CHAPTER 43 Brain Abscess
Brain abscess is defined as a focal intracranial infection that is initiated as an area of cerebritis and evolves into a collection of pus surrounded by a vascularized capsule.1 Given their location, the approach to brain abscesses often presents diagnostic and therapeutic challenges. The following sections review the epidemiology, pathogenesis, etiology, and diagnostic and management approach for this devastating infection.
Epidemiology
Incidence and Risk Factors
Before the advent of infection with human immunodeficiency virus (HIV), brain abscess was not common, with an incidence of 0.3 to 1.3 cases per 100,000 persons per year in the United States.2 This translates to approximately 1500 to 2500 cases per year in the United States, with a higher incidence in developing countries.3 There is a male preponderance of 2 : 1 to 3 : 1, and the median age at infection is between 30 and 40 years.2,4 Differences in age are based on the primary site of infection—when the abscess is from an otitic focus, patients are generally younger than 20 years or older than 40 years, and when secondary to a focus in the paranasal sinuses, most patients are between 30 and 40 years of age. About 25% of brain abscess cases occur in children, mostly secondary to an otitic focus or in those with congenital heart disease; in one review from the University of Virginia Children’s Hospital from 2000 to 2007, an average of only 1.5 children per year were admitted to the inpatient pediatric service with a primary diagnosis of brain abscess.5 Brain abscess may occur after cranial operations. It was reported in only 0.2% of 1587 operations in one study6 and in 10 of 16,540 cranial surgeries performed by 25 neurosurgeons in another review7; although rare, a small percentage of patients will require a repeat operation to treat the infection. In more recent series, brain abscess is more commonly reported in patients who are immunocompromised,2,3 including those infected with HIV, receiving chemotherapy for cancer, receiving immunosuppressive therapy after organ transplantation, or after prolonged use of corticosteroids.
Pathogenesis
Organisms can reach the central nervous system (CNS) by spread from a contiguous source of infection (25% to 50% of cases), hematogenous dissemination (20% to 35% of cases), or trauma1–5,8–16; brain abscess is cryptogenic in about 10% to 35% of patients.2,17 Sources from a contiguous focus of infection include infections in the middle ear, mastoid cells, or paranasal sinuses. Brain abscess that results from otitis media usually localizes to the temporal lobe or cerebellum; in one review, 54% were in the temporal lobe, 44% in the cerebellum, and 2% in both locations.18 Recent series, however, have demonstrated that cases of brain abscess secondary to otitis media have been decreasing, although intracranial complications may be increased in patients in whom appropriate treatment of otitis media is neglected. In patients with brain abscess secondary to paranasal sinusitis, the frontal lobe is the predominant site. When the abscess is a complication of sphenoid sinusitis, the temporal lobe or sella turcica is usually involved. Dental infections, particularly of the molar teeth, can lead to brain abscess, often in the frontal lobe, but temporal lobe extension has been reported.19,20
Hematogenous dissemination to the brain generally leads to multiple, multiloculated abscesses, which are associated with higher mortality than abscesses that result from contiguous foci of infection.2–4 The most common sources in adults are chronic pyogenic lung diseases (especially lung abscess), bronchiectasis, empyema, and cystic fibrosis. Other distant sources of infection include wound and skin infections, osteomyelitis, pelvic infections, and intra-abdominal infections; they can also occur after esophageal dilation or sclerosing therapy for esophageal varices.21–23 Cyanotic congenital heart disease (especially in patients with tetralogy of Fallot and transposition of the great vessels) is another predisposing factor that accounts for 5% to 15% of brain abscess cases. Even higher percentages are reported in pediatric series,24,25 although advances in cardiovascular surgery have led to a decrease in patients with cyanotic congenital heart disease as a predisposing factor.3 Brain abscess occurs in less than 5% of patients with infective endocarditis despite the presence of continuous bacteremia.26–28 There is also a significant likelihood of brain abscess in patients with hereditary hemorrhagic telangiectasia, which is almost always observed in those with coexisting pulmonary arteriovenous malformations, perhaps by allowing septic emboli to cross the pulmonary circulation without capillary filtration29–32; the risk ranges from 5% to 9% and is 1000 times greater than in the general population.
Trauma can lead to brain abscess formation as a result of an open cranial fracture with dural breach or foreign body injury or as a sequela of neurosurgery.33 The incidence of traumatic brain abscess in the civilian population ranges from 2.5% to 10.9%, and reports have included brain abscess secondary to compound depressed skull fractures, dog bites, rooster pecking, tongue piercing, and injuries from lawn darts and pencil tips.34–36 Nosocomial brain abscess has been seen after halo pin insertion,37 after electrode insertion to localize seizure foci,38 and in malignant glioma patients treated by placement of Gliadel wafers in the tumor bed to release carmustine.39 In military populations, the incidence of brain abscess after head trauma ranges from 3% to 17%, and they usually occur secondary to retained bone fragments or contamination of initially uninfected missile sites with bacteria from skin, clothes, or the environment.40 However, the importance of retained bone fragments in the pathogenesis of infection has been questioned. In a study from Croatia of 160 war missile penetrating craniocerebral injuries in which 21 skull base injuries were treated surgically,41 only the accessible retained bone or metallic fragments were removed; the retained foreign bodies did not seem to increase the infection rate except in patients who suffered an in-driven cluster of bone fragments or leakage of cerebrospinal fluid. These findings were confirmed in another retrospective study from Croatia in which 88 patients with brain missile wounds had only accessible bone or metallic fragments removed during intracranial débridement42; there were 9 cases of brain abscess and the presence of retained fragments was not responsible for an increased rate of infection. Similar results were found in another study of 43 patients who survived low-velocity missile injuries to the brain during military conflicts and had retained fragments; suppurative sequelae were seen in 6 patients and only 2 progressed to brain abscess.43
Etiology
Numerous infectious agents have been reported to cause brain abscess. The probable infecting pathogen depends on the pathogenesis of the infection (see earlier) and the presence of various predisposing conditions (Table 43-1). This chapter focuses on important bacterial and fungal causes of brain abscess. Protozoal and helminthic causes (e.g., Trypanosoma cruzi, Taenia solium, Entamoeba histolytica, Schistosoma spp., Microsporidia spp., and Paragonimus spp.) are discussed in other chapters of this book. The most important protozoal cause of brain infection is Toxoplasma gondii, which is seen primarily in patients infected with HIV; this organism and the approach to CNS mass lesions in patients infected with HIV are also discussed in other chapters of this book.
TABLE 43-1 Predisposing Conditions and Probable Etiologic Agents in Brain Abscess
| PREDISPOSING CONDITION | POSSIBLE MICROBIAL CAUSES |
|---|---|
| Otitis media or mastoiditis | Streptococci (aerobic or anaerobic), Bacteroides spp., Prevotella spp., Enterobacteriaceae |
| Sinusitis (frontoethmoidal or sphenoidal) | Streptococci, Bacteroides spp., Enterobacteriaceae, Haemophilus spp., Staphylococcus aureus |
| Dental infection | Mixed Fusobacterium, Prevotella, Actinomyces, and Bacteroides spp.; streptococci |
| Penetrating trauma or secondary to neurosurgical procedure | Staphylococcus aureus, Enterobacteriaceae, Clostridium spp. |
| Lung abscess, empyema, or bronchiectasis | Fusobacterium, Actinomyces, Bacteroides, and Prevotella spp.; streptococci; Nocardia spp. |
| Bacterial endocarditis | Staphylococcus aureus, streptococci |
| Congenital heart disease | Streptococci, Haemophilus spp. |
| Immunocompromised state | |
| Neutropenia | Aerobic gram-negative bacilli, Aspergillus spp., Mucorales, Candida spp., Scedosporium spp. |
| Transplantation | Enterobacteriaceae, Listeria monocytogenes, Nocardia spp., Aspergillus spp., Candida spp., Mucorales, Scedosporium spp., Toxoplasma gondii |
| HIV infection | Listeria monocytogenes, Nocardia spp., Mycobacterium spp., Cryptococcus neoformans, Toxoplasma gondii |
Bacteria
The most common bacterial causes of brain abscess are streptococci (aerobic, anaerobic, and microaerophilic), which are isolated in up to 70% of cases.2,10,44 They include organisms in the Streptococcus anginosus (milleri) group, which normally reside in the oral cavity, appendix, and female genital tract. Staphylococcus aureus is isolated in 10% to 20% of cases, most commonly after cranial trauma or infective endocarditis.2 Enteric gram-negative bacilli (e.g., Proteus spp., Escherichia coli, Klebsiella spp., Pseudomonas aeruginosa, and Enterobacter spp.) are isolated in 23% to 33% of patients; predisposing factors include otitis media, bacteremia, neurosurgical procedures, and the immunocompromised state.2,45,46 Anaerobes (especially Bacteroides and Prevotella spp.) have more often been isolated after proper culture techniques and are found in 20% to 40% of patients, frequently in mixed culture.2,47,48 Multiple organisms are cultured in 14% to 28% of those with positive culture results.2,9–12 The incidence of negative cultures has ranged from 0% to 43%2,3,9–13,15,18,47,49; previous use of antimicrobial therapy may account for these negative culture results.
Other species are less commonly isolated in patients with bacterial brain abscess but should be considered in those with certain underlying conditions. For example, brain abscess caused by Listeria monocytogenes is uncommon (<1% of cases) but accounts for about 10% of cases of CNS listeriosis50,51; Listeria should be considered in patients who are immunocompromised (e.g., leukemia, lymphoma, HIV infection, and conditions requiring corticosteroids or other agents that cause immunosuppression). Salmonella species may cause brain abscess in patients who are bacteremic or in the presence of some compromise of the reticuloendothelial system.52 Brain abscess caused by Nocardia species may occur as part of a disseminated infection in patients with cutaneous or pulmonary disease; most have defects in cell-mediated immunity such as corticosteroid therapy, organ transplantation, HIV infection, or neoplasia.53,54 Rare cases of Nocardia brain abscess have also been seen in pregnant women. Other bacteria that cause brain abscess include Streptococcus pneumoniae, Haemophilus influenzae, Burkholderia pseudomallei, and Actinomyces species.2,16,55,56 When meningitis is caused by certain facultative gram-negative organisms (e.g., Citrobacter diversus), concomitant brain abscess is observed in more than 75% of cases.1,57,58 Mycobacterium tuberculosis and nontuberculous mycobacteria have increasingly been observed to cause brain abscess,1,2,59 with most cases reported in patients with HIV infection60,61; when the caseous core of a CNS tuberculoma liquefies, a tubercular abscess will result.62
Fungi
In recent years, the incidence of fungal brain abscess has been rising as a result of the increased use of corticosteroid therapy, broad-spectrum antimicrobial therapy, and immunosuppressive agents.63–66 Candida species have been the most prevalent fungi but are often not discovered until autopsy; these fungi cause microabscesses, macroabscesses, noncaseating granulomas, and diffuse glial nodules. Risk factors for candidal brain abscess include the use of broad-spectrum antimicrobial agents, corticosteroids, and hyperalimentation; premature birth; malignancy; neutropenia; chronic granulomatous disease; diabetes mellitus; thermal injury; and the presence of a central venous catheter.
CNS aspergillosis is reported in 10% to 20% of patients with invasive disease.63,65,67,68 The lungs are the usual primary site of infection, with dissemination to the CNS occurring by direct extension from an area that is anatomically adjacent to the brain. The most important underlying immune defect in patients with invasive aspergillosis is neutropenia (i.e., in those who have an underlying malignancy), but it may also be seen in patients with hepatic disease, diabetes mellitus, chronic granulomatous disease, Cushing’s syndrome, HIV infection, injection drug use, organ transplantation, and bone marrow transplantation, as well as after craniotomy and in patients receiving chronic corticosteroid therapy.
CNS infections caused by the Mucorales group are among the most fulminant infections known. Diabetes mellitus, usually associated with acidosis, is the most common predisposing condition (≈70% of cases), but disease may also be seen in patients with acidemia from profound systemic illness (e.g., sepsis, severe dehydration, severe diarrhea, chronic kidney disease), hematologic neoplasms, renal transplantation, injection drug use, and use of deferoxamine.65,69 CNS disease results from direct extension from the rhinocerebral form, after open head trauma, or after hematogenous dissemination. Bilateral involvement of the basal ganglia has been reported in injection drug users. Rhizopus arrhizus is the most common isolate.70
Scedosporium species may cause CNS disease in immunocompetent and immunocompromised hosts.65,71–75 These organisms may enter the CNS by direct trauma, hematogenous dissemination, or direct extension from infected sinuses. An association between near-drowning and subsequent CNS infection has been reported because these organisms are present in contaminated water and manure.
