CHAPTER 98 Brain Tumor Stem Cells
Brain tumor stem cells (BTSCs), a small subset of cells found within multiple types of central nervous system (CNS) malignancies, possess two unique qualities that distinguish them from other cells in the tumor stroma: (1) they have all the properties needed to fulfill the criteria for defining stem cells, namely, the ability to self-renew and to differentiate into the three lineages of nervous tissue (astrocytes, oligodendrocytes, and neurons) under appropriate conditions and (2) they have the ability, on injection into the brain of non–immune-competent animals, to initiate brain tumors that recapitulate the growth and infiltrative patterns of the tumor of origin.1,2 The BTSC hypothesis is dependent on the idea that there exists a pool of cells in all brain tumors that contain the necessary genetic programming for tumorigenesis, whereas the remaining cells serve as non–tumor-initiating constituents of the malignant tissue.3 The origin of these BTSCs has yet to be clearly identified, with some implicating abnormal transformation of naturally occurring neural stem cells (NSCs) as the primary source.2 Others speculate that more dedicated cells along the path of differentiation can “revert” and initiate the critical functions of self-regeneration and aberrant proliferation.4 The BTSC hypothesis has some important clinical implications: cancer-initiating stem cells exhibit strong migratory behavior5 and are exquisitely radioresistant and chemoresistant.6 This may serve to explain the local metastatic potential and resistance to treatment of the majority of malignant brain tumors. Rather than attempting to destroy the bulk of the tumor with the antineoplastic agents available today, some research groups have begun to focus on finding a method of selectively targeting this small, yet deadly, pool of tumorigenic BTSCs. Given the numerous similarities existing between normal stem cells and BTSCs, we can potentially exploit the bulk of knowledge about normal stem cell biology to identify possible novel molecular targets that could arrest BTSC proliferation.2 Some examples of therapeutic modalities include inhibitors of the sonic hedgehog (SHH) pathway (required for the proliferation of stem cells),7 induction of terminal differentiation of BTSCs with bone morphogenetic protein [BMP]),8 and disruption of the critical angiogenic niche of BTSCs.9 This chapter primarily reviews the concept of the neurosphere assay, evolution and development of the BTSC hypothesis, basic phenotypic properties of BTSCs, the role of molecular markers implicated in isolating these cells, the intracellular pathways used to maintain “stemness,” their relationship to NSCs, and finally, the clinical application of this hypothesis.
The Neurosphere Assay and Discovery of Adult Neurogenesis
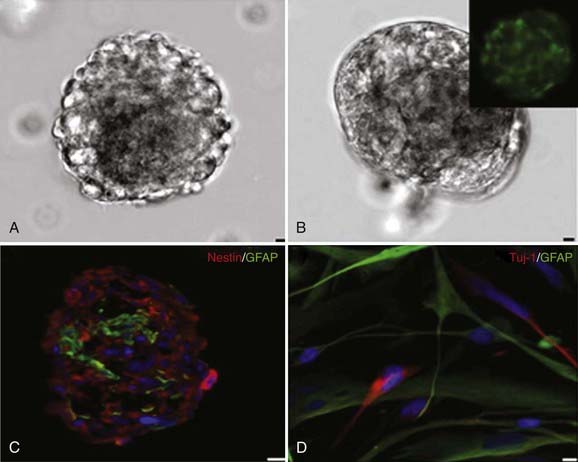
Advent of the neurosphere assay, originally developed for the isolation of adult NSCs, was critical to the identification of cancer-initiating stem cells from human brain tumors. It was originally believed that the adult brain did not retain the ability to produce new neurons in the postnatal period but was rather a static organ.2,10 Altman was the first to challenge this view when he used tritiated thymidine to monitor dividing cells in restricted areas of the neonatal rat brain in 1962.11 Altman was able to show that these dividing cells eventually differentiated into cells that morphologically resembled neurons.11 Kaplan and Hinds confirmed these observations in the 1970s by using electron microscopy to show that the postnatally dividing cells in the brain contained dendrites and axons.12 Introduction of the neurosphere assay, developed by Reynolds and Weiss, allowed in vitro isolation of normal stem cells from adult and fetal brain tissue (Fig. 98-1).13 In this assay, brain tissue is dissociated into single cells and cultured in special media containing various mitogens (i.e., basic fibroblast growth factor, epidermal growth factor).14 Under these conditions, NSCs form a ball of cells called “neurospheres” that contain the parent NSC and its differentiated progeny: neurons, oligodendrocytes, and astrocytes. The self-renewal capacity of these NSCs is confirmed by dissociating the neurospheres and plating individual cells per well with neurosphere media. The neurosphere-forming ability of each cell from the original neurosphere is then assessed to determine “stemness.” This assay allowed identification and isolation of adult NSCs in the subventricular zone (SVZ) of the lateral ventricles and the subgranular layer of the hippocampal dentate gyrus.13,15
It is now recognized that there is a strict hierarchy in the largest of these germinal centers, the SVZ, where adult stem cells systematically differentiate into more dedicated progenitor cells.16 NSCs are found as quiescent type B cells that give rise to the surrounding and more rapidly dividing type C cells. These cells subsequently differentiate into type A cells (neuroblasts), which surround the ventricles, separated only by a single layer of ependymal cells, and directly divide to form new neurons in the adult brain.17 Adult neurogenesis has been implicated in the formation of new neuronal connections and contributes to memory formation in the normal adult brain. More essential for our discussion, adult NSCs are also important because they are believed to undergo malignant transformation into BTSCs.18
Development of the Brain Tumor Stem Cell Hypothesis
For decades, the traditional belief in oncology maintained that human tumors represented a collection of cells in which a majority (or all) had the intrinsic ability to initiate tumorigenesis. The concept that only a small fraction of cells was ultimately responsible for tumorigenesis was originally proposed in 1963, when Bruce and Van Der Gaag recognized the ability of a small number of lymphoma cells to rapidly proliferate and differentiate in vivo.19 The theory was not supported until the advent of more sophisticated research tools that allowed scientists to recognize that not only were leukemia cells clonal in origin20 but also that cancer cells maintained a hierarchical organization similar to embryonic tissues, with only a small subset of proliferating progenitors replenishing the nonregenerating leukemia cells.21,22 This concept quickly spread to other types of malignancies, where it was noted that only a subset of cells from human breast,23 pancreas,24 prostate,25 head and neck,26 and colon27 cancer tissue had the ability to initiate tumorigenesis on transplantation.
