz, P. J. Challenges in mammography: Part 2, Multimodality review of breast augmentation – imaging findings and complications. AJR Am J Roentgenol. 2011; 197:W1031–1045.