Many other fungal species have been reported to cause brain abscess, including Cryptococcus neoformans, the endemic mycoses (Coccidioides spp., Histoplasma spp., and Blastomyces dermatitidis), and many of the dematiaceous fungi. It is beyond the scope of this chapter to review all fungal causes of brain abscess, and more detail can be found from other sources.65
Experimental Models of Infection
Numerous animal models have been developed to study the pathogenesis and pathophysiology of brain abscess.76 Some large-animal models were created by direct implantation of bacteria into the brain; however, these models were limited by lack of reproducibility, they required multiple steps and an agar vehicle to initiate infection, and they were quite expensive. Another method used embolization of contaminated pliable cylinders implanted into the carotid artery but required concomitant cerebral injury for abscess formation, and the accompanying brain infarction caused a high mortality rate even in uninfected control animals.
A better animal model involved the use of mice or rats and consisted of a simple, one-step, easily reproducible procedure for consistent production of brain abscess. Infection was produced by the injection of 1 µL of saline containing a fixed inoculum of bacteria through a bur hole and into the frontal lobe of the brain.77,78 With this model, brain abscess was achieved in a one-step process at a specific site with the injection of bacteria alone, the inoculum could be regulated in terms of both volume and number of organisms, the number of injected bacteria and the number of bacteria that remain viable in the tissue could be quantified at a later time, and there was precise control of the injection site, thereby reducing tissue trauma with minimal (or no) infection in the subarachnoid space. This model also simulated human infection in that the abscess was produced in the white matter at the white and gray matter junction and migrated toward the ventricle, a shift in intracranial contents occurred, and there was minimal histologic evidence of meningitis; the abscess capsule was asymmetric by being more complete on the cortical than on the ventricular side, perhaps because the increased vascularity of normal cortical gray matter allowed greater fibroblast proliferation and collagen helix formation. In addition, development of the abscess paralleled clinical disease with the initial development of cerebritis and massive white matter edema followed by encapsulation.
In another animal model, brain abscess was produced in a rat by direct intracerebral injection of agarose beads laden with S. aureus.79 This method was also easy, reproducible, effective, and associated with a low mortality rate, and the histologic features of these experimental abscesses were similar to those observed in other animal models and in humans. These models have been useful in delineating the early events in brain abscess formation with respect to bacterial virulence factors and the host defense mechanisms involved in brain abscess formation; these concepts are reviewed in greater detail subsequently.
Initiation of Infection
The brain appears to be significantly more sensitive to infection than many other tissues. In a rat model of experimental brain abscess, injection of 104 colony-forming units (CFUs) of S. aureus or 106 CFUs of E. coli failed to cause infection in the skin, but abscess formation in brain tissue was induced by a level as low as 102 CFUs of either organism.80 The brain may also be more susceptible to infection by different organisms; in the experimental rat model, strains of E. coli were more virulent (i.e., led to abscess formation at lower inocula) than P. aeruginosa, S. aureus, or Streptococcus pyogenes.81 In addition, E. coli strains possessing the K1 antigen were more infective than strains without this antigen, thus indicating that certain encapsulated strains may be more virulent in brain abscess formation. Furthermore, inoculation of Bacteroides fragilis or streptococci such as S. intermedius failed to lead to abscess formation in rats even though these organisms account for a high percentage of isolates from brain abscesses in humans; this may be explained by the fact that brain abscess is often the result of a contiguous focus of infection and the synergistic infectivity of mixed populations of anaerobes plus a facultative organism may be necessary to establish infection.82,83 In an experimental dog model of brain abscess formation, inoculation of B. fragilis with Staphylococcus epidermidis in mixed culture caused a virulent reaction,84 although each organism was not tested separately. The role of other bacterial virulence factors in the pathogenesis of brain abscess formation has not been elucidated. However, the role of virulence factor production in the development of brain abscess was demonstrated by the inability of heat-inactivated S. aureus to induce proinflammatory cytokine or chemokine expression in an experimental mouse model; alpha toxin was identified as a key virulence factor for survival of S. aureus in the brain and subsequent development of brain abscess.85
Brain abscess may also develop in patients with bacterial meningitis, a rare complication except in human neonates with meningitis caused by C. diversus.45,46 Pathologically, there is cerebral necrosis and liquefaction, along with vasculitis of small vessels and hemorrhagic necrosis of adjacent tissue with a propensity for contiguous inflammation in the cerebral white matter, which may reflect the effects of endotoxin in the small penetrating vessels in this area; the typical abscess with capsule formation was not present. The pathogenesis was investigated in an infant rat model in which infection was initiated with the production of a high-grade bacteremia, infiltration of the leptomeninges, and subsequent development of ventriculitis.86 Brain abscesses were found exclusively in the periventricular white matter, apparently from disruption of the ventricular ependymal lining with direct extension of the infection into the parenchyma. The virulence factors responsible for the propensity of this organism to cause brain abscess are undefined, although a minor 32-kD outer membrane determinant may be a marker for strains that are more likely to produce ventriculitis and brain abscess87; strains that lacked the 32-kD outer membrane protein caused more bacteremia, meningitis, and death.
Stages of Infection
The temporal course and pathologic consequences of brain abscess were examined in a canine model of infection after inoculation of α-hemolytic streptococci. Four stages of infection were identified (Table 43-2): early cerebritis, late cerebritis, early capsule formation, and late capsule formation.88 These stages are somewhat arbitrary but are useful in the classification and comparison of virulence between different organisms in the production of brain abscess. Similar neuropathologic findings were described in an experimental model of anaerobic brain abscess,84 but capsule formation could not be divided into early and late stages because of delayed encapsulation. S. aureus was found to be more virulent than α-hemolytic streptococci, with a greater amount of necrosis and total area of involvement and a longer course of progression to resolution, time to reach a stable size, and time to contain the necrotic region within a collagenous capsule.89 Inflammation with histologic evidence of extension of inflammation, necrosis, and edema beyond the capsule was also observed, similar to findings after inoculation with B. fragilis. In all these studies, capsule formation was less prominent on the ventricular than on the cortical surface,84,88,89 perhaps because differences in vascularity between the cortical gray and white matter allow greater fibroblast proliferation, which probably explains the tendency for brain abscesses to rupture into the ventricular system rather than into the subarachnoid space. In contrast, the histopathologic sequence of brain abscess formation was examined in an experimental rat model after inoculation of E. coli90; the histopathologic findings supported an alternative hypothesis that brain abscesses tend to rupture intraventricularly because the infectious process is directed along the major white matter tracts (areas of lower tissue resistance) rather than as a result of asymmetric collagen deposition. However, the question of rupture of brain abscess requires further study.
Host Defense Mechanisms
In the experimental models just described, bacteria were inoculated directly into the brain, thus bypassing the brain’s normal host defense mechanisms. Although the brain is generally protected from infection by an intact blood-brain barrier, once infection is established, immune defenses are usually inadequate to control the infection. Local opsonization in the brain is deficient, which allows encapsulated bacteria such as B. fragilis and E. coli to escape efficient phagocytosis; phagocytosis of Bacteroides species also requires heat-labile serum factors such as complement and lysozyme, and these factors are probably absent in the CNS.91,92 The outer membrane components of Bacteroides species may also be important in the inhibition of neutrophil chemotaxis, thereby reducing the host response to brain abscess caused by this organism.93
As shown in Table 43-2, the border around the initial area of inoculation is composed of acute inflammatory cells during the early cerebritis stage, accompanied by the rapid development of a perivascular infiltrate consisting of neutrophils, plasma cells, and mononuclear cells.84,88,89 In an experimental rat model of brain abscess formation, production of the proinflammatory cytokines interleukin-1α (IL-1α), IL-1β, IL-6, and tumor necrosis factor-α (TNF-α) occurred as early as 1 to 6 hours after exposure to S. aureus.94 This was followed by enhanced concentration of the CXC chemokine KC, which correlates with the appearance of neutrophils in the abscess. The importance of neutrophils in the initial containment of S. aureus in the brain was established by the fact that mice transiently depleted of neutrophils, before the implantation of bacteria-laden beads, had higher CNS bacterial burdens than did control animals.95 Both macrophage inflammatory protein-2 (MIP-2) and KC/CXCL1, two neutrophil-attracting CXC chemokines, were significantly elevated in the brain after S. aureus exposure, thus indicating the importance of the CXCR2 ligands MIP-2 and KC, as well as neutrophils, in the acute host response. The continued release of proinflammatory mediators by activated glia and infiltrating peripheral immune cells may potentiate the subsequent recruitment and retention of newly recruited inflammatory cells and glia,96 thereby perpetuating the antibacterial inflammatory response. Recent studies support this persistent immune activation associated with experimental brain abscess in which increased concentrations of IL-1α, TNF-α, and MIP-2 were detected between 14 and 21 days after S. aureus exposure,97 thus suggesting that intervention with anti-inflammatory compounds, subsequent to bacterial neutralization, might minimize damage to the surrounding brain parenchyma. Although Toll-like receptor 2 (TLR2) has an important role in mediating S. aureus–induced activation, additional receptors are also involved in glial responses to S. aureus. With progression to the late cerebritis stage, the acute inflammatory cells become mixed with macrophages and fibroblasts, and reticulin formation surrounds the necrotic center. As the capsule begins to form, increased numbers of fibroblasts and macrophages infiltrate the periphery, and mature collagen is deposited to form a capsule. The necrotic center then continues to decrease in size while marked gliosis develops outside the capsule.
Despite the presence of virulence factors of the organism that resist host defense mechanisms, the host inflammatory response is important in containment of the abscess, as has been examined with the use of immunosuppressed animals. Initial studies in a dog model of experimental brain abscess with S. aureus or Proteus mirabilis demonstrated that the administration of dexamethasone slowed, but did not fully impair, capsule formation.98 In contrast, there was no evidence of capsule formation when dexamethasone was given at the same time as inoculation of either S. pyogenes or S. aureus in another study.99 In an experimental rat model of E. coli brain abscess,90 dexamethasone administration led to a reduction in macrophage and glial responses, collagen deposition, and host survival, with demonstration of an increased number of viable bacteria in the brain abscess. Coadministration of dexamethasone also impaired the lymphocytic and fibroblastic response in a rat model of experimental S. aureus brain abscess,100 although it did not entirely halt the encapsulation or reduce the associated cerebral edema.
Another study in dogs, who were immunosuppressed with azathioprine and prednisone 7 days before the intracerebral inoculation of α-hemolytic streptococci, demonstrated that the immunosuppressed animals manifested a decreased inflammatory response that was characterized by a reduction in neutrophils and macrophages in the lesion, a decrease and delay in collagen deposition, and persistence of viable organisms into the late capsule stage.101 Reduction of neutrophils, plasma cells, lymphocytes, and macrophages in the areas surrounding the necrotic center of the abscess was observed, and cerebritis was also decreased outside the developing capsule. However, gliosis was markedly increased in the area surrounding the collagenous capsule in these immunosuppressed dogs. Although the decreased inflammatory response and edema initially resulted in less mass effect, the eventual size and area of the abscess may have become larger as a result of the diminished host response.
Clinical Findings
The clinical manifestations of brain abscess may run the gamut from indolent to fulminant; most are related to the size and location of the space-occupying lesion within the brain and the virulence of the infecting organism.1–3,8–17 Common symptoms and signs are presented in Table 43-3. Headache is generally observed in 70% to 75% of patients; sudden worsening of the headache, accompanied by new onset of meningismus, may signify rupture of the abscess into the ventricular space.102,103 In one study of 33 consecutive patients with intraventricular rupture of brain abscess, severe headaches and signs of meningeal irritation were prominent findings before rupture, followed by rapid clinical deterioration within 10 days.104 Intraventricular rupture appears to be more likely if the abscess is deep-seated, multiloculated, and in close proximity to the ventricular wall102; a 1-mm reduction of the distance between the ventricle and the abscess increased the rupture rate by 10%. The classic triad of fever, headache, and focal neurological deficits is seen in less than 50% of patients with brain abscess. The specific neurological findings of brain abscess are also defined by location within the CNS (Table 43-4).1–3,9–16,105,106 In immunocompromised patients, the clinical findings may be masked by the diminished inflammatory response.