Ignatova and colleagues described the isolation of neurosphere-forming, bipotent (neuronal/astroglial) precursors from glioblastoma multiforme (GBM).28 This was subsequently confirmed and extended by Singh and coworkers, who also demonstrated that bipotent lineage-restricted progenitors from CNS tumors display short-term self-renewal (three passages in culture).18 Similar findings were reported by Hemmati and coauthors, who described the absence of oligodendrocytes in these cells and also addressed the issue of tumorigenicity by demonstrating tumor formation after intracerebral transplantation of tumor-derived cells.29
Galli and colleagues took these simple observations further by showing that neurosphere-forming cells could also act as cancer-initiating cells and can establish tumors that closely resemble the main histologic, cytologic, and architectural features of the human disease even after multiple serial transplantations.30 This was later confirmed by Singh and associates with a different method of isolating BTSCs (using the NSC marker CD133, to be discussed later).31 Unlike NSC neurospheres, however, BTSC neurospheres consisted of abnormally differentiated cells with a genetic profile similar to that of the original tumor and a significantly increased proliferation rate.18,28–30,32,33 The number of in vitro neurospheres formed in a particular sample of human brain cancer was shown to be correlated with the more rapidly growing, aggressive tumor in vivo, thus suggesting that a poorer clinical outcome was associated with a greater number of brain tumor–perpetuating stem cells.34 These experiments suggested that not all cells within brain tumors had the ability to initiate tumorigenesis.18,29–31,35 Rather, brain tumors represented a hierarchy of malignant cells, with stem cells at the top of the hierarchy “differentiating” into the aberrant tumor stromal cells at the bottom. This basic organization was similar to normal adult neurogenesis, with a small colony of NSCs replenishing the cells of the SVZ11,12 and hippocampus.36 If these BTSCs were “differentiating” into malignant tumor stromal cells, it could be speculated that these less dedicated cells along the path of differentiation must express cell surface markers that vary from their progeny.32 This is supported by recent evidence suggesting that after multiple in vitro passages of primary GBM, the resulting cells are the product of an outgrowth of cell clones that have accumulated profound “de novo” genetic or epigenetic changes, or both. These clones do not share the same genetic profile or growth characteristics of the parent tumor.32
Markers for Neural Stem Cells and Brain Tumor Stem Cells
Nestin is an intermediate filament protein that is produced in both NSCs and progenitor cells during development.37 This molecule has been implicated as important in the morphology and adhesive properties of NSCs and is dramatically downregulated when NSCs differentiate into more committed lines. Interestingly, this marker is upregulated in brain tissue during times of injury, ischemia, and inflammation, which suggests that nestin-labeled cells may be responsible for remodeling of adult brain tissue after damage.38–40 Nestin has also been identified in a variety of human brain tumors, including ependymomas, malignant astrocytomas, oligodendrogliomas,41 and malignant gliomas.42 Nestin-positive cells from multiple brain cancer types have been shown to have significantly enhanced migration and invasive properties43 and readily form neurospheres. More importantly, this marker is a potential candidate for assessing malignant potential, with clinically malignant tissues exhibiting enhanced nestin expression.42 These results suggest that the number of proliferating nestin-labeled cells, or BTSCs, may predict a patient’s clinical outcome.
CD133 (prominin-1) is a cell membrane glycoprotein originally identified on primitive hematopoietic stem cells. It is a five-transmembrane protein that contains two large extracellular loops; it is found on various types of stem cells and known to be downregulated on differentiated cells.44,45 This marker was later identified in murine postnatal brain tissue,45 where cells sorted selectively for CD133 were able to both form neurospheres in vitro and differentiate into both neuronal and glial cell types, thereby fulfilling the definition of an NSC. Although its natural function is unknown, some speculate that CD133 has a role in the dynamic organization of cell surface protrusions. There is mounting evidence suggesting that adult NSCs and BTSCs share this cell surface marker. Singh and coworkers were the first to show that like adult NSCs, CD133-positive cells isolated from human glioblastomas and human medulloblastomas were able to form neurospheres in vitro.31 As few as 100 CD133-positive brain tumor–derived cells were necessary to successfully form tumors in immunodeficient mice, whereas more than 105 CD133-negative cells failed to drive tumor formation.31 Although this evidence points toward CD133 as a putative marker for BTSCs, more recent evidence has complicated the issue. Wang and coauthors reported that CD133-negative cells are also able to form tumors and give rise to CD133-positive cells, which correlated with poor survival in rat models.46 There exists a considerable amount of genetic heterogeneity in brain tumors, and it is likely that there may be multiple convergent pathways leading to the formation of brain tumors. As observed in leukemias and bone marrow stem cells, multiple markers are probably necessary to adequately characterize BTSCs.46,47
Cell of Origin of Brain Tumor Stem Cells
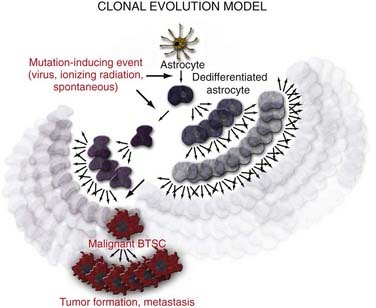
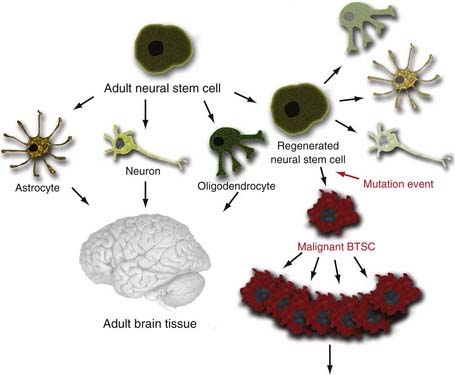
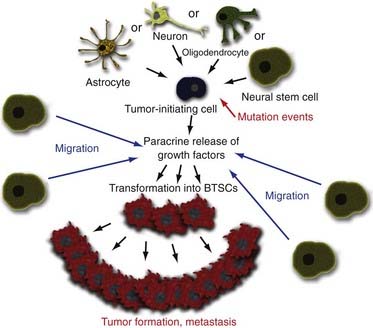
There are three possible explanations for the origins of BTSCs. The traditional clonal evolution hypothesis (Fig. 98-2) argues that brain tumors arise from dedifferentiation of dedicated adult brain cells.48 The subsequent clonal expansion of this single dedifferentiated cell and the accumulation of mutations over time confer the progeny with the ability to proliferate and regenerate in an unregulated manner. This process results in eventual formation of the BTSC, which produces the primary tumor focus and migrates locally to cause metastatic disease. With the discovery of adult NSC populations and the observation that BTSCs behave much like adult NSCs in terms of organization, proliferation, and regeneration, it was hypothesized that abnormal differentiation of adult NSCs may be the direct source of human brain tumors (Fig. 98-3).49 Finally, the progenitor recruitment hypothesis argues that a single tumor progenitor cell of undefined origin has the ability to recruit normally quiescent neural progenitor cells and induce them to proliferate in an unregulated manner via the release of local growth factors (Fig. 98-4). Definitive proof has yet to be found regarding which hypothesis is dominant in the natural pathogenesis of human brain tumors, and there is significant experimental evidence to support all three alternatives.
Considerable evidence supports the idea that dedifferentiation and accumulation of genetic mutations within terminally committed adult brain cells may in fact be the source of human BTSCs. Bachoo and associates were the first to show that mature astrocytes are just as likely as NSCs to contribute to the genesis of glioma if they acquire key genetic mutations, such as loss of the cell cycle regulator Ink4a-Arf.4 GBM can be induced in both NSCs and mature astrocytes by overexpression of the signal transduction protein Ras in an Ink4a-Arf–deficient background. C-myc overexpression in glial fibrillary acidic protein (GFAP)-positive cells is able to induce loss of GFAP expression (astrocyte lineage), which is replaced by nestin-positive markers.4 Finally, Dai and colleagues have shown that when transfecting GFAP-positive cells with a virus expressing platelet-derived growth factor (a molecule that maintains glial precursors in an undifferentiated state), there is a significant increase in the formation of gliomas.50 Transfection of nestin-positive cells with the identical virus also results in the formation of gliomas at a moderately higher rate (40% versus 70%).50 These observations suggest that differentiated cells have the potential to contribute to tumorigenesis if they acquire crucial genetic mutations that allow them to “revert” to a less differentiated state.