TABLE 43-3 Initial Symptoms and Signs in Patients with Brain Abscess
| SYMPTOM OR SIGN | FREQUENCY RANGE (%) |
|---|---|
| Headache | 49-97 |
| Fever | 32-79 |
| Focal neurological deficits* | 23-66 |
| Altered mental status | 28-91 |
| Seizures | 13-35 |
| Nausea and vomiting | 27-85 |
| Nuchal rigidity | 5-41 |
| Papilledema | 9-51 |
* The specific deficit depends on the central nervous system location of the abscess (see Table 43-4).
Data from references 1, 2, 8, 9–17, 102.
TABLE 43-4 Possible Initial Findings in Patients with Brain Abscess Based on Intracranial Location
| INTRACRANIAL LOCATION | FINDING |
|---|---|
| Parietal lobe |
The clinical manifestations of brain abscess may also be defined by the infecting pathogen. For example, patients with nocardial brain abscess may have concomitant pulmonary, skin, or muscle lesions53,54,107; however, the CNS findings are more often nonspecific and associated with fever, headache, and focal neurological deficits defined by location. Patients with Aspergillus brain abscess commonly manifest signs of a stroke syndrome as a result of ischemia or intracerebral hemorrhage, or both, that is referable to the involved areas of the brain.65,67,68 Patients who are severely immunocompromised usually have nonspecific findings shortly before death, whereas those who are less immunocompromised are more likely to have headache and focal neurological deficits, but evidence of aspergillosis in other organ systems is usually apparent.
Patients with rhinocerebral mucormycosis initially have symptoms referable to the eyes or sinuses and complaints of headache, facial pain, diplopia, lacrimation, and nasal stuffiness or epistaxis.65,69 With continued infection and spread to contiguous structures, necrotic lesions appear in the turbinates, nose, paranasal skin, or hard palate; chemosis, proptosis, and external ophthalmoplegia may also occur. Cranial nerve involvement is common, and blindness may occur as a result of invasion of the cavernous sinus, ophthalmic artery, and orbit. Because the organism has a proclivity for blood vessel invasion, thrombosis is a striking feature of the disease. Far advanced disease is suggested by focal deficits such as hemiparesis, seizures, or monocular blindness. In patients with the nonrhinocerebal form, fever, headache, and focal neurological deficits are present in more than half the patients. In one review of 22 cases, half the patients were injection drug users and the basal ganglia was the most commonly involved site.70
Scedosporium apiospermum brain abscess tends to occur in immunocompromised patients or in individuals 15 to 30 days after an episode of near-drowning.72,73 The location tends to be in the cerebrum, cerebellum, or brainstem. Clinical findings include seizures, altered consciousness, headache, meningeal irritation, focal neurological deficits, abnormal behavior, and aphasia.
Diagnosis
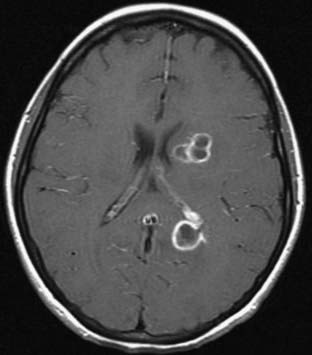
Magnetic resonance imaging (MRI) is the diagnostic neuroimaging procedure of choice in patients with brain abscess; it is more sensitive than computed tomography (CT) and offers significant advantages in the early detection of cerebritis, more conspicuous spread of inflammation into the ventricles and subarachnoid space, and earlier detection of satellite lesions (Figs. 43-1 and 43-2).17,108 On T1-weighted images, the abscess capsule often appears as a discrete rim that is isointense to mildly hyperintense; administration of gadolinium-diethylenetriaminepentaacetic acid helps clearly differentiate the central abscess, surrounding enhancing rim, and cerebral edema. On T2-weighted images, the zone of edema that surrounds the abscess demonstrates marked high signal intensity in which the capsule now appears as an ill-defined hypointense rim at the margin of the abscess. On diffusion-weighted images, restricted diffusion (bright signal) may be seen and may distinguish abscesses from necrotic neoplasms.3 Proton MR spectroscopy is another diagnostic modality that may assist in differentiating between malignant tumors and cerebral abscesses; when combined with diffusion-weighted imaging, MR spectroscopy can significantly increase the diagnostic accuracy of conventional MRI.109 In patients who cannot undergo MRI, CT with and without intravenous contrast enhancement is recommended; there is characteristically a hypodense center with peripheral uniform ring enhancement after the intravenous injection of contrast material.110–112 Other findings include nodular areas and regions of low attenuation without enhancement, the latter finding seen in the early cerebritis stage before abscess formation. In later stages, contrast material no longer differentiates the lucent center, and the CT appearance is similar to that of the early cerebritis stage.
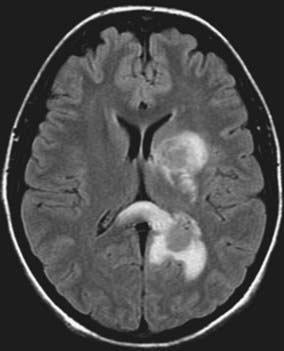
FIGURE 43-2 Axial fluid-attenuated inversion recovery (FLAIR) image in the same patient in Figure 43-1 demonstrating vasogenic edema surrounding each lesion.
(Courtesy of Stanley Lu, MD, Monmouth Medical Center, Long Branch, NJ.)
Neuroimaging may be quite sensitive in defining the findings in patients with fungal brain abscess.65 In patients with CNS aspergillosis, there may be the finding of a cerebral infarct, which typically develops into either single or multiple abscesses; in immunocompromised patients, there may be little or no contrast enhancement on MRI.68 Characteristic changes may be seen on MRI in patients with rhinocerebral mucormycosis, including sinus opacification, erosion of bone, and obliteration of deep fascial planes; cavernous sinus thrombosis may also be apparent. The lack of contrast enhancement in patients with mucormycosis is a poor prognostic sign because it indicates failure of host defense mechanisms to control the offending agent.
A major advance is the ability to perform stereotactic MRI- or CT-guided aspiration to facilitate microbiologic diagnosis.3 Current techniques afford the surgeon rapid, accurate, and safe access to virtually any intracranial point, including those in deep critical areas of the CNS (i.e., the brainstem, cerebellum, and diencephalic structures adjacent to the ventricles). At the time of aspiration, specimens should be sent for Gram stain, routine aerobic and anaerobic culture, modified acid-fast smears, acid-fast smears and culture, and fungal smears and culture. In patients with histopathologic and Gram stain findings suggestive of a bacterial brain abscess but in whom cultures are negative, 16S ribosomal RNA gene sequencing and amplification may be an important adjunct,113 although more data are needed. In patients with Aspergillus brain abscess, appropriate stains may reveal the presence of septate hyphae with acute-angle, dichotomous branching, whereas in patients with mucormycosis, tissue specimens may reveal irregular hyphae with right-angle branching. Histologic preparations of brain abscess specimens caused by Scedosporium species are indistinguishable from those caused by Aspergillus species. The hyphae of dematiaceous fungi may be brownish on hematoxylin-eosin staining but are not distinguishable from those of other molds.
Management
The initial approach to management of a patient with a suspected brain abscess is a multidisciplinary one that involves a neuroradiologist, neurosurgeon, and infectious disease specialist.114 After neuroimaging, if single or multiple ring-enhancing lesions are found, the patient should be taken to surgery and all lesions larger than 2.5 cm excised or aspirated under stereotactic guidance; for abscesses in the early cerebritis stage or if all lesions are 2.5 cm or smaller in diameter, the largest lesion should be aspirated for definitive diagnosis and identification of the organism.115 After aspiration of abscess material and submission of specimens for special stains, histopathologic examination, and culture, empirical antimicrobial therapy should be initiated. The empirical approach to antimicrobial therapy in patients with bacterial brain abscess is based on stains of the aspirated specimen and the probable pathogenesis of infection (Table 43-5).2 The combination of metronidazole plus a third-generation cephalosporin is commonly used14,115,116; in patients in whom S. aureus is also considered a probable pathogen, vancomycin is added pending identification of the organism and in vitro susceptibility testing.117 In patients in whom gram-negative bacilli such as P. aeruginosa is likely, either ceftazidime, cefepime, or meropenem can be used. In patients with no clear predisposing factors, a reasonable regimen to administer is the combination of vancomycin, metronidazole, and a third- or fourth-generation cephalosporin. Once the infecting pathogen is isolated, antimicrobial therapy can be modified for optimal treatment (Table 43-6); recommended dosages of agents in patients with normal renal and hepatic function are shown in Table 43-7.
TABLE 43-5 Predisposing Conditions and Empirical Antimicrobial Therapy in Patients with Presumed Bacterial Brain Abscess
| PREDISPOSING CONDITION | ANTIMICROBIAL THERAPY |
|---|---|
| Otitis media or mastoiditis | Metronidazole + a third-generation cephalosporin* |
| Sinusitis (frontoethmoidal or sphenoidal) | Metronidazole + a third-generation cephalosporin* + vancomycin† |
| Dental infection | Penicillin + metronidazole |
| Penetrating trauma or secondary to a neurosurgical procedure | Vancomycin + a third- or fourth-generation cephalosporin‡ |
| Lung abscess, empyema, or bronchiectasis | Penicillin + metronidazole + a sulfonamide§ |
| Bacterial endocarditis | Vancomycin + gentamicin |
| Congenital heart disease | Third-generation cephalosporin* |
| Unknown | Vancomycin + metronidazole + a third- or fourth-generation cephalosporin‡ |
* Cefotaxime or ceftriaxone; may also use the fourth-generation cephalosporin cefepime.
† Add if infection caused by methicillin-resistant Staphylococcus aureus is suspected, pending results of in vitro susceptibility testing.
‡ Use ceftazidime or cefepime if infection with Pseudomonas aeruginosa is suspected.
§ Trimethoprim-sulfamethoxazole; include if infection caused by Nocardia species is suspected.
TABLE 43-6 Antimicrobial Therapy for Brain Abscess Based on Isolated Pathogen
| ORGANISM | STANDARD THERAPY | ALTERNATIVE THERAPIES |
|---|---|---|
| BACTERIA* | ||
| Actinomyces spp. | Penicillin | Clindamycin |
| Bacteroides fragilis | Metronidazole | Clindamycin |
| Enterobacteriaceae† | Third- or fourth-generation cephalosporin | Aztreonam, meropenem, fluoroquinolone, trimethoprim-sulfamethoxazole |
| Fusobacterium spp. | Penicillin G | Metronidazole |
| Haemophilus spp. | Third-generation cephalosporin‡ | Aztreonam, fluoroquinolone, trimethoprim-sulfamethoxazole |
| Listeria monocytogenes | Ampicillin§ or penicillin G§ | Trimethoprim-sulfamethoxazole |
| Mycobacterium tuberculosis | Isoniazid + rifampin + pyrazinamide + ethambutol | |
| Nocardia spp.‖ | Trimethoprim-sulfamethoxazole | Minocycline, imipenem, meropenem, a third-generation cephalosporin, amikacin |
| Prevotella spp. | Metronidazole | Clindamycin, cefotaxime |
| Pseudomonas aeruginosa | Ceftazidime,§ cefepime,§ or meropenem§ | Aztreonam,§ fluoroquinolone§ |
| Staphylococcus aureus (methicillin sensitive) | Nafcillin or oxacillin | Vancomycin |
| Staphylococcus aureus (methicillin resistant) | Vancomycin | Trimethoprim-sulfamethoxazole,†¶ daptomycin¶ |
| Streptococcus anginosus (milleri) group, other streptococci | Penicillin G | Third-generation cephalosporin,‡ vancomycin |
| FUNGI | ||
| Aspergillus spp. | Voriconazole | Amphotericin B deoxycholate, liposomal amphotericin B, amphotericin B lipid complex, itraconazole,** posaconazole** |
| Candida spp. | Amphotericin B deoxycholate,†† liposomal amphotericin B,†† amphotericin B lipid complex†† | Fluconazole |
| Cryptococcus neoformans | Amphotericin B deoxycholate,†† liposomal amphotericin B,†† amphotericin B lipid complex†† | Fluconazole |
| Mucorales | Amphotericin B deoxycholate, liposomal amphotericin B, amphotericin B lipid complex | Posaconazole** |
| Scedosporium spp. | Voriconazole | Itraconazole,** posaconazole** |
* Depending on the pathogenesis of brain abscess formation (see Table 43-1), combination therapy should be continued for the possibility of a mixed aerobic/anaerobic infection.
† Use of a specific agent depends on in vitro susceptibility testing of the isolated organism.
§ Addition of an aminoglycoside should be considered.