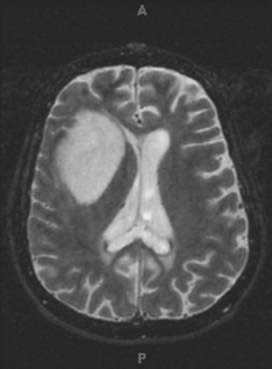
The striking phenotypic similarities between NSCs and BTSCs make it tempting to conclude that BTSCs arise directly from the abnormal differentiation of NSCs. Germinal centers containing adult NSCs have been considered a source of human gliomas because of the clinical observation that gliomas tend to be either periventricular or contiguous with the SVZ (Fig. 98-5).51,52 Strong evidence in animal models supports the idea that regions of the brain with a high degree of cellular proliferation are consequently more susceptible to malignant transformation by chemical or viral exposure.53,54 Rats implanted with a pellet of carcinogenic hydrocarbons were shown to have increased tumor formation when the pellet was placed closer to the NSC-rich SVZ region rather than the cerebral cortex.55 Young rats inoculated with an avian sarcoma virus in the SVZ had a much higher rate of tumor formation than did older rats with a presumably more limited population of NSCs.56 It was also observed that when canine SVZs were inoculated with avian sarcoma, the gliomas that initially formed in the periventricular region eventually grew out of the region into the deep white matter and lost their apparent connection to the SVZ.54 This may serve to explain the presence of gliomas found in humans that seemingly have no apparent connection to the SVZ. Additionally, expression of oncogenes under direction of the nestin (NSC) promoter rather than the GFAP (differentiated astrocyte) promoter resulted in more efficient transformation into glial tumors.57 These observations, combined with the fact that the longevity of NSCs may allow the accumulation of genetic mutations over time, make NSCs an appealing candidate as the originator of human brain tumors.58
Rather than the simplified theory of a single BTSC giving rise to human brain tumors, more complicated pathophysiology may be at work. Morphologic and genetic data suggest that primary brain tumors contain cells of various origins.59 It has previously been established that brain tumors locally release multiple growth factors, or chemoattractants,60 and have been implicated in forming defined angiogenic niches within the tumor stroma.9 Additionally, normal adult NSCs have an uncanny ability to migrate selectively toward brain tumors.61 This suggests that the tumor “cell of origin” may not directly divide to form the tumor mass. Rather, this brain tumor–initiating cell may work to form tumors by locally attracting normal adult NSCs into a special BTSC niche. The tumor-initiating cells then release a hoard of growth factors locally into the surrounding area that induce the once normal NSCs to proliferate in an unregulated manner. This unregulated growth subsequently leads to formation of the tumor mass.
Molecular Pathways Disrupted in Brain Tumor Stem Cells
NSCs and BTSCs share many of the phenotypic properties essential for their maintenance and proliferation, thus making it plausible that these two sets of cells share similar intracellular molecular pathways.1 Numerous genes and signaling pathways have already been described as being involved in the self-renewal properties of stem cells. This section focuses on two of the best characterized pathways that have been implicated in the formation of brain tumors: the SHH and Notch pathways.62–64 These biochemical pathways have been implicated to be important in the pathogenesis of medulloblastomas in particular because of the fact that they play a role in maintaining a small subset of BTSCs in an undifferentiated state and thereby controlling proliferation.49 A different set of molecular pathways (not discussed in this chapter) are thought to be disrupted in gliomas, such as the EGFR/PTEN/Akt/mTOR,65 TP53/MDM2/p14ARF,66 or p16INK4a/RB1 pathway.67
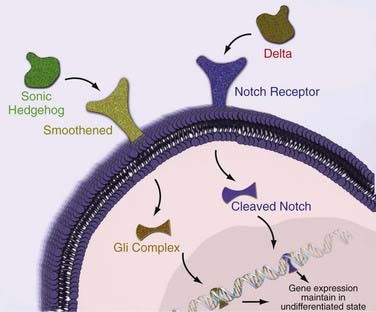
Shh is one of three secreted proteins in the SHH family that plays a key role in mammalian CNS organization during embryogenesis (i.e., regulation of the differentiation pattern of ventral cell types during embryogenesis) and is prominently expressed in the adult cerebellum and hippocampus.68 Shh activates a cell surface receptor that results in the activation of transcription factors of the GLI family (Fig. 98-6).69 This transcription cascade regulates the number of cells with stem cell properties in the germinal centers of the adult brain (SVZ, hippocampus)70–72 while maintaining adult NSCs in an undifferentiated state when injected into chick cerebellum.69 Activation of the SHH pathway and loss of regulation have been shown to result in the formation of medulloblastomas in mice,73 and several human brain tumor lines consistently express GLI genes. Cyclopamine, a plant-derived alkaloid that selectively inhibits the SHH-GLI pathway, significantly reduces the growth of human medulloblastomas and gliomas (Fig. 98-7).7,68