‖ Combination therapy may be required in immunocompromised patients or in those failing standard therapy (see text).
¶ No data on the efficacy of these agents in patients with methicillin-resistant S. aureus brain abscess.
** Consider for use as salvage therapy in nonresponding patients or those intolerant of amphotericin B–based therapies.
†† Addition of flucytosine should be considered.
TABLE 43-7 Recommended Dosages of Antimicrobial Agents in Adults with Brain Abscess and Normal Renal and Hepatic Function*
| ANTIMICROBIAL AGENT | TOTAL DAILY DOSAGE (DOSING INTERVAL IN HOURS) |
|---|---|
| Amikacin† | 15 mg/kg (8) |
| Amphotericin B deoxycholate‡ | 0.6-1 mg/kg (24) |
| Amphotericin B lipid complex | 5 mg/kg (24) |
| Ampicillin | 12 g (4) |
| Aztreonam | 6-8 g (6-8) |
| Cefepime | 6 g (8) |
| Cefotaxime | 8-12 g (4-6) |
| Ceftazidime | 6 g (8) |
| Ceftriaxone | 4 g (12-24) |
| Ciprofloxacin | 800-1200 mg (8-12) |
| Fluconazole | 400-800 mg (24) |
| Flucytosine§ | 100 mg/kg (6) |
| Gentamicin† | 5 mg/kg (8) |
| Isoniazid§ | 300 mg (24) |
| Itraconazole | 400 mg (12) |
| Liposomal amphotericin B | 5 mg/kg (24) |
| Meropenem | 6 g (8) |
| Nafcillin | 9-12 g (4) |
| Oxacillin | 9-12 g (4) |
| Penicillin | 24 million units (4) |
| Posaconazole§ | 800 mg (6-12) |
| Pyrazinamide§ | 15-30 mg/kg (24) |
| Rifampin§ | 600 (24) |
| Tobramycin† | 5 mg/kg (8) |
| Trimethoprim-sulfamethoxazole‖ | 10-20 mg/kg (6-12) |
| Vancomycin¶ | 30-45 mg/kg (8-12) |
| Voriconazole** | 8 mg/kg (12) |
* Unless indicated, therapy is administered intravenously.
† Need to monitor peak and trough serum concentrations.
† Doses up to 1.5 mg/kg per day may be required in patients with aspergillosis or mucormycosis.
‖ Dosage based on trimethoprim component.
¶ Need to monitor trough serum concentrations (maintain at 15 to 20 µg/mL).
** Load with 6 mg/kg every 12 hr for two doses.
Bacterial Brain Abscess
The principles of antimicrobial therapy for bacterial brain abscess are to use agents that are able to penetrate the abscess cavity and have in vitro activity against the isolated pathogen.1,2,4,8,47,118–120 Few studies have examined the penetration of specific antimicrobial agents into brain abscess pus, and some antimicrobial agents that penetrate may be inactivated in a purulent environment. It is also important to note that depending on the pathogenesis, a mixed infection may be present even though only one organism was isolated, thus necessitating the use of more than one antimicrobial agent for therapy. An important agent in the treatment of brain abscess is metronidazole, which has excellent in vitro activity against strict anaerobes, an excellent pharmacokinetic profile, and good oral absorption and penetration into brain abscess cavities116; metronidazole, however, must always be used in combination with an agent effective against streptococci because polymicrobial infections are common in patients with bacterial brain abscess. Vancomycin has also achieved excellent penetration into brain abscess fluid after prolonged therapy, with concentrations that are 90% of those in serum.117 The third-generation cephalosporins are another class of antimicrobial agents that are useful, given their excellent in vitro activity against many etiologic agents that cause brain abscess and their demonstrated success in small clinical studies121,122; when combined with metronidazole and used with surgical therapy (see later) in one study, high doses of cefotaxime were used effectively in the treatment of bacterial brain abscess.123 Imipenem has been used successfully for the treatment of pyogenic and nocardial brain abscess,124,125 although it has been associated with an increased risk for seizures, thus limiting the utility of imipenem in patients with brain abscesses. Meropenem has been efficacious in isolated cases of brain abscess, including one patient with a brain abscess caused by Enterobacter cloacae,126 so this agent may be especially valuable in patients with infections caused by resistant pathogens. The fluoroquinolones have also been used in isolated brain abscess cases given their good CNS penetration127; further studies are needed to demonstrate their efficacy, however.
Surgical therapy is often required for the optimal approach to patients with bacterial brain abscess.5,16,17,49,115,128 Procedures include aspiration after bur-hole placement or complete excision after craniotomy, although no prospective trial comparing these two procedures has ever been performed. Aspiration may be performed under stereotactic neuroimaging guidance, which affords the neurosurgeon rapid, accurate, and safe access to virtually any intracranial point.122,129–132 Stereotactic aspiration is a useful approach even for abscesses located in eloquent or inaccessible regions133; repeat aspiration should be considered if the initial aspiration proves ineffective or partially effective. Intraoperative ultrasound guidance is also helpful for the aspiration of small abscesses and can delineate abscess pockets,134 although endoscopic aspiration is said to be more effective. In one series of 142 patients with brain abscess,13 there were no significant differences in outcome in those who were treated by excision, craniotomy with drainage, or stereotactic drainage, thus indicating that therapy must be individualized for each patient. Recurrence rates after stereotactic aspiration range from 0% to 24%. Complete excision by craniotomy is now infrequently performed because of the success of aspiration and closed drainage techniques, although it may be required for patients with multiloculated abscesses in whom aspiration techniques have failed, for abscesses containing gas, or for abscesses that fail to resolve. Excision is usually required for posttraumatic abscesses that contain foreign bodies or retained bone fragments to prevent recurrence, for abscesses that result from fistulous communications (e.g., secondary to trauma or congenital dermal sinuses), and for those localized to one lobe of the brain and contiguous with a primary focus.17 It has also been suggested that excision is the preferred method of surgical treatment of cerebellar abscesses in children,135,136 given that worse outcomes have been seen in those treated only by aspiration. In patients with intraventricular rupture of a purulent brain abscess who have dilated ventricles and ventriculitis,102,104 rapid evacuation and débridement of the abscess cavity via urgent craniotomy and ventricular drainage should be undertaken, along with intravenous or intrathecal administration of appropriate antimicrobial agents.
Although the optimal approach to brain abscess most often requires a combined medical and surgical approach, certain groups of patients may be treated with medical therapy alone.106,137–139 Such groups include those with medical conditions that increase the risk associated with surgery, multiple abscesses, abscesses in a deep or dominant location, the presence of coexisting meningitis or ependymitis, early reduction of the abscess with clinical improvement after antimicrobial therapy, and abscess size less than 3 cm. However, in one series, no abscess larger than 2.5 cm resolved without surgical therapy.137
The optimal duration of medical treatment of bacterial brain abscess is unclear but has traditionally been 6 to 8 weeks of high-dose intravenous antimicrobial therapy,1,2,17,138 which is often followed by oral antimicrobial therapy for 2 to 3 months if appropriate agents are available; however, the efficacy and necessity for additional oral antimicrobial therapy have not been established. In one study, the authors thought that a combination of surgical aspiration or removal of all abscesses larger than 2.5 cm in diameter, 6 weeks or more of antimicrobial therapy, and weekly neuroimaging to document abscess resolution should lead to cure rates of greater than 90%.140 Courses of 3 to 4 weeks of antimicrobial therapy may be adequate for patients who have undergone surgical excision of the brain abscess, whereas longer courses (up to 12 weeks with parenteral agents) may be required in patients treated with antimicrobial therapy alone. The Infection in Neurosurgery Working Party of the British Society for Antimicrobial Therapy recommends that intravenous therapy be used for 1 to 2 weeks for bacterial brain abscess141; depending on the clinical response, change to an oral regimen can be considered. Although this approach has been used in several series,14,142,143 it cannot be considered standard therapy in most patients with bacterial brain abscess. Repeat neuroimaging studies performed biweekly for up to 3 months after completion of therapy has been suggested to monitor for reexpansion of the abscess or failure of resolution.17,114
Nocardial Brain Abscess
In patients with Nocardia brain abscess, a sulfonamide, with or without trimethoprim, is recommended.54,144–146 Alternative agents with in vitro activity against Nocardia include minocycline, imipenem, amikacin, third-generation cephalosporins, and linezolid,147–150 although in vitro activity may not always correlate with clinical efficacy. In immunocompromised patients or those in whom therapy fails, combination treatment with regimens containing a third-generation cephalosporin or imipenem, along with a sulfonamide or amikacin, should be considered.1,151–155 In patients with brain abscess caused by Nocardia farcinica, a species that may be highly resistant to various antimicrobial agents, successful treatment has included moxifloxacin alone or combined with another agent.156–158
Craniotomy with total excision is difficult in patients with Nocardia brain abscess because these abscesses are often multiloculated.107 In one review of 11 patients with nocardial brain abscess, aspiration alone was effective in 9 patients.159 In contrast, in another series of 3 patients, cure was achieved only after neurosurgical enucleation,160 thus suggesting that an aggressive surgical approach should be used. The duration of therapy in patients with nocardial brain abscess is usually 3 to 12 months,54,161 but it should probably be continued up to 1 year in those who are immunocompromised. Careful follow-up is necessary to monitor for relapse.
Fungal Brain Abscess
Patients with fungal brain abscess, especially those who are immunocompromised, have a high mortality rate despite combined medical and surgical therapy. In patients with candidal brain abscess, recommended therapy is an amphotericin B preparation plus 5-flucytosine65; the efficacy of fluconazole has not been evaluated, but this agent has been used successfully in combination therapy in isolated case reports.162
The therapy of choice for Aspergillus brain abscess is voriconazole.163 In one review that included 19 patients with aspergillosis and cerebral disease, 3 had a partial response to treatment.164 In more recent series, however, the response rate was approximately 35%.68,163 Alternative agents include an amphotericin B preparation, posaconazole, and itraconazole.165–167 Although itraconazole has in vitro activity against Aspergillus species and high-dose therapy has resulted in success in some patients, its unreliable absorption and the modest reported experience in treatment of patients with Aspergillus brain abscess make use of itraconazole more promising as an extension of successful treatment rather than as primary therapy. One patient with an Aspergillus brain abscess was successfully treated with amphotericin B, itraconazole, and interferon gamma, with amphotericin B discontinued after the first 3 weeks of therapy.167 Combination therapies that have shown efficacy are voriconazole in conjunction with either caspofungin or amphotericin B.168–170 Excisional surgery or drainage is a key factor in the successful management of CNS aspergillosis.68,163,171
Patients with CNS mucormycosis should be treated with amphotericin B deoxycholate or a lipid formulation of amphotericin B65,172,173; correction of the underlying metabolic derangements and aggressive surgical débridement are also critical to successful therapy. Because the etiologic agents of mucormycosis invade blood vessels, tissue infarction occurs and impairs the delivery of antifungal agents to the site of infection; this often leaves surgery as the only modality that may effectively eliminate the infecting microorganism. In patients not responding to or intolerant of an amphotericin B formulation, posaconazole can be used as salvage therapy.174 In addition to surgery, hyperbaric oxygen therapy has been reported to be a useful adjunct,175,176 although no randomized, prospective studies have been performed to assess its efficacy.
Surgery is the cornerstone of therapy for brain abscesses caused by Scedosporium species.72 Given the inherent resistance of this organism to amphotericin B, voriconazole is the agent of choice.177 In one review that included 21 patients with CNS disease caused by Scedosporium, 43% had a therapeutic response.178 Combination therapy with voriconazole and terbinafine was also successful in 1 patient with chronic granulomatous disease and a brain abscess caused by Scedosporium prolificans.179 One patient with acute lymphoblastic lymphoma and multiple S. apiospermum brain abscesses who did not respond to itraconazole, amphotericin B, and ketoconazole combined with neurosurgical drainage was successfully treated with posaconazole.180
Adjunctive Therapy
Therapy with corticosteroids should be initiated in brain abscess patients who have associated edema and mass effect, progressive neurological deterioration, or impending cerebral herniation.2,3,17,115 Although use of corticosteroids has not been studied in well-controlled randomized trials and they may lead to a reduction in host defense mechanisms and decrease penetration of some antimicrobial agents into the brain abscess cavity, they may result in improvement of neurological symptoms and signs. High-dose corticosteroid therapy (e.g., dexamethasone, 10 mg every 6 hours) is generally administered initially and then tapered once the patient has stabilized. The use of prolonged courses of corticosteroids is discouraged. These agents may also decrease contrast enhancement of the abscess capsule in the early stages of infection, thereby being a false indicator of radiologic improvement.3
Outcome
Mortality rates in patients with brain abscess in the pre-antibiotic era and into the 1970s were unacceptably high and ranged from 30% to 80%.2,4,8 Since the 1970s, case fatality rates have ranged from 0% to 24%, results attributable to the availability of more effective antimicrobial therapy and the availability of neuroimaging (i.e., CT and MRI), which allows early diagnosis and monitoring of response to therapy.181 In recent series, mortality rates have ranged from 8% to 25%.9–14115 Factors associated with a poor prognosis include a low Glasgow Coma Scale score and the presence of certain underlying diseases and other comorbid conditions. A more favorable outcome was noted in one study of 142 patients when the Glasgow Coma Scale score was higher than 12 and the patients had no evidence of sepsis.12 One important complication that has been associated with poor outcome is intraventricular rupture of the abscess, for which mortality rates have ranged from 27% to 85%.102 In patients who survive the episode of brain abscess, the incidence of neurological sequelae has ranged from 20% to 70%; the prognosis is determined by the rapidity of progression and the patient’s mental status on initial evaluation.182 Long-term sequelae include hemiparesis, persistent visual field defects, cognitive dysfunction, learning disorders, hydrocephalus, and seizures2,3,5; the latter is a long-term risk in approximately 30% to 50% of patients with brain abscesses.
Cranial Subdural Empyema and Epidural Abscess
Epidemiology and Etiology
Cranial subdural empyema refers to a collection of pus between the space of the dura and arachnoid, and it accounts for 15% to 20% of all intracranial infections. The most common predisposing conditions are otorhinologic infections, especially of the paranasal sinuses, which are affected in 40% to 80% of patients.183–189 The mastoid and middle ear are affected in 10% to 20% of patients, who are usually living in geographic locales where patients with otitis media are not properly treated. Other predisposing conditions are skull trauma, neurosurgical procedures, and infection of a preexisting subdural hematoma.190 In one 20-year review of cases of subdural empyema in children,191 it developed in approximately 20% after head trauma or neurosurgery. The infection is metastatic in 5% of cases, usually from a pulmonary source. Meningitis is an important predisposing condition in infants with cranial subdural empyema, which occurs in 2% to 10% of those with bacterial meningitis.187
The etiologic agents in patients with cranial subdural empyema are usually microbial flora from those with chronic sinusitis, and such agents include aerobic streptococci (25% to 45%), staphylococci (10% to 15%), aerobic gram-negative bacilli (3% to 10%), and anaerobic streptococci and other anaerobes (33% to 100% in some series in which careful culturing for anaerobes was performed).185–188,192,193 If the predisposing condition is postoperative or posttraumatic, the usual pathogens are staphylococci and aerobic gram-negative bacilli. Propionibacterium acnes may be isolated from patients after trauma, neurosurgical procedures, or dural allografts.194–196 Operative cultures are reported to be negative in 7% to 53% of patients.189
Cranial epidural abscess refers to a localized collection of pus between the dura mater and overlying skull; because the abscess can cross the cranial dura along emissary veins, an accompanying subdural empyema may also be present.183,197 Therefore, the pathogenesis and bacterial etiology are usually identical to that described in patients with cranial subdural empyema (see earlier).
Clinical Findings
The clinical manifestation of patients with cranial subdural empyema may be rapidly progressive, with symptoms and signs related to increased intracranial pressure, meningeal irritation, or focal cortical inflammation.183–187,189,198 Most patients have fever and headache; vomiting is common as intracranial pressure increases. Altered mental status can occur and progress rapidly to obtundation and coma if the infection is not treated. Focal neurological signs (e.g., hemiparesis and hemiplegia, ocular palsies, dysphasia, homonymous hemianopia, dilated pupils, and cerebellar signs) appear in 24 to 48 hours and progress rapidly, with eventual involvement of the entire cerebral hemispheres, although in one series no focal signs were observed in 41% of 699 patients.187 Seizures occur in 25% to 80% of patients.189 Signs of meningeal irritation are seen in about 80% of patients. In untreated patients, there is rapid neurological deterioration with signs of increased intracranial pressure and cerebral herniation. However, these clinical findings may not be seen in patients in whom subdural empyema develops after cranial surgery or trauma, in those who have previously received antimicrobial therapy, in patients with infected subdural hematomas, or in those with metastatic infection to the subdural space.
The clinical manifestation of cranial epidural abscess may be insidious and is usually overshadowed by the primary focus of infection (whether sinusitis or otitis media).183,197 The findings are generally insidious because the dura is closely opposed to the inner surface of the cranium such that the abscess usually enlarges too slowly to produce a sudden onset of major neurological deficits, as is seen in patients with cranial subdural empyema (see earlier), unless there is deeper intracranial extension. The usual complaints are fever and headache, but the patient may not appear acutely ill, thereby leading to a delay in diagnosis. Eventually, however, focal neurological signs and seizures may develop, and as the abscess enlarges, papilledema and signs of increased intracranial pressure may develop in untreated patients. If the location of the epidural abscess is near the petrous bone, Gradenigo’s syndrome may develop, a condition characterized by the involvement of cranial nerves V and VI and manifested clinically as unilateral facial pain and weakness of the lateral rectus muscle.
Diagnosis
The diagnosis of cranial subdural empyema should be suspected in any patient with meningeal signs and a focal neurological deficit.183–187 MRI is the diagnostic imaging procedure of choice and usually demonstrates a crescentic or elliptical area of hypointensity (on T1 images) below the cranial vault or adjacent to the falx cerebri.189,198,199 Depending on the extent of disease, there may also be an associated mass effect with displacement of midline structures. MRI is superior to CT because it provides better clarity of morphologic detail and may detect the presence of a subdural empyema that is not seen on CT; it is particularly helpful in detecting a subdural empyema at the base of the brain, along the falx cerebri, or in the posterior fossa.
MRI is also the diagnostic imaging procedure of choice in patients with cranial epidural abscess; it usually demonstrates a superficial, circumscribed area of diminished intensity with pachymeningeal enhancement.197 CT is used for imaging bone or if MRI is not available, although MRI is superior in identification and delineation of the collection and is able to differentiate epidural abscesses from sterile effusions or hematomas that may be present in patients who have undergone cranial surgery or suffered head trauma.
Management
Cranial subdural empyema is a surgical emergency because antimicrobial therapy alone does not reliably sterilize the empyema. The goals of surgical therapy are to achieve adequate decompression of the brain and to evacuate the empyema completely.183,198,200,201 The optimal surgical approach is controversial. When comparing craniotomy drainage with drainage after placement of bur holes, some studies have demonstrated a lower mortality rate in patients who have undergone craniotomy, although it may be that the patients who underwent drainage via bur-hole placement were more ill and had a greater surgical risk. If bur-hole drainage is performed, multiple bur holes may be required to allow extensive irrigation. For patients undergoing craniotomy, wide exposure is needed to permit surgical exploration of all areas where empyema is suspected. In a large series of 699 patients in which the efficacy of drainage after CT-guided bur holes was compared with craniectomy or craniotomy drainage,202 mortality rates were higher in patients treated by only drainage via bur holes (23.3%) than in those who underwent craniectomy (11.5%) or craniotomy (8.4%). Patients who underwent drainage via bur holes or craniectomy required more frequent operations to drain recurrent or remaining pus and exhibited higher mortality rates and worse outcomes. Drainage via bur holes or craniectomy is therefore recommended only for patients in septic shock, those with localized parafalcine collections, and children with subdural empyema secondary to meningitis because there is usually no brain swelling and the pus is thin. Despite the surgical approach, however, repeat surgery was required in half the patients treated by bur-hole drainage and a fifth of those initially treated by craniotomy.203
Regardless of the method of drainage, once purulent material is aspirated, initial antimicrobial therapy should be based on the results of Gram staining and the pathogenesis of the infection.189,198 If S. aureus is suspected, vancomycin should be initiated but changed to nafcillin if the organism is found to be susceptible to methicillin. For suspected anaerobes, metronidazole is recommended. If aerobic gram-negative bacilli are suspected, empirical therapy with either ceftazidime, cefepime, or meropenem can be used. Cultures (both aerobic and anaerobic) are needed to guide the use of specific antimicrobial therapy. Depending on the clinical response, antimicrobial therapy should be continued for 3 to 4 weeks after drainage; longer periods of therapy (intravenous or oral) may be needed if the patient has accompanying osteomyelitis. Antimicrobial therapy alone can be considered for patients with cranial subdural empyema who have minimal or no impairment of consciousness, no major neurological deficit, limited extension of the empyema with no midline shift, and early improvement with antimicrobial therapy,186,200 although these patients need careful clinical and neuroimaging monitoring and may require longer courses of antimicrobial therapy.
Management of cranial epidural abscess also requires a combined medical and surgical approach.183,197,200,204 Empirical antimicrobial therapy is similar to that for cranial subdural empyema. For surgical drainage, craniotomy or craniectomy is generally preferred over bur-hole placement or aspiration of purulent material through the scalp. Antimicrobial therapy is usually continued for 3 to 6 weeks or longer (i.e., 6 to 8 weeks) after drainage if the patient also has underlying osteomyelitis.
Cavusoglu H, Kaya RA, Turkmenoglu ON, et al. Brain abscess: analysis of results in a series of 51 patients with a combined surgical and medical approach during an 11-year period. Neurosurg Focus. 2008;24(6):E9.
Dupuis-Girod S, Giraud S, Decullier E, et al. Hemorrhagic hereditary telangiectasia (Rendu-Osler disease) and infectious diseases: an underestimated association. Clin Infect Dis. 2007;44:841-845.
Eckburg PB, Montoya JG, Vosti KL. Brain abscess due to Listeria monocytogenes: five cases and a review of the literature. Medicine (Baltimore). 2001;80:223-235.
Erdogan E, Cansever T. Pyogenic brain abscess. Neurosurg Focus. 2008;24(6):E2.
Hagensee ME, Bauwens JE, Kjos B, et al. Brain abscess following marrow transplantation: experience at the Fred Hutchinson Cancer Center, 1984-1992. Clin Infect Dis. 1994;19:402-408.
Hakan T. Management of bacterial brain abscesses. Neurosurg Focus. 2008;24(6):E4.
Infection in Neurosurgery Working Party of the British Society for Antimicrobial Chemotherapy. The rational use of antibiotics in the treatment of brain abscess. Br J Neurosurg. 2000;14:525-530.
Jansson AK, Enbland P, Sjolin J. Efficacy and safety of cefotaxime in combination with metronidazole for empirical treatment of brain abscess in clinical practice: a retrospective study of 66 consecutive cases. Eur J Clin Microbiol Infect Dis. 2004;23:7-14.
Kocherry XG, Hegde T, Sastry KVR, et al. Efficacy of stereotactic aspiration in deep-seated and eloquent-region intracranial pyogenic abscesses. Neurosurg Focus. 2008;24(6):E13.
Lee GYF, Daniel RT, Brophy BP, et al. Surgical treatment of nocardial brain abscesses. Neurosurgery. 2002;51:668-672.
Lee TH, Chang WN, Thung-Ming S, et al. Clinical features and predictive factors of intraventricular rupture in patients who have bacterial brain abscess. J Neurol Neurosurg Psychiatry. 2007;78:303-309.
Lu CH, Chang WN, Lin YC, et al. Bacterial brain abscess: microbiological features, epidemiological trends and therapeutic outcomes. Q J Med. 2002;95:501-509.
Mamelak AN, Mampalam TJ, Obana WG, et al. Improved management of multiple brain abscesses: a combined surgical and medical approach. Neurosurgery. 1995;36:76-86.
Mampalam TJ, Rosenblum ML. Trends in the management of bacterial brain abscesses: a review of 102 cases over 17 years. Neurosurgery. 1988;23:451-458.
Mathisen GE, Johnson JP. Brain abscess. Clin Infect Dis. 1997;25:763-781.
McClelland SIII, Hall WA. Postoperative central nervous system infection: incidence and associated factors in 2111 neurosurgical procedures. Clin Infect Dis. 2007;45:55-59.
Nathoo N, Nadvi SS, Gouws E, et al. Craniotomy improves outcomes for cranial subdural empyemas: computed tomography–era experience with 699 patients. Neurosurgery. 2001;49:872-878.
Nathoo N, Nadvi SS, van Dellen JR, et al. Intracranial subdural empyemas in the era of computed tomography: a review of 699 cases. Neurosurgery. 1999;44:529-535.