Notch signaling has also been implicated in the formation of brain tumors. When activated by Delta family proteins, the Notch receptor releases an intracellular domain that subsequently activates helix-loop-helix (HLH) transcription factors (see Fig. 98-6). Notch signaling has been shown to be required for maintenance of NSCs in the adult brain, with Notch-deficient mice exhibiting enhanced neuronal and glial differentiation.74 Consequently, experimental evidence has shown that Notch signaling is essential for the growth and maintenance of medulloblastomas.75 Blockade of the Notch pathway in medulloblastomas results in decreased proliferation and increased neuronal differentiation of cells.75 More importantly, when injected into mice, there was a reduced frequency of tumor formation from the Notch-inhibited batch of cells than from the Notch-active cells, thus suggesting that Notch signaling is essential for tumorigenesis.76 Researchers noted that when blocking the Notch cascade, the number of CD133-positive cells was reduced fivefold.76 This suggests that the loss of tumorigenesis was due to depletion of BTSCs as a result of the inability to maintain an undifferentiated state with the help of Notch.76
Clinical Implications and Future Directions
BTSCs are emerging as an important new target for the treatment of human brain tumors. The BTSC hypothesis predicts that even if a tumor is reduced in size by surgical or chemical means, if the stem cell regenerating that tumor is spared, the tumor will regrow and the patient will relapse.58 The failure of current chemotherapy and radiotherapy in some patients may reflect the resistance pattern of these particular BTSCs to standard treatments. Glioma, for example, is clinically treated with external beam radiation therapy and a concurrent alkylating agent (temozolomide), with survival of patients remaining at a dismal 14.9 months.77,78 Experimental evidence has demonstrated that after subjecting mice xenografts to external beam irradiation, the population of CD133-positive cells was increased, whereas the CD133-negative cells were diminished, thus suggesting that irradiation fails to destroy the BTSCs.79 Furthermore, Bao and colleagues demonstrated that CD133-positive cells were able to activate the DNA damage checkpoint in response to irradiation and exhibited enhanced repair of radiation-induced DNA damage when compared with their CD133-negative counterparts.79 Additionally, Hegi’s group was able to show that CD133-positive cells exhibited reduced silencing of the DNA repair gene MGMT80 and that inactivation of this gene by methylation was an independent prognostic indicator for survival.81
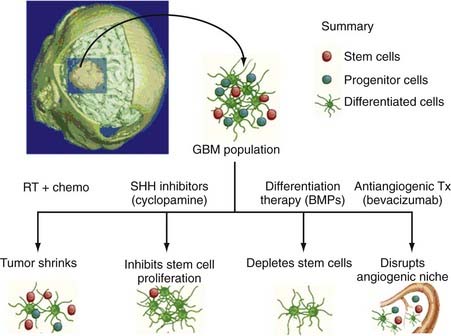
Emphasis on diminishing the population of tumorigenic BTSCs rather than the nonproliferative/transiently proliferative constituents of the tumor stroma can theoretically result in enhanced survival. Identifying the specific intracellular pathways that are used by BTSCs for growth and proliferation may lead to the identification of chemical agents that can selectively block these pathways and destroy BTSCs. Alternatively, inducing the differentiation of BTSCs into more benign, non–tumor-initiating astrocytes has been suggested as a therapeutic strategy (Fig. 98-7). Piccirillo and colleagues have shown that BMP can reduce the number of CD133-positive cells, thereby effectively blocking tumor growth and resulting in enhanced survival of mice by nearly 80% (Fig. 98-7).8
Recent evidence has also suggested that the vasculature of brain tumors creates special niches in which BTSCs are encouraged to flourish and perpetuate.9 Increasing the number of endothelial cells in xenografts of brain tumors resulted in the expansion of BTSCs, whereas inhibition of endothelial cell production by small interfering RNA (siRNA)-based vascular endothelial cell growth factor inhibitors resulted in a significant reduction in these tumorigenic stem cells.9 This suggests that the BTSC niche may be critical for the maintenance of malignancies and that disruption of this critical niche may be a potential therapeutic option (Fig. 98-7).
Practical applications may soon come from the study of BTSCs. The BTSC neurosphere assay has the potential to help tailor chemotherapy regimens for individual patients.81 The kinetic properties of BTSCs seen in vitro may aid in diagnosis and help advise the surgeon about the optimal aggressiveness of treatment. Phillips and coworkers have described certain genetic signatures in BTSCs that predict poor survival,82 and Hau and colleagues have also recently described the methylation status of various DNA repair genes as a practical prognostic indicator.81
Aboody KS, Brown A, Rainov NG, et al. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci U S A. 2000;97:12846.
Altman J. Autoradiographic study of degenerative and regenerative proliferation of neuroglia cells with tritiated thymidine. Exp Neurol. 1962;5:302.
Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319.
Alvarez-Buylla A, Seri B, Doetsch F. Identification of neural stem cells in the adult vertebrate brain. Brain Res Bull. 2002;57:751.
Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756.
Calabrese C, Poppleton H, Kocak M, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69.