Osborn MK, Steinberg JP. Subdural empyema and other suppurative complications of paranasal sinusitis. Lancet Infect Dis. 2007;7:62-67.
Pandey P, Umesh S, Bhat D, et al. Cerebellar abscesses in children: excision or aspiration? J Neurosurg Pediatr. 2008;1:31-34.
Peleg AY, Husain S, Qureshi ZA. Risk factors, clinical characteristics, and outcome of Nocardia infection in organ transplant recipients: a matched case-control study. Clin Infect Dis. 2007;44:1307-1314.
Seydoux C, Francioli P. Bacterial brain abscess: factors influencing mortality and sequelae. Clin Infect Dis. 1992;15:394-401.
Stephanov S. Surgical treatment of brain abscess. Neurosurgery. 1988;22:724-730.
Tseng JH, Steng MY. Brain abscess in 142 patients: factors influencing outcome and mortality. Surg Neurol. 2006;65:557-562.
Walsh TJ, Anaissie EJ, Denning DW, et al. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:327-360.
1 Mathisen GE, Johnson JP. Brain abscess. Clin Infect Dis. 1997;25:763-781.
2 Kastenbauer S, Pfister HW, Wispelwey B, et al. Brain abscess. In: Scheld WM, Whitley RJ, Marra CM, editors. Infections of the Central Nervous System. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2004:479-507.
3 Erdogan E, Cansever T. Pyogenic brain abscess. Neurosurg Focus. 2008;24(6):E2.
4 Heilpern KL, Lorber B. Focal intracranial infections. Infect Dis Clin North Am. 1996;10:879-898.
5 Sheehan JP, Jane JAJr, Ray DKR, et al. Brain abscess in children. Neurosurg Focus. 2008;24(6):E6.
6 McClelland SIII, Hall WA. Postoperative central nervous system infection: incidence and associated factors in 2111 neurosurgical procedures. Clin Infect Dis. 2007;45:55-59.
7 Dashti SR, Barharvahdat H, Spetzler RF, et al. Operative intracranial infection following craniotomy. Neurosurg Focus. 2008;24(6):E10.
8 Kaplan K. Brain abscess. Med Clin North Am. 1985;69:345-360.
9 Xiao F, Tseng MY, Teng LJ, et al. Brain abscess: clinical experience and analysis of prognostic factors. Surg Neurol. 2005;63:442-450.
10 Prasad KN, Mishra AM, Gupta D, et al. Analysis of microbial etiology and mortality in patients with brain abscess. J Infect. 2006;53:221-227.
11 Hakan T, Ceran N, Erdem I, et al. Bacterial brain abscesses: an evaluation of 96 cases. J Infect. 2006;52:359-366.
12 Tseng JH, Steng MY. Brain abscess in 142 patients: factors influencing outcome and mortality. Surg Neurol. 2006;65:557-562.
13 Tonon E, Scotton PG, Gallucci M, et al. Brain abscess: clinical aspects of 100 patients. Int J Infect Dis. 2006;10:103-109.
14 Carpenter J, Stapleton S, Holliman R. Retrospective analysis of 49 cases of brain abscess and review of the literature. Eur J Clin Microbiol Infect Dis. 2007;26:1-11.
15 Yang SY. Brain abscess: a review of 400 cases. J Neurosurg. 1981;55:794-799.
16 Chun CH, Johnson JD, Hofstetter M, et al. Brain abscess: a study of 45 consecutive cases. Medicine. 1986;65:415-431.
17 Lu CH, Chang WN, Lui CC. Strategies for the management of bacterial brain abscess. J Clin Neurosci. 2006;13:979-985.
18 Sennaroglu L, Sozeri B. Otogenic brain abscess: review of 41 cases. Otolaryngol Head Neck Surg. 2000;123:751-755.
19 Corson MA, Postlethwaite KP, Seymour RA. Are dental infections a cause of brain abscess? Case report and review of the literature. Oral Dis. 2001;7:61-65.
20 Vargas J, Hernandez M, Silvestri C, et al. Brain abscess due to Arcanobacterium haemolyticum after dental extraction. Clin Infect Dis. 2006;42:1810-1811.
21 Kuman P, Mehta SK, Deri BI, et al. Pyogenic meningitis and cerebral abscesses after endoscopic injection sclerotherapy. Am J Gastroenterol. 1991;86:1672-1674.
22 Algood L, Boon P, DeVos M, et al. Brain abscess after esophageal dilatation for stenosis. Clin Neurol Neurosurg. 1992;94:169-172.
23 Gaini S, Grand M, Michelsen J. Brain abscess after esophageal dilatation: case report. Infection. 2008;36:71-73.
24 Park SC, Neeches WH. The neurologic complications of congenital heart disease. Neurol Clin. 1993;11:441-462.
25 Takeshita M, Kagawa M, Yato S, et al. Current treatment of brain abscess in patients with congenital cyanotic heart disease. Neurosurgery. 1997;41:1270-1279.
26 Pruitt AA, Rubin RHJ, Karchmer AW, et al. Neurologic complications of bacterial endocarditis. Medicine (Baltimore). 1978;57:329-343.
27 Tunkel AR, Kaye D. Neurologic complications of infective endocarditis. Neurol Clin. 1993;11:419-440.
28 Tunkel AR, Pradhan SK. Central nervous system infections in injection drug users. Infect Dis Clin North Am. 2002;16:589-605.
29 Press OW, Ramsey PG. Central nervous system infections associated with hereditary hemorrhagic telangiectasia. Am J Med. 1984;77:86-92.
30 Gelfand MS, Stephens DS, Howell EI, et al. Brain abscess: association with pulmonary arteriovenous fistula and hereditary hemorrhagic telangiectasia—Report of three cases. Am J Med. 1988;85:718-720.
31 Dupuis-Girod S, Giraud S, Decullier E, et al. Hemorrhagic hereditary telangiectasia (Rendu-Osler disease) and infectious diseases: an underestimated association. Clin Infect Dis. 2007;44:841-845.
32 Sell B, Evans J, Horn D. Brain abscess and hereditary hemorrhagic telangiectasia. South Med J. 2008;101:618-625.
33 Tunkel AR, Turtz AR. Posttraumatic infection of the central nervous system. In: Evans RW, editor. Neurology and Trauma. 2nd ed. Oxford: Oxford University Press; 2006:628-638.
34 Foy P, Schair M. Cerebral abscesses in children after pencil tip injuries. Lancet. 1980;2:662-663.
35 Tay JS, Garland JS. Serious head injuries from lawn darts. Pediatrics. 1987;79:260-263.
36 Martinello RA, Cooney EL. Cerebellar brain abscess associated with tongue piercing. Clin Infect Dis. 2003;36:e32-e34.
37 Saeed MU, Dacuycuy AC, Kennedy DJ. Halo pin insertion–associated brain abscess. Spine. 2007;32:E271-E274.
38 Yee-Guardino S, Danziger-Isakov L, Knouse M, et al. Nosocomially acquired Pseudomonas stutzeri brain abscess in a child: case report and review. Infect Control Hosp Epidemiol. 2006;27:630-632.
39 McGovern PC, Lautenbach E, Brennan PJ, et al. Risk factors for postcraniotomy surgical site infection after 1,3-bis (2-chloroethyl)-1-nitrosourea (Gliadel) wafer placement. Clin Infect Dis. 2003;36:759-765.
40 Rish BL, Careness WF, Dillon JD, et al. Analysis of brain abscess after penetrating craniocerebral injuries in Vietnam. Neurosurgery. 1981;9:535-541.
41 Splavski B, Sisljagic V, Peric LJ, et al. Intracranial infection as a common complication following war missile skull base injury. Injury. 2000;31:233-237.
42 Hecimovic I, Dmitrovic B, Kurbel S, et al. Intracranial infection after missile brain wound: 15 war cases. Zentralblatt Neurochir. 2000;61:95-102.
43 Bhatoe HS. Retained intracranial splinters: a follow up study in survivors of low intensity military conflicts. Neurol India. 2001;49:29-32.
44 Su TM, Lin YC, Lu CH, et al. Streptococcal brain abscess: analysis of clinical features in 20 patients. Surg Neurol. 2001;56:189-194.
45 Lu CH, Chang WN, Lin YC, et al. Bacterial brain abscess: microbiological features, epidemiological trends and therapeutic outcomes. Q J Med. 2002;95:501-509.
46 Rau CS, Chang WN, Lin YC, et al. Brain abscess caused by aerobic gram-negative bacilli: clinical features and therapeutic outcomes. Clin Neurol Neurosurg. 2002;105:60-65.
47 De Louvois J, Gortvai P, Hurley R. Bacteriology of abscesses of the central nervous system: a multicentre prospective study. Br Med J. 1977;2:981-984.
48 De Louvois J. The bacteriology and chemotherapy of brain abscess. J Antimicrob Chemother. 1978;4:395-413.
49 Mampalam TJ, Rosenblum ML. Trends in the management of bacterial brain abscesses: a review of 102 cases over 17 years. Neurosurgery. 1988;23:451-458.
50 Eckburg PB, Montoya JG, Vosti KL. Brain abscess due to Listeria monocytogenes: five cases and a review of the literature. Medicine (Baltimore). 2001;80:223-235.
51 Clauss HE, Lorber B. Central nervous system infection with Listeria monocytogenes. Curr Infect Dis Rep. 2008;10:300-306.
52 Kuruvath S, Basu S, Elwitigala JP, et al. Salmonella enteritidis brain abscess in a sickle cell disease patient: case report and review of the literature. Int J Infect Dis. 2008;12:298-302.
53 Lerner PI. Nocardiosis. Clin Infect Dis. 1996;22:891-905.
54 Peleg AY, Husain S, Qureshi ZA. Risk factors, clinical characteristics, and outcome of Nocardia infection in organ transplant recipients: a matched case-control study. Clin Infect Dis. 2007;44:1307-1314.
55 Grigoriadis E, Gold WL. Pyogenic brain abscess caused by Streptococcus pneumoniae: case report and review. Clin Infect Dis. 1997;25:1108-1112.
56 Smego RAJr, Foglia G. Actinomycosis. Clin Infect Dis. 1998;26:1255-1263.
57 Kline MW. Citrobacter meningitis and brain abscess in infancy: epidemiology, pathogenesis, and treatment. J Pediatr. 1988;113:430-434.
58 Morgan MG, Stuart C, Leonard AT, et al. Citrobacter diversus brain abscess: case reports and molecular epidemiology. J Med Microbiol. 1992;36:273-278.
59 Kumar R, Pandey CK, Bose N, et al. Tuberculous brain abscess: clinical presentation, pathophysiology and treatment (in children). Childs Nerv Syst. 2002;18:118-123.
60 Farrar DJ, Flanigan TP, Gordon NM, et al. Tuberculous brain abscess in a patient with HIV infection: case report and review. Am J Med. 1997;102:297-301.
61 Monno L, Carbonara S, Costa D, et al. Cerebral lesions in two patients with AIDS: the possible role of Mycobacterium kansasii. Clin Infect Dis. 1996;22:1130-1131.
62 Zugar A. Tuberculosis. In: Scheld WM, Whitley RJ, Marra CM, editors. Infections of the Central Nervous System. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2004:441-459.
63 Salaki JS, Louria DB, Chmel H. Fungal and yeast infections of the central nervous system: a clinical review. Medicine (Baltimore). 1984;63:108-132.
64 Hagensee ME, Bauwens JE, Kjos B, et al. Brain abscess following marrow transplantation: experience at the Fred Hutchinson Cancer Center, 1984-1992. Clin Infect Dis. 1994;19:402-408.
65 Cortez KJ, Walsh TJ. Space-occupying fungal lesions. In: Scheld WM, Whitley RJ, Marra CM, editors. Infections of the Central Nervous System. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2004:713-734.
66 Walsh TJ, Hier DB, Caplan LP. Fungal infections of the central nervous system: comparative analysis of risk factors and clinical signs in 57 patients. Neurology. 1985;35:1654-1657.
67 Denning DW. Invasive aspergillosis. Clin Infect Dis. 1998;26:781-805.
68 Ruhnke M, Kofla G, Otto K, et al. CNS aspergillosis. Recognition, diagnosis and management. CNS Drugs. 2007;21:659-676.
69 Sugar AM. Mucormycosis. Clin Infect Dis. 1992;14:S126-S129.
70 Stave GM, Heimberger T, Kerkering TM. Zygomycosis of the basal ganglia in intravenous drug users. Am J Med. 1989;86:115-117.