Chaichana KL, McGirt MJ, Frazier J, et al. Relationship of glioblastoma multiforme to the lateral ventricles predicts survival following tumor resection. J Neuro Oncol. 2008;89:219.
Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17:5046.
Fomchenko EI, Holland EC. Origins of brain tumors—a disease of stem cells? Nat Clin Pract Neurol. 2006;2:288.
Galli R, Binda E, Orfanelli U, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011.
Holland EC, Hively WP, DePinho RA, et al. A constitutively active epidermal growth factor receptor cooperates with disruption of G1 cell-cycle arrest pathways to induce glioma-like lesions in mice. Genes Dev. 1998;12:3675.
Ignatova TN, Kukekov VG, Laywell ED, et al. Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia. 2002;39:193.
Kaplan MS, Hinds JW. Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science. 1977;197:1092.
Quinones-Hinojosa A, Chaichana K. The human subventricular zone: a source of new cells and a potential source of brain tumors. Exp Neurol. 2007;205:313.
Quinones-Hinojosa A, Sanai N, Soriano-Navarro M, et al. Cellular composition and cytoarchitecture of the adult human subventricular zone: a niche of neural stem cells. J Comp Neurol. 2006;494:415.
Ruiz i Altaba A, Sanchez P, Dahmane N. Gli and hedgehog in cancer: tumours, embryos and stem cells. Nat Rev Cancer. 2002;2:361.
Sanai N, Alvarez-Buylla A, Berger MS. Neural stem cells and the origin of gliomas. N Engl J Med. 2005;353:811.
Singh SK, Clarke ID, Hide T, et al. Cancer stem cells in nervous system tumors. Oncogene. 2004;23:7267.
Singh SK, Clarke ID, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821.
Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396.
Stecca B, Ruiz i Altaba A. Brain as a paradigm of organ growth: Hedgehog-Gli signaling in neural stem cells and brain tumors. J Neurobiol. 2005;64:476.
Wang J, Sakariassen PO, Tsinkalovsky O, et al. CD133 negative glioma cells form tumors in nude rats and give rise to CD133 positive cells. Int J Cancer. 2008;122:761.
Wang JC, Dick JE. Cancer stem cells: lessons from leukemia. Trends Cell Biol. 2005;15:494.
Yuan X, Curtin J, Xiong Y, et al. Isolation of cancer stem cells from adult glioblastoma multiforme. Oncogene. 2004;23:9392.
1 Galderisi U, Cipollaro M, Giordano A. Stem cells and brain cancer. Cell Death Differ. 2006;13:5.
2 Quinones-Hinojosa A, Chaichana K. The human subventricular zone: a source of new cells and a potential source of brain tumors. Exp Neurol. 2007;205:313.
3 Clarke MF, Dick JE, Dirks PB, et al. Cancer stem cells—perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339.
4 Bachoo RM, Maher EA, Ligon KL, et al. Epidermal growth factor receptor and Ink4a/Arf: convergent mechanisms governing terminal differentiation and transformation along the neural stem cell to astrocyte axis. Cancer Cell. 2002;1:269.
5 Xie Z, Chin LS. Molecular and cell biology of brain tumor stem cells: lessons from neural progenitor/stem cells. Neurosurg Focus. 2008;24(3-4):E25.
6 Sakariassen PO, Immervoll H, Chekenya M. Cancer stem cells as mediators of treatment resistance in brain tumors: status and controversies. Neoplasia. 2007;9:882.
7 Bar EE, Chaudhry A, Lin A, et al. Cyclopamine-mediated hedgehog pathway inhibition depletes stem-like cancer cells in glioblastoma. Stem Cells. 2007;25:2524.
8 Piccirillo SG, Reynolds BA, Zanetti N, et al. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature. 2006;444:761.
9 Calabrese C, Poppleton H, Kocak M, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69.
10 Quinones-Hinojosa A, Sanai N, Soriano-Navarro M, et al. Cellular composition and cytoarchitecture of the adult human subventricular zone: a niche of neural stem cells. J Comp Neurol. 2006;494:415.
11 Altman J. Autoradiographic study of degenerative and regenerative proliferation of neuroglia cells with tritiated thymidine. Exp Neurol. 1962;5:302.
12 Kaplan MS, Hinds JW. Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science. 1977;197:1092.
13 Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707.
14 Reynolds BA, Tetzlaff W, Weiss S. A multipotent EGF-responsive striatal embryonic progenitor cell produces neurons and astrocytes. J Neurosci. 1992;12:4565.
15 Alvarez-Buylla A, Seri B, Doetsch F. Identification of neural stem cells in the adult vertebrate brain. Brain Res Bull. 2002;57:751.
16 Lois C, Garcia-Verdugo JM, Alvarez-Buylla A. Chain migration of neuronal precursors. Science. 1996;271:978.
17 Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17:5046.
18 Singh SK, Clarke ID, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821.
19 Bruce WR, Van Der Gaag H. A quantitative assay for the number of murine lymphoma cells capable of proliferation in vivo. Nature. 1963;199:79.