71 Travis LB, Roberts GD, Wilson WR. Clinical significance of Pseudallescheria boydii: a review of ten years’ experience. Mayo Clin Proc. 1985;60:531-537.
72 Berenguer J, Diaz-Mediavilla J, Urra D, et al. Central nervous system infection caused by Pseudallescheria boydii. Rev Infect Dis. 1989;11:890-896.
73 Dworzack DL, Clark RB, Borkowski WJ, et al. Pseudallescheria boydii brain abscess: association with near-drowning and efficacy of high-dose, prolonged miconazole therapy in patients with multiple abscesses. Medicine (Baltimore). 1989;68:218-224.
74 Kershaw P, Freeman R, Templeton D, et al. Pseudallescheria boydii infection of the central nervous system. Arch Neurol. 1990;47:468-472.
75 Lamaris G, Chamilos G, Lewis RE, et al. Scedosporium infection in a tertiary care cancer center: a review of 25 cases from 1989-2006. Clin Infect Dis. 2006;43:1580-1584.
76 Tunkel AR, Scheld WM. Pathogenesis and pathophysiology of bacterial infections. In: Scheld WM, Whitley RJ, Durack DT, editors. Infections of the Central Nervous System. 2nd ed. Philadelphia: Lippincott-Raven; 1997:297-312.
77 Winn HR, Rodeheaver G, Moore P, Wheeler CB. A new model for experimental brain abscess in rats and mice. Surg Forum. 1978;29:500-502.
78 Winn HR, Mendes M, Moore P, et al. Production of experimental brain abscess in the rat. J Neurosurg. 1979;51:685-690.
79 Flaris NA, Hickey WF. Development and characterization of an experimental model of brain abscess in the rat. Am J Pathol. 1992;141:1299-1307.
80 Mendes M, Moore P, Wheeler CB, et al. Susceptibility of brain and skin to bacterial challenge. J Neurosurg. 1980;52:772-775.
81 Costello GT, Heppe R, Winn HR, et al. Susceptibility of brain to aerobic, anaerobic, and fungal organisms. Infect Immun. 1983;41:535-539.
82 Onderonk AB, Kasper DL, Cisneros RL, et al. The capsular polysaccharide of Bacteroides fragilis as a virulence factor: comparison of the pathogenic potential of encapsulated and unencapsulated strains. J Infect Dis. 1977;136:82-89.
83 Onderonk AB, Kasper DL, Mansheim BJ, et al. Experimental animal models for anaerobic infections. Rev Infect Dis. 1979;1:291-301.
84 Britt RH, Enzmann DR, Placone RCJr, et al. Experimental anaerobic brain abscess: computerized tomographic and neuropathological correlations. J Neurosurg. 1984;60:1148-1159.
85 Kielian T, Cheung A, Hickey WF. Diminished virulence of an alpha-toxin mutant of Staphylococcus aureus in experimental brain abscess. Infect Immun. 2001;69:6902-6911.
86 Kline MW, Kaplan SL, Hawkins EP, et al. Pathogenesis of brain abscess formation in an infant rat model of Citrobacter diversus bacteremia and meningitis. J Infect Dis. 1988;157:106-112.
87 Kline MW, Mason EOJr, Kaplan SL. Characterization of Citrobacter diversus strains causing neonatal meningitis. J Infect Dis. 1988;157:101-105.
88 Britt RH, Enzmann DR, Yeager AS. Neuropathological and computerized tomographic findings in experimental brain abscess. J Neurosurg. 1981;55:590-603.
89 Enzmann DR, Britt RR, Obana WG, et al. Experimental Staphylococcus aureus brain abscess. Am J Neuroradiol. 1986;7:395-402.
90 Neuwelt EA, Lawrence MS, Blank NK. Effect of gentamicin and dexamethasone on the natural history of the rat Escherichia coli brain abscess model with histopathological correlation. Neurosurgery. 1984;15:475-483.
91 Casciato DA, Rosenblatt JE, Goldberg LS, et al. In vitro interaction of Bacteroides fragilis with polymorphonuclear leukocytes and serum factors. Infect Immun. 1975;11:337-342.
92 Ingham HR, Sisson PR, Middleton RL, et al. Phagocytosis and killing of bacteria in aerobic and anaerobic conditions. Med Microbiol. 1981;14:391-399.
93 Adamu SA, Sperry JF. Polymorphonuclear neutrophil chemotaxis induced and inhibited by Bacteroides spp. Infect Immun. 1981;33:806-810.
94 Kielian T, Hickey WF. Proinflammatory cytokine, chemokine, and cellular adhesion molecule expression during the acute phase of experimental brain abscess development. Am J Pathol. 2000;157:647-658.
95 Kielian T, Barry B, Hickey WF. CXC chemokine receptor-2 ligands are required for neutrophil-mediated host defense in experimental brain abscesses. J Immunol. 2001;166:4634-4643.
96 Kielian T, Esen N, Bearden ED. Toll-like receptor 2 (TLR2) is pivotal for recognition of Staphylococcus aureus peptidoglycan but not intact bacteria by microglia. Glia. 2005;49:567-576.
97 Kielian T, Esen N, Liu S, et al. Minocycline modulates neuroinflammation independently of its antimicrobial activity in Staphylococcus aureus–induced brain abscess. Am J Pathol. 2007;171:1199-1214.
98 Long WD, Meacham WF. Experimental method for producing brain abscess in dogs with evaluation of the effect of dexamethasone and antibiotic therapy on the pathogenesis of intracerebral abscesses. Surg Forum. 1968;19:437-438.
99 Quartey GRC, Johnston JA, Rozdilsky B. Decadron in the treatment of cerebral abscess: an experimental study. J Neurosurg. 1976;45:301-310.
100 Yildizhan A, Pasaoglu A, Kandemir B. Effect of dexamethasone on various stages of experimental brain abscess. Acta Neurochir. 1989;96:141-148.
101 Obana WG, Britt RH, Placone RC, et al. Experimental brain abscess development in the chronically immunosuppressed host: computerized tomographic and neuropathologic correlations. J Neurosurg. 1986;65:382-391.
102 Lee TH, Chang WN, Thung-Ming S, et al. Clinical features and predictive factors of intraventricular rupture in patients who have bacterial brain abscess. J Neurol Neurosurg Psychiatry. 2007;78:303-309.
103 Zeidman SM, Geisler FH, Olivi A. Intraventricular rupture of a purulent brain abscess: case report. Neurosurgery. 1995;36:189-193.
104 Takeshita M, Kawamata T, Izawa M, et al. Prodromal signs and clinical factors influencing outcome in patients with intraventricular rupture of purulent brain abscess. Neurosurgery. 2001;48:310-317.
105 Dake MD, McMurdo SK, Rosenblum ML, et al. Pyogenic abscess of the medulla oblongata. Neurosurgery. 1986;18:370-372.
106 Carpenter JL. Brain stem abscesses: cure with medical therapy, case report, and review. Clin Infect Dis. 1994;18:219-226.
107 Mamelak AN, Obana WG, Flaherty JF, et al. Nocardial brain abscess: treatment strategies and factors influencing outcome. Neurosurgery. 1994;35:622-631.
108 Zimmerman RA, Wong AM, Girard N. Imaging of intracranial infections. In: Scheld WM, Whitley RJ, Marra CM, editors. Infections of the Central Nervous System. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2004:31-55.
109 Kapsalaki EZ, Gotsis ED, Fountas KN. The role of proton magnetic resonance spectroscopy in the diagnosis and categorization of cerebral abscesses. Neurosurg Focus. 2008;24(6):E7.
110 Osenbach RK, Loftus CM. Diagnosis and management of brain abscess. Neurosurg Clin North Am. 1992;3:403-420.
111 Miller ES, Psrilal SD, Uttley D. CT scanning in the management of intracranial abscess: a review of 100 cases. Br J Neurosurg. 1988;2:439-446.
112 Zimmerman RD, Weingarten K. Neuroimaging of cerebral abscesses. Neuroimaging Clin North Am. 1991;1:1-16.
113 Petti CA, Simmon KE, Bender J, et al. Culture-negative intracerebral abscesses in children and adolescents from Streptococcus anginosus group infection: a case series. Clin Infect Dis. 2008;46:1578-1580.
114 Mamelak AN, Mampalam TJ, Obana WG, et al. Improved management of multiple brain abscesses: a combined surgical and medical approach. Neurosurgery. 1995;36:76-86.
115 Hakan T. Management of bacterial brain abscesses. Neurosurg Focus. 2008;24(6):E4.
116 Alderson D, Strong AJ, Ingham MR, et al. Fifteen year review of the mortality of brain abscess. Neurosurgery. 1981;8:1-6.
117 Levy RM, Gutin PH, Baskin DS, et al. Vancomycin penetration of a brain abscess: case report and review of the literature. Neurosurgery. 1986;18:633-636.
118 De Louvois J. Antimicrobial chemotherapy in the treatment of brain abscess. J Antimicrob Chemother. 1983;11:205-207.
119 Black P, Graybill JR, Charache P. Penetration of brain abscess by systemically administered antibiotics. J Neurosurg. 1973;38:705-709.
120 De Louvois J, Gortvai P, Hurley R. Antibiotic treatment of abscesses of the central nervous system. Br Med J. 1977;2:985-987.
121 Green HT, O’Donoghue MAT, Shaw MDM, et al. Penetration of ceftazidime into intracranial abscesses. J Antimicrob Chemother. 1989;24:431-436.
122 Skrap M, Melatini A, Vassallo A, et al. Stereotactic aspiration and drainage of brain abscesses: experience with 9 cases. Minim Invasive Neurosurg. 1996;39:108-112.
123 Sjolin J, Lilja A, Eriksson N, et al. Treatment of brain abscess with cefotaxime and metronidazole: prospective study on 15 consecutive patients. Clin Infect Dis. 1993;17:857-863.
124 Aseni V, Carton JA, Maradona JA, et al. Imipenem therapy of brain abscesses. Eur J Clin Microbiol Infect Dis. 1996;15:653-657.
125 Aseni V, Carton JA, Maradona JA, et al. Therapy of brain abscess with imipenem—A safe therapeutic choice? J Antimicrob Chemother. 1996;37:200-203.
126 Meis JFGM, Groot-Loonen J, Hoogkamp-Korstanje JAA. A brain abscess due to multiply-resistant Enterobacter cloacae successfully treated with meropenem. Clin Infect Dis. 1995;20:1567.
127 Wessalowksi R, Thomas L, Kivit J, et al. Multiple brain abscesses caused by Salmonella enteritidis in a neonate: successful treatment with ciprofloxacin. Pediatr Infect Dis J. 1993;12:683-688.
128 Stephanov S. Surgical treatment of brain abscess. Neurosurgery. 1988;22:724-730.
129 Dyste GN, Hitchon PW, Menezes AH, et al. Stereotaxic surgery in the treatment of multiple brain abscesses. J Neurosurg. 1988;69:188-194.
130 Itakura T, Yokote H, Ozaki F, et al. Stereotactic operation for brain abscess. Surg Neurol. 1987;28:196-200.
131 Shahzadi S, Lozano AM, Bernstein M, et al. Stereotactic management of bacterial brain abscesses. Can J Neurol Sci. 1996;23:34-39.
132 Laborde G, Klimek L, Harders A, Gilsbach J. Frameless stereotactic drainage of intracranial abscesses. Surg Neurol. 1993;40:16-21.
133 Kocherry XG, Hegde T, Sastry KVR, et al. Efficacy of stereotactic aspiration in deep-seated and eloquent-region intracranial pyogenic abscesses. Neurosurg Focus. 2008;24(6):E13.
134 Strowitzki M, Schwerdtfeger K, Steudel WI. Ultrasound-guided aspiration of brain abscess through a single burr hole. Minim Invasive Neurosurg. 2001;44:135-140.
135 Frazier JL, Ahn ES, Jallo GI. Management of brain abscesses in children. Neurosurg Focus. 2008;24(6):E8.
136 Pandey P, Umesh S, Bhat D, et al. Cerebellar abscesses in children: excision or aspiration? J Neurosurg Pediatrics. 2008;1:31-34.
137 Rosenblum ML, Hoff JT, Norman D, et al. Nonoperative treatment of brain abscesses in selected high-risk patients. J Neurosurg. 1980;52:217-225.
138 Boom WH, Tuazon CU. Successful treatment of multiple brain abscesses with antibiotics alone. Rev Infect Dis. 1985;7:189-199.
139 Fulgham JR, Wijdicks EFM, Wright AJ. Cure of a solitary brainstem abscess with antibiotic therapy: case report. Neurology. 1996;46:1451-1454.