20 Fialkow PJ, Singer JW, Adamson JW, et al. Acute nonlymphocytic leukemia: heterogeneity of stem cell origin. Blood. 1981;57:1068.
21 Griffin JD, Linch D, Sabbath K, et al. A monoclonal antibody reactive with normal and leukemic human myeloid progenitor cells. Leuk Res. 1984;8:521.
22 Wang JC, Dick JE. Cancer stem cells: lessons from leukemia. Trends Cell Biol. 2005;15:494.
23 Al-Hajj M, Wicha MS, Benito-Hernandez A, et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983.
24 Esposito I, Kleeff J, Bischoff SC, et al. The stem cell factor–c-kit system and mast cells in human pancreatic cancer. Lab Invest. 2002;82:1481.
25 Collins AT, Berry PA, Hyde C, et al. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946.
26 Prince ME, Sivanandan R, Kaczorowski A, et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci U S A. 2007;104:973.
27 Ricci-Vitiani L, Lombardi DG, Pilozzi E, et al. Identification and expansion of human colon-cancer–initiating cells. Nature. 2007;445:111.
28 Ignatova TN, Kukekov VG, Laywell ED, et al. Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia. 2002;39:193.
29 Hemmati HD, Nakano I, Lazareff JA, et al. Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci U S A. 2003;100:15178.
30 Galli R, Binda E, Orfanelli U, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011.
31 Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396.
32 Lee J, Kotliarova S, Kotliarov Y, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9:391.
33 Tunici P, Bissola L, Lualdi E, et al. Genetic alterations and in vivo tumorigenicity of neurospheres derived from an adult glioblastoma. Mol Cancer. 2004;3:25.
34 Zeppernick F, Ahmadi R, Campos B, et al. Stem cell marker CD133 affects clinical outcome in glioma patients. Clin Cancer Res. 2008;14:123.
35 Yuan X, Curtin J, Xiong Y, et al. Isolation of cancer stem cells from adult glioblastoma multiforme. Oncogene. 2004;23:9392.
36 Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319.
37 Zimmerman L, Parr B, Lendahl U, et al. Independent regulatory elements in the nestin gene direct transgene expression to neural stem cells or muscle precursors. Neuron. 1994;12:11.
38 Duggal N, Schmidt-Kastner R, Hakim AM. Nestin expression in reactive astrocytes following focal cerebral ischemia in rats. Brain Res. 1997;768:1.
39 Li Y, Chopp M. Temporal profile of nestin expression after focal cerebral ischemia in adult rat. Brain Res. 1999;838:1.
40 Sahin Kaya S, Mahmood A, Li Y, et al. Expression of nestin after traumatic brain injury in rat brain. Brain Res. 1999;840:153.
41 Tohyama T, Lee VM, Rorke LB, et al. Nestin expression in embryonic human neuroepithelium and in human neuroepithelial tumor cells. Lab Invest. 1992;66:303.
42 Dahlstrand J, Collins VP, Lendahl U. Expression of the class VI intermediate filament nestin in human central nervous system tumors. Cancer Res. 1992;52:5334.
43 Rutka JT, Ivanchuk S, Mondal S, et al. Co-expression of nestin and vimentin intermediate filaments in invasive human astrocytoma cells. Int J Dev Neurosci. 1999;17:503.
44 Kania G, Corbeil D, Fuchs J, et al. Somatic stem cell marker prominin-1/CD133 is expressed in embryonic stem cell–derived progenitors. Stem Cells. 2005;23:791.
45 Weigmann A, Corbeil D, Hellwig A, et al. Prominin, a novel microvilli-specific polytopic membrane protein of the apical surface of epithelial cells, is targeted to plasmalemmal protrusions of non-epithelial cells. Proc Natl Acad Sci U S A. 1997;94:12425.
46 Wang J, Sakariassen PO, Tsinkalovsky O, et al. CD133 negative glioma cells form tumors in nude rats and give rise to CD133 positive cells. Int J Cancer. 2008;122:761.
47 Sulman E, Aldape K, Colman H. Brain tumor stem cells. Curr Probl Cancer. 2008;32:124.
48 Nowell PC. The clonal evolution of tumor cell populations. Science. 1976;194:23.
49 Nicolis SK. Cancer stem cells and “stemness” genes in neuro-oncology. Neurobiol Dis. 2007;25:217.
50 Dai C, Celestino JC, Okada Y, et al. PDGF autocrine stimulation dedifferentiates cultured astrocytes and induces oligodendrogliomas and oligoastrocytomas from neural progenitors and astrocytes in vivo. Genes Dev. 2001;15:1913.
51 Chaichana KL, McGirt MJ, Frazier J, et al. Relationship of glioblastoma multiforme to the lateral ventricles predicts survival following tumor resection. J Neuro Oncol. 2008;89:219.
52 Sanai N, Alvarez-Buylla A, Berger MS. Neural stem cells and the origin of gliomas. N Engl J Med. 2005;353:811.
53 Hopewell JW. The subependymal plate and the genesis of gliomas. J Pathol. 1975;117:101.
54 Vick NA, Lin MJ, Bigner DD. The role of the subependymal plate in glial tumorigenesis. Acta Neuropathol. 1977;40:63.
55 Hopewell JW, Wright EA. The importance of implantation site in cerebral carcinogenesis in rats. Cancer Res. 1969;29:1927.
56 Dana D, Copeland DDB. Influence of age at inoculation on avian oncornavirus-induced brain tumor incidence, tumor morphology, and postinoculation survival in F344 rats. Cancer Res. 1977;37:1657.
57 Holland EC, Hively WP, DePinho RA, et al. A constitutively active epidermal growth factor receptor cooperates with disruption of G1 cell-cycle arrest pathways to induce glioma-like lesions in mice. Genes Dev. 1998;12:3675.
58 Singh SK, Clarke ID, Hide T, et al. Cancer stem cells in nervous system tumors. Oncogene. 2004;23:7267.
59 Fomchenko EI, Holland EC. Origins of brain tumors—a disease of stem cells? Nat Clin Pract Neurol. 2006;2:288.
60 van der Valk P, Lindeman J, Kamphorst W. Growth factor profiles of human gliomas. Do non-tumour cells contribute to tumour growth in glioma? Ann Oncol. 1997;8:1023.
61 Aboody KS, Brown A, Rainov NG, et al. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci U S A. 2000;97:12846.
62 Bhardwaj G, Murdoch B, Wu D, et al. Sonic hedgehog induces the proliferation of primitive human hematopoietic cells via BMP regulation. Nat Immunol. 2001;2:172.
63 Lee HY, Kleber M, Hari L, et al. Instructive role of Wnt/beta-catenin in sensory fate specification in neural crest stem cells. Science. 2004;303:1020.
64 Varnum-Finney B, Xu L, Brashem-Stein C, et al. Pluripotent, cytokine-dependent, hematopoietic stem cells are immortalized by constitutive Notch1 signaling. Nat Med. 2000;6:1278.
65 Kita D, Yonekawa Y, Weller M, et al. PIK3CA alterations in primary (de novo) and secondary glioblastomas. Acta Neuropathol. 2007;113:295.
66 Watanabe K, Sato K, Biernat W, et al. Incidence and timing of p53 mutations during astrocytoma progression in patients with multiple biopsies. Clin Cancer Res. 1997;3:523.
67 Nakamura M, Watanabe T, Klangby U, et al. p14ARF deletion and methylation in genetic pathways to glioblastomas. Brain Pathol. 2001;11:159.
68 Dahmane N, Sanchez P, Gitton Y, et al. The Sonic Hedgehog–Gli pathway regulates dorsal brain growth and tumorigenesis. Development. 2001;128:5201.
69 Stecca B, Ruiz i Altaba A. Brain as a paradigm of organ growth: Hedgehog-Gli signaling in neural stem cells and brain tumors. J Neurobiol. 2005;64:476.
70 Lai K, Kaspar BK, Gage FH, et al. Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nat Neurosci. 2003;6:21.
71 Machold R, Hayashi S, Rutlin M, et al. Sonic hedgehog is required for progenitor cell maintenance in telencephalic stem cell niches. Neuron. 2003;39:937.
72 Rao G, Pedone CA, Del Valle L, et al. Sonic hedgehog and insulin-like growth factor signaling synergize to induce medulloblastoma formation from nestin-expressing neural progenitors in mice. Oncogene. 2004;23:6156.
73 Ruiz i Altaba A, Sanchez P, Dahmane N. Gli and hedgehog in cancer: tumours, embryos and stem cells. Nat Rev Cancer. 2002;2:361.
74 Hitoshi S, Alexson T, Tropepe V, et al. Notch pathway molecules are essential for the maintenance, but not the generation, of mammalian neural stem cells. Genes Dev. 2002;16:846.
75 Hallahan AR, Pritchard JI, Hansen S, et al. The SmoA1 mouse model reveals that notch signaling is critical for the growth and survival of sonic hedgehog–induced medulloblastomas. Cancer Res. 2004;64:7794.
76 Fan X, Matsui W, Khaki L, et al. Notch pathway inhibition depletes stem-like cells and blocks engraftment in embryonal brain tumors. Cancer Res. 2006;66:7445.
77 Roa W, Brasher PM, Bauman G, et al. Abbreviated course of radiation therapy in older patients with glioblastoma multiforme: a prospective randomized clinical trial. J Clin Oncol. 2004;22:1583.
78 Velema JP, Percy CL. Age curves of central nervous system tumor incidence in adults: variation of shape by histologic type. J Natl Cancer Inst. 1987;79:623.
79 Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756.
80 Beier D, Rohrl S, Pillai DR, et al. Temozolomide preferentially depletes cancer stem cells in glioblastoma. Cancer Res. 2008;68:5706.
81 Hau P, Stupp R, Hegi ME. MGMT methylation status: the advent of stratified therapy in glioblastoma? Dis Markers. 2007;23:97.
82 Phillips HS, Kharbanda S, Chen R, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9:157.