140 Cavusoglu H, Kaya RA, Turkmenoglu ON, et al. Brain abscess: analysis of results in a series of 51 patients with a combined surgical and medical approach during an 11-year period. Neurosurg Focus. 2008;24(6):E9.
141 Infection in Neurosurgery Working Party of the British Society for Antimicrobial Chemotherapy. The rational use of antibiotics in the treatment of brain abscess. Br J Neurosurg. 2000;14:525-530.
142 Skoutelis AT, Gogos CA, Maraziotis TE, et al. Management of brain abscesses with sequential intravenous/oral antibiotic therapy. Eur J Clin Microbiol Infect Dis. 2000;19:332-335.
143 Jansson AK, Enbland P, Sjolin J. Efficacy and safety of cefotaxime in combination with metronidazole for empirical treatment of brain abscess in clinical practice: a retrospective study of 66 consecutive cases. Eur J Clin Microbiol Infect Dis. 2004;23:7-14.
144 Wallace RJJr, Septimus EJ, Williams TWJr, et al. Use of trimethoprim-sulfamethoxazole for treatment of infections due to Nocardia. Rev Infect Dis. 1982;4:315-325.
145 Smego RAJr, Moeller MB, Gallis HA. Trimethoprim-sulfamethoxazole therapy for Nocardia infections. Arch Intern Med. 1983;143:711-718.
146 Overkamp D, Waldmann B, Lins T, et al. Successful treatment of brain abscess caused by Nocardia in an immunocompromised patient after failure of co-trimoxazole. Infection. 1992;20:365-366.
147 Wallace RJJr, Steele LC, Sumter G, et al. Antimicrobial susceptibility patterns of Nocardia asteroides. Antimicrob Agents Chemother. 1988;32:1776-1779.
148 Berkey P, Moore D, Rolston K. In vitro susceptibilities of Nocardia species to newer antimicrobial agents. Antimicrob Agents Chemother. 1988;32:1078-1079.
149 Brown-Elliott BA, Warad SC, Crist CJ, et al. In vitro activities of linezolid against multiple Nocardia species. Antimicrob Agents Chemother. 2001;45:1295-1297.
150 Moylett EH, Pacheco SE, Brown-Elliott BA, et al. Clinical experience with linezolid for the treatment of Nocardia infection. Clin Infect Dis. 2003;36:313-318.
151 Krone A, Schaal KP, Brawanski A, et al. Nocardial cerebral abscess cured with imipenem/amikacin and enucleation. Neurosurg Rev. 1989;12:333-340.
152 Kim J, Minamoto GY, Hoy CD, et al. Presumptive cerebral Nocardia asteroides infection in AIDS: treatment with ceftriaxone and minocycline. Am J Med. 1991;90:656-658.
153 Garlando F, Bodmer T, Lee C, et al. Successful treatment of disseminated nocardiosis complicated by cerebral abscess with ceftriaxone and amikacin: case report. Clin Infect Dis. 1992;15:1039-1040.
154 Jansen C, Frenay HM, Vandertop WP, et al. Intracerebral Nocardia asteroides abscess treated by neurosurgical aspiration and combined therapy with sulfadiazine and cefotaxime. Clin Neurol Neurosurg. 1991;93:253-255.
155 Fleetwood IG, Embil JM, Ross IB. Nocardia asteroides cerebral abscess in immunocompetent hosts: report of three cases and review of surgical recommendations. Surg Neurol. 2000;53:605-610.
156 Fihman V, Bercot B, Mateo J, et al. First successful treatment of Nocardia farcinica brain abscess with moxifloxacin. J Infect. 2006;52:e99-e102.
157 Fellows GA, Kalsi PS, Martin AJ. Nocardia farcinica brain abscess in a patient without immunocompromise. Br J Neurosurg. 2007;21:301.
158 Kandasamy J, Iqbal HJ, Cooke RPD, et al. Primary Nocardia farcinica brain abscess with secondary meningitis and ventriculitis in an immunocompetent patient, successfully treated with moxifloxacin. Acta Neurochir (Wien). 2008;150:505-506.
159 Lee GYF, Daniel RT, Brophy BP, et al. Surgical treatment of nocardial brain abscesses. Neurosurgery. 2002;51:668-672.
160 Valarezo J, Cohen JE, Valarezo L, et al. Nocardial cerebral abscess: report of three cases and review of the current neurosurgical management. Neurol Res. 2003;25:27-30.
161 Filice GA, Simpson GL. Management of Nocardia infections. In: Remington JS, Swartz MN, editors. Current Clinical Topics in Infectious Diseases. New York: McGraw-Hill; 1984:49-64.
162 Kamitsuka MD, Nugent NA, Conrad PD, et al. Candida albicans brain abscesses in a premature infant treated with amphotericin B, flucytosine and fluconazole. Pediatr Infect Dis J. 1995;14:329-331.
163 Walsh TJ, Anaissie EJ, Denning DW, et al. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:327-360.
164 Denning DW, Ribaud P, Milpied N, et al. Efficacy and safety of voriconazole in the treatment of acute invasive aspergillosis. Clin Infect Dis. 2002;34:563-571.
165 Denning DW, Stevens DA. Antifungal and surgical treatment of invasive aspergillosis: review of 2,121 published cases. Rev Infect Dis. 1990;12:1147-1201.
166 Sanchez C, Mauri E, Dalmau D, et al. Treatment of cerebral aspergillosis with itraconazole: do high doses improve the prognosis? Clin Infect Dis. 1995;21:1485-1487.
167 Saulsbury FT. Successful treatment of Aspergillus brain abscess with itraconazole and interferon-gamma in a patient with chronic granulomatous disease. Clin Infect Dis. 2001;32:e137-139.
168 Gubler C, Wildi SM, Imhof A, et al. Disseminated invasive aspergillosis with cerebral involvement treated with caspofungin and voriconazole. Infection. 2007;35:364-366.
169 Ehrmann S, Bastides F, Gissot V, et al. Cerebral aspergillosis in the critically ill: two cases of successful medical treatment. Intensive Care Med. 2005;31:738-742.
170 Damaj G, Ivanov V, Le Brigand B, et al. Rapid improvement of disseminated aspergillosis with caspofungin/voriconazole combination in an adult leukemic patient. Ann Hematol. 2004;83:390-393.
171 Goodman ML, Coffey RJ. Stereotactic drainage of Aspergillus brain abscess with long-term survival: case report and review. Neurosurgery. 1989;24:96-99.
172 Ochi JW, Harris JP, Feldman JI, et al. Rhinocerebral mucormycosis: results of aggressive surgical debridement and amphotericin B. Laryngoscope. 1988;98:1339-1342.
173 Talmi YP, Goldschmied-Reouven A, Bakon M, et al. Rhino-orbital and rhino-orbital-cerebral mucormycosis. Otolaryngol Head Neck Surg. 2002;127:22-31.
174 van Burik AH, Hare RS, Solomon HF, et al. Posaconazole is effective as salvage therapy in zygomycosis: a retrospective summary of 91 cases. Clin Infect Dis. 2006;42:e61-e65.
175 Couch L, Theilen F, Mader JT. Rhinocerebral mucormycosis with cerebral extension successfully treated with adjunctive hyperbaric oxygen therapy. Arch Otolaryngol Head Neck Surg. 1988;114:791-794.
176 Ferguson BJ, Mitchell TG, Moon R, et al. Adjunctive hyperbaric oxygen for treatment of rhinocerebral mucormycosis. Rev Infect Dis. 1988;10:551-559.
177 Nesky MA, McDougal EC, Peacock JEJr. Pseudallescheria boydii brain abscess successfully treated with voriconazole and surgical drainage: case report and literature review of central nervous system pseudallescheriasis. Clin Infect Dis. 2000;31:673-677.
178 Troke P, Aguirrebengoa K, Arteaga C, et al. Treatment of scedosporiosis with voriconazole: clinical experience with 107 patients. Antimicrob Agents Chemother. 2008;52:1743-1750.
179 Bhat SV, Paterson DL, Rinaldi MG, et al. Scedosporium prolificans brain abscess in a patient with chronic granulomatous disease: successful combination therapy with voriconazole and terbinafine. Scand J Infect Dis. 2007;39:87-90.
180 Mellinghoff IK, Winston DJ, Mukwaya G, et al. Treatment of Scedosporium apiospermum brain abscess with posaconazole. Clin Infect Dis. 2002;34:1648-1650.
181 Rosenblum ML, Joff JT, Norman D, et al. Decreased mortality from brain abscesses since advent of computerized tomography. J Neurosurg. 1978;49:658-668.
182 Seydoux C, Francioli P. Bacterial brain abscess: factors influencing mortality and sequelae. Clin Infect Dis. 1992;15:394-401.
183 Silverberg AL, DiNubile MJ. Subdural empyema and cranial epidural abscess. Med Clin North Am. 1985;69:361-374.
184 Coonrad JD, Dans PE. Subdural empyema. Am J Med. 1972;53:85-91.
185 Kaufman DM, Miller MH, Steigbigel NH. Subdural empyema: analysis of 17 recent cases and review of the literature. Medicine (Baltimore). 1975;54:485-498.
186 Dill SR, Cobbs CG, McDonald CK. Subdural empyema: analysis of 32 cases and review. Clin Infect Dis. 1995;20:372-386.
187 Nathoo N, Nadvi SS, van Dellen JR, et al. Intracranial subdural empyemas in the era of computed tomography: a review of 699 cases. Neurosurgery. 1999;44:529-535.
188 Kaufman DM, Litman N, Miller MH. Sinusitis-induced subdural empyema. Neurology. 1983;33:123-132.
189 Osborn MK, Steinberg JP. Subdural empyema and other suppurative complications of paranasal sinusitis. Lancet Infect Dis. 2007;7:62-67.
190 Nathoo N, Nadvi SS, van Dellen JR. Traumatic cranial empyemas: a review of 55 patients. Br J Neurosurg. 2000;14:326-330.
191 Wu TJ, Chiu NC, Huang FY. Subdural empyema in children—20-year experience in a medical center. J Microbiol Immunol Infect. 2008;41:62-67.
192 Yoshikawa TT, Chow AW, Guze LB. Role of anaerobic bacteria in subdural empyema: report of four cases and review of 327 cases from the English literature. Am J Med. 1975;58:99-104.
193 Brook I. Aerobic and anaerobic bacteriology of intracranial abscesses. Pediatr Neurol. 1992;8:210-214.
194 Jallo GI, Koslow M, Hanna BA, et al. Propionibacterium as a cause of postneurosurgical infection in patients with dural allografts: report of three cases. Neurosurgery. 1999;44:1138-1141.
195 Chu RM, Tummala RP, Hall WA. Focal intracranial infections due to Propionibacterium acnes: report of three cases. Neurosurgery. 2001;49:717-720.
196 Barkhoudarian G, Hoff JT, Thompson BG. Propionibacterium infection associated with bovine pericardium dural allograft. J Neurosurg. 2005;103:182-185.
197 Kastenbauer S, Pfister HW, Scheld WM. Epidural abscess. In: Scheld WM, Whitley RJ, Marra CM, editors. Infections of the Central Nervous System. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2004:509-521.
198 Hartman BJ, Helfgott DC, Weingarten K. Subdural empyema and suppurative intracranial phlebitis. In: Scheld WM, Whitley RJ, Marra CM, editors. Infections of the Central Nervous System. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2004:523-535.
199 Levy ML, Wieder BH, Schneider J, et al. Subdural empyema of the cervical spine: clinicopathologic correlates and magnetic resonance imaging. J Neurosurg. 1993;79:929-935.
200 Bockova J, Rigamonti D. Intracranial empyema. Pediatr Infect Dis J. 2000;19:735-737.
201 Yilmaz N, Kiymaz N, Yilmaz C, et al. Surgical treatment outcome of subdural empyema. Pediatr Neurosurg. 2006;42:293-298.
202 Nathoo N, Nadvi SS, Gouws E, et al. Craniotomy improves outcomes for cranial subdural empyemas: computed tomography–era experience with 699 patients. Neurosurgery. 2001;49:872-878.
203 Mauser HW, van Houwelingen HC, Tulleken CA. Factors affecting the outcome of subdural empyema. J Neurol Neurosurg Psychiatry. 1987;50:1136-1141.
204 Hlavin ML, Kaminski HJ, Fenstermaker RA, White RJ. Intracranial suppuration: a modern decade of postoperative subdural empyema and epidural abscess. Neurosurgery. 1994;34:974-980.